Abstract
Although a wide assortment of agents is currently available for the treatment of depression, this disorder remains poorly managed in a large proportion of patients. Traditional antidepressant treatments target the biogenic amine systems. However, a growing body of evidence is implicating the involvement of neuropeptides in depression, especially the neurokinin substance P. This study evaluated the effects of selective antagonists of the tachykinin NK1, NK2, and NK3 receptors in the forced swim test, a commonly used screen for antidepressants. Rats were given CP-96,345 (2S, 3S)-cis-2-(diphenylmethyl)-N-[(2-methoxyphenyl)-methyl]-1-azabicyclo[2.2.2]octan-3-amine, SR 48968 (S)-N-methyl-N[4-(4-acetylamino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)-butyl]benzamide, or SR 142801 (S)-(N)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl) piperidin-3-yl) propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide, antagonists of the NK1, NK2, and NK3 receptors, respectively, at doses of 2.5, 5, and 10 mg/kg, intraperitoneally (i.p.). The time of immobility during the forced swim test was used as an indicator of antidepressant activity of the antagonists. All antagonists decreased immobility times. CP-96,345 and SR 142801 showed dose-related effects; SR 48968 had its maximum effect at 2.5 mg/kg. The magnitude of the effects of the neurokinin receptor antagonists was approximately the same as that of amitriptyline and desipramine, two traditional antidepressants, both given at 10 mg/kg, i.p. This study provides comparative data on the relative effectiveness of NK1, NK2, and NK3 receptor antagonists in this screen for antidepressant drug activity.
Keywords: Forced swim test; Depression; Neurokinin receptor antagonist; CP-96,345; SR 48968; SR 142801
1. Introduction
Depression is a pervasive mood disorder with current estimates of lifetime prevalence ranging from 5% to 20%, and a mortality rate of about 15% due to suicide (Baby et al., 1999). Many patients have benefited from traditional antidepressant therapies, which typically involve altering levels of biogenic amines [serotonin (5-HT), noradrenaline, and dopamine]. However, a subset of depressive patients does not show adequate improvement, or experiences intolerable side effects, with conventional antidepressants (Maubach et al., 1999). It has recently been shown that the neuropeptide, substance P, may be involved in the neuropathology of this disorder and could possibly open new avenues for alternative therapeutic interventions.
Substance P is a tachykinin that is widely distributed in the central nervous system and has been implicated in various conditions including pain (Henry, 1976) and control of autonomic output (Backman and Henry, 1984). It is also found in peripheral tissues where it has been implicated in inflammatory bowel disease and asthma (Quartara and Maggi, 1998). Substance P exerts its effects mainly through binding to the G protein-coupled tachykinin NK1 receptor with high affinity, but it can also activate the tachykinin NK2 and NK3 receptors (reviewed in Harrison and Geppetti, 2001).
Evidence suggesting or supporting a role for substance P in mood disorders includes increased serum substance P levels in major depression (Bondy et al., 2003), elevated levels of substance P in the cerebrospinal fluid of depressed patients (Rimón et al., 1984), and an antidepressant-induced decrease in substance P levels in the rat brain (Shirayama et al., 1996). Other lines of evidence that may implicate substance P in depression include the expression of the NK1 receptor on tyrosine hydroxylase-positive cell bodies in the locus coeruleus of the rat (Hahn and Bannon, 1998), and the coexistence of substance P and serotonin in the dorsal raphe nuclei of humans (Sergeyev et al., 1999). The potential therapeutic benefit of pharmacological agents targeting substance P neurotransmission was most emphatically shown by Kramer et al. (1998), who found that administration of the NK1 receptor antagonist MK-869 (2-(R)-(1-(R)-3,5-bis(trifluoromethyl)phenylehoxy)-3-(S)-(4-fluoro)phenyl-4-(3-oxo-1,2,4-triazol-5-yl)methylmorpholine) produces a strong antidepressant effect in humans with moderate to severe major depressive disorder.
To our knowledge, the possible antidepressant effect of selective NK2 and NK3 receptor antagonists has not been thoroughly investigated. Accordingly, the present experiments assessed the effects of selective NK1, NK2, and NK3 receptor antagonists in the forced swim test. Originally described by Porsolt et al. (1977), the forced swim test is a behavioural test that has been used for over 20 years as a screen for potential antidepressant drugs. In this test, a rat is placed into a tank of water from which escape is impossible; following a period of active swimming (presumably reflecting the attempt to escape), the rat adopts a characteristic immobile posture where it simply tries to maintain its head above water (Porsolt et al., 1977). Most clinically effective antidepressants have been shown to reduce the overall amount of time in which the rat remains immobile, and, in general, this effect is not observed with most other psychoactive agents that do not possess antidepressant activity (reviewed in Borsini and Meli, 1988).
