Abstract
BACKGROUND
Studies in Europe have suggested that injectable diacetylmorphine, the active ingredient in heroin, can be an effective adjunctive treatment for chronic, relapsing opioid dependence.
METHODS
In an open-label, phase 3, randomized, controlled trial in Canada, we compared injectable diacetylmorphine with oral methadone maintenance therapy in patients with opioid dependence that was refractory to treatment. Long-term users of injectable heroin who had not benefited from at least two previous attempts at treatment for addiction (including at least one methadone treatment) were randomly assigned to receive methadone (111 patients) or diacetylmorphine (115 patients). The primary outcomes, assessed at 12 months, were retention in addiction treatment or drug-free status and a reduction in illicit-drug use or other illegal activity according to the European Addiction Severity Index.
RESULTS
The primary outcomes were determined in 95.2% of the participants. On the basis of an intention-to-treat analysis, the rate of retention in addiction treatment in the diacetylmorphine group was 87.8%, as compared with 54.1% in the methadone group (rate ratio for retention, 1.62; 95% confidence interval [CI], 1.35 to 1.95; P<0.001). The reduction in rates of illicit-drug use or other illegal activity was 67.0% in the diacetylmorphine group and 47.7% in the methadone group (rate ratio, 1.40; 95% CI, 1.11 to 1.77; P=0.004). The most common serious adverse events associated with diacetylmorphine injections were overdoses (in 10 patients) and seizures (in 6 patients).
CONCLUSIONS
Injectable diacetylmorphine was more effective than oral methadone. Because of a risk of overdoses and seizures, diacetylmorphine maintenance therapy should be delivered in settings where prompt medical intervention is available. (ClinicalTrials.gov number, NCT00175357.)
Opioid dependence, most commonly manifested as heroin dependence, is a chronic relapsing condition1 that is estimated to affect more than 1 million persons in North America.2,3 The risks of opioid dependence include fatal overdoses, infections (including endocarditis, human immunodeficiency virus infection, and hepatitis C virus infection), social disintegration, violence, and crime. The associated burdens on communities include medical, public health, and criminal-justice costs as well as public disorder and crimes against property.
Methadone, the standard opioid-substitution treatment, has been shown to reduce major risks associated with untreated opioid dependence in patients who are willing to undergo and are successfully retained in treatment.4–6 However, 15 to 25% of the most adversely affected persons do not have a good response to this treatment.7 Such persons are either not retained in methadone maintenance treatment for very long or continue to use illicit opioids while in treatment.8,9 European studies have suggested that injectable di-acetylmorphine, the active ingredient in heroin, can be an effective adjunctive maintenance treatment for such persons.10–13 To investigate this possibility in North America, we conducted a randomized, controlled trial comparing injectable diacetylmorphine with oral methadone. Because of financial and logistical barriers in the United States, the trial could be conducted only in Canada.
METHODS
STUDY DESIGN, SETTING, AND PARTICIPANTS
The North American Opiate Medication Initiative (NAOMI) was an open-label, phase 3, randomized, controlled trial conducted in Montreal, Quebec, and Vancouver, British Columbia, from March 2005 through July 2008. Inclusion criteria consisted of opioid dependence (meeting three or more of seven criteria listed in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition,14 including tolerance or withdrawal), an age of 25 years or older, opioid use for at least 5 years, daily opioid injection, and no change in city of residence for at least 1 year. Participants must have had a minimum of two previous treatments for opioid dependence, including at least one attempt at methadone maintenance treatment in which they received 60 mg or more of methadone daily for at least 30 days during a 40-day period. Also, participants could not have been enrolled in methadone maintenance treatment within the previous 6 months. Exclusion criteria were severe medical or psychiatric conditions that are contraindications for diacetylmorphine, pregnancy, and involvement in the criminal justice system that could have resulted in extended incarceration during the study period.
RANDOMIZATION
A computer-generated randomization list of permuted blocks of two, four, and six was used. Patients were assigned to receive diacetylmorphine, methadone, or hydromorphone in a 45:45:10 ratio. Randomization was stratified according to center and according to the number of previous methadone treatments (two or fewer vs. three or more). Eligible participants were instructed to go to the treatment clinic on the following Monday morning, at which time they were first informed of their treatment assignment.
