Abstract
Here we describe the use of data independent acquisition (DIA) on a Q-Exactive mass spectrometer for the detection and quantification of peptides in complex mixtures using the Skyline Targeted Proteomics Environment (freely available on-line at http://skyline.maccosslab.org). The systematic acquisition of MS/MS spectra by DIA is in contrast to DDA where the acquired MS/MS spectra are only suitable for identification of a stochastically sampled set of peptides. Similar to selected reaction monitoring (SRM), peptides can be quantified from DIA data using targeted chromatogram extraction. Unlike SRM, data acquisition is not constrained to a pre-determined set of target peptides. In this protocol, a spectral library is generated using data dependent acquisition (DDA), and chromatograms are extracted from the DIA data for all peptides in the library. Similar to SRM, quantification using DIA data is based on the area under the curve of extracted MS/MS chromatograms. Additionally, a quality control method suitable for DIA based on targeted MS/MS acquisition is detailed. Not including time spent acquiring data, and time for database searching, the procedure takes about 1–2 hours to complete. Typically, data acquisition requires roughly 1–4 hours per sample and a database search will take 0.5–2 hours to complete.
INTRODUCTION
Data independent acquisition1,2 (DIA) is a relatively new mass spectrometry-based technique for systematically collecting tandem mass spectrometry data3. A dataset acquired by DIA has been described as a “molecular snapshot” of the sample4 because the data can be queried for any detectable peptide. Both the presence of a peptide and changes in abundance across samples can be monitored2. Therefore, DIA is well-suited for applications where a researcher needs to measure hundreds of proteins (or more), or a researcher desires the flexibility to investigate multiple hypotheses without having to acquire additional data sets. This protocol describes how to acquire and analyze mass spectrometry data using DIA for the analysis of peptides.
Method setup for DIA is simpler when compared with targeted acquisition approaches like selected reaction monitoring (SRM) or parallel reaction monitoring (PRM), which require complex scheduling to measure beyond about 50 peptides5–7.
DIA has been used almost exclusively in “bottom-up” mass spectrometry analyses where proteins are digested into peptides using a proteolytic enzyme (e.g., trypsin) prior to analysis8. In contrast to intact proteins, the resulting peptides have reduced physiochemical diversity, resulting in simplified sample preparation and increased mass spectrometric sensitivity9. After digestion, peptides are separated by reversed-phase high performance liquid chromatography (RP-HPLC) and emitted directly into a mass spectrometer via an electrospray ionization (ESI) interface10. On trapping instruments (such as the Q-Exactive), ions transmitted into the mass spectrometer are collected over a short period (often less than 100 milliseconds) and analyzed by mass spectrometry (MS) or tandem mass spectrometry (MS/MS)11. An MS spectrum consists of the mass-to-charge ratios (m/z) and signal intensity (charges/second) of the collected intact peptide ions. An MS/MS spectrum consists of m/z versus intensity of fragment ions derived from peptide precursors that had been isolated by mass prior to fragmentation using collisions with an inert gas (Figure 1). In complex samples (e.g. mammalian), quantification using the MS/MS signal is generally more sensitive than using the MS signal alone due to increased selectivity 2,4,12,13. In complex samples, there is a greater likelihood that an MS signal for a peptide will have interference in the form of chemical noise from another analyte in the sample with the same m/z (e.g. a peptide with the same amino acid composition but different sequence) or a very close m/z that cannot be resolved by the mass analyzer. In these cases, quantification using the MS/MS signal will be more sensitive because the more selective fragment ion measurements will be less prone to chemical noise interference. For low complexity samples (e.g. simple prokaryotes, selectively enriched samples), accurate mass measurement of intact peptide mass, combined with chromatographic retention time, can be sufficiently selective for quantitative measurements14. However, for larger proteomes (e.g., mammalian) selective quantification requires the additional measurement of peptide fragment ions.
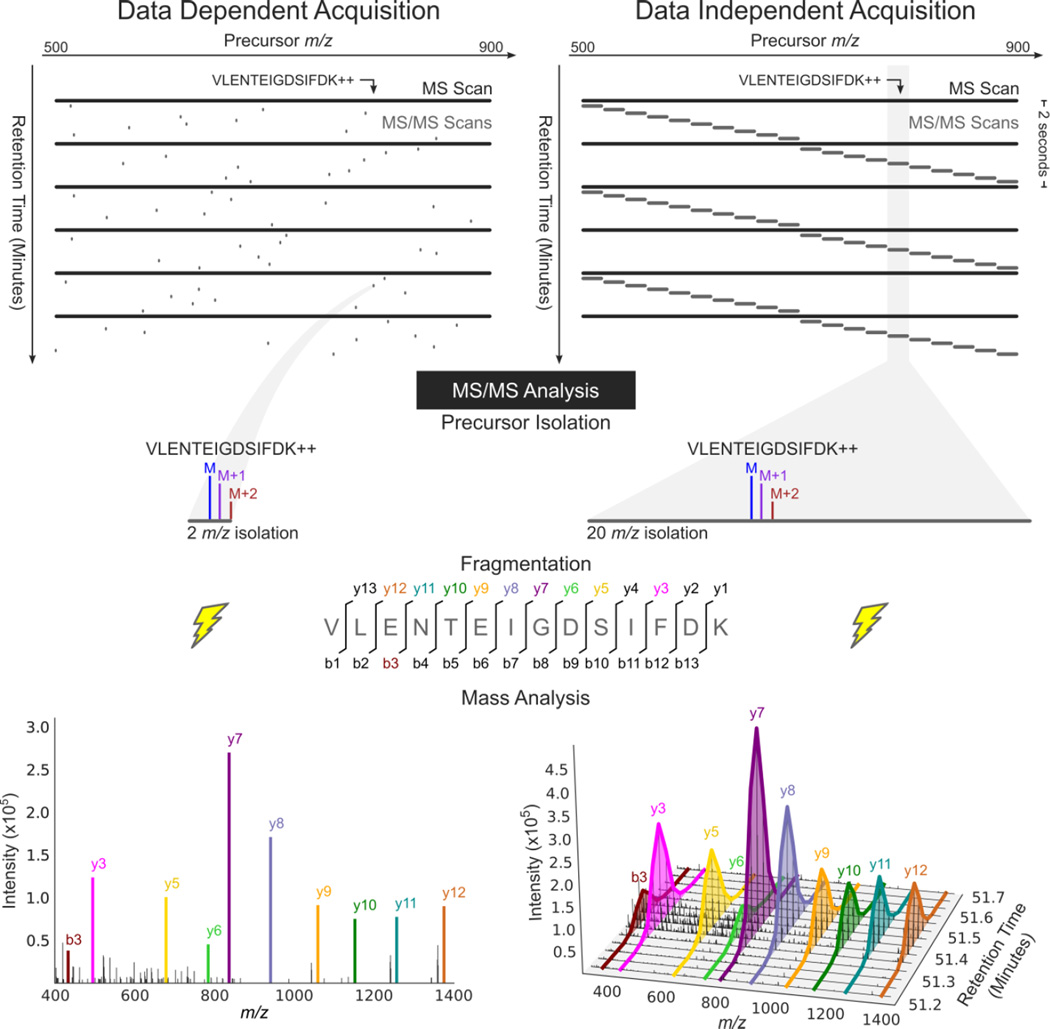
Figure 1. MS/MS analysis in data dependent acquisition and data independent acquisition.
DDA acquires MS/MS scans with narrow isolation windows centered on peptide precursors detected in an MS scan. DIA acquires MS/MS scans with wide isolation windows that do not target any particular peptide precursor. Instead, the scans are arranged side-by-side to collectively cover a desired precursor m/z range (500–900 m/z here) comprehensively. Fragment ion information for the peptide precursor VLENTEIGDSIFDK++ is present in a single MS/MS spectrum in a DDA analysis, but can be extracted over time from DIA data and used for quantification due to the repetitive MS/MS sampling cycle of DIA.
There are many possible strategies for collecting data independent acquisition data3. The DIA technique detailed here provides a starting point suitable for many applications. This method acquires both MS and MS/MS data for all molecular species between m/z 500 and 900. The mass spectrometer is programmed to acquire a repeated cycle of 20 MS/MS scans with contiguous isolation windows, each being 20 m/z wide. The first MS/MS spectrum contains fragments derived from peptide precursor ions isolated from the range from m/z 500–520, the next m/z 520–540, and so on until the 20th MS/MS scan which analyzes m/z 880–900 (Figure 1). An MS scan is acquired every 10th MS/MS scan. On a Q-Exactive, this cycle repeats every ~2 seconds, which is fast enough to perform analysis of peptides on a chromatographic time scale.
To detect and quantify a peptide, mass chromatograms (m/z-specific signal intensity over time) are extracted for a set of likely precursor charge states (e.g. +2, +3, +4 for tryptic peptides) and their associated fragment ions2,4,15. This targeted chromatogram extraction produces data that can be analyzed in fundamentally the same way as SRM data. However, DIA data acquisition differs from SRM because the precursor isolation window for MS/MS scans is much wider, and does not target any specific precursor. In this way, DIA combines untargeted data acquisition with targeted data analysis4. The quantitative metric for each peptide is calculated using the background subtracted area under the curve of the precursor or fragment ion chromatograms over the peptide elution. Changes in these peak areas can be statistically evaluated using packages such as MSStats16, used in conjunction with Skyline17, to determine which of a set of assayed peptides have a statistically significant increase or decrease in abundance between samples.
It is useful, although not strictly necessary, to have prior knowledge of when a peptide elutes from the chromatography column (i.e., retention time) to detect and quantify the peptide using DIA data1,18. In this regard, having a spectral library can aid in the analysis of DIA data because it contains retention time information and reference MS/MS spectra for each peptide. In this protocol, a spectral library is generated from data dependent acquisition (DDA) data acquired on each type of biological sample to aid in the interpretation of DIA data. However, pre-existing spectral libraries can also be used.
The Skyline software platform aids in both the acquisition and interpretation of data in this protocol. Skyline is a familiar tool to many for the generation of instrument methods for SRM data acquisition and interpretation of the resulting data. However, it is also capable of interpreting DIA data in a similar manner that it is used for SRM. Because both SRM and DIA data are based on the analysis of MS/MS chromatograms (selected and extracted respectively), the processing (chromatogram peak integration) and visualization of data acquired using these two methods is very similar within Skyline (Figure 1). Therefore, the DIA workflow described in this protocol should be familiar to those with experience using Skyline for SRM. This familiarity combined with the availability (free and open-source), vendor-neutrality, extensive documentation, and active support of Skyline make it an attractive option for processing DIA data. There are other software platforms (ex. ABSciex PeakView, Biognosys Spectronaut, OpenSWATH19, Thermo Pinpoint, and Waters Protein Lynx Global Server) that support the interpretation of DIA data, although only a subset can currently analyze the data acquired using the technique in this protocol (OpenSWATH and Pinpoint).
Experimental design
There is no “one size fits all” DIA method. Some key considerations in designing an optimal DIA method are sample complexity, chromatography conditions, the mass spectrometer being used, and the desired sensitivity. The method presented in this protocol is a recommended starting point for analysis of a complex sample (yeast lysate) on a Q-Exactive using HPLC-MS/MS. However, there are many parameters of a DIA method that may need to be adjusted depending on the experiment. Some of the key performance characteristics of a DIA method are:
MS/MS isolation width
The MS/MS isolation width alters the precursor selectivity of the method. With wide isolation windows, precursor selectivity is low, many precursors are co-fragmented, and highly chimeric MS/MS spectra are generated20. These highly chimeric spectra have increased chemical noise which compromises the sensitivity of the method. The more complex a sample is, the more sensitivity will be improved by reducing the MS/MS isolation width12. This protocol uses an isolation width of 20 m/z. This should be increased if the sample is low complexity, a larger m/z range needs to be analyzed, or the better sampling of each chromatographic peak is required (Table 1)and decreased if greater sensitivity is required.
Table 1. Guide to modifying the DIA method.
| Goal | Selectivity |
m/z Range Covered |
Number of Sample Injections |
Chromatographic Sampling Rate |
|---|---|---|---|---|
| Increase sensitivity | ||||
| Reduce isolation width | ↑ | ↓ | ||
| Reduce isolation width and use multiple injections per sample |
↑ | ↑ | ||
| Increase max ion inject time* | ↓ | |||
| Increase resolving power and max ion inject time* |
↑ | ↓↓ | ||
| Sample more peptides | ||||
| Use multiple injections per sample |
↑ | ↑ | ||
| Increase isolation width | ↓ | ↑ | ||
|
Improve chromatogram sampling |
||||
| Increase isolation width | ↓ | ↑ | ||
| Reduce m/z range covered | ↓ | ↑ |
Instruments with an ion trap mass analyzer that use automatic gain control (e.g. Thermo Scientific LTQ, Velos, Orbitrap, and Exactive series).