In addition to assessing the effects of NK1, NK2, or NK3 receptor antagonists, two clinically effective tricyclic antidepressants, amitriptyline and desipramine, were used as positive controls. These drugs inhibit reuptake of neurotransmitters (amitriptyline being a serotonin and noradrenaline reuptake inhibitor, and desipramine being a noradrenaline reuptake inhibitor) at the presynaptic nerve terminal, increasing their availability in the synapse (Mongeau et al., 1997). Several studies have shown that the forced swim test is sensitive to acute administration of both of these antidepressants (Kitada et al., 1981; Miyauchi et al., 1981; Porsolt et al., 1977; Satoh et al., 1984). The effects of CP-96,345 (2S, 3S)-cis-2-(diphenyl-methyl)-N -[(2-methoxyphenyl)-methyl]-1-azabicy-clo[2.2.2]octan-3-amine, a nonpeptide NK1 receptor antagonist (Snider et al., 1991); SR 48968 (S)-N-methyl-N[4-(4-acetylamino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)-butyl]benzamide, an NK2 receptor antagonist; and SR 142801 (S)-(N)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl) piperidin-3-yl) propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide, an NK3 receptor antagonist, were compared with those of the positive controls. In addition, the effects of the three neurokinin receptor antagonists were compared to the effects of lorazepam, an agent with anxiolytic but not antidepressant properties. Thus, the drug, lorazapam, was used as a negative control for these experiments.
2. Materials and methods
2.1. Animals
Male Sprague–Dawley rats weighing 300–400 g (Charles River, Quebec, Canada) were housed in pairs and maintained on a 12 h:12 h light/dark cycle, with food and water available ad libitum. All procedures complied with the Guidelines for the Care and Use of Experimental Animals, Volumes I and II, of the Canadian Council on Animal Care, and were approved by the Animal Care Committee of McGill University.
2.2. Drugs
All drugs were administered intraperitoneally (i.p.) in a volume of 1 ml/kg body weight. Except where indicated, drugs were dissolved in saline. Amitriptyline HCl and desipramine HCl (Sigma, St. Louis, MO, USA) were each given at a dose of 10 mg/kg. CP-96,345 (a gift from Pfizer Central Research, Groton, CT, USA) was given at doses of 2.5, 5, or 10 mg/kg, whereas the inactive enantiomer CP-96,344 (a gift from Pfizer Central Research) was given at a dose of 5 mg/kg. SR 48968 and SR 142801 (gifts from Sanofi-Synthelabo, Montpellier, France) were each given at doses of 2.5, 5, or 10 mg/kg, and were dissolved in 10% and 25% dimethyl sulfoxide (DMSO; Sigma) in saline, respectively. Lorazepam (Wyeth-Ayerst, Montreal, Quebec, Canada) was given at a dose of 0.5 mg/kg. The control group received no treatment or saline, while the vehicle group received 25% DMSO in saline. All injections, with the exception of lorazepam, were administered 24, 5, and 1 h prior to the 5-min test on day 2 (see Section 2.3). Lorazepam was given once daily for 5 days before the 5-min test (to induce tolerance to the sedative effects of this compound; File, 1981), and, on the day of the test, it was given at 5 and 1 h before the start of the test.
2.3. Forced swim test procedure
The test performed is based on the original method described by Porsolt et al. (1977, 1978). On day 1 (conditioning, pretest session), rats were individually placed in a clear Plexiglass cylinder (29 cm in diameter and 50 cm in height) containing 30 cm of water (25+0.5 °C) and left to swim for 15 min. No injections were administered prior to this session, with the exception of the lorazepam-treated group. Upon removal from the water, rats were towel-dried, administered the first of the three injections, placed under a heating lamp for 15–30 min, and finally returned to their home cage. Twenty-four hours later, the rats were tested under the same conditions for 5 min (test session). Rats were judged to be immobile when neither hind leg was moving, and the rat was slightly hunched forward. All rats were observed for mobility before and following the swim test, on both days.
2.4. Statistics
Data were analyzed using a one-way between-subjects analysis of variance (ANOVA) with Tukey’s HSD test for post-hoc comparisons.