INTERVENTIONS
A total of 111 participants were randomly assigned to receive oral methadone, and 115 participants were randomly assigned to receive injectable di-acetylmorphine (diacetylmorphine hydrochloride, DiaMo Narcotics). In addition, 25 participants were randomly assigned to receive injectable hydromorphone instead of diacetylmorphine, for validation of the self-reported use of illicit heroin by means of urine testing. The investigators and participants were aware of whether the assigned study drug was oral methadone or one of the injectable drugs, but diacetylmorphine and hydromorphone were administered in a double-blind fashion. The injectable medications were self-administered under supervision in the treatment clinics up to three times daily, with a maximum daily dose of 1000 mg of diacetylmorphine. Patients receiving injectable medications could at any time switch partially or totally to oral methadone if such a switch was deemed appropriate by the patient and his or her physician. Methadone dosages and delivery were based on best practices and current clinical practice guidelines.15 Methadone was dispensed at the study clinic (in Vancouver only), other clinics, or community pharmacies on a daily basis. All patients were offered a comprehensive range of psychosocial and primary care services in keeping with Health Canada best practices.15 Study treatments were provided for 12 months, followed by a 3-month period during which injectable drugs were tapered in participants who were still receiving them, and treatment in these patients was switched to conventional therapies such as methadone. The study followed Good Clinical Practice guidelines16 and was approved by the Therapeutic Products Directorate of Health Canada and by the institutional review board at each site. All participants provided written informed consent.
OUTCOME MEASURES
Evaluations were performed at baseline and at 3, 6, 9, and 12 months at a separate research office that operated independently from the treatment clinic in each city. The first primary outcome was retention in addiction treatment at 12 months (defined as receipt of the study medication on at least 10 of the 14 days before the 12-month assessment, or confirmation of retention in any other treatment program or abstinence from opioids during this interval). Retention was assessed with the use of detailed data on daily prescription-drug use and, when possible, with the use of administrative data and pharmacy and physician records. The second primary outcome was reduction in illicit-drug use or other illegal activities. On the basis of composite scores on the European Addiction Severity Index17 (see the Supplementary Appendix, available with the full text of this article at NEJM.org), patients were considered to have a response at 12 months if they had an improvement of at least 20% from the baseline score for illicit-drug use or legal status (or both). In addition, to rule out deterioration in other variables, a patient with a response could have a decrease of 10% or more on at most one of the remaining composite scores. All participants lost to follow-up were considered not to have been retained in treatment and not to have had a response.
To ensure safety, participants who received injectable medication were assessed for 15 minutes before and 30 minutes after the injection period at every visit. Serious adverse events pertain to the 12-month treatment period and the 3-month transition period (see the Supplementary Appendix for additional details).
STATISTICAL ANALYSIS
Sample-size requirements for the study were calculated on the basis of the two primary outcomes, each at a two-sided 2.5% type I error rate. We determined that 114 patients per group would yield 80% power to detect prespecified absolute increases of 20 percentage points in outcome rates in the diacetylmorphine group as compared with the methadone group, with assumed rates in the latter group ranging from 25% to 50%.
Retention and response rates were calculated on an intention-to-treat basis and compared with the use of a two-sample test of proportions (chi-square test). Rate ratios and 95% confidence intervals were calculated for both primary outcomes. Logistic-regression models were used to compare subgroups (i.e., the Vancouver group vs. the Montreal group). Analysis of covariance of the treatment effect, with adjustment for baseline measures, was used for secondary analyses of continuous data. There were no interim analyses. All reported P values are two-sided and not adjusted for multiple testing.
RESULTS
PATIENTS
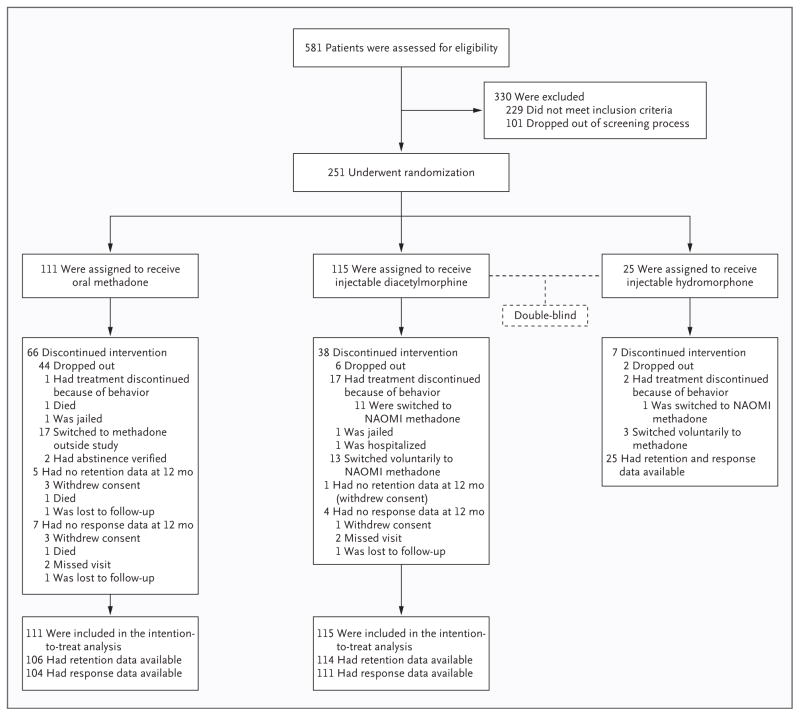
Of the 581 patients who were eligible for screening (Fig. 1), a total of 251 provided written informed consent and were randomly assigned to treatment. A total of 111 patients received oral methadone (44.2%), 115 patients received injectable diacetylmorphine (45.8%), and 25 patients received injectable hydromorphone (10.0%). We obtained 12-month retention data on 245 of 251 participants (97.6%) and response data on 240 of 251 participants (95.6%). The baseline characteristics of the groups were similar (Table 1). The severity of the opioid dependence in enrolled patients was indicated by long histories of injectable drug use, extensive involvement in criminal activity, and multiple attempts at treatment.
