Abstract
Background
Heroin addiction is a chronic relapsing disease, best treated with opioid-agonist substitution therapy such as methadone maintenance. However, a subset of the most severely affected individuals do not benefit sufficiently from this treatment. The North American Opiate Medication Initiative (NAOMI) is a randomized clinical trial (RCT) to evaluate the hypothesis that pharmaceutical-grade heroin, diacetylmorphine (DAM) is more effective in retaining patients and improving their outcomes than Methadone Maintenance Treatment (MMT) among those with chronic, refractory injection opioid dependence.
Purpose/Methods
The study aimed at randomizing 253 participants to two intervention arms: (1) MMT alone or (2) injectable opioids (DAM or hydromorphone) plus adjunctive MMT if deemed appropriate. The planned study duration was 3 years, with a 1-year intake period, 1 year of treatment, and an additional year of follow-up. The NAOMI trial was initiated in March 2005 at two Canadian sites (Vancouver and Montreal). This was the first multicenter RCT in North America to compare the relative efficacy of these different therapeutic strategies. We discuss the rationale behind the NAOMI study design, as well as the scientific and political issues and methodological challenges arising from the conduct of a trial that involves the prescription of a controlled substance to individuals with dependence on that substance.
Limitations
Restrictive entry criteria led to the exclusion of many otherwise eligible participants, slowing recruitment into the study. Inability to offer DAM treatment beyond 12 months led to artificial boundary effects in the trial.
Conclusions
Addiction treatment research navigates between science and politics, and evidence-based medicine is many times confronted by moral beliefs. Political considerations influence study design to a further degree than in RCTs treating less-stigmatized disorders with more-reputable medications.
Background
Opioid dependence, most commonly manifested as heroin dependence, is a chronic, relapsing disease [1]. Opioids such as heroin activate opioid receptors in the brain (and the body), and this stimulation results in feelings of euphoria, triggering the pleasure circuit and releasing excess amounts of dopamine, leading to substance dependence or addiction [2]. Once physically dependent, individuals suffer severe withdrawal symptoms including nausea, vomiting, sweating, abdominal pain, and agitation if the drug is not taken [3]. Injection-drug use places users at risk of contracting HIV, Hepatitis C, and other blood-borne pathogens. Co-morbid mental illness, cardio-pulmonary conditions, and serious bacterial infections are also common in populations with opioid dependence, and the possibility of overdose leads to greatly elevated mortality risk. Chronic opioid dependence is also associated with poor psychosocial functioning, given that it is generally accompanied by unemployment, imprisonment, poor housing conditions, and illegal activities [4,5].
Opioid addiction treatment
Strategies to treat and stabilize the problems associated with illicit opioid use are many and varied. Treatment ranges from abstinence (with or without medications) to substitution treatment, with or without concomitant psychosocial support or psychiatric therapy. Recent reviews suggest that substitution treatment is the most effective approach [6–8]. In Western countries methadone is the most widely used opioid agonist in substitution treatment. Other alternatives are currently available depending on the country: buprenorphine [9], morphine [10,11], dihydrocodeine [12,13] and diacetylmorphine [14–16] among others.
Methadone Maintenance Treatment (MMT)
Methadone is a long-acting synthetic opioid agonist that is easily absorbed when taken orally and has a half-life of approximately 25 h, allowing once-daily administration. MMT provides methadone orally on a regular (usually daily) basis to the patient [17]. Studies have suggested methadone is successful in blocking the effects of opioid withdrawal symptoms and reducing major risks, harms, and costs associated with untreated opioid dependence in patients attracted into and successfully retained in methadone treatment [6,18,19]. Best treatment outcomes have generally been correlated with a number of program factors, including high level and quality of psychosocial-care services, duration of treatment retention, sufficient methadone dosing, and a basic level of patient identification with program rules and staff [20–22].
However, MMT is not always successful at retaining patients in treatment for prolonged periods. For example, in British Columbia only 52% of MMT patients are retained for at least a year [23] in line with estimates of retention found in a National Institute of Drug Abuse review [24]. Other programs show higher retention rates, between 60 and 80%, such as in Ontario [25], in a low-threshold program in Montreal [26], and in most of the European Countries [27,28]. A variety of reasons for patients not being successfully maintained in MMT have been documented. The most important reason is likely the lack of adherence to best practices and clinical guidelines in prescribing MMT, with many patients receiving less than the minimum effective maintenance dose of 60 mg per day [29]. Other reasons include difficulties with side effects such as nausea, numbness, severe withdrawal, and depression, and the poor street reputation of MMT among some drug users [30,31]. Alternative treatment modalities are needed for opioid-dependent individuals who cannot be attracted into or productively retained in conventional forms of opioid-addiction treatment.
Heroin-Assisted Treatment (HAT)
Diacetylmorphine (DAM) is the active opioid found in illicit ‘street’ heroin and can be manufactured as a pure pharmaceutical product. In light of the suboptimal success of conventional treatments, several countries have recently begun to provide diacetylmorphine to treatment-resistant opioid users as an alternative treatment modality. Where available, DAM is provided in clinics where patients receive prescribed doses, up to three times per day, self-injected under the supervision of healthcare staff.
Several randomized trials and cohort studies have shown that HAT – provided in specialized clinics – is feasible, safe, and effective when treating long-term chronic injecting opioid users for whom the available treatments are not effective [15,16,32–36]. Patients treated with DAM improved in a number of dimensions, including reduction of illicit heroin and cocaine use, decreased criminal activity, and improvements in physical and mental health.