3. Results
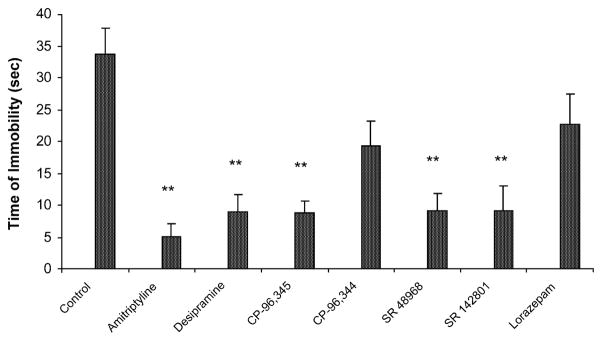
There was no overt difference in locomotor behaviour before or after either swim in any animal. The effects of CP-96,345 (tachykinin NK1 receptor antagonist), CP-96,344 (inactive enantiomer of CP-96,345), SR 48968 (tachykinin NK2 receptor antagonist), SR 142801 (tachykinin NK3 receptor antagonist), amitriptyline (positive control), desipramine (positive control), and lorazepam (negative control) on immobility time during the 5-min test are shown in Fig. 1. Data from rats given no treatment, saline, or 25% DMSO vehicle were pooled into one group (“control”) because these groups showed nearly identical immobility times. A one-way between-subjects ANOVA conducted on the immobility times of the eight groups revealed a significant group effect (P<0.0001). Tukey’s post-hoc pairwise comparison tests revealed that animals administered CP-96,345, SR 48968, SR 142801, amitripty-line, and desipramine displayed significantly lower immobility times relative to the control group (all P<0.01). There were no differences between the amitriptyline-, desipramine-, CP-96,345-, SR 48968-, and SR 142801-treated groups (all P>0.05). There were no significant differences between the control group and the lorazepam-treated group (P>0.05), nor between the control group and the group treated with CP-96,344 (P>0.05).
Fig. 1.
Effect of administration of amitriptyline (10 mg/kg, n=9), desipramine (10 mg/kg, n=8), CP-96,456 (5 mg/kg, n=8), CP-96,344 (5 mg/kg, n=8), SR 48968 (5 mg/kg, n=8), SR 142801 (5 mg/kg, n=8), and lorazepam (0.5 mg/kg, n=8) on immobility time during the 5-min test session of the forced swim test (n=32 for control group). Data are expressed as mean±S.E.M. **P<0.01 compared to control group.
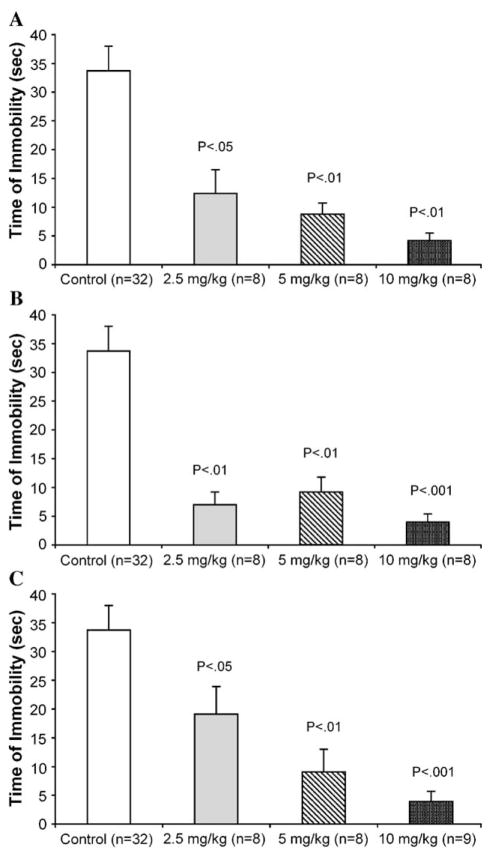
Each neurokinin antagonist was given at three doses: 2.5, 5, and 10 mg/kg (Fig. 2). CP-96,345 decreased immobility time at all doses, and showed a dose-related effect (P<0.05 for 2.5 mg/kg, P<0.01 for 5 mg/kg, and P<0.01 for 10 mg/ kg) when compared to the control group (Fig. 2A). SR 48968 also decreased immobility scores at each of the doses given (P<0.01 for 2.5 mg/kg, P<0.01 for 5 mg/kg, and P<0.001 for 10 mg/kg, when compared to controls), and showed a near maximum effect at the lowest dose (Fig. 2B). SR 142801 also showed a dose-related effect on decreases in immobility time, but only at 5 mg/kg (P<0.01) and 10 mg/kg (P<0.001; Fig. 2C).