Figure 1. Randomization, Treatment, and Outcomes.
Behaviors that led to discontinuation of treatment were attempts to take the drug out of the clinic, threats, or intimidation. NAOMI denotes North American Opiate Medication Initiative.
Table 1.
Baseline Characteristics of the Patients.*
| Variable | Methadone (N = 111) |
Diacetylmorphine (N = 115) |
Hydromorphone (N = 25) |
Total (N = 251) |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| City — no. (%) | ||||
|
| ||||
| Montreal | 26 (23.4) | 27 (23.5) | 6 (24.0) | 59 (23.5) |
|
| ||||
| Vancouver | 85 (76.6) | 88 (76.5) | 19 (76.0) | 192 (76.5) |
|
| ||||
| Age — yr | 39.3±9.4 | 39.7±7.6 | 41.6±8.8 | 39.7±8.6 |
|
| ||||
| Male sex — no. (%) | 65 (58.6) | 73 (63.5) | 16 (64.0) | 154 (61.4) |
|
| ||||
| Aboriginal descent — no. (%) | 28 (25.2) | 25 (21.7) | 7 (28.0) | 60 (23.9) |
|
| ||||
| School education — yr | 11.0±2.2 | 10.8±2.6 | 11.2±2.7 | 10.9±2.4 |
|
| ||||
| Homeless or living in shelter or single-occupancy hotel room — no. (%) | 80 (72.1) | 88 (76.5) | 15 (60.0) | 183 (72.9) |
|
| ||||
| Charged in lifetime for any crime — no. (%) | 103 (92.8) | 110 (95.7) | 24 (96.0) | 237 (94.4) |
|
| ||||
| Illegal activities other than illicit-drug use in previous mo — no. (%) | 81 (73.0) | 87 (75.7) | 17 (68.0) | 185 (73.7) |
|
| ||||
| Health | ||||
|
| ||||
| Chronic medical problem — no. (%) | 56 (50.5) | 64 (55.7) | 14 (56.0) | 134 (53.4) |
|
| ||||
| HCV-positive — no. (%)† | 69 (62.2) | 74 (64.3) | 15 (60.0) | 158 (62.9) |
|
| ||||
| HIV-positive — no. (%)† | 12 (10.8) | 11 (9.6) | 1 (4.0) | 24 (9.6) |
|
| ||||
| Previous drug treatments — no. | 11.2±12.7 | 11.4±10.7 | 9.0±6.7 | 11.1±11.4 |
|
| ||||
| Previous attempts at methadone maintenance treatment — no. | 3.2±2.0 | 3.2±1.8 | 2.8±0.9 | 3.2±1.8 |
|
| ||||
| Drug use | ||||
|
| ||||
| Duration of injection-drug use — yr | 16.3±10.3 | 16.1±9.4 | 19.1±9.8 | 16.5±9.8 |
|
| ||||
| Use of illicit drugs during previous mo — no. of days | ||||
|
| ||||
| Heroin‡ | 27.4±5.7 | 26.6±7.3 | 26.3±8.4 | 26.9±6.8 |
|
| ||||
| Opioids‡ | 7.4±10.5 | 9.5±11.5 | 9.9±11.8 | 8.6±11.1 |
|
| ||||
| Cocaine powder‡ | 4.1±7.7 | 5.4±9.7 | 7.1±10.0 | 5.0±8.9 |
|
| ||||
| “Crack” cocaine§ | 12.7±13.3 | 15.1±13.2 | 8.5±10.3 | 13.4±13.1 |
Plus–minus values are means ±SD. HCV denotes hepatitis C virus, and HIV human immunodeficiency virus.