Chromatographic sampling rate
The sampling rate is often referred to as the “duty cycle” of the method; in the context of DIA, we define the chromatographic sampling rate as how frequently over time a particular precursor will be sampled by MS or MS/MS as it elutes. To accurately measure the signal for a precursor, at least 8 (and preferably more than 10) measurements must be made as the precursor elutes21. Therefore, if the average width of a chromatographic peak is ~30 seconds, the duty cycle must be 3 seconds or less to measure 10 points across each peak. In this protocol, each precursor is sampled every 2 seconds by MS/MS and every second by MS.
m/z range covered
The range of precursor m/z that is analyzed by MS/MS in the experiment. In this protocol, the m/z range covered is 500–900 m/z because this region is very peptide-dense in a tryptic digest22. This region is roughly two times as peptide dense as the rest of the m/z range23. However, there are still peptides that will fall outside of this range that would require an expanded m/z range to cover. The m/z range may need to be reduced or expanded depending on the scan speed and ion optics23 of the mass spectrometer used, desired assay sensitivity, and/or required chromatographic sampling rate (Table 1).
Resolving power
The resolving power is the ability of the mass analyzer to resolve two nearby peaks. High resolving power can improve quantitation by resolving nearby interfering MS or MS/MS peaks from the desired signal peak24. On low resolving power mass analyzers, such as a linear ion trap, it may be necessary to decrease the MS/MS isolation width (increase precursor selectivity) to measure peptides in a complex sample12,20. Additionally, on Fourier-transform (FT-ICR, Orbitrap) and ion trap mass analyzers25, the resolving power impacts the spectrum acquisition rate (higher resolving power reduces spectrum acquisition rate) . On Fourier-transform mass analyzers, this is due to a longer transient acquisition required for higher resolving power. On ion trap instruments, this is due to a slower scan speed (kDa/sec) required for higher resolving power. In this protocol, the resolving power is set to 17,500 at 200 m/z for the MS/MS scans, and 35,000 at 200 m/z for MS scans.
Mass accuracy
Mass measurement accuracy is a metric for how close a measured m/z is to its theoretical value. High mass measurement accuracy increases confidence that the signal measured is qualitatively the correct target peptide of interest24,26–28
AGC Target and Maximum ion injection time
These parameters apply to ion-trapping instruments only. The AGC target is the target number of charges placed in the trap for an MS or MS/MS scan. It is preferable to “fill” the trap to its maximum capacity (limited by space-charging effects) to achieve the greatest sensitivity and dynamic range. However, it is also necessary to impose a maximum inject time – the maximum amount of time spent filling the trap in an attempt to reach the AGC target – to maintain the required scan rate. In this protocol, the AGC target for MS and MS/MS scans is set to 1,000,000 ions and the maximum injection time is set to 55 milliseconds for MS and “auto” for MS/MS scans respectively (see Supplementary Note).
Number of sample injections
In this protocol, the analysis is completed in a single sample injection. However, if multiple sample injections (i.e. multiple liquid chromatography runs) are used for each sample, selectivity can be improved by reducing the MS/MS isolation width, or the m/z range covered can be increased.
The DIA method described in this protocol can be altered to improve sensitivity, cover more peptides, or increase the chromatogram sampling rate (Table 1). The protocol is not specific for any particular liquid chromatography setup. However, it is optimal for an HPLC separation that generates peaks with an average full-width at half max (FWHM) of ~15 seconds. If the peak width is expected to be much different, the protocol should be modified (Table 1).
Similarly, the protocol is not specific to a particular quality control (QC) standard. However, if too many QC peptides are analyzed in a single method, the chromatographic sampling rate may suffer. The QC sample and HPLC setup used to generate example data (see ANTICIPATED RESULTS) are described in the Supplementary Methods.
Because Skyline is a vendor-neutral platform, it can be used to generate DIA methods and analyze data for almost any DIA capable mass spectrometer. However, the optimal parameters for the DIA method will vary depending on the scan rate, resolving power, dynamic range, and sensitivity of the mass analyzer.
To generate a spectral library, data dependent acquisition (DDA) data is acquired on each type of sample. Not all samples need to be analyzed in the generation of the spectral library. However, the diversity of samples being analyzed should be represented in the set of samples chosen for a spectral library so each potential protein of interest is present in at least one of the samples included in the library. The DDA parameters used in this protocol may not be optimal depending on the type of sample being used and chromatography conditions. Optimization of DDA parameters for analysis of complex samples on a Q-Exactive is a topic that has been explored previously29 and is outside the scope of this manuscript. SEQUEST30 is used for database searching combined with Percolator31 to calculate the statistical significance of peptide-spectrum matches. However, there are many other database search pipelines that are supported by Skyline and can be used in lieu of SEQUEST and Percolator. The tools currently supported are PeptideProphet, SpectrumMill, OMSSA, PEAKS DB, Morpheus, X! Tandem, Mascot, Protein Pilot, ID Picker (Myrimatch), PRIDE, MaxQuant, Proteome Discoverer, Scaffold, ByOnic, MSGF+, and ProteinLynx Global Server. More information can be found at http://proteome.gs.washington.edu/software/bibliospec.
Comparison with other methods
The performance of the DIA technique presented in this protocol is difficult to compare to the performance of other DIA techniques because the performance of any method is dependent on the sample being analyzed and liquid chromatography conditions. However, these techniques can be compared based on the key metrics presented in the Experimental Design section.
SWATH
The technique presented in this protocol is most similar to the SWATH4 technique implemented on quadrupole time-of-flight (Q-TOF) instrumentation. The SWATH method uses a sequence of 32 MS/MS scans with a 26 m/z wide isolation window to cover the m/z range from 400–1200 m/z. The method presented in this protocol has greater precursor selectivity (20 m/z wide solation windows), but analyzes a smaller m/z range (500–900 m/z). Additionally, the SWATH method has a longer duty cycle (32 scans @ 10Hz -- ~3.2 seconds) compared to the method in this protocol (22 scans @ 10 Hz -- ~2 seconds). The resolving power of the SWATH experiment is 18,500 compared to 17,500 on the method in this protocol. Because the resolving power of the Orbitrap decreases with increasing m/z32, SWATH has greater resolving power for higher m/z analytes (roughly 3-fold at 1,000 m/z).
MSE
The original MSE technique alternates between “high energy” and “low energy” scans on a Q-TOF instrument1,33. The high energy scans are MS/MS scans with no precursor isolation, and the low energy scans are MS scans. MSE has much lower precursor selectivity than the method in this protocol, but has a much faster duty cycle (~0.3 seconds) which makes the technique compatible with ultra performance liquid chromatography (UPLC). Modern implementations of MSE use ion mobility separation prior to peptide fragmentation to improve precursor selectivity34.
Selected Reaction Monitoring (SRM)
Rather than broadly interrogating a wide precursor m/z range, SRM35,36 acquires MS/MS data on a pre-selected set of hundreds of peptide precursor targets (assuming prior knowledge of retention time)6,7. Both the acquisition and analysis of SRM data are targeted, whereas for DIA data, only analysis is targeted. Due to the targeted acquisition of SRM data, the acquired data is informative only for the pre-selected targets. DIA data is unhindered by this constraint because the data can be queried for any peptide in a wide m/z range. Therefore, if a large number of peptides need to be analyzed (e.g. interrogation of large signaling pathways or classes of proteins), or the peptides that need to be analyzed may change in the course of data analysis (e.g. iterative hypothesis testing), DIA is the preferred method. However, if an investigator is only interested in a restricted set of peptides, SRM is expected to provide more accurate and sensitive measurements than the DIA protocol presented here37.
The DIA method in this protocol uses much wider isolation windows (20 m/z) than is typical for SRM (0.2- 1 m/z). Because of the greater isolation window width, the majority of DIA spectra will be chimeric which can potentially lead to more interference in the chromatograms extracted from DIA data than SRM. However, the higher resolving power of the Orbitrap mass analyzer (17,500 @ 200 m/z) relative to the quadrupole mass filter typically used for SRM (~200–300 @ 200 m/z) recovers some of the selectivity lost in the precursor isolation4,37 relative to SRM. In both SRM and DIA, peptides can be quantified in spite of chimeric spectra (DIA) or co-isolation (SRM) so long as there are transitions selective for the peptide of interest with enough signal for quantification4,6.
Data Dependent Acquisition (DDA)
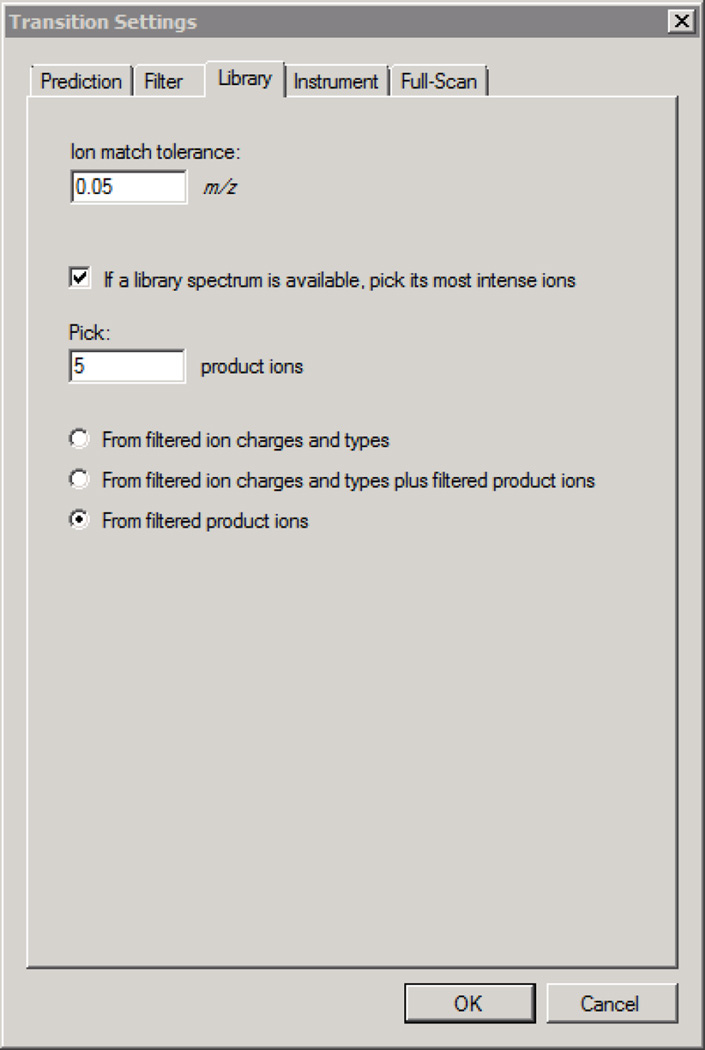
In DDA38, MS/MS scans are acquired on a subset of precursors detected in an MS “survey” scan. DDA cycles between acquisition of an MS survey scan and acquisition of MS/MS scans targeting the top-N precursors detected in the most recent survey scan. Because the MS/MS scans have a narrow isolation width (usually ~2 m/z) centered on a detected precursor, they are less chimeric than DIA spectra and are more amenable to database searching to assign a peptide sequence to each spectrum. Quantification can be performed by integrating precursor ion chromatograms extracted from the MS signal, spectral counting, or measurement of MS/MS reporter ions from samples with isobarically tagged peptides39. However, quantification by extracted fragment ion chromatograms as in SRM and DIA analysis is not possible due to incomplete and irregular sampling of chromatographic peaks by MS/MS (Figure 1). This caveat limits quantification in complex mixtures where integration of fragment ion chromatograms is more selective and therefore more sensitive than MS-based quantification40.
DDA is a powerful technique for the identification of peptides in a sample but is less effective for the detection of any particular peptide of interest. To detect a peptide, the MS/MS data must be queried for the signal specific to that peptide. Because the MS/MS data in a DDA experiment is sampled stochastically, it is impossible to determine whether a peptide with no matching spectra is non-detectable, or detectable but not sampled by MS/MS.
Limitations
As with any mass spectrometry-based technique, many peptides will fall below the limit of detection of DIA. It may be necessary to enrich the sample for proteins or peptides of interest prior to analysis, or to use fractionation techniques to improve sensitivity further. Even then, many peptides and proteins may still remain below the limit of detection41.
