Abstract
Microglia, the main phagocytes of the central nervous system (CNS), are involved in the surveillance and maintenance of nervous tissue. During normal tissue homeostasis, microglia migrate within the CNS, phagocytose dead cells and tissue debris, and modulate synapse pruning and spine formation via controlled phagocytosis. In the event of an invasion by a foreign body, microglia are able to phagocytose the invading pathogen and process it proteolytically for antigen presentation. Internalized substrates are incorporated and sorted within the endocytic pathway and thereafter transported via complex vesicular routes. When targeted for degradation, substrates are delivered to acidic late endosomes and lysosomes. In these, the enzymatic degradation relies on pH and enzyme content. Endocytosis, sorting, transport, compartment acidification and degradation are regulated by complex signaling mechanisms, and these may be altered during aging and pathology. In this review, we discuss the endocytic pathway in microglia, with insight into the mechanisms controlling lysosomal biogenesis and pH regulation. We also discuss microglial lysosome function associated with Alzheimer’s disease (AD) and the mechanisms of amyloid-beta (Aβ) internalization and degradation. Finally, we explore some therapies currently being investigated to treat AD and their effects on microglial response to Aβ, with insight in those involving enhancement of lysosomal function.
Keywords: lysosome, endocytosis, microglia, TFEB, MITF, amyloid-beta, Alzheimer’s disease
1. Introduction
The mononuclear phagocytic cell population in vertebrates includes monocytes, macrophages, dendritic cells, Langerhans cells, microglia and osteoclasts (Arandjelovic and Ravichandran, 2015; Nakamichi et al., 2013). Phagocytes are responsible for the clearance of infectious agents, dead cells and tissue debris and are involved in immune response. Monocytes circulate in the blood and can enter organs and become macrophages in normal conditions as well as in response to stimuli including infectious agents and inflammatory signals. However, an important fraction of the macrophages is tissue-specific. These tissue-resident macrophages derive from primitive macrophages generated from early erythro-myeloid progenitors in the yolk sac, and maintain their presence in tissues by slow self-renewal (Davies et al., 2013; Hoeffel and Ginhoux, 2015). Microglia, the resident macrophages of the CNS, derive exclusively from primitive macrophages in the yolk sac, populate the CNS before and shortly after birth and self-renew locally with a slow turnover rate throughout life (Ginhoux and Prinz, 2015; Gomez Perdiguero et al., 2015; Nakamichi et al., 2013).
Microglia constitute 10–15% of brain cells and play a critical role in tissue surveillance and maintenance of CNS homeostasis. Microglia are very motile within the CNS and engage in the phagocytosis of apoptotic cells and debris, participate in tissue repair, and modulate synapse pruning and spine formation, among other important functions. Microglia also help to initiate the immune response against infectious agents by acting as antigen-presenting cells (Ransohoff and Cardona, 2010).
Generally, phagocytic cells internalize a wide variety of extracellular material via several mechanisms collectively named endocytosis. Internalized material follows branching vesicular transport pathways, and some internalized material is delivered to acidic late endosomes and lysosomes, where degradation occurs. Sorting of internalized substrates and targeting to degradative acidic organelles is regulated by complex sorting mechanisms.
Moreover, as resident CNS phagocytes, microglia play a role in many neurodegenerative diseases. AD, the most common form of dementia, accounts for 60 to 80% of all cases worldwide. One of the main histopathological hallmarks of AD is the presence of amyloid plaques in specific areas of the brain. However, in the majority of AD cases, it is unclear whether faster production or slower clearance of Aβ species is responsible for plaque accumulation.
In this review, we discuss the endocytic pathway in the context of phagocytic cells, and specifically microglia, with insight into the mechanisms regulating microglial lysosome biogenesis. We also discuss microglial lysosome function in the context of aging and AD, with focus on the various mechanisms of Aβ internalization and degradation. Finally, we explore some therapies currently being investigated to treat AD and their effects on microglial activity and response to Aβ.
2. The endocytic pathway in phagocytic cells
Endocytic processes are involved in the internalization of nutrients, antigen presentation, regulation of cell-surface receptor expression, cellular cholesterol homeostasis, maintenance of cell polarity and removal of pathogens, among other functions (Mukherjee et al., 1997). In the following section we discuss the main endocytic routes in phagocytic cells. Figure 1 summarizes the various mechanisms of endocytosis in the context of microglia.