These infections were self-reported.
More than 95% of this drug was injected.
More than 95% of this drug was smoked.
DOSAGE
Overall, patients received diacetylmorphine a median of 2 times per day (interquartile range, 2 to 3) and collectively received a total of 89,924 diacetylmorphine injections. After excluding each participant’s initial 90 days of dose adjustment, the average daily dose of diacetylmorphine received was 392.3 mg when the drug was prescribed alone. Patients who were prescribed methadone maintenance treatment alone in NAOMI received a mean daily dose of 96.0 mg. For the 30 patients who were prescribed diacetylmorphine with methadone at some point during treatment (median number of days, 210; interquartile range, 113 to 262), the mean daily dose of diacetylmorphine was 365.5 mg and the mean daily dose of methadone was 34.0 mg.
PRIMARY OUTCOMES
For the outcome of a reduction in illicit-drug use or other illegal activities, 67.0% of the patients in the diacetylmorphine group were classified as having a response, as compared with 47.7% of patients in the methadone group (rate ratio, 1.40; 95% confidence interval [CI], 1.11 to 1.77; P = 0.004) (Table 2). The rate of retention in treatment for addiction in the diacetylmorphine group was 87.8%, as compared with 54.1% in the methadone group (rate ratio, 1.62; 95% CI, 1.35 to 1.95; P<0.001). Of the patients assigned to diacetylmorphine, 23 (20.0%) switched to methadone. Neither primary outcome showed significant treatment-by-center interaction. Retention and response rates among patients in the hydromorphone group were 88% and 64%, respectively.
Table 2.
Primary Outcomes at 12 Months.*
| Variable | Methadone (N = 111) |
Diacetylmorphine (N = 115) |
Rate Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| number (percent) | ||||
|
| ||||
| Reduction in illicit-drug use or other illegal activities | 53 (47.7) | 77 (67.0) | 1.40 (1.11–1.77) | 0.004 |
|
| ||||
| Reduction in illicit-drug use alone | 15 (13.5) | 26 (22.6) | ||
|
| ||||
| Reduction in other illegal activities alone | 6 (5.4) | 1 (0.9) | ||
|
| ||||
| Reduction in both illicit-drug use and other illegal activities | 32 (28.8) | 50 (43.5) | ||
|
| ||||
| Retention in addiction treatment | 60 (54.1) | 101 (87.8) | 1.62 (1.35–1.95) | <0.001 |
|
| ||||
| NAOMI diacetylmorphine | NA | 77 (67.0) | ||
|
| ||||
| NAOMI methadone maintenance treatment | 45 (40.5) | 21 (18.3) | ||
|
| ||||
| Other methadone maintenance treatment | 13 (11.7) | 2 (1.7) | ||
|
| ||||
| Other, nonmethadone treatment | 0 | 0 | ||
|
| ||||
| Abstinence | 2 (1.8) | 1 (0.9) | ||
Rate ratios are for the diacetylmorphine group as compared with the methadone group. NA denotes not applicable, and NAOMI North American Opiate Medication Initiative.
INDIVIDUAL SCORES IN THE NINE DOMAINS OF THE RESPONSE OUTCOME
The composite scores for drug use and for illegal activities according to the European Addiction Severity Index improved in both groups (Table 3). The diacetylmorphine group had significant improvement in six of the seven remaining subscales, as compared with improvement in two subscales in the methadone group. After adjustment for baseline values, the scores in the diacetylmorphine group improved significantly more than the scores in the methadone group in four of the composite scores, including that for drug use.
Table 3.
Subscale Composite Scores Based on the European Addiction Severity Index, from Baseline Assessment through 12 Months.