Fig. 2.
Dose–response relationship of tachykinin receptor antagonists (2.5, 5, and 10 mg/kg) on time of immobility during the 5-min test session of the forced swim test: (A) NK1 antagonist, CP-96,345; (B) NK2 antagonist, SR 48968; (C) NK3 antagonist, SR 142801. Data are expressed as mean±S.E.M. P values given are with respect to control.
4. Discussion
The forced swim test is a well-validated and extensively used screen for compounds with antidepressant activity. Using this paradigm, it was shown here that treatment with the tachykinin receptor antagonists CP-96,345, SR 48968, and SR 142801 decreased the time of immobility in the forced swim test when compared to controls. The effects of these antagonists were similar to those produced by amitriptyline or desipramine—two well-established antidepressants. The lack of effect of the clinically effective anxiolytic lorazepam in this test indicates that the results observed with the neurokinin antagonists and the antidepressants are not false-positives. To our knowledge, this is the first attempt to evaluate the effects of blockade of each of the three neurokinin receptors in the rat forced swim test in a single study.
The first report of efficacy of an NK1 receptor antagonist, MK-869, in humans with major depressive disorder was shown by Kramer et al. (1998). Although these findings indicated a therapeutic potential of a neurokinin antagonist, it was not a surprise, considering the ubiquitous nature of substance P distribution in the central nervous system (Maggi, 1995) and its involvement in a number of mental dysfunctions (Quartara and Maggi, 1998). Simultaneously, there were studies in rats showing the analgesic effectiveness of transdermal amitriptyline (Haderer et al., 2003), and the antihyperalgesic and analgesic actions of amitriptyline after mild thermal injury (Oatway et al., 2003). In humans, amtriptyline is used as a therapy for chronic pain, including postherpetic neuralgia, diabetic neuropathy, chronic non-cancer pain, and fibromyalgia (Bryson and Wilde, 1996). The effectiveness of antidepressant drugs as analgesics, and the antidepressant property of an antinociceptive agent, suggest a common mechanism of action for most pharmacological agents used in the treatment of these purportedly distinct pathological syndromes.
The anatomical localization of substance P has been shown in areas of the brain thought to mediate affect. These include the striatum, nucleus accumbens, hippocampus, and the lateral nucleus of the hypothalamus (reviewed in Quartara and Maggi, 1998). The anatomical colocalization of substance P with serotonin in the raphe nuclei (Chan-Palay et al., 1978) and the excitation of locus coeruleus neurons by substance P (Guyenet and Aghajanian, 1977), which can be inhibited by CP-96,345 (McLean et al., 1991), may be important in explaining the role of substance P in affective behaviour. In terms of function, there is evidence to show that tachykinin NK1 receptor interference, either by antagonists or genetic disruption, leads to an increased firing of 5-HT neurons in the dorsal raphe nucleus and the desensitization of the autoinhibitory 5-HT1A receptor (Santarelli et al., 2001). Similarly, Froger et al. (2001) found a desensitization of 5-HT1A autoreceptors following knockout of the NK1 receptor—an effect comparable to that caused by chronic selective serotonin reuptake inhibitors (SSRIs; Froger et al., 2001). By these mechanisms, then, it should not be surprising that administration of an NK1 receptor antagonist would have the same outcome effect as administration of an SSRI.
The interaction between substance P-ergic and mono-aminergic systems is especially important in view of the fact that many antidepressant agents act by inhibiting the transport proteins for noradrenaline and/or serotonin, thus raising the synaptic concentrations of these neurotransmitters (Owens et al., 1996). In addition, a recent study reported that NK1 receptor activation in the rat dorsal raphe nucleus excites a population of 5-HT neurons via glutamatergic transmission (Valentino et al., 2003). Thus, there is ample evidence to suggest a more complex relationship between substance P-ergic and monoaminergic systems than simple anatomical coexistence.
Interestingly, recent evidence suggests that the NK1 receptor may be involved in the response to stress, as shown by behavioural studies in knockout mice (De Felipe et al., 1998). Several studies have shown that alterations in substance P levels in the hippocampus, striatum, periaque-ductal gray, and septum occur following stressors such as immobilization and isolation in the rat (Brodin et al., 1994; Rosen et al., 1992; Takayama et al., 1986). Further, NK1 receptor internalization was shown in the basolateral amygdala following maternal separation and immobilization in guinea pig pups and gerbils, respectively (Smith et al., 1999). A stressful experience such as forced swimming would be expected to cause the release of substance P in the brain and may account for the activity of neurokinin receptor antagonists in the forced swim test.