It is possible1,2,15,18,42,43, but challenging, to detect peptides directly from DIA data without the aid of a spectral library or a retention time calibration standard. When fragment ion chromatograms have been extracted for a peptide precursor, there are often multiple potential peaks in retention time where fragment ions co-elute, making it difficult to select the correct peak for the peptide13. Additionally, it is difficult to assign statistical confidence in the detection of a peptide once the correct peak has been selected. For these reasons, this protocol includes a step to generate a spectral library using data dependent acquisition combined with a database search. The spectral library is a set of MS/MS spectra that have been confidently matched to a peptide sequence using an automated database search algorithm. The spectral library contains information about the expected retention time of the peptide, and the expected fragment ion abundance ratios which aid in selection of the correct peak for a peptide.
Unfortunately, the set of peptides that can be analyzed in the DIA data using this protocol is limited to those that are present in the spectral library (identified by DDA). A similar technique to counter this limitation is to generate a single, deep spectral library for an organism using extensive sample fractionation and use that library for all future DIA analyses of that organism44.
The collision energy used for peptide fragmentation can be optimized based on the charge state of a selected precursor in DDA and SRM experiments45,46. In DIA experiments, the collision energy cannot be optimized by charge state because many precursors are co-fragmented without any particular precursor being the target for MS/MS analysis. In this protocol, the collision energy is optimized for a precursor charge state of +2 (“Default charge state” setting in Xcalibur method editor). Peptides with other charge states may not fragment as well and high m/z fragment ions from peptides with charge states greater than +2 may not be measured.
MATERIALS
EQUIPMENT
Q-Exactive mass spectrometer with Tune software version 2.3 SP1
Nano-flow liquid chromatography system (see example in Supplementary Methods)
PC running Windows XP or later with Skyline v. 2.6. Skyline is downloaded and installed by following the steps in Box 1.
REAGENTS
Quality control47 (QC) sample
The quality control sample is used to monitor the performance of the liquid chromatography setup and mass spectrometer throughout an experiment. The sample is analyzed by LC-MS/MS 3–5 times at the beginning of the experiment to establish a baseline. After this, the sample is analyzed roughly every 5 liquid chromatography runs and compared to the baseline in order to detect any issues that may arise such as: spray instability, excessive retention time or peak area variability, irregularities in chromatographic peak shape, contaminants, excessive sample carry-over, a reduction in mass measurement accuracy, or poor peptide fragmentation. The quality control sample should be a mixture containing at least 6, but preferably 10 known peptides with elution times spread across the entire separation gradient and a strong signal in both MS and MS/MS scans. A complex sample such as yeast lysate may be used, or a simpler mixture containing a set of synthetic peptides is also appropriate. Preferably, the QC sample should be easy to prepare reproducibly in large quantities that can be frozen as single use aliquots.
QC Sample Preparation
Solubilize bovine serum albumin (Sigma) in 50 mM ammonium bicarbonate (ABC) buffer and quantify using the BCA protein assay (Pierce). Dry and resolubilize 200 µg in 50 µL 8 M urea containing 200 mM ABC. Reduce the disulfides using 10 mM tris(2-carboxyethyl)phosphine) (TCEP) for one hour at room temperature and then alkylate using 11 mM of iodoacetamide for 20 minutes also at room temperature. Add 150 µL of water to dilute the urea to 2 M, then add Promega trypsin at a substrate to enzyme ratio of 50:1 and digest overnight at 37 °C. Stop the digestion by adding trifluoroacetic acid (TFA), and then desalt using an MCX cartridge (Waters). Dry the desalted digest using vacuum centrifugation, and combine the resolubilized BSA tryptic peptides (200 fmol/µL) with 50 fmol/µL of the Peptide Retention Time Calibration Standard (Pierce).
(The preparation of the QC sample used for demonstration in the “ANTICIPATED RESULTS” is slightly different – four additional bovine proteins are included – and is described in Supplementary Methods.)
Protein digests for analysis by DIA and/or DDA. These are the biological samples which will be used to test a hypothesis based on the detection and quantification of peptides in the samples. Sample preparation is the same for DDA and DIA analysis, but in most cases, only a subset of the samples will be analyzed by DDA (see “EXPERIMENTAL DESIGN”). This protocol assumes that the samples were digested with trypsin, and are mass spectrometry compatible (free or minimal amount of detergents, salts, metabolites, lipids, nucleic acids, sugars, and other contaminants): The preparation of a yeast tryptic digest used for demonstration in the “ANTICIPATED RESULTS” is described in Supplementary Methods.
PROCEDURE
Box 1 Installing Skyline on your PC
Navigate to http://skyline.maccosslab.org on a PC running Windows
Select Download and Install: Skyline 2.6 (or the latest version). In Windows Explorer or on the Start menu, right-click Computer and click Properties. If System Type is listed as 64-bit Operating system, install 64-bit Skyline. Otherwise, install 32-bit.
Follow the prompts to complete the installation.
<CRITICAL Skyline version 3.1 or greater is not compatible with Windows XP.
End of Box 1
Generate Quality Control Instrument Method
-
1
Open Skyline and click on Blank Document to start a new document.
-
2
Click Settings → Peptide Settings and select the Digestion tab
-
3
Set the Background proteome option to None, the enzyme used for digestion, and the maximum number of missed cleavages that are to be allowed in a peptide being analyzed
-
4
Select the Library tab at the top of the Peptide Settings window and, if there are any libraries listed, make sure they are unchecked
-
5
Perform either option A or B depending on whether the quality control sample contains structural or isotope modifications (option A) or not (option B)
-
The quality control sample contains structural or isotope modifications
Select the Modification tab at the top of the Peptide Settings window
Click the Edit list… button to the right of “Isotope modifications” or “Structural modifications”
Click Add…, and select the name of the modification if it is present, otherwise, enter a name and populate the form manually
Click OK and check the box next to the added modification, click OK again to return to the main Skyline window
-
The quality control sample does not contain any structural or isotope modifications
Select the Modification tab at the top of the Peptide Settings window
Uncheck any checked boxes in the “Isotope modifications” and “Structural modifications” lists
Click OK to return to the main Skyline window
-
-
6
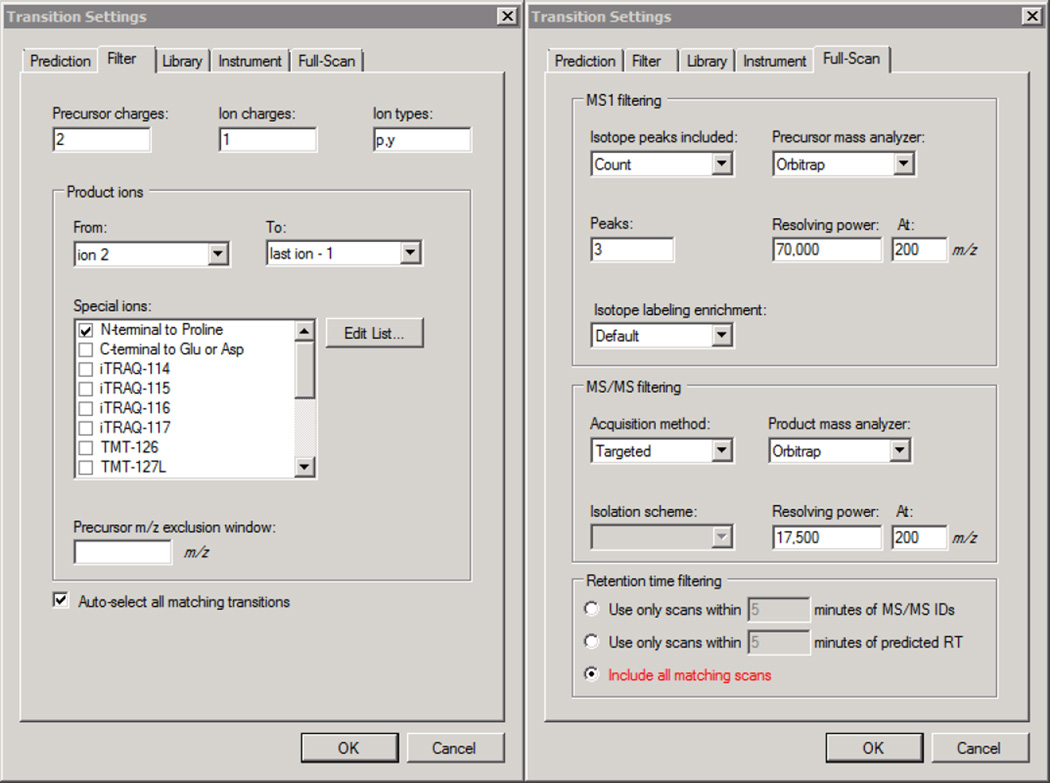
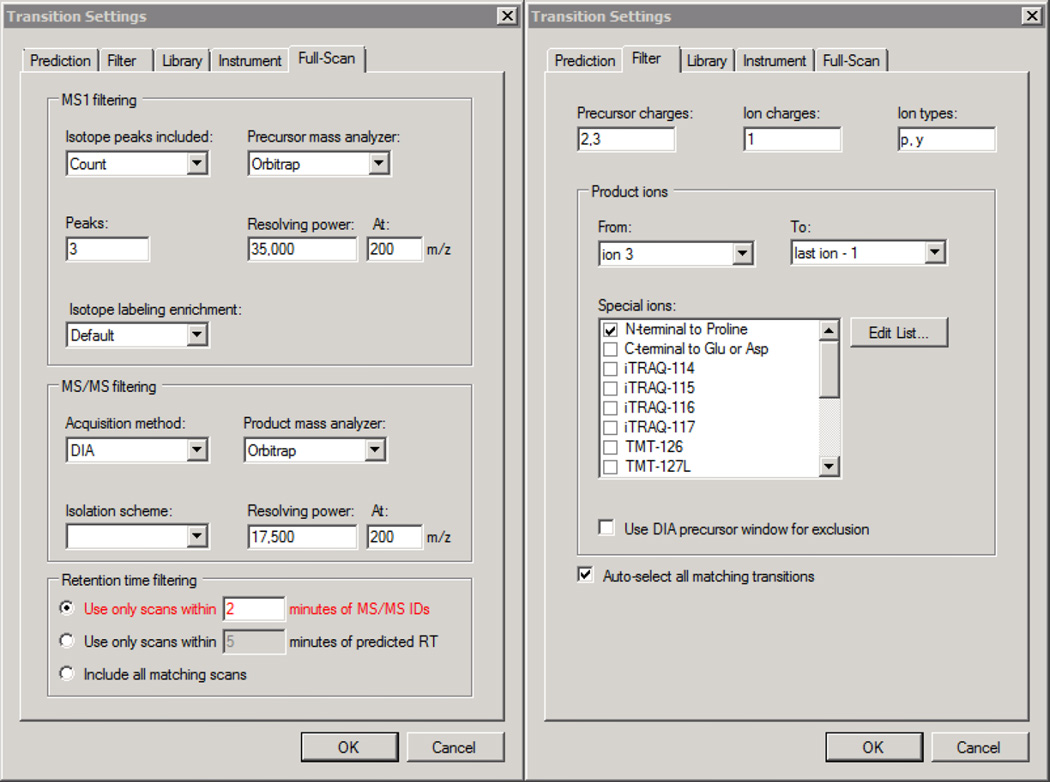
Click Settings → Transition Settings → Filter and populate the fields to match Figure 2. If additional precursor charge states are to be analyzed, add more comma separated charges to the Precursor charges box.
-
7
Click the Full Scan tab, populate the fields in this window to match those in Figure 2 and click OK
-
8
Add the peptides in the QC mixture to the document by clicking Edit → Insert → Peptides, entering the QC peptides to be analyzed into the window, and clicking Insert.
-
9
(optional) Refining precursors for QC samples with isotope modifications (steps 9–11) If peptides with isotope modifications are being analyzed, Click Edit → Expand All → Peptides.