| Variable and Study Group | Baseline Score | Score at 3 Mo | Score at 6 Mo | Score at 9 Mo | Score at 12 Mo | Estimated Mean Change from Baseline to 12 Mo | P Value | Between-Group Difference in Adjusted Mean Scores | P Value† |
|---|---|---|---|---|---|---|---|---|---|
| Illicit-drug use | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.53 | 0.29 | 0.31 | 0.31 | 0.26 | −0.23 | <0.01 | −0.07 | <0.01 |
|
| |||||||||
| Methadone | 0.54 | 0.41 | 0.39 | 0.36 | 0.33 | −0.16 | <0.01 | ||
|
| |||||||||
| Illegal activities | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.37 | 0.17 | 0.21 | 0.22 | 0.20 | −0.17 | <0.01 | −0.03 | 0.12 |
|
| |||||||||
| Methadone | 0.35 | 0.24 | 0.23 | 0.22 | 0.18 | −0.12 | <0.01 | ||
|
| |||||||||
| Medical status | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.38 | 0.32 | 0.29 | 0.29 | 0.26 | −0.09 | <0.01 | −0.06 | 0.06 |
|
| |||||||||
| Methadone | 0.37 | 0.36 | 0.36 | 0.37 | 0.29 | −0.02 | 0.59 | ||
|
| |||||||||
| Psychiatric status | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.21 | 0.15 | 0.17 | 0.17 | 0.16 | −0.05 | <0.01 | −0.06 | <0.01 |
|
| |||||||||
| Methadone | 0.18 | 0.22 | 0.21 | 0.21 | 0.20 | 0.03 | 0.07 | ||
|
| |||||||||
| Economic status | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.91 | 0.87 | 0.86 | 0.88 | 0.88 | −0.04 | <0.01 | 0.03 | 0.22 |
|
| |||||||||
| Methadone | 0.89 | 0.81 | 0.83 | 0.85 | 0.84 | −0.07 | <0.01 | ||
|
| |||||||||
| Employment satisfaction | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.25 | 0.13 | 0.10 | 0.08 | 0.10 | −0.15 | <0.01 | −0.05 | 0.02 |
|
| |||||||||
| Methadone | 0.26 | 0.17 | 0.19 | 0.15 | 0.11 | −0.11 | <0.01 | ||
|
| |||||||||
| Family relations | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.09 | 0.06 | 0.06 | 0.05 | 0.07 | −0.04 | <0.05 | −0.02 | 0.21 |
|
| |||||||||
| Methadone | 0.08 | 0.08 | 0.08 | 0.08 | 0.06 | −0.01 | 0.60 | ||
|
| |||||||||
| Social relations | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.13 | 0.06 | 0.09 | 0.08 | 0.09 | −0.05 | <0.01 | −0.03 | 0.05 |
|
| |||||||||
| Methadone | 0.10 | 0.13 | 0.10 | 0.09 | 0.08 | 0.00 | 0.83 | ||
|
| |||||||||
| Alcohol use | |||||||||
|
| |||||||||
| Diacetylmorphine | 0.04 | 0.02 | 0.03 | 0.03 | 0.04 | −0.01 | 0.35 | −0.01 | 0.11 |
|
| |||||||||
| Methadone | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.00 | 0.82 | ||
Subscale scores in the European Addiction Severity Index range from 0 to 1, with higher scores indicating more severe problems.
P values are for the between-group difference in mean scores adjusted for baseline.
USE OF ILLICIT OPIOIDS AND COCAINE
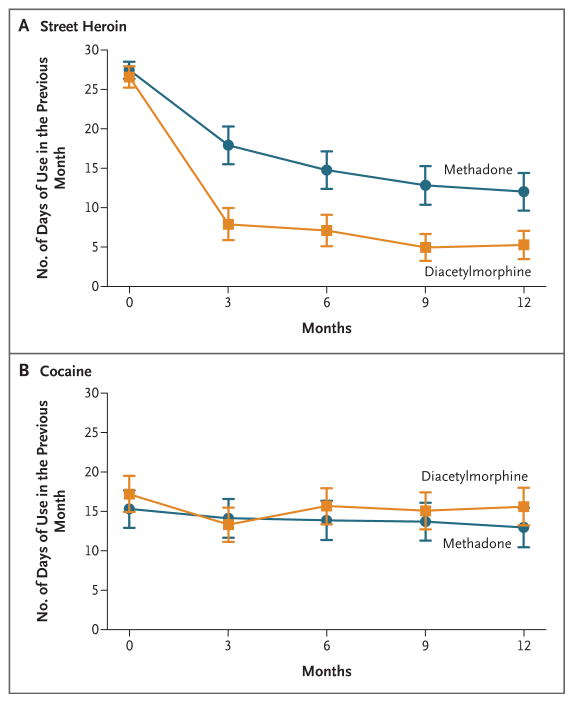
The mean number of days of use of illicit heroin in the previous month decreased from 26.6 to 5.3 in the diacetylmorphine group and from 27.4 to 12.0 in the methadone group (P<0.001). The number of days of cocaine use did not change significantly in either group (Fig. 2). Both groups spent a median of $1,200 (U.S. dollars) on illicit drugs in the month before baseline; at 12 months, this amount was reduced to $320 in the diacetylmorphine group and $400 in the methadone group.
Figure 2. Number of Days of Illicit-Drug Use in the Previous Month, According to Treatment Assignment.