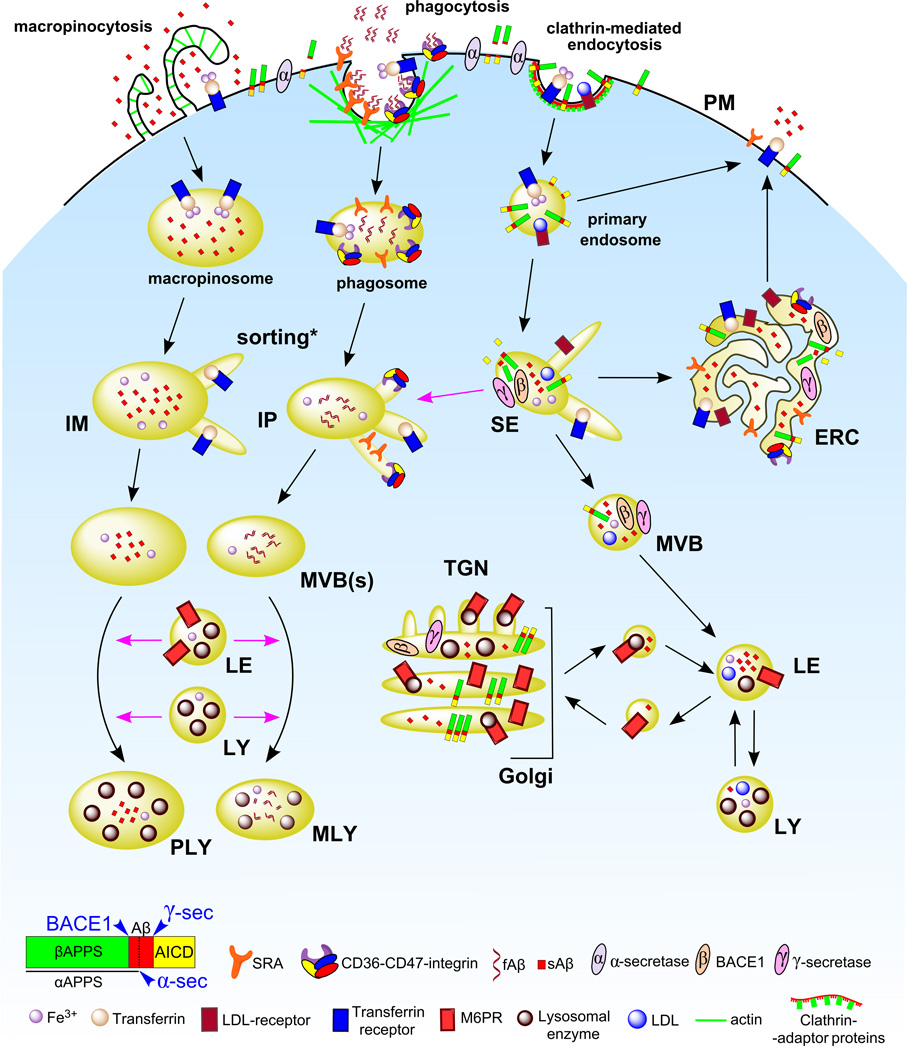
Figure 1. Proposed endocytic pathways in phagocytic cells.
Endocytic mechanisms, namely macropinocytosis, phagocytosis and clathrin-mediated endocytosis, are depicted on the PM. Following receptor-mediated endocytosis, substrates bound to receptors undergo sorting in SEs and receptors are recycled back to the PM either directly from nascent endosomes or via the ERC, whereas nascent phagosomes and macropinosomes fuse with SEs and undergo a similar sorting process (indicated by an asterisk). SEs as well as intermediate phagosomes / macropinosomes containing sorted cargo (IPs and IMs, respectively) mature and adopt a multivesicular morphology (MVBs) where an additional sorting process takes place. Substrates in MVBs eventually reach LEs, which receive enzymes and membrane proteins from the TGN via vesicular trafficking, and the LY. Most degradation occurs in these acidic compartments. In contrast, IPs and IMs acquire enzymatic cargo by fusion with LEs and LYs, giving rise to phagolysosomes and macropinolysosomes. APP cellular trafficking: Surface APP is cleaved by α-secretase followed by extracellular release of the αAPP fragment. However, an important fraction of APP is incorporated into the endocytic pathway by clathrin-mediated endocytosis and processed mainly in SEs and the ERC by BACE1 and γ-secretase, leading to secretion of AICD to the cytosol and Aβ to the lumen of the compartments. Trafficking routes are indicated by black arrows. Fusion events are indicated by pink arrows. Abbreviations: PM, plasma membrane. SE, sorting endosome. ERC, endocytic recycling compartment. IP, intermediate phagosome. IM, intermediate macropinosome. MVB, multivesicular body. TGN, trans-Golgi network. LE, late endosome. LY, lysosome. PLY, phagolysosome. MLY, macropinolysosome. SRA, scavenger receptor class A. APP, amyloid precursor protein. αAPPs, soluble APP N-terminal fragment from α-secretase cleavage. βAPPs, soluble APP N-terminal fragment from β-secretase cleavage. AICD, APP intracellular domain. sAβ, soluble Aβ. fAβ, fibrillar Aβ. LDL, low density lipoprotein. M6PR, mannose-6-phosphate receptors.