In view of these facts, it is not surprising to see the role of neurokinin receptors in depression. Although the NK1 antagonist, CP-96,345, used in the present study has relatively low affinity for the rat NK1 receptor (reviewed in Maggi, 1995), it has been shown that this compound is able to cross the blood–brain barrier and reverse the effects of centrally administered substance P in the rat (Yashpal et al., 1993).
The presence of central tachykinin NK2 receptors may be low compared with peripheral binding sites. However, these receptors are indeed present in discrete layers of the frontal cortex, hippocampus, and raphe nuclei (Hagan et al., 1993), and are functionally significant because antagonists to these receptors are active in rodent models of anxiety (Hagan and McLean, 1993; Stratton et al., 1993; Walsh et al., 1995). The efficacy of SR 48968, the NK2 receptor antagonist used in the present study, has previously been shown in the rat, including the inhibition of nociception-induced activation of thalamic neurons (Santucci et al., 1993). The antidepressant-like activity of SR 48968 was demonstrated by a reduction of immobility times in the forced swim test in mice and rats, and by inhibition of separation-induced vocalizations in guinea pig pups (Steinberg et al., 2001).
The distribution of tachykinin NK3 receptors includes many brain areas involved in the control of affect, similar to NK1 receptor distribution. For instance, high densities of the NK3 receptor are found in forebrain areas such as the amygdala, prefrontal cortex, bed nucleus of the stria terminalis, and hippocampus, all involved in execution of emotional expression (Dam et al., 1990; Ribeiro-da-Silva et al., 2000). NK3 binding sites have also been shown in the raphe nuclei (Dam et al., 1990), and NK3 binding sites are decreased in the median raphe nucleus when treated with 5,7-dihydroxytryptamine (Stoessl and Hill, 1990), thus providing a possibility of neurokinin and serotonergic interactions.
It is possible that the effect of NK3 receptor blockade on immobility is mediated partly through an interaction with noradrenaline, serotonin, and/or other neurotransmitters in the brain. For instance, the NK3 agonist, senktide, applied onto guinea pig locus coeruleus slices increases firing of noradrenergic neurons, and intracerebroventricular administration of senktide increases noradrenaline release in the medial prefrontal cortex of guinea pigs (Jung et al., 1996). SR 142801 blocked these responses completely (Jung et al., 1996). Intracisternal administration of NK3 receptor agonists, senktide and L-363,851, in mice induces head twitches and forepaw treading seen as in stimulation by serotonergic mechanisms (Stoessl et al., 1987, 1988). Moreover, these responses are blocked by 5-HT1 and 5-HT2 receptor antagonists (Stoessl et al., 1987). Similar responses including wet dog shakes and forepaw treading were observed in rats given senktide, and these effects were abolished by 5-HT receptor antagonists and p-chlorophenylalanine-induced depletion of 5-HT (Stoessl et al., 1990). Clearly, additional work will be needed in order to determine the precise mechanism(s) through which SR 142801 exerts an antidepressant effect in the forced swim test.
The results of the present study demonstrating an antidepressant-like effect in the forced swim test by SR 142801 are in apparent conflict with another report showing that the NK3 receptor agonist, aminosenktide, had similar effects in the forced swim test in some mice (Panocka et al., 2001). It is difficult to account for this difference because the mice were selected over 43 generations for differences in opioid-mediated analgesia.
In conclusion, all three neurokinin receptors, substance P-preferring NK1, NKA-preferring NK2, and NKB-preferring NK3 receptors, are localized in areas of the brain that are critical to the expression of affective behaviours. Moreover, the anatomical coexistence of these receptors with serotonin provides ample opportunity for interaction between neurokinins and serotonergic systems. As many antidepressants act by increasing the synaptic availability of serotonin, these interactions are of importance. However, at the present time, it is unclear whether the effects of these antagonists are mediated through a modulation of monoaminergic neurotransmission, or whether they might occur through an amine-independent mechanism. Therefore, it is possible that a mechanism other than, or in addition to, the monoamine system may be involved in the pathogenesis of depression. The findings in the present study provide evidence for a therapeutic potential of neurokinin antagonists as future antidepressants. Furthermore, as all three neurokinin receptor antagonists led to the same “antidepressant” effect, it is possible that concomitant blockade of more than one receptor type may result in additional effects or even in synergism.