The numbers of days of illicit heroin use (Panel A) and cocaine use (Panel B) are shown. The I bars represent 95% confidence intervals.
In the group of 25 patients who were randomly assigned to the hydromorphone group, there were 46 follow-up visits during the 12-month study period at which no use of illicit heroin in the previous 30 days was reported (among 17 different persons). None of the 46 urine samples obtained at these visits tested positive for 6-monoacetylmorphine or morphine. Of 23 patients in the hydromorphone group who were asked at 12 months which drug they thought they were receiving, none reported that the drug was definitely hydromorphone.
SERIOUS ADVERSE EVENTS
A total of 79 serious adverse events were reported in 54 patients: 18 in the methadone group, 51 in the diacetylmorphine group, and 10 in the hydromorphone group (Table 4). One death occurred during the observation period — a patient randomly assigned to receive methadone died from an opioid overdose, according to a police report. None of the serious adverse events in the methadone group were considered to be related to the administration of study methadone during the study period. Overall, infections, overdoses, and seizures were the most frequent serious adverse events reported, the latter two being the most frequent events related to diacetylmorphine. All overdoses related to diacetylmorphine that required treatment with naloxone were classified as being life-threatening and hence serious adverse events. All these events resolved without sequelae or hospital admission. Seven of the 10 patients who required naloxone subsequently reported that they had also used other drugs, such as benzodiazepines or cocaine, at the time of the overdose. All seven seizures that occurred at the clinic were classified as having some relationship to the study medication: two occurred in a patient with a history of epilepsy and the remaining five occurred in patients who had used cocaine or benzodiazepines before the seizure.
Table 4.
Serious Adverse Events Related to the Study Medications.
| Serious Adverse Events | Methadone (N = 111) |
Diacetylmorphine (N = 115) |
Hydromorphone (N = 25) |
|
|---|---|---|---|---|
| no./total no.(%) | ||||
|
| ||||
| Not judged by investigators to be related to study drug | 18/18 (100) | 27/51 (53) | 5/10 (50) | |
|
| ||||
| Judged to be related to study drug | 0/18 | 24/51 (47) | 5/10 (50) | |
|
| ||||
| Sepsis and other infections | 0/0 | 2/24 (8) | 0/5 | |
|
| ||||
| Cocaine-induced psychosis | 0/0 | 1/24 (4) | 0/5 | |
|
| ||||
| Seizures* | 0/0 | 7/24 (29) | 0/5 | |
|
| ||||
| Diseases of the respiratory system | 0/0 | 1/24 (4) | 0/5 | |
|
| ||||
| Abscesses and cellulitis | 0/0 | 1/24 (4) | 2/5 (40) | |
|
| ||||
| Suicidal ideation | 0/0 | 0/24 | 1/5 (20) | |
|
| ||||
| Fractures | 0/0 | 1/24 (4) | 0/5 | |
|
| ||||
| Opioid overdoses* | 0/0 | 11/24 (46) | 2/5 (40) | |
A total of 20 overdoses and seizures occurred in 18 patients.
DISCUSSION
In this trial, patients assigned to receive injectable diacetylmorphine were more likely to stay in treatment and to reduce their use of illegal drugs and other illegal activities than patients assigned to receive oral methadone. These findings are consistent with the results of European studies that suggest greater effectiveness of diacetylmorphine than methadone as maintenance treatment for long-term, treatment-refractory opioid use.10,12,13 Two of these trials showed no differences between groups in the rate of retention in treatment for addiction. However, the fact that control patients were eligible to receive diacetylmorphine at the end of the study period may have introduced a bias in the observed retention rates. In addition, patients currently enrolled in methadone maintenance treatment were eligible for the European trials but not for the present study. Although the definitions of clinical response varied among the trials, all of them considered the same variables (drug use, illegal activities, health, and social adjustment) and showed greater effectiveness of diacetylmorphine than of methadone for maintenance treatment.
Secondary analyses showed that both groups had significant improvement in many of the variables that were evaluated. The diacetylmorphine group had greater improvements with respect to medical and psychiatric status, economic status, employment situation, and family and social relations. These results are particularly noteworthy in view of the nature of the population and the time frame. The fact that patients who received diacetylmorphine had significant improvement in these areas suggests a positive treatment effect beyond a reduction in illicit-drug use or other illegal activities.
The lack of effective pharmacologic treatments for cocaine addiction poses a considerable challenge for the treatment of opioid addiction in patients who use more than one drug.9 One argument against diacetylmorphine maintenance is that patients receiving free diacetylmorphine might increase their spending on cocaine, other drugs, or both. Such an increase has not been reported in other studies.10,12,13,18 Moreover, we observed an important overall reduction in the money spent on illicit drugs in both groups.