-
10
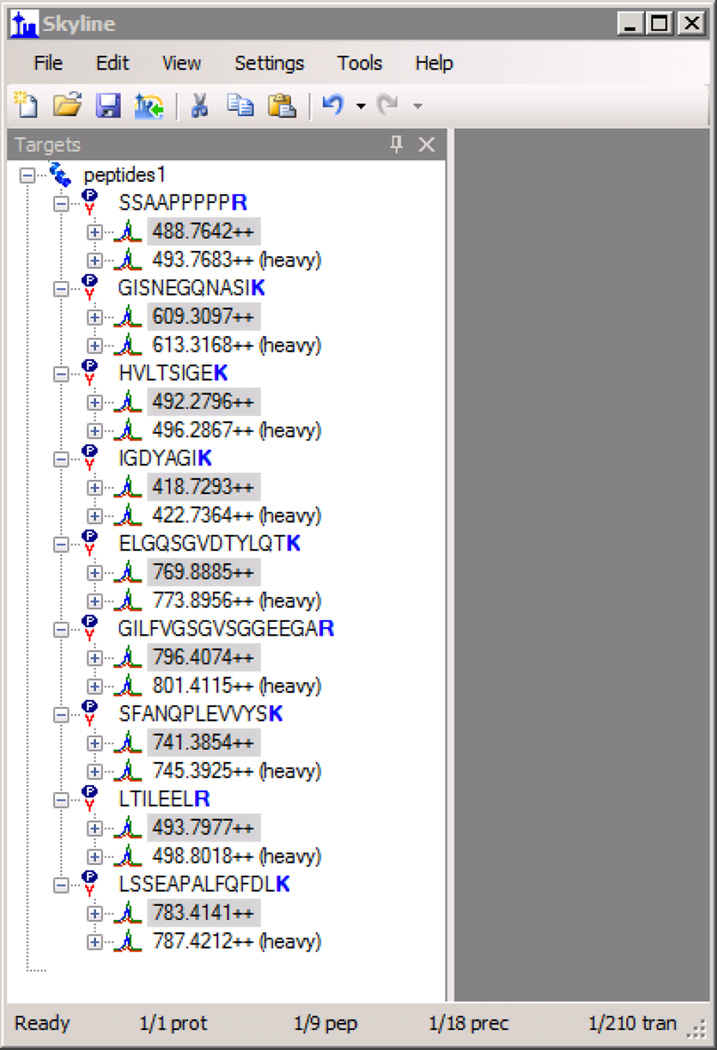
Hold down the Ctrl key and select all of the “light” peptide precursors in the Targets pane of the main Skyline window for peptides that have isotope modifications, but for which you do not wish to measure the unmodified analyte (example in Figure 3). Press the Delete key to remove them.
-
11
Hold down the Ctrl key and select all of the “heavy” peptide precursors in the Targets pane of the main Skyline window for peptides that do not have isotope modifications. Press the Delete key to remove them.
-
12
Continue steps towards generating a Quality Control Instrument Method Save the Skyline document by selecting File → Save As…
-
13
Export an isolation list by clicking File → Export → Isolation List… and clicking OK
-
14
Open XCalibur and select Instrument Setup to open the method editor
-
15
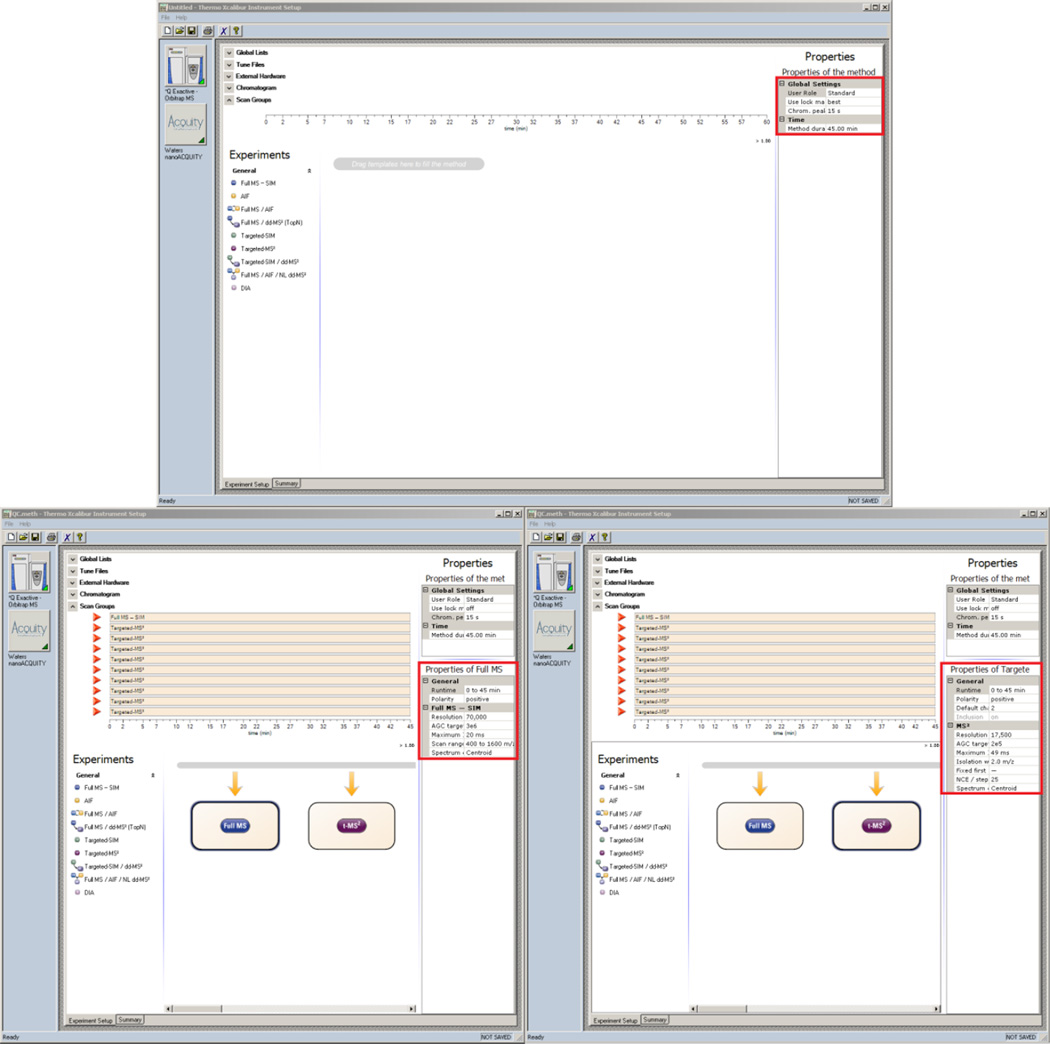
Populate the Method Duration and Chromatographic Peak Width (FWHM) fields to match the chromatography method being used (Figure 4 top)
-
16
Drag and drop a Full MS-SIM scan event from under the Experiments heading to the timeline
-
17
Set the properties of the Full MS scan to match those in Figure 4 (bottom left)
-
18
Drag and drop N Targeted-MS2 scans from under the Experiments heading to the timeline where N is the number of QC peptides being analyzed
-
19
Edit the Runtime of all scan events in the method by holding down the Ctrl key, selecting each scan under the Scan Groups heading, releasing the Ctrl key, and entering the Runtime to match the full method duration
-
20
Select all of the targeted-MS2 scans and set the properties to match Figure 4 (bottom right)
-
21
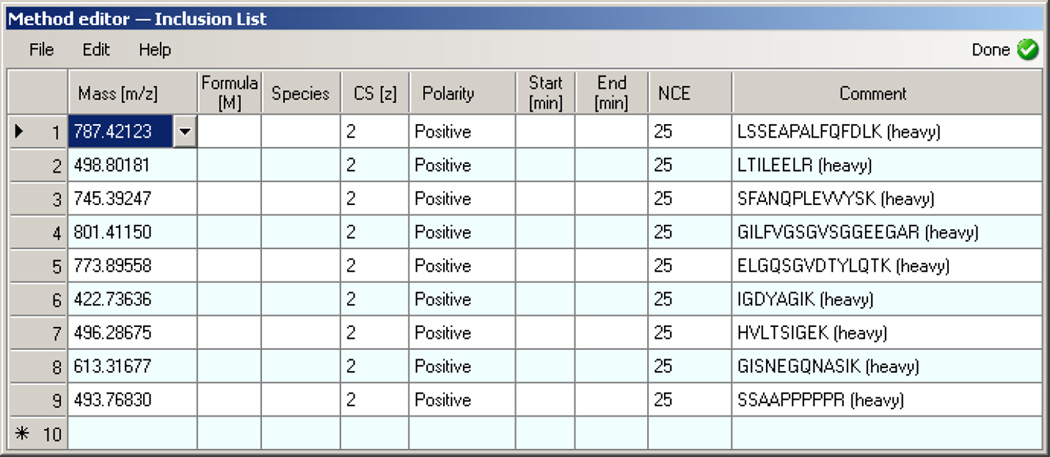
Click on Global Lists heading and select Inclusion. Select File → Import and import the isolation list exported from Skyline in Step 13 (Figure 5).
-
22
Click on the Tune Files heading and select a Base Tune File to be used in the Properties pane
-
23
Enter settings for a liquid chromatography method (not covered in this protocol)
-
24
Save the method by selecting File → Save As…
Figure 2. Skyline - QC Transition Filter and Full-Scan Settings.
Figure 3. Skyline - Light Targets Selected.
Figure 4. Xcalibur - QC Method Setup.
Figure 5. Xcalibur - Insert Inclusion List.
Generate Data Independent Acquisition Instrument Method
-
25
Create a new document in Skyline by clicking File → New
-
26
Select Settings → Transition Settings and populate the fields in the Full-Scan tab to match Figure 6 (left)
-
27
If chromatography is expected to drift by more than +/− 2 minutes, change the “Use only scans within XX minutes” setting to reflect this
-
28
In the drop down box under “Isolation scheme”, select <Add…>
-
29
Enter the name “20mzDIA”, select Prespecified isolation windows, and click the Calculate… button
-
30
Enter “Start m/z:” 500, “End m/z:” 900, “Window width:” 20, and check Optimize window placement and click OK
-
31
Click OK in the “Edit Isolation Scheme” dialog.
-
32
Populate the fields in the Filter tab to match Figure 6 (right) and click OK.
-
33
Export an isolation list by clicking File → Export → Isolation List… → OK
-
34
Save the Skyline document by clicking File → Save As…
-
35
Open XCalibur and select Instrument Setup to open the method editor
-
36
Populate the Method Duration and Chromatographic Peak Width (FWHM) fields to match the chromatography method being used and the results that are typically obtained using it.
-
37
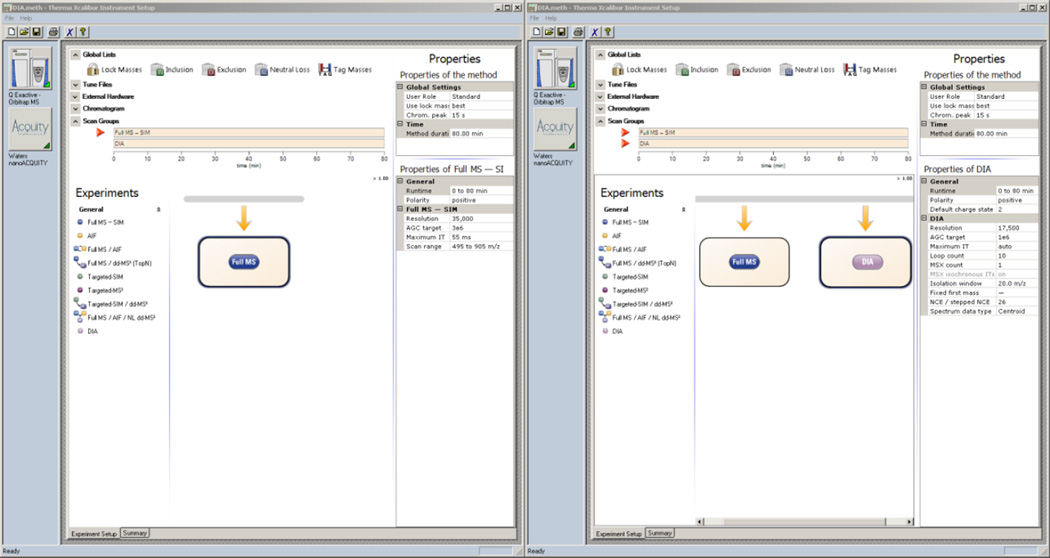
Drag and drop a Full MS-SIM scan event from under the Experiments heading to the timeline
-
38
Set the properties of the Full MS scan to match those in Figure 7A (set Runtime to match the Method duration)
-
39
Drag and drop a DIA scan event from under the Experiments heading to the timeline
-
40
Set the properties of the DIA scan to match those in Figure 7B (set Runtime to match the Method duration)
-
41
Click on Global Lists heading and select Inclusion. Select File → Import and import the isolation list exported from Skyline in Step 33.
-
42
Click on the Tune Files heading and select a Base Tune File to be used in the Properties pane
-
43
Enter settings for a liquid chromatography method (not covered in this protocol)
-
44
Save the method by selecting File → Save As…
Figure 6. Skyline - DIA Full-Scan and Filter Settings.
Figure 7. Xcalibur - DIA MS and MS2 Settings.