Acknowledgments
This work was supported by grants from the Stairs Foundation of McGill University and the Canadian Institutes of Health Research. The generous gifts of CP-96,345 and CP-96,344 from Pfizer Central Research, and SR 48968 and SR 142801 from Sanofi-Synthelabo are gratefully acknowledged.
References
- Baby S, Nguyen M, Tran D, Raffa RB. Substance P antagonists: the next breakthrough in treating depression? J Clin Pharm Ther. 1999;24:461–469. doi: 10.1046/j.1365-2710.1999.00257.x. [DOI] [PubMed] [Google Scholar]
- Backman SB, Henry JL. Effect of substance P and thyrotropin-releasing hormone on sympathetic preganglionic neurones in the upper thoracic intermediolateral nucleus of the cat. Can J Physiol Pharm. 1984;62:248–251. doi: 10.1139/y84-038. [DOI] [PubMed] [Google Scholar]
- Bondy B, Baghai TC, Minov C, Schule C, Schwarz MJ, Zwanzger P, Rupprecht R, Moller H. Substance P serum levels are increased in major depression: preliminary results. Biol Psychiatry. 2003;53:538–542. doi: 10.1016/s0006-3223(02)01544-5. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Brodin E, Rosen A, Schott E, Brodin K. Effects of sequential removal of rats from a group cage, and of individual housing of rats, on substance P, cholecystokinin and somatostatin levels in the periaqueductal grey and limbic regions. Neuropeptides. 1994;26:253–260. doi: 10.1016/0143-4179(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Bryson HM, Wilde MI. Amitriptyline: a review of its pharmacological properties and therapeutic use in chronic pain states. Drugs Aging. 1996;8:459–476. doi: 10.2165/00002512-199608060-00008. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Jonsson G, Palay SL. Serotonin and substance P coexist in neurons of the rat’s central nervous system. Proc Natl Acad Sci U S A. 1978;75:1582–1586. doi: 10.1073/pnas.75.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TV, Escher E, Quirion R. Visualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H]senktide. Brain Res. 1990;506:175–179. doi: 10.1016/0006-8993(90)91218-6. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JMA, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:339–394. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- File SE. Rapid development of tolerance to the sedative effects of lorazepam and triazolam in rats. Psychopharmacology (Berl) 1981;73:240–245. doi: 10.1007/BF00422410. [DOI] [PubMed] [Google Scholar]
- Froger N, Gardier AM, Moratalla R, Alberti I, Lena I, Boni C, De Felipe C, Rupniak NM, Hunt SP, Jacquot C, Hamon M, Lanfumey L. 5-Hydroxytryptamine (5-HT)IA autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization. J Neurosci. 2001;21:8188–8197. doi: 10.1523/JNEUROSCI.21-20-08188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Aghajanian GK. Excitation of neurons in the nucleus locus coeruleus by substance P and related peptides. Brain Res. 1977;4:178–184. doi: 10.1016/0006-8993(77)90144-5. [DOI] [PubMed] [Google Scholar]
- Haderer A, Gerner P, Kao G, Srinivasa V, Wang GK. Cutaneous analgesia after transdermal application of amitriptyline versus lidocaine in rats. Anesth Analg. 2003;96:1707–1710. doi: 10.1213/01.ANE.0000060456.91215.90. [DOI] [PubMed] [Google Scholar]
- Hagan RM, McLean S. Vineyard peptide conference bears fruit. Trends Pharmacol Sci. 1993;14:315–318. doi: 10.1016/0165-6147(93)90001-z. [DOI] [PubMed] [Google Scholar]
- Hagan RM, Beresford IJM, Stables J, Dupere J, Stubbs CM, Elliott PJ, Sheldrick RLG, Chollet A, Kawashima E, McElroy AB, Ward P. Characterisation, CNS distribution and function of NK2 receptors studied using potent NK2 receptors antagonists. Regul Pept. 1993;46:9–19. doi: 10.1016/0167-0115(93)90005-s. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Bannon MJ. Tachykinin NK1 receptor antagonists enhance stress-induced c-fos in rat locus coeruleus. Eur J Pharmacol. 1998;348:155–160. doi: 10.1016/s0014-2999(98)00150-2. [DOI] [PubMed] [Google Scholar]