Overdoses and seizures were the two most common serious adverse events related to diacetylmorphine.19,20 Sixteen of the 115 patients randomly assigned to receive diacetylmorphine had a life-threatening overdose or seizure during the study. Because the study included close medical supervision, these serious adverse events were promptly treated, and all patients recovered. Heroin is a respiratory depressant, and heroin injection is less safe than oral treatments.21,22 When injected opioids are taken under the supervision of health care staff, overdoses and seizures have been shown to be effectively treated.10,12,13,22 Had these overdoses occurred under circumstances in which no medical help was immediately available, as would be the case with the use of illicit heroin, the outcomes might have been worse. Our safety data suggest that diacetylmorphine should be delivered in settings where prompt medical intervention is available.
The investigators and patients were aware of the assignment to methadone maintenance treatment versus diacetylmorphine. Although a double-blind comparison was theoretically possible with the use of placebo injections and placebo methadone, the investigators and patients probably would have been aware of the group assignments, given the different pharmacokinetic properties of the study drugs. Moreover, such a protocol would have eliminated the natural advantages of methadone maintenance treatment (e.g., once-daily oral administration) that might have led to its greater acceptance.
Concerns have been raised about reliance on self-reported data in studies involving injection-drug users. However, the Addiction Severity Index has been widely used in North America and has consistently shown validity and reliability in studies of addiction treatment.23 Evaluations based on self-report have been shown to be reliable when conducted by people with no control or power over the treatment process,24 as in the present study. Self-reported nonuse of illicit heroin was confirmed by means of urine testing at 100% of 46 visits in the group of patients randomly assigned to receive hydromorphone (the double-blind portion of the study).
It could be argued that another opioid-substitution medication, such as buprenorphine, might have been used as a comparison drug instead of methadone. The available evidence does not support this suggestion: a recent Cochrane review concluded that buprenorphine maintenance was significantly less effective than methadone maintenance.25 Thus, methadone maintenance remains the standard treatment and the proper comparison drug for an experimental substitution therapy.
Some persons probably volunteered for the study in the hope of being assigned to receive diacetylmorphine; hence, we anticipated a substantial dropout rate in the methadone group. To partially address this possibility, patients who dropped out of the treatment program were allowed to return to the clinic at any time during the 12-month period. We also offered an optimized methadone program that addressed the likely problems that participants had encountered in previous attempts at methadone maintenance treatment, such as long waiting times, limited primary care and psychosocial services, punitive treatment approaches, and suboptimal dosing. Nevertheless, a question can be raised with respect to the validity of an intention-to-treat comparison in a trial when a significant treatment dropout rate is observed in the control group. Such a comparison is valid, in our view, if the dropout rate reflects the true lack of acceptance that would be observed if methadone maintenance treatment alone were offered in the clinical setting.
An unexpected finding was that patients who received the injectable drugs could not accurately determine whether they were receiving diacetylmorphine or hydromorphone. We also observed similar outcomes with these two drugs, although the study was not powered for such a comparison. Should hydromorphone be proved to be non-inferior to diacetylmorphine, the benefits of injectable opioid maintenance might be achievable without the emotional and regulatory barriers often presented by heroin maintenance.
In this trial, both diacetylmorphine treatment and optimized methadone maintenance treatment resulted in high retention and response rates. Methadone, provided according to best-practice guidelines, should remain the treatment of choice for the majority of patients. However, there will continue to be a subgroup of patients who will not benefit even from optimized methadone maintenance. Prescribed, supervised use of diacetylmorphine appears to be a safe and effective adjunctive treatment for this severely affected population of patients who would otherwise remain outside the health care system.
Supplementary Material
Acknowledgments
Supported by grants from the Canadian Institutes of Health Research, the Canada Foundation for Innovation, the Canada Research Chairs Program, the University of British Columbia, Providence Health Care, the University of Montreal, Centre de Recherche et Aide aux Narcomanes, the Government of Quebec, Vancouver Coastal Health, and the BC Centre for Disease Control.
We thank N. Laliberté, C. Gartry, K. Sayers, P.-A. Guevremont, P. Schneeberger, J. Chettiar, K. Lock, J. Lawlor, P. Pelletier, S. Maynard, M.-I. Turgeon, G. Brunelle, A. Chan, S. MacDonald, T. Corneil, J. Geller, B. Nosyk, S. Jutha, S. Chai, M. Piacsezna, S. Sizto, many other staff members, and members of the data and safety monitoring board (A. Marlatt, N. El-Guebaly, J. Raboud, and D. Roy) for their dedication; the many U.S. scientists who contributed to the early design discussions but ultimately were unable to participate in the trial; and the NAOMI trial participants.