HAT is not simply a pharmacotherapy; it is a treatment approach that is situated within a context involving neighborhood factors, the local drug scene, housing, policing, medical care, and other treatment services. Its role and effectiveness is entangled with the ancillary services available, drug policies, and treatment philosophy. Most of the European countries adopted a pragmatic public health perspective after the rapid spread of HIV among injecting populations. This resulted in a significant expansion and wider availability of MMT, with a clear harm-reduction philosophy [27]. The expansion and availability of MMT in Canada in particular and North America in general, has been slower and, with exceptions, situated within a more repressive and controlling philosophy [37]. Due to the importance of context, the North American Opiate Medication Initiative (NAOMI) was designed to investigate the effectiveness of HAT in the North American context, that is, at several Canadian and US cities. In the end, however, the study could only be conducted in Canada. The following sections detail the specific objectives of the study, study design, patient population, and trial management including clinic and research office operations. We conclude with a discussion of some of the key scientific and political issues in the design and conduct of the study.
Objectives
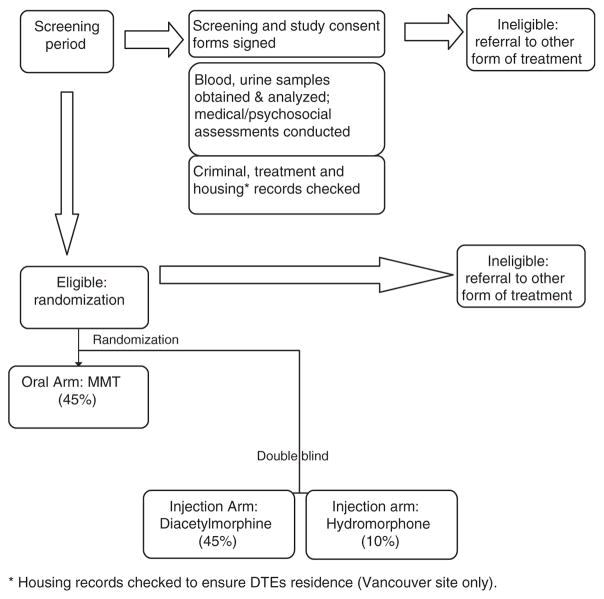
The general objective of the NAOMI trial is to determine whether the closely supervised provision of injectable, pharmaceutical-grade heroin (in combination with oral methadone if deemed appropriate) is more effective than MMT alone in recruiting, retaining, and benefiting chronic, opioid-dependent, injection-drug users (IDUs) who have not sufficiently benefited from current standard treatment options (Figure 1).
Figure 1.
Schematic for NAOMI trial
The underlying theory is that HAT may perform better at attracting and retaining individuals in treatment. The resulting ongoing contact with physicians, nurses, and counsellors together with decreases in the use of illicit heroin and illegal activity are hypothesized to have a stabilizing effect on these individuals. In time, it is hoped that they may progress to the point of transitioning to other forms of treatment such as MMT or abstinence-based treatment. For some, this might occur within the first 12 months of treatment; for others, it might not.
Study hypotheses and design
NAOMI is a two-center (Montreal and Vancouver) open, phase III randomized controlled clinical trial that enrols injection heroin users who are not benefiting from other treatments. Following a screening period for study eligibility, 45% of participants are randomized to an ‘optimized’ version of oral methadone treatment, which serves as the control arm. Fifty-five percent of participants are randomized to the injection arm. Within the injection arm, 45% receive injectable DAM and the remaining 10% receive injectable hydromorphone (HDM, Dilaudid™), which is pharmacologically similar to DAM in order to validate self-reported illicit heroin use. Participants in the injection arm are eligible to receive MMT as an adjunct or alone, if desired, at any time during the treatment period. HAT should not be viewed simply as the thrice-daily self-administration of pharmaceutical heroin but rather as a flexible treatment approach. The study is being conducted following Good Clinical Practice Guidelines and in accordance with the Declaration of Helsinki.
Choice of endpoints
Two primary outcomes were declared from the study. The first of these is retention in treatment at 12 months. Retention in treatment has been shown to be associated with treatment success [38]. Unlike other trials, in this context, it is important to note that patients can have successful outcomes without being retained on treatment protocol. For example, a patient might become abstinent, an injection patient might transition to MMT alone, or an MMT patient might choose to receive methadone at a community clinic instead of the study clinic. Such outcomes are considered to be positive and considered as part of the definition of retention. Thus, a patient is considered to be ‘retained’ if he or she received study medication on at least 10 of the 14 days prior to the 12-month assessment or is confirmed to have been in any other formal treatment program or abstinent during this 2-week interval. Retention is assessed through detailed study medication usage data collected on a daily basis at the treatment clinic, and by administrative data and pharmacy and physician records where possible.
The second primary outcome measures response to treatment in terms of decreases in the use of illicit drugs and illegal activities. Based on composite scores from the European Addiction Severity Index (EuropASI) [39], patients are considered responders at 12 months if they display a 20% improvement in illicit drug use or legal status scores from baseline. In addition, to protect against negative effects, a responder is allowed a deterioration of 10% or greater at the most, on one of the seven remaining composite scores. All participants lost to follow-up are considered nonretained and nonresponders.