2.1. Pinocytosis
Pinocytosis, originally described by Lewis (Lewis, 1931), is a process by which the plasma membrane (PM) forms vesicles that engulf extracellular fluid in a non-selective way. In macropinocytosis, large vesicles (up to 5 µm in diameter) are formed by actin-dependent membrane ruffles whose tips fall back to the PM (Hewlett et al., 1994; Lim and Gleeson, 2011). Fusion of the tips with the PM forms a new vesicle that contains extracellular fluid. Bone-marrow derived macrophages (BMMs) (Norbury et al., 1995), dendritic cells (Sallusto et al., 1995) and microglia (Booth and Thomas, 1991; Fitzner et al., 2011; Mandrekar et al., 2009) are capable of constitutive macropinocytosis. Pinocytotic behavior was described in microglia more than 20 years ago (Booth and Thomas, 1991).
Macropinocytosis allows cells to survey and internalize large amounts of the extracellular milieu in search of cell debris, damaged proteins, apoptotic cells and pathogens, most of which are degraded. For instance, macrophages can internalize up to 200% of their surface area per hour by macropinocytosis (Steinman et al., 1976). Antigens contained in macropinosomes in antigen presenting cells can become associated with major histocompatibility complex (MHC) complexes for antigen presentation (Sallusto et al., 1995). Macropinocytosis has also been associated with pathogen entry (reviewed in (Lim and Gleeson, 2011)).
2.2. Phagocytosis
Phagocytosis is the process by which cells internalize, by vesicular engulfment, large particles (typically ≥5 µm) such as apoptotic cells, foreign bodies and pathogens (Flannagan et al., 2012). Professional phagocytes such as microglia and macrophages are able to migrate to specific areas by chemotaxis and clear foreign bodies by phagocytosis (described in detail in section 2.6). The phagocytosed particle is recognized by specific receptors on the PM of the phagocytic cell. Moreover, for an immune response, phagocytic cells can act as antigen presenting cells (Flannagan et al., 2012).
There are a variety of receptors involved in phagocytosis. Pattern-recognition receptors (PRRs) recognize specific pathogen-associated molecular patterns (PAMPs) found in bacteria or fungi. In microglia, PAMPs are recognized by Toll-like receptors (TLRs) as well as by scavenger receptor A (SRA) and scavenger receptor class B - CD36 (Flannagan et al., 2012; Koenigsknecht-Talboo and Landreth, 2005; Paresce et al., 1996) among others.
In contrast to PRRs, opsonic receptors (ORs) recognize foreign bodies (or antigens) coated with immunoglobulins (IGs) (Janeway, 2001). The most studied opsonic receptors are the Fc receptor, which binds to the Fc region of immunoglobulin G (IgG), and receptors of the complement system such as CR1, CR3 and CR4 (Flannagan et al., 2012). Surface receptors CD45, CD11b, and CD11c are also expressed in microglia and macrophages (extensively reviewed in (Ransohoff and Cardona, 2010; Saijo and Glass, 2011)).
Upon recognition by the appropriate receptor, additional receptors are recruited to the phagocytic cup (Flannagan et al., 2012). This requires lateral mobility of receptors (Holowka et al., 2007) and continuous binding of new receptors to the foreign body for complete sealing of the phagocytic cup. Receptor binding elicits complex signaling cascades leading to actin polymerization, extension of actin filaments, and actin association with myosins, allowing for the progression of the phagocytic cup (Freeman and Grinstein, 2014). PM lipids also play an important role in the formation of the phagocytic vesicle (Botelho et al., 2000; Corrotte et al., 2006). For a thorough review of phagocytosis see (Flannagan et al., 2012; Gordon, 2016).
2.3. Receptor-mediated endocytosis
In all nucleated vertebrate cells, a significant fraction of internalized PM and extracellular fluid enters the cell by receptor-mediated endocytosis (RME) via clathrin-coated pits (Mukherjee et al., 1997). Clathrin-independent mechanisms involving caveolae and other membrane invaginations have been discussed elsewhere (Le Roy and Wrana, 2005).
When macromolecules destined for RME are recognized by their transmembrane receptors, they become concentrated in patch-like indented regions of the PM called clathrin coated pits. These invaginate to form vesicles of ∼150 nm diameter coated with clathrin and other proteins (Kirchhausen et al., 2014; Mukherjee et al., 1997). Adaptor proteins facilitate the recruitment of clathrin to the PM for coated-pit formation (Maxfield and McGraw, 2004; McMahon and Boucrot, 2011). These coated vesicles engulf the ligands, bound to their respective membrane receptors, along with extracellular fluid. Coated vesicle formation involves several accessory proteins including epsins, endophilin, dynamin and amphiphysin, which are responsible for membrane bending and fission (Merrifield and Kaksonen, 2014).