Footnotes
Dr. Brissette reports receiving consulting and lecture fees from Schering-Plough. No other potential conflict of interest relevant to this article was reported.
References
- 1.Cami J, Farre M. Drug addiction. N Engl J Med. 2003;349:975–86. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- 2.World drug report 2008. Vienna: United Nations Office on Drugs and Crime; 2008. [Google Scholar]
- 3.Popova S, Rehm J, Fischer B. An overview of illegal opioid use and health services utilization in Canada. Public Health. 2006;120:320–8. doi: 10.1016/j.puhe.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309:997–1001. doi: 10.1136/bmj.309.6960.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2003;2:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- 6.Gowing L, Farrell M, Bornemann R, Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2004;4:CD004145. doi: 10.1002/14651858.CD004145.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–7. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein MF, Deren S, Kang SY, Des Jarlais DC, Magura S. Evaluation of an alternative program for MMTP drop-outs: impact on treatment re-entry. Drug Alcohol Depend. 2002;66:181–7. doi: 10.1016/s0376-8716(01)00199-5. [DOI] [PubMed] [Google Scholar]
- 9.Termorshuizen F, Krol A, Prins M, Geskus R, van den Brink W, van Ameijden EJ. Prediction of relapse to frequent heroin use and the role of methadone prescription: an analysis of the Amsterdam Cohort Study among drug users. Drug Alcohol Depend. 2005;79:231–40. doi: 10.1016/j.drugalcdep.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 10.van den Brink W, Hendriks VM, Blanken P, Koeter MW, van Zwieten BJ, van Ree JM. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ. 2003;327:310. doi: 10.1136/bmj.327.7410.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehm J, Gschwend P, Steffen T, Gutzwiller F, Dobler-Mikola A, Uchtenhagen A. Feasibility, safety, and efficacy of injectable heroin prescription for refractory opioid addicts: a follow-up study. Lancet. 2001;358:1417–23. doi: 10.1016/S0140-6736(01)06529-1. [DOI] [PubMed] [Google Scholar]
- 12.March JC, Oviedo-Joekes E, Perea-Milla E, Carrasco F. Controlled trial of prescribed heroin in the treatment of opioid addiction. J Subst Abuse Treat. 2006;31:203–11. doi: 10.1016/j.jsat.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry. 2007;191:55–62. doi: 10.1192/bjp.bp.106.026112. [DOI] [PubMed] [Google Scholar]
- 14.Diagnostic and statistical manual of mental disorders, 4th ed: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 15.Health Canada. Best practices in methadone maintenance treatment. Ontario: Minister of Public Works and Government Services Canada; 2002. [Google Scholar]
- 16.ICH Harmonised Tripartite Guideline: guideline for good clinical practice. London: European Medicines Agency; 2002. [Google Scholar]
- 17.Kokkevi A, Hartgers C. EuropASI: European adaptation of a multidimensional assessment instrument for drug and alcohol dependence. Eur Addict Res. 1995;1:208–10. [Google Scholar]
- 18.Blattler R, Dobler-Mikola A, Steffen T, Uchtenhagen A. Decreasing intravenous cocaine use in opiate users treated with prescribed heroin. Soz Praventivmed. 2002;47:24–32. doi: 10.1007/BF01318402. [DOI] [PubMed] [Google Scholar]
- 19.Manuel traitement avec prescription d’héroine: directives, recommandations, informations. Berne, Switzerland: Office Fédéral de la Santé Publique; 2004. [Google Scholar]
- 20.Brust JC. Seizures and substance abuse: treatment considerations. Neurology. 2006;67(Suppl 4):S45–S48. doi: 10.1212/wnl.67.12_suppl_4.s45. [DOI] [PubMed] [Google Scholar]
- 21.Ladewig D, Dursteler-MacFarland KM, Seifritz E, Hock C, Stohler R. New aspects in the treatment of heroin dependence with special reference to neurobiological aspects. Eur Psychiatry. 2002;17:163–6. doi: 10.1016/s0924-9338(02)00644-2. [DOI] [PubMed] [Google Scholar]
- 22.Stoermer R, Drewe J, Dursteler-Mac Farland KM, et al. Safety of injectable opioid maintenance treatment for heroin dependence. Biol Psychiatry. 2003;54:854–61. doi: 10.1016/s0006-3223(03)00290-7. [DOI] [PubMed] [Google Scholar]
- 23.Thomas McLellan A, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict. 2006;15:113–24. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- 24.Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51:253–63. doi: 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 25.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;2:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.