Primary hypotheses
Among patients randomized to receive DAM (with or without methadone), the proportion who enter treatment and are retained as defined above at 12 months, will be higher than those randomized to receive oral methadone alone under an intent-to-treat analysis.
Among patients randomized to receive DAM (with or without methadone), the proportion who demonstrate a positive response to treatment at 12 months will be higher than those randomized to receive oral methadone alone under an intent-to-treat analysis.
Study population
The target population of the NAOMI study is long-term opioid dependent individuals who have tried conventional treatments in the past, who are not currently in treatment, and who currently regularly inject illicit heroin. The detailed inclusion and exclusion criteria are seen in Table 1. The objective is to reach opioid-dependent individuals who are currently not being reached by the treatment system.
Table 1.
NAOMI inclusion and exclusion criteria
| NAOMI inclusion criteria |
| Opioid dependence (DSM-IV) |
| Minimum 25 years of age |
| 5 years or more of opioid use |
Regular opioid injection:
|
| Minimum of 1-year residence in site/city location |
Minimum of two previous opiate addiction treatments:
|
No enrolment in any other opioid substitution program within the previous 6 months:
|
| Willingness and ability to adhere to study protocol and follow-up schedule |
| Documentation of fulfillment of the above study criteria (prison records, treatment records, cohort study enrolment, urine sampling) |
| Provide written and informed consent |
| NAOMI exclusion criteria |
| Severe medical or psychiatric conditions that are contra-indicated for heroin treatment |
| Pregnancy upon study entry |
| Current justice system involvement that is likely to result in an extended period of incarceration (more than 4 months) during the study period |
| Serum bilirubin > 2.5 × normal |
| Stage II or greater hepatic encephalopathy |
| Chronic respiratory disease resulting in resting respiratory rate > 20/min |
| Bipolar mood disorder, schizophrenia or other psychotic disorder with active psychotic symptoms refractory to medical management within the previous 6 months |
| Major depression requiring electroconvulsive therapy within the previous 12 months |
It is noteworthy that NAOMI participants are required to have a minimum of two previous treatment attempts, one of which must have been MMT. Furthermore, to avoid engaging patients for whom previous MMT experiences might not have included an adequate treatment episode, the trial required at least one prior MMT episode with a dosage of 60 mg or more per day for a minimum of 30 days in a 40-day period.
Sample size
Sample-size requirements for the study were calculated based on the major outcomes of retention and response. Because two primary variables were declared, each was evaluated at an alpha threshold of 0.025. We determined that 114 evaluable patients per group would yield 80% power to detect absolute increases of 20% in retention and response rates in the experimental group (injectable DAM) as the control rate (oral methadone) ranges from 25 to 50%. Because all participants lost to follow-up are considered not retained and nonresponders, oversampling for individuals lost to follow-up is not required as such participants are assumed to be failure events.
Randomization is stratified by the center and the number of prior treatment episodes to control for initial differences between treatment groups. All randomizations are assigned on an individual basis and performed using a block randomization technique with variable block size. Randomization tables are used by the data center, located in Vancouver. Coordinators at each clinic site call a dedicated phone line at the data center to determine treatment allocation. The data center also provides treatment assignment electronically to the pharmacies preparing the injection drugs at each site.
Recruitment and screening
Within each of the two cities, there are two separate facilities: a treatment clinic and a research center. The treatment clinic houses all of the clinical staff and treatment activities. The research center is responsible for recruitment and screening and for conducting all baseline and follow-up assessments.
Within each city, the treatment clinic and research center are operated independently for two main reasons. First, for the intent-to-treat analysis to be valid in a study with significant expected levels of treatment drop-out, it is imperative that the research follow-up be as close to complete as possible, independent of treatment retention. Second, it is critical that the participants provide honest answers to sensitive research questions about ongoing illicit drug use and criminal activity. In their past experience, however, admissions of such behaviors to treatment providers may have precipitated negative impacts on their treatment. By reassuring them that their research responses will not be shared with the treatment clinic and cannot in any way influence their ongoing treatment, we hope to encourage forthright reporting [40].
Participant recruitment is conducted primarily through the use of street outreach and dedicated phone lines. To initiate treatment, eligible individuals are instructed to visit the treatment clinic the following Monday morning at which time they are informed of their treatment allocation.
Blinding
It is not possible to blind the oral methadone arm vs. injection drug arm because placebo heroin would be instantly recognized. However, the administration of DAM vs. HDM is blinded within the injection arm. Identical pre-filled syringes of the injection drugs are provided to the treatment clinic by the pharmacy. Pharmacologically equivalent doses of DAM and HDM are provided over a dose range, allowing blinded dose adjustment by treatment providers.
Treatment and transition
Study interventions are provided for 12 months followed by a 3-month period during which participants still being treated with injection drugs are tapered and transitioned to conventional therapies such as methadone. This transition must occur given that HAT is not a legal therapy outside the context of the trial. Those on MMT alone at 12 months are transferred to an existing methadone program or another treatment modality of their choice. The 12-month research visit at which the primary outcome measures are assessed is conducted before any tapering or transition begins.