Within seconds following vesicle internalization, the clathrin coat and the proteins involved in vesicle formation are removed in an energy-dependent step to be reused in new cycles of RME (Lemmon, 2001). The resulting uncoated vesicles (primary endosomes) fuse with each other and with transient early endosomes denominated sorting endosome (SEs). The process involves small GTPase regulatory proteins (Rab proteins) as well as SNARE complexes, which mediate vesicle fusion (reviewed in (Maxfield and McGraw, 2004; McMahon and Boucrot, 2011)).
By concentrating receptors and ligands, clathrin-mediated endocytosis carries out the internalization of nutrients, pathogens, antigens, growth factors and receptors (Le Roy and Wrana, 2005) without the need to internalize a large volume of extracellular fluid. Clathrin-coated pits have been found in microglia processes and synaptic elements (Tremblay et al., 2010). After incorporation into the endocytic pathway, the various receptors and cargoes follow specific trafficking routes that determine their cellular fate.
2.4. Sorting endosomes and the endocytic recycling compartment
The SE is localized at the periphery of the cell and has tubulovesicular morphology. At the mildly acidic pH (5.9–6.0) of the SE (Maxfield and McGraw, 2004), many ligands such as low density lipoproteins (LDL) dissociate from their receptors. The sorting of ligands and receptors is based in part on a geometry-based sorting process (Maxfield and McGraw, 2004; Mukherjee et al., 1997). Membrane constituents, including many recycling receptors, are removed from the SEs in narrow diameter tubules and transported to the recycling compartment or to the PM directly (Maxfield and McGraw, 2004). Soluble contents, including ligands released from their receptors, are generally retained in the more spherical parts of the SEs as they mature into a late endosome (LE).
The endocytic recycling compartment (ERC) is an organelle with a tubular morphology and associated varicosities that is often concentrated near the microtubule organizing center and typically has a lumenal pH of 6.4–6.5 (reviewed in (Maxfield and McGraw, 2004)). The ERC contains transferrin associated with its receptor and other unbound receptors that are recycled to the PM, but it does not contain ligands targeted to LEs and lysosomes (LYs) (Yamashiro et al., 1984). Vesicles are continuously budding from the ERC and reaching the PM where recycled receptors participate in new rounds of endocytosis. Some ERC contents are delivered to the trans Golgi network (TGN) (Lin et al., 2004; Mallet and Maxfield, 1999; Wilcke et al., 2000).
2.5. Late endosomes / lysosomes
SEs acquire newly endocytosed vesicles for a finite period of time (approx 5–10 min) and subsequently are translocated toward the center of the cell by microtubule-associated transport (Dunn and Maxfield, 1992; Lakadamyali et al., 2006). As they mature, vesicles often acquire a multivesicular morphology. The endosomal sorting complex required for transport (ESCRT), composed of ESCRT-0/I/II/III and the AAA ATPase Vps4 complexes, is responsible for the inward budding and detachment of endosomal membrane containing ubiquitylated receptors, which become tagged at the PM, and their sorting into multivesicular bodies (MVBs) (Henne et al., 2011; Hurley, 2015). ESCRT complexes are recruited from the cytosol to the endosomes in a sequential manner; ESCRT-0 initiates the pathway by binding to ubiquitylated substrates in endosomal membranes, followed by binding of ESCRT-I and II to the cargo and subsequent sequestration and sorting by ESCRT-III, which drives vesicle budding. ESCRT-III is finally disassembled by Vps-Vta1 complex (reviewed in (Henne et al., 2011)). Cargo sorted into MVBs by the ESCRT machinery is selectively targeted for degradation in LEs and LYs.
In some instances, MVBs secrete their intraluminal vesicles (ILVs) to the extracellular milieu by fusion of the limiting membrane with the cell’s PM, in a process also mediated by the ESCRT complex (Hurley, 2015). The secreted vesicles, called exosomes (30–100nm in diameter), contain a variety of membrane-associated and cytosolic proteins, mRNAs and miRNAS among components that play important roles in cell-to-cell signaling (Budnik et al., 2016). Neuronal and glial cells secrete exosomes loaded with various cargoes. Importantly, microglial exosomes contain MHC class II complexes (Potolicchio et al., 2005) probably as means to rapidly propagate and present antigens under pathological conditions (Prada et al., 2013).
As MVBs reach the perinuclear region, they adopt a complex structure with internal membranes typical of LEs, become acidified to pH 5–5.5 and acquire Rab9 as well as LY-associated membrane proteins (LAMPs). Many of the lysosomal hydrolases are tagged with mannose-6-phosphate in the Golgi, and they are delivered to LEs by mannose-6-phosphate receptors (M6PRs) via vesicular transport from the TGN (Luzio et al., 2014). The lower pH of the LEs facilitates dissociation of the enzymes from the M6PRs and allows full activation of their enzymatic activity (Majumdar et al., 2011; Mukherjee et al., 1997; Nixon, 2013).