- Harrison S, Geppetti P. Substance P. Int J Biochem Cell Biol. 2001;33:555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- Henry JL. Effects of substance P on functionally identified units in cat spinal cord. Brain Res. 1976;114:439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- Jung M, Michaud JC, Steinberg R, Barnouin MC, Hayar A, Mons G, Souilhac J, Emonds-Alt X, Soubrie P, Le Fur G. Electrophysiological, behavioural and biochemical evidence for activation of brain noradrenergic systems following neurokinin NK3 receptor stimulation. Neuroscience. 1996;74:403–414. doi: 10.1016/0306-4522(96)00150-9. [DOI] [PubMed] [Google Scholar]
- Kitada Y, Miyauchi T, Satoh A, Satoh S. Effects of antidepressants in the rat forced swimming test. Eur J Pharmacol. 1981;72:145–152. doi: 10.1016/0014-2999(81)90269-7. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, Reines SA, Liu G, Snavely D, Wyatt-Knowles E, Hale JJ, Mills SG, MacCoss M, Swain CJ, Harrison T, Hill RG, Hefti F, Scolnick EM, Cascieri MA, Chicchi GG, Sadowski S, Williams AR, Hewson L, Smith D, Carlson EJ, Hargreaves RJ, Rupniak NMJ. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- Maubach KA, Rupniak NMJ, Kramer MS, Hill RG. Novel strategies for pharmacotherapy of depression. Curr Opin Chem Biol. 1999;3:481–488. doi: 10.1016/S1367-5931(99)80070-2. [DOI] [PubMed] [Google Scholar]
- McLean S, Ganong AH, Seeger TF, Bryce DK, Pratt KG, Reynolds LS, Siok CJ, Lowe JA., III Activity and distribution of binding sites in brain of a nonpeptide substance P (NK1) receptor antagonist. Science. 1991;251:437–439. doi: 10.1126/science.1703324. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Kitada Y, Satoh S. Effects of acutely and chronically administered antidepressants on the brain regional 3-methoxy-4-hydroxyphenylethyleneglycol sulfate in the forced swimming rat. Life Sci. 1981;29:1921–1928. doi: 10.1016/0024-3205(81)90525-7. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Blier P, de Montigny C. The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res Brain Res Rev. 1997;23:145–195. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- Oatway M, Reid A, Sawynok J. Peripheral antihyperalgesic and analgesic actions of ketamine and amitriptyline in a model of mild thermal injury in the rat. Anesth Analg. 2003;97:168–173. doi: 10.1213/01.ane.0000067406.52093.bf. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Stout SC, Mulchahey JJ, Plotsky PM. Molecular and neurobiological mechanisms in the treatment of psychiatric disorders. In: Tasman A, Kay J, Lieberman JA, editors. Textbook of Psychiatry. Saunders; Philadelphia: 1996. [Google Scholar]
- Panocka I, Massi M, Lapo I, Swiderski T, Kowalczyk M, Sadowski B. Antidepressant-type effect of the NK3 tachykinin receptor agonist aminosenktide in mouse lines differing in endogenous opioid system activity. Peptides. 2001;22:1037–1042. doi: 10.1016/s0196-9781(01)00438-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Quartara L, Maggi CA. The tachykinin NK1 receptor: distribution and pathophysiological roles. Neuropeptides. 1998;32:1–49. doi: 10.1016/s0143-4179(98)90015-4. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, McLeod AL, Krause JE. Neurokinin receptors in the CNS. In: Quirion R, Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 2000. pp. 195–240. [Google Scholar]
- Rimón R, Le Grevés P, Nyberg F, Heikkila L, Salmela L, Terenius L. Elevation of substance P-like peptides in the CSF of psychiatric patients. Biol Psychiatry. 1984;19:509–516. [PubMed] [Google Scholar]
- Rosen A, Brodin K, Eneroth P, Brodin E. Short-term restraint stress and s.c saline injection alter the tissue levels of substance P and cholecystokinin in the periaqueductal grey and limbic regions of rat brain. Acta Physiol Scand. 1992;146:341–348. doi: 10.1111/j.1748-1716.1992.tb09428.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille EL, Blier P, Hen R, Heath MJS. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc Natl Acad Sci U S A. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci V, Gueudet C, Edmonds-Alt X, Breliere JC, Soubrie P, LeFur G. The NK2 receptor antagonist SR48968 inhibits thalamic responses evoked by thermal but not mechanical nociception. Eur J Pharmacol. 1993;237:143–146. doi: 10.1016/0014-2999(93)90104-p. [DOI] [PubMed] [Google Scholar]