Research assessments
Apart from the daily visits to the clinic for medication, study participants return to the research center for face-to-face outcome interviews/assessments every 3 months during the first year and at 18 and 24 months. To ensure a high follow-up rate, in addition to financial incentives provided for research visits, an outreach team experienced in working with the target population maintains contact with participants and actively works to ensure that follow-up appointments are kept. It is important to note that the financial incentives are in place for research follow-up and not for retention in treatment. It is made clear that a subject’s treatment status will not impact their participation in the research portion of the study.
Discussion
A randomized controlled trial of an illegal substance in a very troubled and stigmatized group of individuals who are dependent on that substance presents a number of unique ethical, regulatory, logistical, methodological, and political challenges. These have affected the present study as well as the European trials that preceded it [41].
Patient safety
Regulatory agencies and ethics review boards are appropriately concerned with patient safety. In the context of human experimentation, the question they raise is whether the experimental medication can be administered safely to human volunteers compared to nonadministration. Heroin is a respiratory depressant and opponents of heroin prescription have used the observation of decreased oxygen saturation following heroin injection to argue that heroin administration is less safe than nonadministration [42,43]. Technically this is correct. However, analysis of patient safety should take into account that in the absence of the trial, all participants would be injecting street heroin. In our view, a more appropriate comparison is the relative safety of the administration of precise doses of pharmaceutical heroin prescribed by physicians, supervised by nurses, and conducted under sterile conditions vs. administration of unknown doses of adulterated street heroin in filthy conditions often in isolation and sometimes with used syringes, increasing the risk of untreated overdose and HIV and HCV infection. Rather than nonadministration, it is the latter to which prospective trial participants are continuously exposed in the absence of the experimental medication.
Choice of comparator
The use of placebo as a control therapy in opioid addiction treatment trials faces an ethical barrier as untreated patients are at high risk of morbidity and mortality [44]. A cautionary example can be found in a Swedish RCT of buprenorphine in which 20% of the placebo-control group had died by 12 months as against none in the experimental group [45].
Given the need for an active comparator, substitution therapies with long-acting oral agonist opioids have been shown to be the most effective available treatment [7,18,46]. Methadone maintenance treatment is commonly used in North America and is seen as the most effective available treatment [19,45–53].
However, given that patients had already attempted methadone treatment at least once in the past, a question can be raised as to the validity of the comparison. Is the study not biased if the control group is randomized to receive a therapy that has previously failed and in which patients may have considerable disinterest? From the methodological standpoint, the ideal way to address this would be to conduct the study in patients who have not previously received MMT. However, this would change the trial into an investigation of HAT as first-line substitution therapy. The reality is that policy-makers and ethics boards would find the concept of offering HAT to individuals who had not previously attempted oral substitution with methadone at least once, as unpalatable. Conducting the trial in treatment-naïve individuals, while methodologically sound, is politically unacceptable.
To address the issue of the comparator, we chose to offer an optimized MMT program that corrected many of the deficiencies participants had likely encountered in previous experiences with MMT such as long waiting times, limited primary care or psychosocial services, and suboptimal dosing. As such, the trial addresses a highly relevant clinical question regarding treatment strategy: for individuals who have previously not benefited from conventional treatments including MMT, is it better to offer one more attempt at MMT (in an optimal program) or should one offer HAT ?
Lack of equipoise and treatment retention
While there may be equipoise regarding HAT and MMT in the clinical and scientific community, this is not the case among individuals with opioid dependence who have not benefited from MMT in the past. Focus groups conducted prior to NAOMI demonstrated that a proportion of this target group have essentially rejected MMT as a treatment option [31]. In a survey of untreated opioid users in Toronto who were offered immediate admission to MMT, 33% would have rejected it outright and 19% were ambivalent [54].
Generally, it is difficult to engage participants in various types of addiction research and the potential of randomization to placebo or control treatment is seen as one of the most significant barriers [55]. In the case of HAT with MMT as the comparator, the risk of dropout in the control arm is further amplified.
One way to address this is use of a crossover design in which control individuals are promised a switch to the investigational therapy after a pre-specified period, as occurred in the trial of HAT in the Netherlands [56]. One difficulty with this is that the promise of HAT at the end of control treatment may act as a protocol-driven incentive for control individuals to enter into and adhere to MMT, biasing the retention and response rates upward from those that would be observed if optimized MMT were the only treatment option available in the clinical setting. The NAOMI study thus avoids a bias in other HAT trials due to the incentive provided by a crossover design.
Some of this bias is likely present in NAOMI even in the absence of the crossover incentive. It is clear that some individuals come forward to be randomized into NAOMI only in the hope that they will be allocated to HAT. Of those allocated to MMT, some drop out immediately but others end up staying and benefiting from MMT. This biases the retention and response rates in the control group upward because it is not clear whether any of these individuals would have been attracted to come forward for MMT had the possibility of HAT not been present.
All this said, the lack of equipoise was recognized as an issue during the design of NAOMI and the investigators were aware of the likelihood of significant early drop-outs in the control arm. This was partially mitigated by allowing treatment drop-outs to return at a later date and still meet the criteria for response and retention.
The question arises as to the validity of an intent-to-treat comparison of retention and response in a trial when significant treatment drop-outs are expected in the control arm. The answer, in our view, is that such a comparison is valid if this level of drop-out reflects a true lack of acceptance on the part of the participants similar to that which would be observed if the control strategy alone were offered in the real clinical setting.
On the other hand, validity would indeed be threatened if a large number of treatment drop-outs were also lost to research follow-up. However, contrary to common misconceptions about the ability of our patient population to comply with research protocols, the rate of loss to research follow-up in NAOMI to date has been only 5%.