LEs and LYs contain more than 100 membrane proteins (Schwake et al., 2013) and 70 hydrolytic enzymes (Lubke et al., 2009) that facilitate the degradation of macromolecules delivered via endocytic, phagocytic and autophagic pathways (Luzio et al., 2014). A large fraction of degradation probably occurs in LEs (Mukherjee et al., 1997). Nevertheless, molecules that are difficult to degrade remain in LEs that mature to become LYs.
Relating to phagocytosis and macropinocytosis, both phagosomes and macropinosomes mature in a process very similar to endosome maturation (Flannagan et al., 2012; Garin et al., 2001). Following internalization, early macropinosomes and phagosomes fuse with early endosomal compartments. The vesicles contain transferrin receptors, which are removed during maturation. As they remodel into maturing phagosomes or macropinosomes, intermediate phagosomes (IP) or macropinosomes (IM) adopt a multivesicular morphology (MVBs) and thereafter acquire LAMPs and enzymatic cargo by fusion with existing LEs and LYs. Eventually, the vesicles adopt a morphology that resembles that of LEs, followed by a decrease in Rab7 and a further increase in LAMPs as endosomes mature further (Racoosin and Swanson, 1993).
2.6. Mechanisms of microglia phagocytosis in the healthy brain
In healthy conditions, microglia undertake several functions related to maintaining tissue homeostasis (Sierra et al., 2013; Siskova and Tremblay, 2013). Among these, microglia are involved in ‘controlled phagocytosis’, a process by which the cells engage in the noninflammatory clearance of apoptotic cells and cell debris as part of their scavenging/clearing role within the CNS. Controlled phagocytosis is a focus of current research due to its potential implications in AD and epilepsy, among other CNS diseases (Brown and Neher, 2014; Sierra et al., 2013).
Apoptotic cells release ‘find me’ signals, ligands that act as powerful chemoattractants to recruit microglia that initiate phagocytosis of the cell corpses. Well-characterized ‘find-me’ signals are ATP and UDP, recognized by purinergic receptors P2X4 and P2X7 or P2Y6 and P2Y12, which are expressed by microglia (Haynes et al., 2006; Koizumi et al., 2007; Ohsawa et al., 2007). Following recruitment, ‘eat me’ signals or ligands such as phosphatidylserine (PS), which becomes exposed in the outer leaflet of the plasma membrane during apoptosis, calreticulin and complement components C1q and C3, are recognized by specific microglia surface receptors, which initiate actin-associated cytoskeleton remodeling, engulfment and phagocytosis. Internalized material is digested in acidic compartments by mechanisms described in sections 2.3 through 2.5, followed by the subsequent post-digestion responses such as release of anti-inflammatory mediators. These processes have been extensively reviewed (Brown and Neher, 2014; Sierra et al., 2013). A number of receptors involved in the recognition of ‘eat me’ signals have recently been described, including triggering receptor expressed on myeloid cells 2 (TREM2) (Takahashi et al., 2005), MerTK and Axl (Grommes et al., 2008), among others.
TREM2 promotes intracellular protein tyrosine phosphorylation (Takahashi et al., 2005; Wunderlich et al., 2013). Although ligands for TREM2 have not yet been unambiguously identified, there is evidence of direct binding of the receptor to a number of phospholipids, including PS, and anionic and zwitterionic non-phosphate lipids, released by damaged neurons and myelin, respectively (Cantoni et al., 2015; Wang et al., 2015) as well as to apoE (Atagi et al., 2015). Upon binding to ligands, TREM2 initiates phagocytosis via its signaling adaptor protein TYROBP/DNAX protein of 12 KDa (DAP12) (Wunderlich et al., 2013). TREM2 activation has been shown to increase phagocytosis in microglia and decrease their inflammatory profile (Hsieh et al., 2009), whereas the R47H TREM2 variant has been associated with increased risk for late onset AD (Guerreiro et al., 2013). Recently, it has been shown that TREM2 lipid sensing sustains the microglial response in a mouse model of AD, and that the R47H variant impairs the detection of lipid ligands by the receptor, suggesting a link to defective phagocytosis in AD (Wang et al., 2015).
Mer and Axl are members of the TAM (Tyro, Axl and Mer) receptor tyrosine kinase family and regulate the innate immune response in dendritic cells and macrophages (Lemke, 2013). Mer is abundantly expressed by microglia whereas Axl levels in the CNS are low (Fourgeaud et al., 2016; Gautier et al., 2012). Both receptors engage PS indirectly, via binding to soluble opsonins protein-S (protS) and growth-arrest-specific 6 (Gas6), the latter being the only ligand to which Axl has been shown to bind, and both are expressed by apoptotic cells and microglia (Ishimoto et al., 2000; Lew et al., 2014). Recently, Fourgeaud and collaborators demonstrated that removal of Axl and Mer by genetic ablation in CX3CR1 mice caused a striking increase in uncleared apoptotic cell bodies in brain areas undergoing adult neurogenesis (Fourgeaud et al., 2016). In their study, both protS and Gas6 promoted microglial phagocytosis, suggesting that the process, although with some contribution from Axl, is mainly driven by Mer.