Generate Data Dependent Acquisition Instrument Method
-
45
Open XCalibur and select Instrument Setup to open the method editor
-
46
Populate the Method Duration and Chromatographic Peak Width (FWHM) fields to match the chromatography method being used
-
47
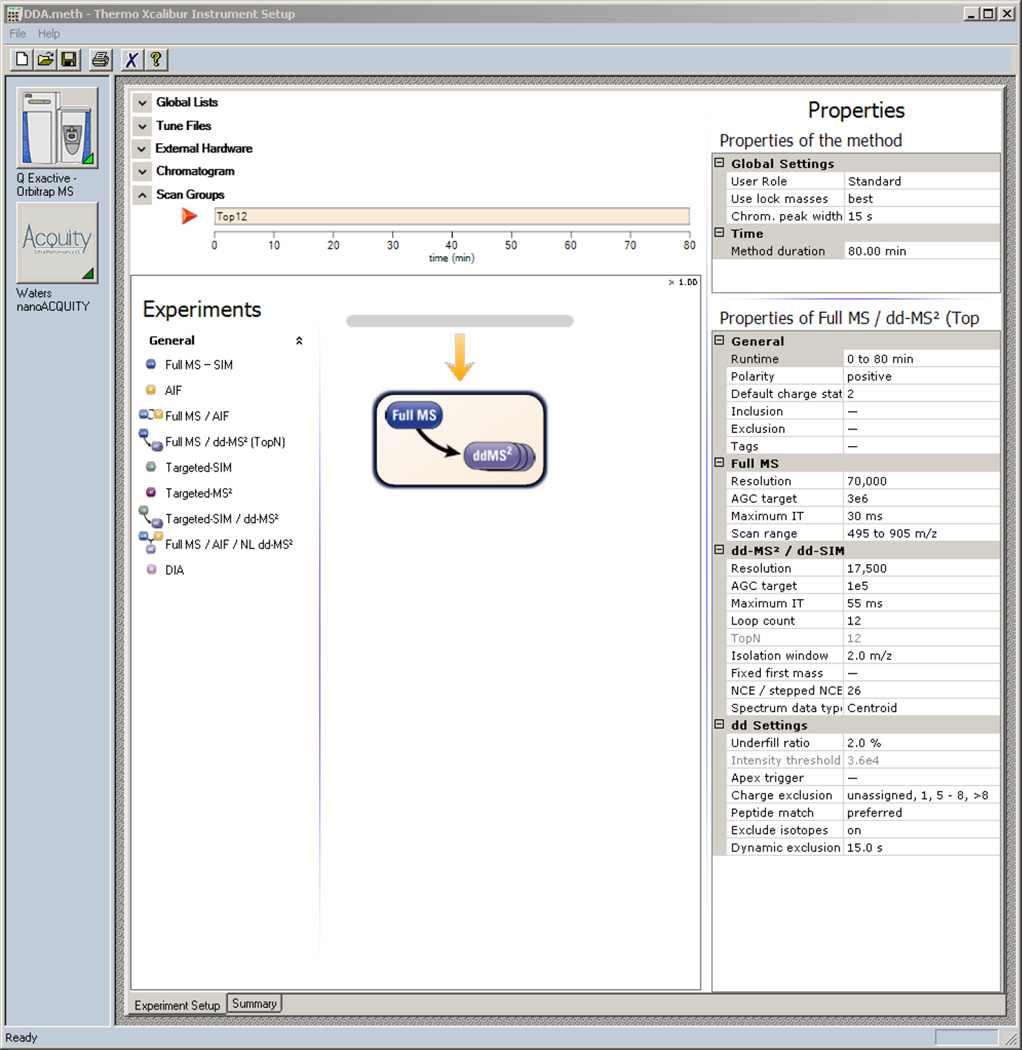
Drag and drop a Full MS-ddMS2 scan event from under the Experiments heading to the timeline
-
48
Set the properties of the Full MS – ddMS2 scan to match those in Figure 8 (set Runtime to match the Method duration)
-
49
Click on the Tune Files heading and select a Base Tune File to be used in the Properties pane
-
50
Enter settings for a liquid chromatography method (not covered in this protocol)
-
51
Save the method by selecting File → Save As…
Figure 8. Xcalibur - DDA Method.
Run Quality Control Samples
-
52
Acquire data on a QC sample in 4–6 replicate LC runs.
-
53
Open the QC Skyline document that was saved in Step 12
-
54
Select File → Import → Results… and click OK. Select all of the raw data files from all of the QC runs by holding down the Shift key to select a range of files and select Open. If a dialog box appears prompting about removing a common prefix, select Do not remove. The data should import in 5 minutes or less.
-
55
Select View → Peak Areas → Replicate Comparison and View → Retention Times → Replicate Comparison to view plots of the peak area (example in Figure 9A) and retention time (example in Figure 9B) of the currently selected peptide in each QC run. Click on each peptide and verify that the retention time and peak areas for each peptide are stable.
-
56
Close the peak area and retention time windows, click View → Auto-Zoom → Best Peak to zoom in on the chromatogram peak for the peptide. Click View → Transitions → Split Graph to view MS chromatograms and MS/MS chromatograms separately (example in Figure 10). Select each peptide and verify that the chromatographic peak shape is acceptable, and that the mass measurement accuracy (annotated above the peak) is reasonable.
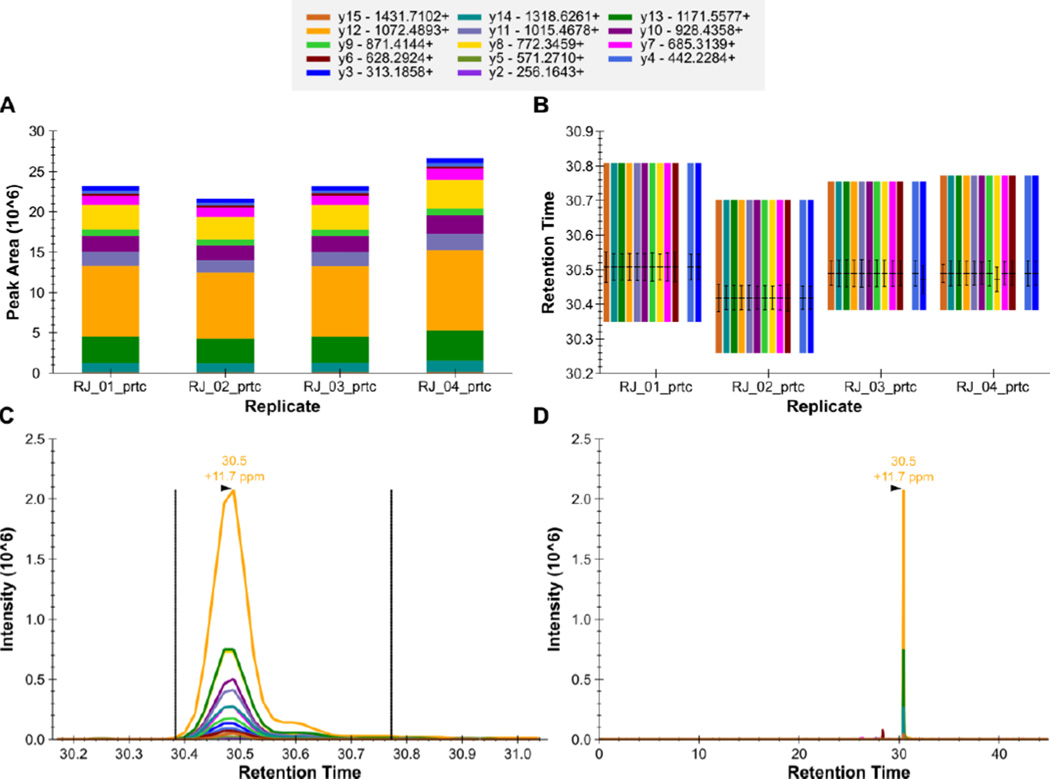
Figure 9. Skyline - QC Peptide GILFVGSGVSGGEEGAR++.
The total fragment ion signal (integrated over time) for the peptide GILFVGSGVSGGEEGAR++ is plotted as a bar for each of four QC replicate injections (A). The contribution from each individual fragment ion is displayed as a different color in the bars. The retention time of the peptide is plotted for each of the four replicate injections as a group of vertical bars (B). Within each group, there are colored bars, one for each transition measured for the peptide. Each bar starts and stops at the integration boundaries (in retention time) of the detected chromatographic peak. The retention time at the peak, and full-width at half max (FWHM) for each transition peak are overlaid on each transition as a horizontal and vertical black line, respectively. The extracted signal for the peptide from a single replicate is plotted in C and D at different levels of zoom, with the mass measurement error and retention time of the most intense transition annotated above the peak. The vertical lines on either side of the peak in C indicate the integration boundaries for the peak.
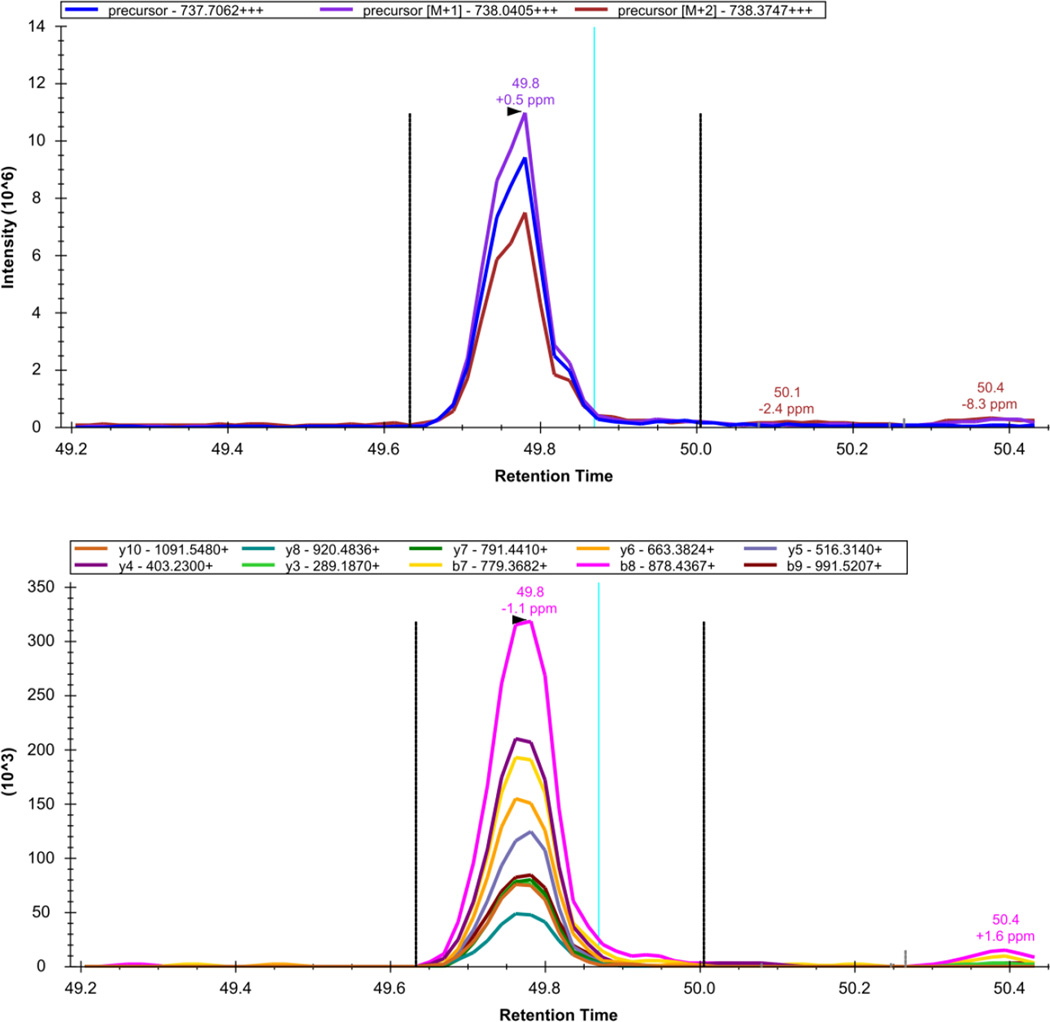
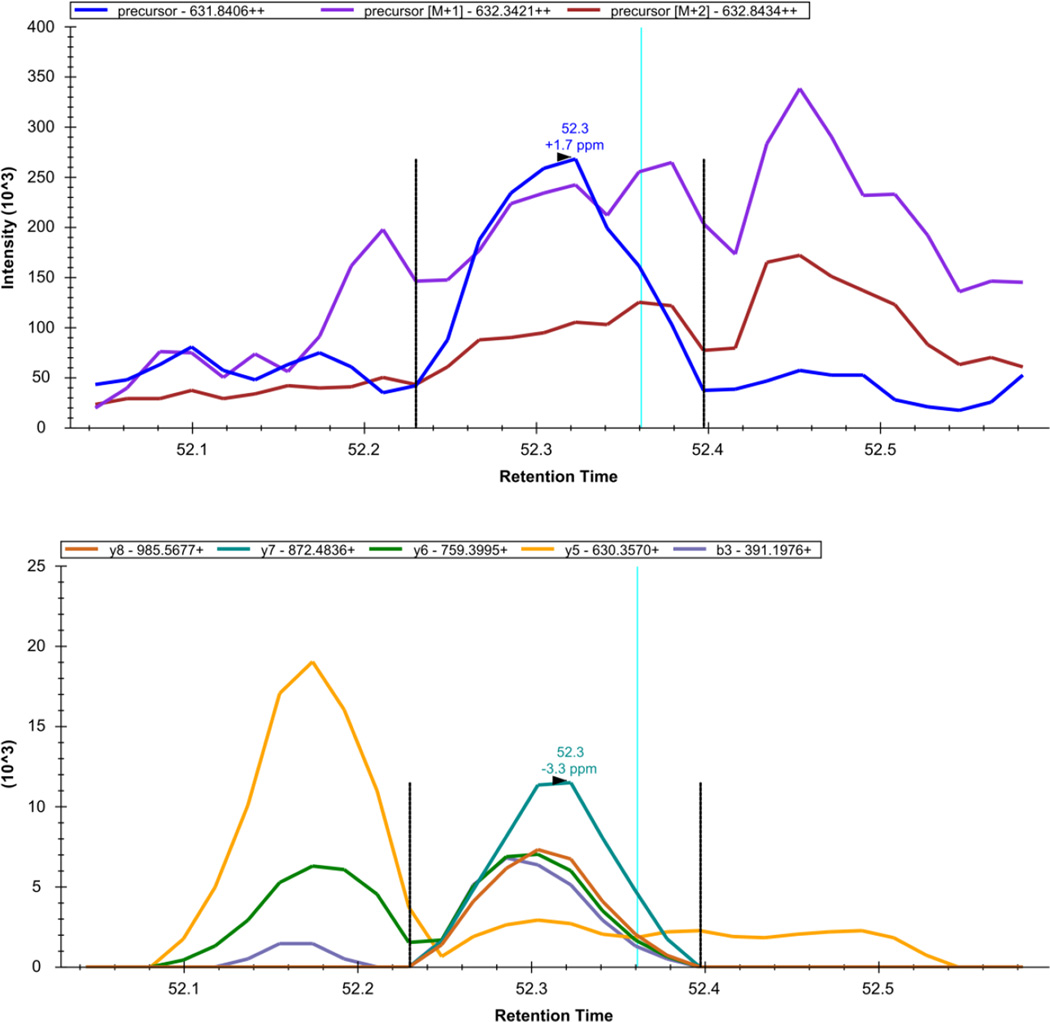
Figure 10. Skyline - DIA Data: LGEHNIDVLEGNEQFINAAK+++.