Treatment discontinuation and compassionate access
Medically prescribed heroin for addiction treatment is not available in Canada outside the context of the NAOMI trial which was approved with a treatment period of 12–15 months. The discontinuation of the injection treatment, if proven to be effective, evokes well-founded ethical concerns [57]. With the exception of Canada, in all the other HAT studies patients could continue receiving DAM under compassionate use after the trial period [14]. To this point, Health Canada has not authorized the NAOMI clinics to continue the treatment with DAM to those benefiting from treatment under its Special Access Program (compassionate use). As a result, we have to be very clear in our ethics applications and in our informed consent process with participants that HAT will not be available outside the context of the study and will therefore be provided for no more than 12–15 months. Beyond the clear ethical concerns, this timeline affects trial validity by creating boundary effects that are discussed later.
The attribution and ascertainment of adverse events
The issue of adverse events in a population with multiple co-morbidities is complex. Many of the NAOMI participants have ongoing medical conditions that are associated with their underlying drug use, poor nutrition, and unstable housing. To confound matters further, adverse effects of illicit street-heroin use are similar to the effects of the study medications. In addition, many participants are also found to take other prescribed medications and use street drugs such as cocaine and black-market benzodiazepines. This makes the attribution of causal relationships with study medications exceedingly difficult.
With regard to ascertainment, a significant bias in comparisons of adverse-event incidence will occur in such a population if one group is seen more frequently than another. In NAOMI, retained injection patients are interviewed and observed up to three times per day. They are screened each time by a nurse to ensure that administration of the injection medication is safe. On the other hand, some methadone patients visit the clinic at the most only once per day for only a few minutes, and some receive their medication at community pharmacies and are thus not even seen daily. More frequent and intense contact with participants allocated to injection leads to increased ascertainment of health events, especially in a population where health events occur frequently. Thus, the validity of comparisons of adverse events between injection and MMT individuals is questionable. This bias is less pronounced in the case of serious adverse events (SAEs) because it is more likely that the study team would become aware of hospitalizations and deaths.
Boundary effects
Clearly, treatment effects can be obscured if the duration of a trial is insufficient for some effects to become manifest. In the case of NAOMI, the treatment period was limited to 12 months for logistical, financial, and political reasons. However, longer-term observations from European trials demonstrate that treatment periods greater than 1 year are required for some individuals [34,58,59]. This type of limitation is present in many clinical trials involving chronic therapies of chronic conditions.
In the case of NAOMI, as noted earlier, participants understand that DAM is not available outside the context of the study and will therefore be provided for no more than 12–15 months. We provided assurances that we would make every effort to switch participants to the treatment of their choice during months 12–15. Nevertheless, we observed that the realization that treatment will change after 12 months causes increasing anxiety among some participants as they progress toward the end of their treatment year. In some cases, this anxiety leads to some behaviors such as increased illicit drug use that have a negative impact on treatment response measures at 12 months. Such a boundary effect is not inherent in the treatment strategy, but is rather a protocol-driven artifact caused by the inability to offer the treatment beyond a limited period within the trial. Such an effect would not be observed if this treatment strategy were offered in clinical practice because no such artificial end would necessarily be imposed. As such, it biases the treatment effect toward the null.
Science and politics in study implementation
Initial discussions regarding a North American HAT trial began in 1998, and six sites across Canada and the United States were planned. As development of the study proposal progressed, all of the US investigators eventually removed their sites from active consideration because of political considerations and lack of an identifiable US funding source. The loss of US sites increased the recruitment requirements of the remaining Canadian sites.
Though the proposal for a three-site Canadian study was approved by the Canadian Institutes of Health Research in 2001, many tasks remained that required an additional 3 years. It was necessary to raise additional funds not covered by the research grant including renovation, security, and medication costs. Multiple approvals from Health Canada for the use of DAM had to be obtained as well as approval for import of the medication from the International Narcotics Control Board. The clinics in Vancouver and Montreal had to be constructed according to the specifications of Health Canada for inventory and security systems aimed at ensuring safety and eliminating the possibility of diversion of the drug to the black market.
Inclusion criteria for the study were influenced not only by the science of HAT but also by the politics. The city of Vancouver, in granting the Vancouver site’s development permit, insisted that recruitment be limited to within a mile of the treatment clinic. The goal was to reassure the local community that the study would not increase crime and public disorder in the neighborhood by drawing people with heroin dependence from other parts of the city (the so-called ‘honeypot effect’, which was not experienced within European cities conducting HAT trials [41]). This inclusion criterion was not incorporated for any valid scientific or safety reason, and significantly slowed recruitment at the Vancouver site.
An important scientific question is whether individuals currently in a MMT program that is not effective (e.g., they continue to use significant amounts of street heroin) would benefit from a switch to HAT as opposed to continued MMT. NAOMI could have addressed this question if such individuals had been included in the trial, but this was considered politically untenable. As such, the trial was restricted to those who had not been in any treatment program in the previous 6 months. None of the European HAT trials imposed this restriction, instead, some of them required that volunteers had to be on MMT to be eligible [32,60]. This restriction in NAOMI not only limited the clinical question being addressed, but it led to recruitment challenges – hundreds of willing participants who contacted the study were screened out because they were currently or recently in MMT programs [61]. By the end of the recruitment period, only 22% of all prospective participants who had contact with NAOMI recruiters were randomized into the study. As a result, recruitment into NAOMI took much longer than anticipated as has been the case in previous trials [36,62].