2.7. Regulators of microglial survival and homeostasis in the CNS
The macrophage colony-stimulating factor cytokine (M-CSF or CSF1) is a key mediator in the differentiation of myeloid progenitor cells into various phagocytic cell types through binding to its receptor, the colony-stimulating factor-1 receptor (CSF-1R, but also known as CD115) (Pollard, 2009). Other cytokines such as interleukin-3 (IL3 or multi-CSF), interleukin-4 (IL4), granulocyte macrophage colony-stimulating factor (GM-CSF or CSF-2) or interleukin-5 (IL5) also play a role in macrophage development and have been described elsewhere (Nakamichi et al., 2013; Pollard, 2009).
CSF-1R is expressed by all tissue macrophages (Hume, 2015) and by some neurons (Chitu et al., 2015) and is necessary for microglial viability (Elmore et al., 2014). CSF-1R depletion either with antibodies against the receptor (MacDonald et al., 2010), receptor inhibitors (Elmore et al., 2014) or by genetic ablation (Dai et al., 2002) in mice leads to a removal of tissue macrophages and microglia. Differentiation of microglia and their presence in the CNS is highly dependent on CSF-1R expression (Ginhoux and Prinz, 2015) and its ligand, M-CSF, is mainly expressed by neurons within the CNS (Nandi et al., 2012; Wang et al., 2012). However, depletion of M-CSF expression does not lead to the same depletion of macrophages as removal of CSF-1R (Dai et al., 2002), suggesting the existence of additional ligands that might compensate for the lack of this cytokine.
A second cytokine ligand for the CSF-1R receptor, interleukin-34 (IL34), has been identified (Lin et al., 2008). IL34 is also mainly produced by neurons. Its genetic ablation led to removal of an important fraction of microglia in the cortex, hippocampus and olfactory bulbs but not in the cerebellum or brain stem, where M-CSF is primarily expressed during development. Importantly, other phagocyte populations such as monocytes, dendritic cells and most tissue macrophages remained almost intact, thus suggesting a role for IL34 in the specific development of microglia (Greter et al., 2012; Wang et al., 2012). While IL34 regulates the development and maintenance of microglia in forebrain structures, M-CSF seems to control microglia populations in white matter areas, and, to a lesser extent, in the cortex and cerebellum (Kondo and Duncan, 2009; Nandi et al., 2012). Lastly, M-CSF and CSF-1R-deficient mice present brain abnormalities and die before adulthood (Ginhoux et al., 2010), whereas brains in mice deficient in IL34 are largely normal (Wang et al., 2012), suggesting that only M-CSF, through CSF-1R signaling, is responsible for overall brain development (Chitu et al., 2016). M-CSF is highly expressed in the yolk sac, and it may contribute to the expansion of microglial precursors, whereas IL34 does not control embryonic development of microglia (Ginhoux et al., 2010; Greter et al., 2012).
2.8. Mechanisms regulating lysosomal biogenesis in microglia
Transcription of lysosomal enzymes, membrane proteins and proton pumps is critical for the final steps of vesicle maturation. In phagocytic cells, lysosomal biogenesis is regulated by a number of transcription factors. In the present section we discuss the MiTF/TFE family of transcription factors and their associated signaling cascades, which are summarized in Figure 2.