The extracted precursor (top) and fragment ion (bottom) signal extracted for the peptide precursor LGEHNIDVLEGNEQFINAAK+++ from DIA data acquired on a yeast sample digest using this protocol are plotted. The vertical lines on either side of the peak indicate the integration boundaries for the peak. The vertical blue line shows the retention time of the peptide identification contained in the spectral library generated from DDA data. The mass measurement error and retention time of the most intense transition (fragment ion data) or isotopic peak of (precursor data) for the peptide precursor are annotated above the chromatographic peak.
Run Samples
-
57
Acquire data on the samples to be analyzed, at least one DDA dataset should be acquired for each sample type for generating a spectral library. It is recommended that QC samples be analyzed every 4–5 injections to monitor system performance48.
Build a Spectral Library
-
58
Search the DDA data using an automated database search algorithm such as SEQUEST or Mascot
-
59
Open the Skyline file saved in Step 34 and click Settings → Peptide Settings. Click on the Library tab, and select Build…
-
60
Type in a name for the library, and select an Output Path where the created library will be stored. Uncheck the Keep redundant library checkbox, and enter a cut-off score of 0.99. The Lab Authority should be a unique identifier for the lab that generated the library (ex. proteome.gs.washington.edu). Click Next.
-
61
Select Add Files… and add the files generated from the database search. Select Finish. A progress bar will appear on the bottom status bar of Skyline, and a notification will appear when the library generation is complete.
-
62
Select the checkbox next to the library that was just built
-
63
Click on Explore… and verify that the library that was just built is displayed in the Spectral Library Explorer. Verify that the spectra have retention time information, shown as “RT: …” in the lower right portion of the window, beneath the spectrum plot
-
64
Select Library under Pick peptides matching to only insert peptides into the document that were identified by DDA. Click OK.
-
65
Select Settings → Transition Settings, and click on the Library tab.
-
66
Enter the settings in Figure 11 to select transitions for each peptide precursor based on the library MS/MS spectra. Click OK to close the Transition Settings dialog.
-
67
OPTIONAL: Learn more about the spectral library explorer by following the tutorial at https://skyline.gs.washington.edu/labkey/tutorial_library_explorer.url
Figure 11. Skyline - DIA Settings for Spectral Library Refinement of Transitions.
Optional: Build a Background proteome
<CRITICAL> If there is sequence information (ex. open reading frame sequences or expressed sequence tags for the organism being analyzed) for proteins that could be potentially present in the measured samples, a background proteome can be defined to improve data analysis in Skyline. Improvements include easier insertion of proteins into the Skyline document, automated retrieval of metadata for proteins from UniProt, and the ability to inspect peptide uniqueness.
-
68
Click Settings → Peptide Settings, select the Digestion tab, and select “<Add…>” under Background Proteome.
-
69
Enter a name for the background proteome such as “yeast”, click the “Create…” button and find a location for the background proteome to be stored.
-
70
Select “Add File…” and select a .fasta file containing the protein sequences for the organism(s) being studied
-
71
Select OK to finish generating the background proteome, click OK again to close the Peptide Settings dialog
Import Data into Skyline
-
72
Enter the names and sequences of proteins to be analyzed. This step can be performed using option A, B, C, or D depending what information you have about these protein sequences.
-
A .fasta file of protein sequences to be analyzed is available
Select Edit →Insert→FASTA, copy the contents of the .fasta file into the dialog, and click Insert
-
All proteins with peptides contained in the spectral library are to be analyzed
Select View → Spectral Libraries, check the Associate proteins check-box if a background proteome was generated in Steps 68–71, and select Add all…
-
A list of protein sequences to be analyzed is available
Select Edit → Insert → Proteins to open a dialog that can be used to enter proteins to be analyzed
-
A list of peptide sequences to be analyzed is available
Select Edit → Insert → Peptides to open a dialog that can be used to enter peptides to be analyzed
-
-
73
Select File→Import→Results, click OK, and select the files containing DIA data to be analyzed, click Open
-
74
As the data imports, a progress bar will appear at the bottom of the Skyline window, or a progress graph will pop-up. Once the data have finished importing, clicking on an individual peptide will show chromatograms for that peptide. To view the MS and MS/MS data for the peptide (example in Figure 10), select View → Transitions → All and View → Transitions → Split Graph. Peak integration boundaries are indicated as vertical dashed lines and can be manually refined if necessary by clicking and dragging beneath the x-axis or clicking and dragging the boundaries themselves. To see peptide ID times from the DDA data indicated, right-click a chromatogram graph and make sure Peptide ID Times → From Other Runs is selected
-
75
To compare the signal for a peptide across samples, select View → Peak Areas → Replicate Comparison to generate a plot of the integrated peptide peak area in each sample (example in Figure 9A)
Optional: Export Results from Skyline
-
76
Select File → Export → Report →Edit List… →Add…
-
77
Enter a name for the report in the View Name box
-
78
Add the following items to the report by navigating to them in the hierarchy and selecting the checkbox next to them:
-
-
Proteins:Protein Name
-
-
Replicates:Replicate Name
-
-
Proteins:Peptides:Peptide Sequence
-
-
Proteins:Peptides:Precursors:Precursor Charge
-
-
Proteins:Peptides:Precursors:Precursor Mz
-
-
Proteins:Peptides:Precursors:Transitions:Fragment Ion
-
-
Proteins:Peptides:Precursors:Transitions:Product Charge
-
-
Proteins:Peptides:Precursors:Transitions:Product Mz
-
-
Proteins:Peptides:Precursors:Transitions:Transition Results:Area
-
-
-
79
Click OK, and click OK in the Edit Reports dialog box
-
80
In the Export Report dialog box, select the report that was just generated and click Export, choose a location to save the report to and click Save to export a comma-separated-value (.csv) file that is easy to parse and can be viewed using most spreadsheet software.
-
81
OPTIONAL: Generate more advanced reports by following the tutorial at (https://skyline.gs.washington.edu/labkey/tutorial_custom_reports.url)
TIMING
Box 1 Installing Skyline – 5 minutes
Generate Quality Control Instrument Method – 20 minutes
Generate Data Independent Acquisition Instrument Method – 15 minutes
Generate Data Dependent Acquisition Instrument Method – 10 minutes
Run Quality Control Samples – ~6 hours, heavily dependent on LC setup
Run Samples – Depends on sample count and LC setup
Build a Spectral Library – Database search time + 5 minutes
Optional: Build a Background proteome – 5 minutes
Import Data into Skyline – 0.5–10 minutes per data file
Optional: Export Results from Skyline – 5 minutes
TROUBLESHOOTING
Troubleshooting guidelines can be found in Table 2.
Table 2.
Troubleshooting
| Step | Problem | Possible Reason | Solution |
|---|---|---|---|
| 38 | Option “auto” is not available |
Q-Exactive instrument software is out of date |
Update instrument software or enter “50 ms” |
| 55 | Peak areas or retention times are not stable |
Peak is not selected correctly in some replicates |
Compare plots across replicates, verify that same peak is selected in all, correct peak integration by clicking on the correct peak |
| Poorly performing LC column |
Replace chromatography column / diagnose problem with the LC column |
||
| 56 | Peaks appear undersampled or as a single spike |
Slow duty cycle | Reduce the number of QC peptides being measured or measure QC peptides using the DIA method |
| Poor mass measurement accuracy |
Instrument has not been calibrated recently |
Calibrate the mass analyzer according to vendor protocol |
|
| 61 | Error message appears |
Incompatible file | Make sure the files being used to generate the spectral library are a compatible format. Compatible formats are listed in the Experimental design section |
| 73 | Out of memory exception appears |
Too many peptides analyzed or not enough memory |
Re-import with less peptides included in the document or less transitions per peptide, or try importing on a computer with more RAM |
| Peptide shows no chromatograms |
Peptide was not analyzed in the DIA method |
Check if the peptide precursor m/z is included in the MS or MS/MS scan range of the method, if it is, double check transitions settings |
|
| Chromatograms appear, but there is no peak |
Peptide is not detected | Modify the DIA acquisition method to improve sensitivity (see Table 1) | |
| Spectral library identification is a false positive |
Change Transitions Settings → Full-Scan → Retention Time Filtering to “All Matching Scans” and re-import the data by pressing Ctrl+R and selecting Re-import for the relevant files. After re-import, check to see if there is a peak for the peptide |
||
| Peptide peaks appear undersampled or as a single spike |
Slow duty cycle | Modify the DIA acquisition method to improve chromatogram sampling (see Table 1) |
|
| 75 | Inconsistent peak area across replicate samples |
Peak is not selected correctly in some replicates |
Compare plots across replicates, verify that same peak is selected in all, correct the peak integration by clicking on the correct peak and manually adjusting integration boundaries if necessary |
| Many peptide peaks are not selected properly |
Peptides are low abundance, mixture is very complex |
Try using a more advanced peak picking model as in (https://skyline.gs.washington.edu/labkey/tutorial_peak_picking.url) or manually refine peak integrations |
ANTICIPATED RESULTS
Quality Control Sample
The peak areas and retention times of all of the standard peptides should be reproducible by the end of the quality control (QC) runs (Figure 9 and Figure 12) with good peak shape and stable spray (changes in ion current due to fluctuations in electrospray ionization). The expected level of reproducibility will vary based on the liquid chromatography setup being used. The mass measurement error should be as expected for the instrument being used. In the QC data from Figure 9 and Figure 12, the mass measurement error (~11 ppm) is consistently worse than expected for a Q-Exactive (<10 ppm) indicating that the instrument should be calibrated.