Lessons learned
We have already learned much about the successes and deficiencies of the various design choices and procedures. On the positive side, the decision to include the double-blind comparison involving hydromorphone has proved to be a good one and sets NAOMI apart from the European studies. The HDM group was intended to verify the use of street heroin in urine samples. To our surprise, participants do not appear to be able to distinguish if they are receiving HDM and outcomes appear similar with both formulations. This design choice will not only allow for validation of self-reported illicit heroin use but it also opens the door to future studies testing HDM (a licensed medication) as a treatment for our target population. In addition, use of methadone that is optimized as a treatment has resulted in retention rates that are higher than we anticipated, suggesting that we have addressed at least some of the deficiencies that may have previously been experienced. Some of the most important lessons concern the strategies for recruitment of participants and the independence of the research and the clinical teams. An active recruitment carried out by well-trained outreach staff with good ties to the community that serves our target population was crucial to recruiting the necessary number of participants. The independence of the research group from the treatment providers has allowed for more valid self-reporting of illicit drug use. Moreover, despite the marginalized nature of the study participants, the research team has been able to obtain outcome data from over 95% of randomized individuals, irrespective of whether or not they were retained in treatment.
On the negative side, the restriction of participation to only those not receiving MMT in the previous 6 months and the demand of at least 60 mg dosage in a previous MMT attempt posed significant challenges to recruitment. These criteria led to the exclusion of many otherwise eligible participants, slowing recruitment and creating negative feelings in the community about the trial.
Conclusion
Addiction-treatment research navigates between science and politics, and evidence-based medicine is many times confronted by moral beliefs. Political considerations influence study design to a farther degree than in RCTs treating less-stigmatized disorders and involving more reputable medications. It should be emphasized that HAT is aimed at reaching and treating the most vulnerable subgroup of opioid-dependent persons, with the highest prevalence of health and psychosocial problems associated with their dependence. For these patients, alternative treatments are needed, and only through well-designed studies can we determine their effectiveness.
Acknowledgments
The NAOMI study is funded by the Canadian Institutes of Health Research (CIHR). Dr Schechter is a tier I Canada Research Chair.
References
- 1.Hser YI, Huang D, Chou CP, Anglin MD. Trajectories of heroin addiction: growth mixture modeling results based on a 33-year follow-up study. Eval Rev. 2007;31:548–63. doi: 10.1177/0193841X07307315. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–8. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD. Longitudinal patterns of drug injection behavior in the ALIVE Study cohort,1988–2000: description and determinants. Am J Epidemiol. 2003;158:695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- 5.March JC, Oviedo-Joekes E, Romero M. Drugs and social exclusion in ten European cities. Eur Addict Res. 2006;12:33–41. doi: 10.1159/000088581. [DOI] [PubMed] [Google Scholar]
- 6.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2003:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- 7.Amato L, Davoli M, Perucci AC, et al. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28:321–9. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Van den Brink W, Haasen C. Evidence-based treatment of opioid-dependent patients. Can J Psychiatry. 2006;51:635–46. doi: 10.1177/070674370605101003. [DOI] [PubMed] [Google Scholar]
- 9.Mattick R, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Kraigher D, Jagsch R, Gombas W, et al. Use of slow-release oral morphine for the treatment of opioid dependence. Eur Addict Res. 2005;11:145–51. doi: 10.1159/000085550. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell TB, White JM, Somogyi AA, Bochner F. Switching between methadone and morphine for maintenance treatment of opioid dependence: impact on pain sensitivity and mood status. Am J Addict. 2006;15:311–5. doi: 10.1080/10550490600754374. [DOI] [PubMed] [Google Scholar]
- 12.Strain EC, Walsh SL, Bigelow GE. Blockade of hydromorphone effects by buprenorphine/naloxone and buprenorphine. Psychopharmacology (Berl) 2002;159:161–6. doi: 10.1007/s002130100920. [DOI] [PubMed] [Google Scholar]
- 13.Krausz M, Verthein U, Degkwitz P, Haasen C, Raschke P. Maintenance treatment of opiate addicts in Germany with medications containing codeine–results of a follow-up study. Addiction. 1998;93:1161–7. doi: 10.1046/j.1360-0443.1998.93811614.x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer B, Oviedo-Joekes E, Blanken P, et al. Heroin-assisted treatment (HAT) a decade later: a brief update on science and politics. J Urban Health. 2007;84:552–62. doi: 10.1007/s11524-007-9198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehm J, Gschwend P, Steffen T, Gutzwiller F, Dobler-Mikola A, Uchtenhangen A. Feasibility, safety, and efficacy of injectable heroin prescription for refractory opioid addicts: a follow-up study. Lancet. 2001;358:1417–23. doi: 10.1016/S0140-6736(01)06529-1. [DOI] [PubMed] [Google Scholar]