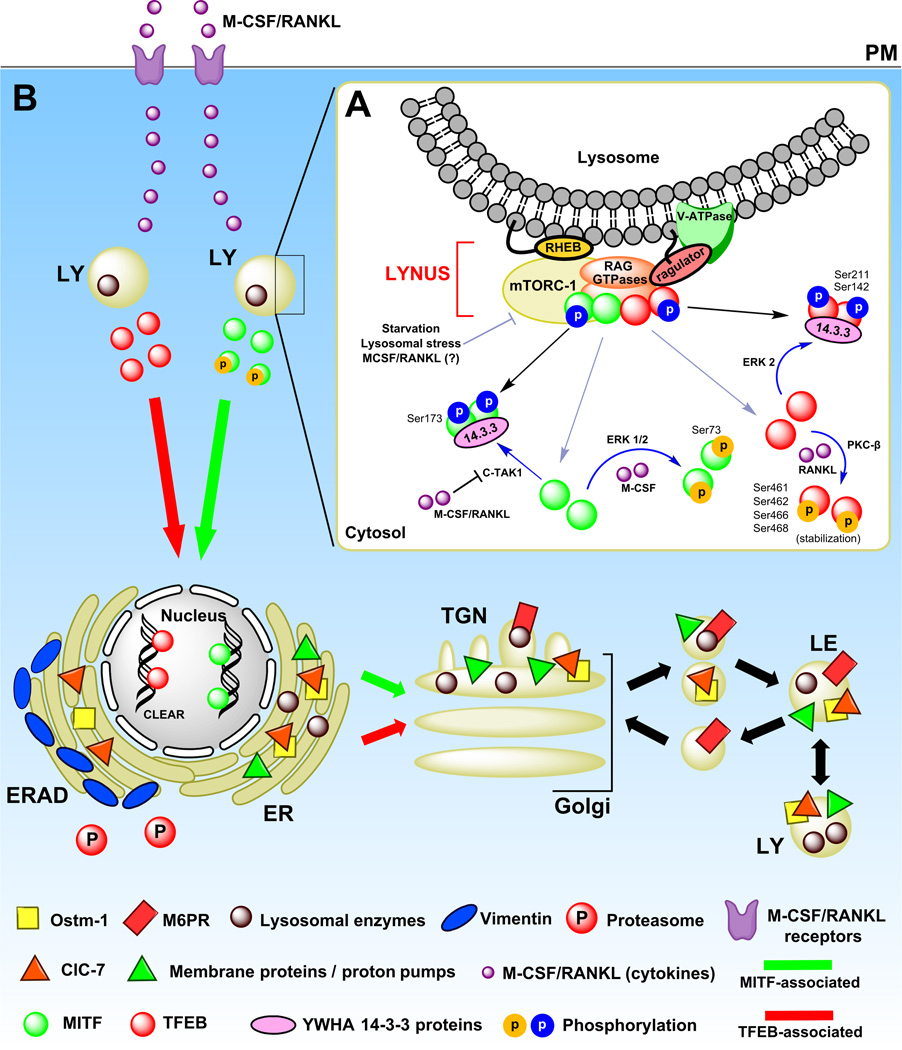
Figure 2. Proposed mechanisms of lysosomal biogenesis in microglia.
(A) TFEB and MITF localization is dependent on their phosphorylation sites and states. In fully fed cells, TFEB (and probably MITF too) associates with active RAG GTPases in the LYNUS machinery at the surface of lysosomes and becomes phosphorylated by mTORC-1 at multiple sites including Ser211 and Ser142 (phosphorylation indicated by blue circles) followed by release to the cytosol and complex formation with 14-3-3 chaperones (indicated by black lines and arrowheads) (Martina and Puertollano, 2013; Settembre et al., 2013b). C-TAK1 and ERK1/2 have also been suggested to phosphorylate MITF and TFEB at Ser173 and Ser142, respectively, and promote their cytosolic stabilization by complex formation with 14-3-3 proteins (indicated by blue lines and arrowheads). Under certain conditions such as starvation or cellular stress, mTORC-1 detaches from LYNUS and no longer phosphorylates TFEB likely leading to destabilization of TFEB-14-3-3 complexes and promoting the nuclear translocation of the factor (indicated by grey lines and arrowheads). MITF may be regulated in a similar manner (Martina and Puertollano, 2013; Roczniak-Ferguson et al., 2012; Settembre et al., 2013b). In addition, following uptake by specific PM receptors, M-CSF and RANKL are also able to promote nuclear translocation of MITF by inhibiting C-TAK1 (which in turn destabilizes MITF-14-3-3 complexes) and/or promoting ERK 1/2-associated phosphorylation of the factor at Ser73 (Bronisz et al., 2006; Weilbaecher et al., 2001). RANKL alone has been shown to increase TFEB nuclear levels by preventing its degradation via PKC-β-associated phosphorylation at multiple serine residues (phosphorylation indicated by yellow circles), which seems to stabilize TFEB in the cytosol (Ferron et al., 2013). (B). Following nuclear mobilization, MITF and TFEB (depicted by green and red circles/arrows, respectively) promote the transcription of lysosomal genes (Meadows et al., 2007; Sardiello et al., 2009; Settembre et al., 2012). Newly synthesized lysosomal proteins pass through the ER and the Golgi and reach acidic late endosomes by vesicular trafficking (depicted by triangles, circles, rectangles and squares in the various organelles, and described in the legend). Abbreviations: M-CSF, macrophage-colony stimulating factor. RANKL, receptor activator of nuclear factor kappa-B ligand. LY, lysosome. MITF, microphthalmia-associated transcription factor. TFEB, transcription factor EB. C-TAK1, Cdc25C-associated kinase 1. MAPK, Mitogen-activated protein kinases. mTORC1, mammalian target of rapamycin complex 1. LYNUS, LY nutrient sensing machinery.