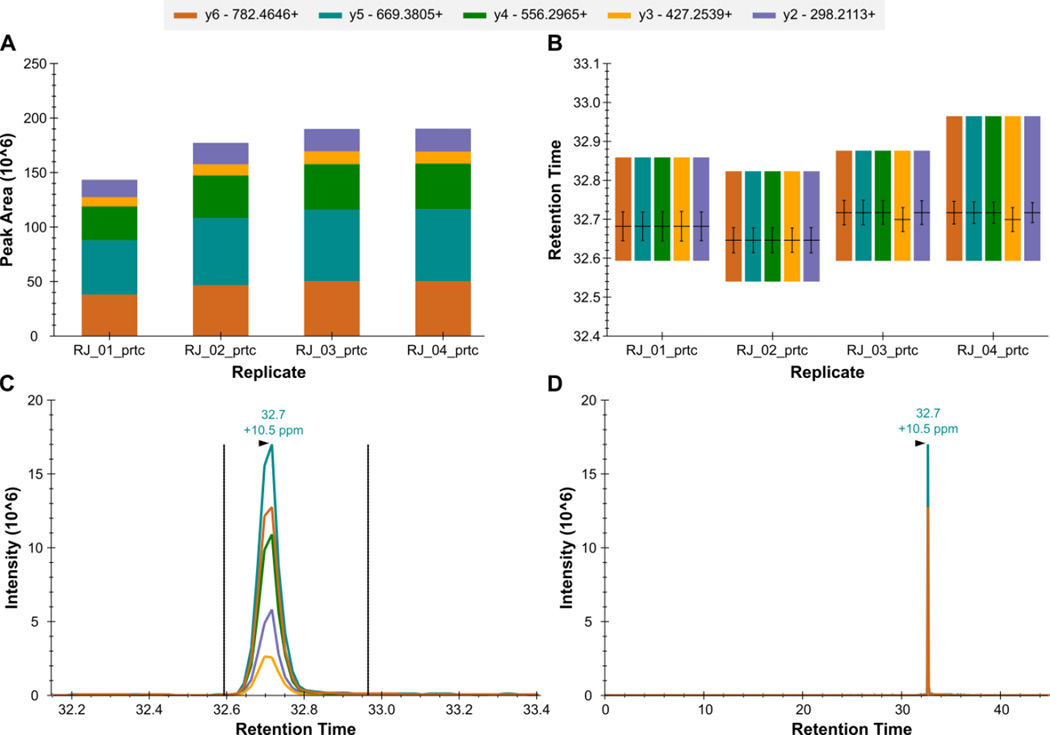
Figure 12. Skyline - QC Peptide LTILEELR++.
The total fragment ion signal (integrated over time) for the peptide LTILEELR++ is plotted as a bar for each of four QC replicate injections (A). The contribution from each individual fragment ion is displayed as a different color in the bars. The retention time of the peptide is plotted for each of the four replicate injections as a group of vertical bars (B). Within each group, there are colored bars, one for each transition measured for the peptide. Each bar starts and stops at the integration boundaries (in retention time) of the detected chromatographic peak. The retention time at the peak, and full-width at half max (FWHM) for each transition peak are overlaid on each transition as a horizontal and vertical black line, respectively. The extracted signal for the peptide from a single replicate is plotted in C and D at different levels of zoom, with the mass measurement error and retention time of the most intense transition annotated above the peak. The vertical lines on either side of the peak in C indicate the integration boundaries for the peak.
DIA Data
The results of the DIA data will strongly depend on the sample being analyzed and the equipment being used to analyze the sample. In a complex mixture such as a yeast lysate, the lower limit of quantification for peptides is generally expected to be in the atto- to femtomolar range. In a mixture as complex as a yeast lysate, many peptide peaks will be well-behaved (Figure 10), but others will require further refinement. For example, some peaks may have strong interference in the precursor signal (Figure 13). Other peptides may additionally have strong interference in the some of the transitions being measured, which should be removed from consideration prior to analysis (Figure 14). Finally, some peptides may fragment poorly, and show only a good signal from measurement of precursor ions (Figure 15). Note that in Figures 10, and 13–15 up to 10 fragment ions were extracted for each peptide (compared to 5 in Step 66) to more strongly illustrate these behaviors. Similar to an SRM experiment, many peptides are expected to be undetectable or have a very low measured intensity due to factors independent of the acquisition method such as inefficient ionization by electrospray or incomplete digestion. Conversely, it is preferable to select a set of peptides that respond well in the mass spectrometer (“proteotypic”) for analysis6,49,50. For each peptide, the sensitivity and accuracy of quantification can be improved by manually removing transitions with strong interference6. For a detailed tutorial on manual transition refinement from DIA data, we refer the reader to the “Exploring DIA Results” section of the Skyline DIA Tutorial (https://skyline.gs.washington.edu/labkey/tutorial_dia.url).
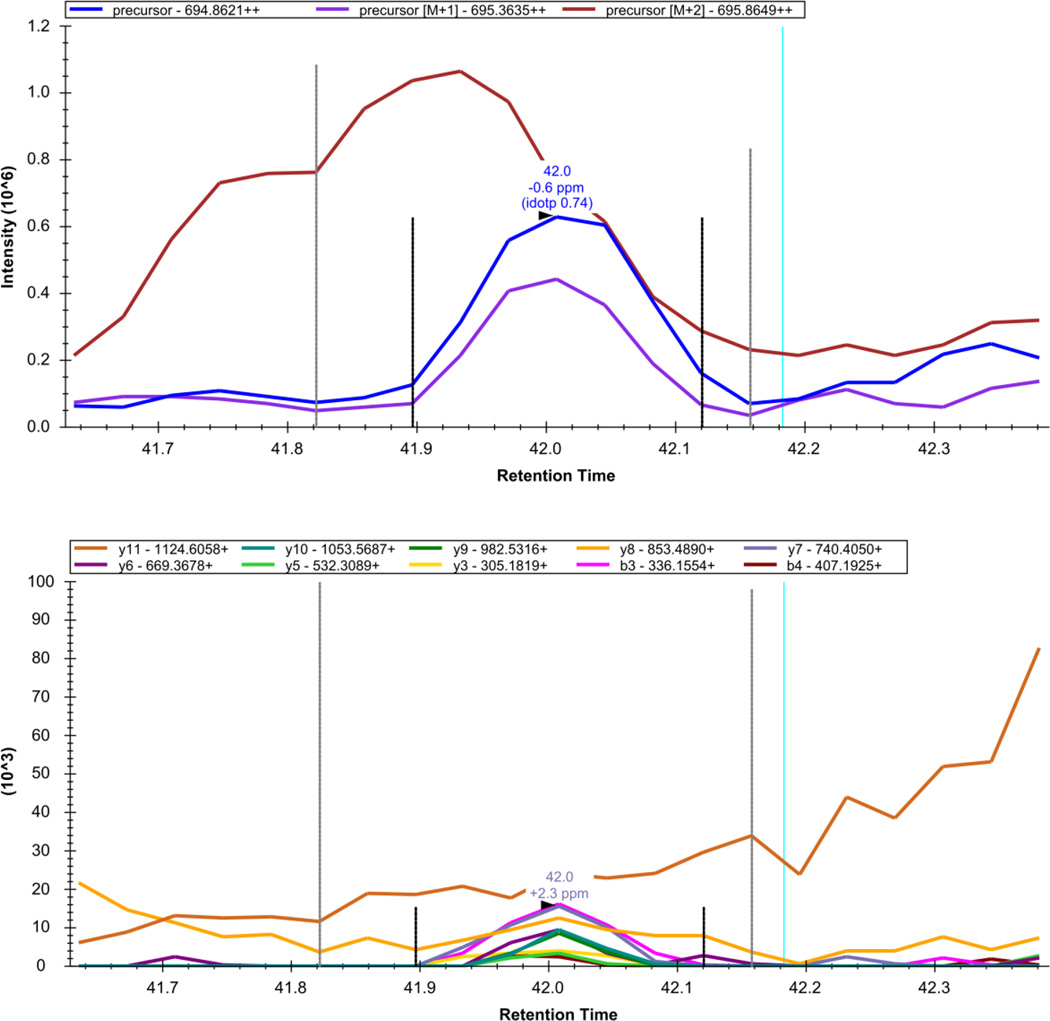
Figure 13. Skyline - DIA Data: NYIIEELNVR++.
The extracted precursor (top) and fragment ion (bottom) signal extracted for the peptide precursor NYIIEELNVR++ from DIA data acquired on a yeast sample digest using this protocol are plotted. The vertical lines on either side of the peak indicate the integration boundaries for the peak. The vertical blue line shows the retention time of the peptide identification contained in the spectral library generated from DDA data. The mass measurement error and retention time of the most intense transition (fragment ion data) or isotopic peak of (precursor data) for the peptide precursor are annotated above the chromatographic peak.
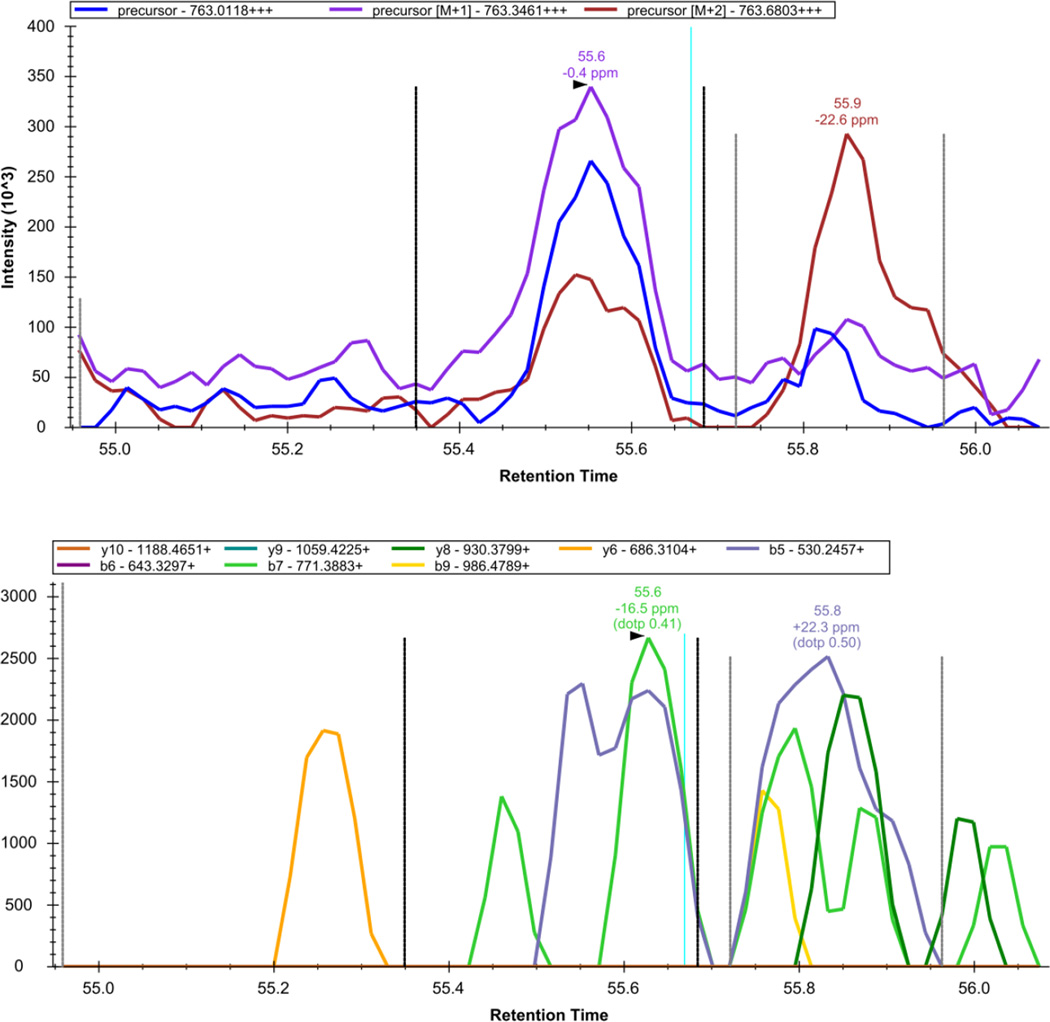
Figure 14. Skyline - DIA Data: TYAAEIAHNISAK.
The extracted precursor (top) and fragment ion (bottom) signal extracted for the peptide precursor TYAAEIAHNISAK++ from DIA data acquired on a yeast sample digest using this protocol are plotted. The vertical lines on either side of the peak indicate the integration boundaries for the peak. The vertical blue line shows the retention time of the peptide identification contained in the spectral library generated from DDA data. The mass measurement error and retention time of the most intense transition (fragment ion data) or isotopic peak of (precursor data) for the peptide precursor are annotated above the chromatographic peak.