- 16.Haasen C, Verthein U, Degkwitz P, et al. Heroin-assisted treatment for opioid dependence: Randomised controlled trial. Br J Psychiatry. 2007;191:55–62. doi: 10.1192/bjp.bp.106.026112. [DOI] [PubMed] [Google Scholar]
- 17.Seidenberg A, Honegger U. Methadon, Heroin und andere Opioide – Medizinisches Manual für die ambulante opioid-gestützte Behandlung [Methadone, Heroin and other Opiods –Medical Manual for Outpatient Opioid – Supported Treatment] Verlag Hans Huber; Bern: 1998. [Google Scholar]
- 18.Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309:997–1001. doi: 10.1136/bmj.309.6960.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology (Berl) 1999;145:162–74. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- 20.Mayet S, Farrell M, Ferri M, Amato L, Davoli M. Psychosocial treatment for opiate abuse and dependence. Cochrane Database Syst Rev. 2005:CD004330. doi: 10.1002/14651858.CD004330.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Dose-response effects of methadone in the treatment of opioid dependence. Ann Intern Med. 1993;119:23–7. doi: 10.7326/0003-4819-119-1-199307010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Caplehorn RM, Lumley TS, Irwig L, Saunders JB. Changing attitudes and beliefs of staff working in methadone maintenance programs. Aust N Z J Public Health. 1998;22:505–8. doi: 10.1111/j.1467-842x.1998.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JF, Warren LD. Client retention in the British Columbia Methadone Program, 1996–1999. Can J Public Health. 2004;95:104–9. doi: 10.1007/BF03405776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute on Drug Abuse. Methadone Maintenance Treatment: Translating Research into Policy. NIDA Research Monograph; Washington, DC: 1995. [Google Scholar]
- 25.Strike CJ, Gnam W, Urbanoski K, et al. Factors predicting 2-year retention in methadone maintenance treatment for opioid dependence. Addictive Behaviors. 2005;30(5):1025–8. doi: 10.1016/j.addbeh.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Perreault M, Heroux MC, White ND, et al. Treatment retention and evolution of clientele in a low threshold methadone substitution treatment program in Montreal. Can J Public Health. 2007;98:33–6. doi: 10.1007/BF03405382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrell M, Howes S, Verster AD, Davoli M. Reviewing current practice in drug substitution treatment in the European Union. EMCDDA; Louxembourg: 2000. [Google Scholar]
- 28.Wittchen HU, Apelt SM, Soyka M, et al. Feasibility and outcome of substitution treatment of heroin-dependent patients in specialized substitution centers and primary care facilities in Germany: a naturalistic study in 2694 patients. Drug Alcohol Depend. 2008;95:245–57. doi: 10.1016/j.drugalcdep.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Blaney T, Craig RJ. Methadone maintenance. Does dose determine differences in outcome? J Subst Abuse Treat. 1999;16:221–8. doi: 10.1016/s0740-5472(98)00031-2. [DOI] [PubMed] [Google Scholar]
- 30.Zule WA, Desmond DP. Attitudes toward methadone maintenance: implications for HIV prevention. J Psychoactive Drugs. 1998;30:89–97. doi: 10.1080/02791072.1998.10399674. [DOI] [PubMed] [Google Scholar]
- 31.Fischer B, Chin AT, Kuo I, Kirst M, Vlahov D. Canadian illicit opiate users’ views on methadone and other opiate prescription treatment: an exploratory qualitative study. Subst Use Misuse. 2002;37:495–522. doi: 10.1081/ja-120002807. [DOI] [PubMed] [Google Scholar]
- 32.van den Brink W, Hendriks VM, Blanken P, et al. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ. 2003;327:310. doi: 10.1136/bmj.327.7410.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartnoll RL, Mitcheson MC, Battersby A, et al. Evaluation of heroin maintenance in controlled trial. Arch Gen Psychiatry. 1980;37:877–84. doi: 10.1001/archpsyc.1980.01780210035003. [DOI] [PubMed] [Google Scholar]
- 34.Guttinger F, Gschwend P, Schulte B, Rehm J, Uchtenhagen A. Evaluating long-term effects of heroin-assisted treatment: the results of a 6-year follow-up. Eur Addict Res. 2003;9:73–9. doi: 10.1159/000068811. [DOI] [PubMed] [Google Scholar]
- 35.Perneger TV, Giner F, del Rio M, Mino A. Randomised trial of heroin maintenance programme for addicts who fail in conventional drug treatments. BMJ. 1998;317:13–8. doi: 10.1136/bmj.317.7150.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.March JC, Oviedo-Joekes E, Perea-Milla E, Carrasco F. Controlled trial of prescribed heroin in the treatment of opioid addiction. J Subst Abuse Treat. 2006;31:203–11. doi: 10.1016/j.jsat.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Fischer B, Rehm J, Blitz-Miller T. Injection drug use and preventive measures: a comparison of Canadian and western European jurisdictions over time. CMAJ. 2000;162:1709–13. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Friedmann PD, Gerstein DR. Does retention matter? Treatment duration and improvement in drug use. Addiction. 2003;98:673–84. doi: 10.1046/j.1360-0443.2003.00354.x. [DOI] [PubMed] [Google Scholar]
- 39.Kokkevi A, Hartgers C. EuropASI: European adaptation of a multidimensional assessment instrument for drug and alcohol dependence. European Addiction Research. 1995;1:208–10. [Google Scholar]