- Satoh H, Mori J, Shimomura K, Ono T, Kikuchi H. Effect of zimeldine, a new antidepressant, on the forced swimming test in rats. Jpn J Pharmacol. 1984;35:471–473. doi: 10.1254/jjp.35.471. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Hökfelt T, Hurd Y. Serotonin and substance P coexist in dorsal raphe neurons of the human brain. NeuroReport. 1999;10:3967–3970. doi: 10.1097/00001756-199912160-00044. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Mitsushio H, Takashima M, Ichikawa H, Takahashi K. Reduction of substance P after chronic antidepressants treatment in the striatum, substantia nigra and amygdala of the rat. Brain Res. 1996;739:70–78. doi: 10.1016/s0006-8993(96)00812-8. [DOI] [PubMed] [Google Scholar]
- Smith DW, Hewson L, Fuller P, Williams AR, Wheeldon A, Rupniak NMJ. The substance P antagonist L-760,735 inhibits stress-induced NK1 receptor internalization in the basolateral amygdala. Brain Res. 1999;848:90–95. doi: 10.1016/s0006-8993(99)01976-9. [DOI] [PubMed] [Google Scholar]
- Snider RM, Constantine JW, Lowe JA, III, Longo KP, Lebel WS, Woody HA, Drozda SE, Desai MC, Vinick FJ, Spencer RW, Hess HJ. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991;251:435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- Steinberg R, Alonso R, Griebel G, Bert L, Jung M, Oury-Donat F, Poncelet M, Gueudet C, Desvignes C, Le Fur G, Soubrie P. Selective blockade of neurokinin-2 receptors produces antidepressant-like effects associated with reduced corticotropin-releasing factor function. J Pharmacol Exp Ther. 2001;299:449–458. [PubMed] [Google Scholar]
- Stoessl AJ, Hill DR. Autoradiographic visualization of NK-3 tachykinin binding sites in the rat brain, utilizing [3H]senktide. Brain Res. 1990;534:1–7. doi: 10.1016/0006-8993(90)90105-k. [DOI] [PubMed] [Google Scholar]
- Stoessl AJ, Dourish CT, Young SC, Williams BJ, Iversen SD, Iverson LI. Senktide, a selective neurokinin B-like agonist, elicits serotonin-mediated behaviour following intracisternal administration in the mouse. Neurosci Lett. 1987;80:321–326. doi: 10.1016/0304-3940(87)90475-7. [DOI] [PubMed] [Google Scholar]
- Stoessl AJ, Dourish CT, Iversen SD. The NK-3 tachykinin receptor agonist senktide elicits 5-HT-mediated behaviour following central or peripheral administration in mice and rats. Br J Pharmacol. 1988;94:285–287. doi: 10.1111/j.1476-5381.1988.tb11527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoessl AJ, Dourish CT, Iversen SD. Pharmacological characterization of the behavioural syndrome induced by the NK-3 tachykinin agonist senktide in rodents: evidence for mediation by endogenous 5-HT. Brain Res. 1990;517:111–116. doi: 10.1016/0006-8993(90)91015-9. [DOI] [PubMed] [Google Scholar]
- Stratton SC, Beresford IJ, Harvey FJ, Turpin MP, Hagan RM, Tyers MB. Anxiolytic activity of tachykinin NK2 receptor antagonists in the mouse light–dark box. Eur J Pharmacol. 1993;250:R11–R12. doi: 10.1016/0014-2999(93)90042-g. [DOI] [PubMed] [Google Scholar]
- Takayama H, Ota Z, Ogawa N. Effect of immobilization stress on neuropeptides and their receptors in rat central nervous system. Regul Pept. 1986;15:239–248. doi: 10.1016/0167-0115(86)90065-0. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bey V, Pernar L, Commons KG. Substance P acts through local circuits within the rat dorsal raphe nucleus to alter serotonergic neuronal activity. J Neurosci. 2003;23:7155–7159. doi: 10.1523/JNEUROSCI.23-18-07155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Stratton SC, Harvey FJ, Beresford IJ, Hagan RM. The anxiolytic-like activity of GR159897, a non-peptide NK2 receptor antagonist, in rodent and primate models of anxiety. Psychopharmacology. 1995;121:186–191. doi: 10.1007/BF02245629. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Radhakrishnan V, Coderre TJ, Henry JL. CP-96,345, but not its stereoisomer, CP-96,344, blocks the nociceptive responses to intrathecally administered substance P and to noxious thermal and chemical stimuli in the rat. Neuroscience. 1993;52:1039–1047. doi: 10.1016/0306-4522(93)90550-y. [DOI] [PubMed] [Google Scholar]