Figure 15. Skyline - DIA Data: VSLDDLQQSIEEDEDHVQST.
The extracted precursor (top) and fragment ion (bottom) signal extracted for the peptide precursor VSLDDLQQSIEEDEDHVQST+++ from DIA data acquired on a yeast sample digest using this protocol are plotted. The vertical lines on either side of the peak indicate the integration boundaries for the peak. The vertical blue line shows the retention time of the peptide identification contained in the spectral library generated from DDA data. The mass measurement error and retention time of the most intense transition (fragment ion data) or isotopic peak of (precursor data) for the peptide precursor are annotated above the chromatographic peak.
Statement of Responsibility
JDE, BM, RJ, YX, and MM developed and optimized the protocol. JDE drafted the text of the manuscript. RJ prepared samples and acquired data presented in “Anticipated Results”.
Supplementary Material
Acknowledgments
Financial support for this work was provided from National Institutes of Health grants P41 GM103533, R01 GM103551, and U54 HG008097.
Footnotes
Competing Financial Interest
MJM is a paid consultant for Thermo Fisher Scientific.
YX is an employee of Thermo Fisher Scientific.
The MacCoss lab receives research support from Thermo Fisher Scientific.
Supplementary Note: Automated Gain Control on a Q-Exactive
A description of how the settings for maximum injection time, automated gain control target, and resolving power impact the scan rate on a Q-Exactive (Thermo Scientific; Bremen, Germany) mass spectrometer.
Supplementary Methods: Quality Control and Yeast Sample Preparation and Analysis
Details on the preparation of quality control and yeast samples analyzed to generate example data presented in the ANTICIPATED RESULTS section. Additionally, the high performance liquid chromatography (HPLC) system and method used in these analyses is described.
Contributor Information
Jarrett D. Egertson, Email: jegertso@uw.edu.
Brendan MacLean, Email: brendanx@proteinms.net.
Richard Johnson, Email: rj8@uw.edu.
Yue Xuan, Email: yue.xuan@thermofisher.com.
References
- 1.Purvine S, Eppel J-T, Yi EC, Goodlett DR. Shotgun collision-induced dissociation of peptides using a time of flight mass analyzer. Proteomics. 2003;3:847–850. doi: 10.1002/pmic.200300362. [DOI] [PubMed] [Google Scholar]
- 2.Venable JD, Dong M-Q, Wohlschlegel J, Dillin A, Yates JR. Automated approach for quantitative analysis of complex peptide mixtures from tandem mass spectra. Nat. Methods. 2004;1:39–45. doi: 10.1038/nmeth705. [DOI] [PubMed] [Google Scholar]
- 3.Law KP, Lim YP. Recent advances in mass spectrometry: data independent analysis and hyper reaction monitoring. Expert Rev. Proteomics. 2013;10:551–566. doi: 10.1586/14789450.2013.858022. [DOI] [PubMed] [Google Scholar]
- 4.Gillet LC, et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics MCP. 2012;11 doi: 10.1074/mcp.O111.016717. O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stahl-Zeng J, et al. High Sensitivity Detection of Plasma Proteins by Multiple Reaction Monitoring of N-Glycosites. Mol. Cell. Proteomics. 2007;6:1809–1817. doi: 10.1074/mcp.M700132-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol. Syst. Biol. 2008;4:222. doi: 10.1038/msb.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Percy AJ, Chambers AG, Yang J, Hardie DB, Borchers CH. Advances in multiplexed MRM-based protein biomarker quantitation toward clinical utility. Biochim. Biophys. Acta BBA - Proteins Proteomics. 2014;1844:917–926. doi: 10.1016/j.bbapap.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 9.Chait BT. Mass Spectrometry: Bottom-Up or Top-Down? Science. 2006;314:65–66. doi: 10.1126/science.1133987. [DOI] [PubMed] [Google Scholar]
- 10.Yates JR, Ruse CI, Nakorchevsky A. Proteomics by Mass Spectrometry: Approaches, Advances, and Applications. Annu. Rev. Biomed. Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 11.Payne AH, Glish GL. In: Methods in Enzymology. Burlingame AL, editor. Vol. 402. Academic Press; 2005. pp. 109–148. [DOI] [PubMed] [Google Scholar]
- 12.McLafferty FW. Tandem Mass Spectrometry. John Wiley & Sons Inc; 1983. [Google Scholar]
- 13.Egertson JD, et al. Multiplexed MS/MS for improved data-independent acquisition. Nat. Methods. 2013;10:744–746. doi: 10.1038/nmeth.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norbeck AD, Monroe ME, Adkins JN, Smith RD. The utility of accurate mass and LC elution time information in the analysis of complex proteomes. J. Am. Soc. Mass Spectrom. 2005;16:1239–1249. doi: 10.1016/j.jasms.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisbrod CR, Eng JK, Hoopmann MR, Baker T, Bruce JE. Accurate Peptide Fragment Mass Analysis: Multiplexed Peptide Identification and Quantification. J. Proteome Res. 2012;11:1621–1632. doi: 10.1021/pr2008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clough T, Thaminy S, Ragg S, Aebersold R, Vitek O. Statistical protein quantification and significance analysis in label-free LC-MS experiments with complex designs. BMC Bioinformatics. 2012;13(S6) doi: 10.1186/1471-2105-13-S16-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLean B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Bourne PE, Bandeira N. MixGF: spectral probabilities for mixture spectra from more than one peptide. Mol. Cell. Proteomics MCP. 2014 doi: 10.1074/mcp.O113.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Röst HL, et al. OpenSWATH enables automated, targeted analysis of data-independent acquisition MS data. Nat. Biotechnol. 2014;32:219–223. doi: 10.1038/nbt.2841. [DOI] [PubMed] [Google Scholar]
- 20.Gallien S, Duriez E, Demeure K, Domon B. Selectivity of LC-MS/MS analysis: Implication for proteomics experiments. J. Proteomics. 2013;81:148–158. doi: 10.1016/j.jprot.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DE, Hayes JM. Systematic errors in gas chromatography-mass spectrometry isotope ratio measurements. Anal. Chem. 1976;48:1375–1382. [Google Scholar]
- 22.King NL, et al. Analysis of the Saccharomyces cerevisiae proteome with PeptideAtlas. Genome Biol. 2006;7:R106. doi: 10.1186/gb-2006-7-11-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canterbury JD, Merrihew GE, MacCoss MJ, Goodlett DR, Shaffer SA. Comparison of Data Acquisition Strategies on Quadrupole Ion Trap Instrumentation for Shotgun Proteomics. J. Am. Soc. Mass Spectrom. 2014;25:2048–2059. doi: 10.1007/s13361-014-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scigelova M, Hornshaw M, Giannakopulos A, Makarov A. Fourier Transform Mass Spectrometry. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M111.009431. M111.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonscher KR, Yates III JR. The Quadrupole Ion Trap Mass Spectrometer—A Small Solution to a Big Challenge. Anal. Biochem. 1997;244:1–15. doi: 10.1006/abio.1996.9877. [DOI] [PubMed] [Google Scholar]
- 26.Clauser KR, Baker P, Burlingame AL. Role of accurate mass measurement (+/− 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal. Chem. 1999;71:2871–2882. doi: 10.1021/ac9810516. [DOI] [PubMed] [Google Scholar]
- 27.Zubarev RA, Håkansson P, Sundqvist B. Accuracy Requirements for Peptide Characterization by Monoisotopic Molecular Mass Measurements. Anal. Chem. 1996;68:4060–4063. [Google Scholar]
- 28.Marshall AG, Hendrickson CL, Jackson GS. Fourier transform ion cyclotron resonance mass spectrometry: A primer. Mass Spectrom. Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.Randall SM, Cardasis HL, Muddiman DC. Factorial Experimental Designs Elucidate Significant Variables Affecting Data Acquisition on a Quadrupole Orbitrap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2013;24:1501–1512. doi: 10.1007/s13361-013-0693-y. [DOI] [PubMed] [Google Scholar]
- 30.Eng JK, McCormack AL, Yates III JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 31.Käll L, Canterbury JD, Weston J, Noble WS, MacCoss MJ. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods. 2007;4:923–925. doi: 10.1038/nmeth1113. [DOI] [PubMed] [Google Scholar]
- 32.Perry RH, Cooks RG, Noll RJ. Orbitrap mass spectrometry: instrumentation, ion motion and applications. Mass Spectrom Rev. 2008;27:661–699. doi: 10.1002/mas.20186. [DOI] [PubMed] [Google Scholar]
- 33.Plumb RS, et al. UPLC/MS(E); a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Commun. Mass Spectrom. RCM. 2006;20:1989–1994. doi: 10.1002/rcm.2550. [DOI] [PubMed] [Google Scholar]
- 34.Moon MH, Myung S, Plasencia M, Hilderbrand AE, Clemmer DE. Nanoflow LC/ion mobility/CID/TOF for proteomics: analysis of a human urinary proteome. J. Proteome Res. 2003;2:589–597. doi: 10.1021/pr034018v. [DOI] [PubMed] [Google Scholar]
- 35.Yost RA, Enke CG. Selected ion fragmentation with a tandem quadrupole mass spectrometer. J. Am. Chem. Soc. 1978;100:2274–2275. [Google Scholar]
- 36.Yost RA, Enke CG. Triple quadrupole mass spectrometry for direct mixture analysis and structure elucidation. Anal. Chem. 1979;51:1251–1264. doi: 10.1021/ac50048a002. [DOI] [PubMed] [Google Scholar]
- 37.Bensimon A, Heck AJR, Aebersold R. Mass Spectrometry-Based Proteomics and Network Biology. Annu. Rev. Biochem. 2012;81:379–405. doi: 10.1146/annurev-biochem-072909-100424. [DOI] [PubMed] [Google Scholar]
- 38.Stahl DC, Swiderek KM, Davis MT, Lee TD. Data-controlled automation of liquid chromatography/tandem mass spectrometry analysis of peptide mixtures. J. Am. Soc. Mass Spectrom. 1996;7:532–540. doi: 10.1016/1044-0305(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 39.Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal. Bioanal. Chem. 2007;389:1017–1031. doi: 10.1007/s00216-007-1486-6. [DOI] [PubMed] [Google Scholar]
- 40.McLafferty FW. Tandem mass spectrometry. Science. 1981;214:280–287. doi: 10.1126/science.7280693. [DOI] [PubMed] [Google Scholar]
- 41.Anderson NL, Anderson NG. The Human Plasma Proteome History, Character, and Diagnostic Prospects. Mol. Cell. Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 42.Geromanos SJ, et al. The detection, correlation, and comparison of peptide precursor and product ions from data independent LC-MS with data dependant LC-MS/MS. PROTEOMICS. 2009;9:1683–1695. doi: 10.1002/pmic.200800562. [DOI] [PubMed] [Google Scholar]
- 43.Bern M, et al. Deconvolution of Mixture Spectra from Ion-Trap Data-Independent-Acquisition Tandem Mass Spectrometry. Anal. Chem. 2010;82:833–841. doi: 10.1021/ac901801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schubert OT, et al. The Mtb Proteome Library: A Resource of Assays to Quantify the Complete Proteome of Mycobacterium tuberculosis. Cell Host Microbe. 2013;13:602–612. doi: 10.1016/j.chom.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLean B, et al. Effect of Collision Energy Optimization on the Measurement of Peptides by Selected Reaction Monitoring (SRM) Mass Spectrometry. Anal. Chem. 2010;82:10116–10124. doi: 10.1021/ac102179j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Ficarro SB, Li S, Marto JA. Optimized Orbitrap HCD for Quantitative Analysis of Phosphopeptides. J. Am. Soc. Mass Spectrom. 2009;20:1425–1434. doi: 10.1016/j.jasms.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Bereman MS. Tools for monitoring system suitability in LC MS/MS centric proteomic experiments. PROTEOMICS n/a–n/a. 2014 doi: 10.1002/pmic.201400373. [DOI] [PubMed] [Google Scholar]
- 48.Bereman MS, et al. Implementation of statistical process control for proteomic experiments via LC MS/MS. J. Am. Soc. Mass Spectrom. 2014;25:581–587. doi: 10.1007/s13361-013-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallick P, et al. Computational prediction of proteotypic peptides for quantitative proteomics. Nat. Biotechnol. 2007;25:125–131. doi: 10.1038/nbt1275. [DOI] [PubMed] [Google Scholar]
- 50.Stergachis AB, MacLean B, Lee K, Stamatoyannopoulos JA, MacCoss MJ. Rapid empirical discovery of optimal peptides for targeted proteomics. Nat. Methods. 2011;8:1041–1043. doi: 10.1038/nmeth.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.