- 40.Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51:253–63. doi: 10.1016/s0376-8716(98)00028-3. discussion 267–8. [DOI] [PubMed] [Google Scholar]
- 41.Bammer G, van den Brink W, Gschwend P, Hendriks V, Rehm J. What can the Swiss and Dutch trials tell us about the potential risks associated with heroin prescribing? Drug Alcohol Rev. 2003;22:363–71. doi: 10.1080/0959523031000154517. [DOI] [PubMed] [Google Scholar]
- 42.Ladewig D, Dursteler-MacFarland KM, Seifritz E, Hock C, Stohler R. New aspects in the treatment of heroin dependence with special reference to neurobiological aspects. Eur Psychiatry. 2002;17:163–6. doi: 10.1016/s0924-9338(02)00644-2. [DOI] [PubMed] [Google Scholar]
- 43.Stoermer R, Drewe J, Dursteler-Mac Farland KM, et al. Safety of injectable opioid maintenance treatment for heroin dependence. Biol Psychiatry. 2003;54:854–61. doi: 10.1016/s0006-3223(03)00290-7. [DOI] [PubMed] [Google Scholar]
- 44.Bland JM, Kerr D. Fifth revision of Declaration of Helsinki. Clause 29 forbids trials from using placebos when effective treatment exists. BMJ. 2002;324:975. [PubMed] [Google Scholar]
- 45.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361:662–8. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 46.Anonymous National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280:1936–43. [PubMed] [Google Scholar]
- 47.Lepere B, Gourarier L, Sanchez M, et al. Reduction in the number of lethal heroin overdoses in France since 1994. Focus on substitution treatments. Ann Med Interne (Paris) 2001;152(Suppl 3):IS5–12. [PubMed] [Google Scholar]
- 48.van Ameijden EJ, Langendam MW, Coutinho RA. Dose-effect relationship between overdose mortality and prescribed methadone dosage in low-threshold maintenance programs. Addict Behav. 1999;24:559–63. doi: 10.1016/s0306-4603(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 49.Brugal MT, Domingo-Salvany A, Puig R, et al. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and aids in a cohort of heroin users in Spain. Addiction. 2005;100:981–9. doi: 10.1111/j.1360-0443.2005.01089.x. [DOI] [PubMed] [Google Scholar]
- 50.Fischer B, Popova S, Rehm J, Ivsins A. Drug-related overdose deaths in British Columbia and Ontario, 1992–2004. Can J Public Health. 2006;97:384–7. doi: 10.1007/BF03405347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Healey A, Knapp M, Marsden J, Gossop M, Stewart D. Criminal outcomes and costs of treatment services for injecting and non-injecting heroin users: evidence from a national prospective cohort survey. J Health Serv Res Policy. 2003;8:134–41. doi: 10.1258/135581903322029476. [DOI] [PubMed] [Google Scholar]
- 52.Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515–32. doi: 10.1046/j.1360-0443.1998.9345157.x. [DOI] [PubMed] [Google Scholar]
- 53.Torrens M, San L, Martinez A, et al. Use of the Nottingham Health Profile for measuring health status of patients in methadone maintenance treatment. Addiction. 1997;92:707–16. [PubMed] [Google Scholar]
- 54.Fischer B, Gliksman L, Rehm J, Daniel N, Medved W. Comparing opiate users in methadone treatment with untreated opiate users: results of a follow-up study with a Toronto opiate user cohort. Can J Public Health. 1999;90:299–303. doi: 10.1007/BF03404513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomson CL, Morley KC, Teesson M, Sannibale C, Haber PC. Issues with recruitment to randomised controlled trials in the drug and alcohol field: a literature review and Australian case study. Drug Alcohol Rev. 2008;27:115–22. doi: 10.1080/09595230701829561. [DOI] [PubMed] [Google Scholar]
- 56.van den Brink W, Hendriks VM, Blanken P, Huijsman IA, van Ree JM. Medical Co-prescription of Heroin: Two Randomized Controlled Trials. Central Committee on the Treatment of Heroin Addicts (CCBH); Netherlands: 2002. [Google Scholar]
- 57.Small DR, Drucker E. Policy makers ignoring science Scientists Ignoring policy: the medical ethical challenges of heroin treatment. Harm Reduct J. 2006;3:16. doi: 10.1186/1477-7517-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verthein U, Bonorden-Kleij K, Degkwitz P, et al. Long-term effects of heroin-assisted treatment in Germany. Addiction. 2008;103:960–6. doi: 10.1111/j.1360-0443.2008.02185.x. discussion 967–8. [DOI] [PubMed] [Google Scholar]
- 59.Oviedo-Joekes E, March JC, Romero M, Perea-Milla E. The Andalusian trial on heroin assisted treatment: a 2 years follow-up. Drug Alcohol Rev. 2009 doi: 10.1111/j.1465-3362.2009.00100.x. In press. [DOI] [PubMed] [Google Scholar]
- 60.Lintzeris N, Strang J, Metrebian N, et al. Methodology for the randomised injecting opioid treatment trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UK. Harm Reduct J. 2006;3:28. doi: 10.1186/1477-7517-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oviedo-Joekes E, Brissette S, Marsh DC, et al. A randomized trial of diacetylmorphine vs. methadone for treatment-refractory opioid addiction. New Engl J Med. 2009 doi: 10.1056/NEJMoa0810635. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haasen C, Vertheim U, Degkwitz P. The German Model Project for Heroin Assisted Treatment of Opioid Dependent Patients. A Multicentric, Randomized, Controlled Treatment Study. Centre for Interdisciplinary Addiction Research of Hambur University (ZIS); Germany: 2006. [Google Scholar]