Transcription factor EB (TFEB), a protein belonging to the MiTF/TFE family of transcription factors also composed of TFE3, TFEC and the microphthalmia-associated transcription factor (MITF), is a master regulator of lysosomal protein transcription in many cell types (Sardiello et al., 2009; Settembre et al., 2011) and participates in the regulation of lysosomal biogenesis in microglia (Tanaka et al., 2013). TFEB associates with the LY nutrient sensing machinery (LYNUS), composed of the kinase complex mammalian target of rapamycin complex 1 (mTORC1), RAG GTPase proteins, the vacuolar ATPase complex (V-ATPase), the ragulator complex, which anchors RAGs to the lysosomal membrane, and the small GTPase RAS homologue enriched in brain (RHEB) which is involved in mTORC-1 activation, along with other proteins (Settembre et al., 2013b). Under high nutrient conditions, TFEB associated with RAG GTPases at the surface of lysosomes and undergoes phosphorylation at several residues such as Ser211 and Ser142 by mTORC-1 (Settembre et al., 2013b). Phosphorylated TFEB is then released to the cytosol, where it associates with YWHA (14-3-3) family of proteins (Martina et al., 2012), which act as chaperones, thus stabilizing TFEB in the cytosol and probably masking the RAG-binding domain in TFEB (Martina and Puertollano, 2013). Extracellular signal-regulated kinase 2 (ERK2), a mitogen-activated protein kinase (MAPK), has also been shown to phosphorylate TFEB at Ser142 and promote its cytosolic stabilization (Settembre et al., 2012). However, during cellular starvation (absence of amino acids) or lysosomal stress, mTORC-1 and RAG GTPases are inactivated and TFEB no longer undergoes mTORC-1-associated phosphorylation. As a consequence, the binding site established in TFEB by mTORC-1 phosphorylation, which facilitated TFEB and 14-3-3 complex formation, is no longer available, and TFEB diffuses from the surface of lysosomes to undergo nuclear translocation (Settembre et al., 2013a; Settembre et al., 2011). Once in the nucleus, TFEB binds to CLEAR motifs, 10bp domains present in a number of LY-associated genes, promoting their transcription (Sardiello et al., 2009; Settembre et al., 2013a). Additionally, in osteoclasts, receptor activator of nuclear factor kappa-B ligand (RANKL) alone has been shown to increase TFEB nuclear levels by preventing its degradation, via phosphorylation at Ser461 and/or Ser462, Ser466 and Ser468 by protein kinase C-β (PKC-β), which stabilized the factor in the cytosol (Ferron et al., 2013).
MITF, also belonging to the MiTF/TFE family, is closely related to TFEB (Martina et al., 2014). MITF is involved in the terminal differentiation of developmentally unrelated cell types such as osteoclasts and melanocytes. Animals bearing dominant-negative mutations in the MITF gene present a severe osteopetrotic phenotype (Weilbaecher et al., 2001). In bone marrow–derived macrophages (BMMs), MITF is complexed with 14-3-3 proteins in the cytosol, and experiments conducted in RAW 264.7 macrophages suggested that phosphorylation at Ser173 by Cdc25C-associated kinase 1 (C-TAK1) was necessary in order to maintain MITF-14-3-3 association (Bronisz et al., 2006). However, like TFEB, MITF is also capable of nuclear translocation. Upon treatment with RANKL and M-CSF, C-TAK-induced phosphorylation of MITF is inactivated, leading to destabilization of the 14-3-3-MITF complexes and promotion of nuclear translocation of the factor (Bronisz et al., 2006). In primary osteoclasts, M-CSF has also been shown to promote nuclear translocation of MITF via phosphorylation at Ser73 by ERK 1/2 followed by recruitment of the transcriptional coactivator p300 (Weilbaecher et al., 2001). Interestingly, a consensus MAPK phospho-acceptor serine is conserved within MITF, TFE, TFEC and TFEB (Weilbaecher et al., 2001). Hence, M-CSF may be able to induce transcriptional activity via both MITF and TFEB. p38 MAPK and phosphoinositide-3 kinase (PI3K) have also been associated with MITF-mediated maturation and gene expression in cells of myeloid lineage (Carey et al., 2016; Ma et al., 2011). It is important to note that, so far, 9 MITF isoforms have been identified (Martina et al., 2014). Therefore, some of the mechanisms described in the literature may be isoform-specific.
Work carried out in HeLa and ARPE cells indicated that, like TFEB, MITF also associates with RAG GTPases in the LYNUS, and it has been suggested that the factor may undergo regulation in a manner similar to TFEB (Martina and Puertollano, 2013; Roczniak-Ferguson et al., 2012). Moreover, homodimerization and heterodimerization between MITF, TFEB, TFEC and TFE3 is required for binding to DNA and transcriptional activation of target genes (Martina et al., 2014).
M-CSF has been shown to promote lysosomal biogenesis in osteoclasts and BMMs (Jaworowski et al., 1999; Meadows et al., 2007; Motyckova et al., 2001). As discussed in sections 2.7, neurons and microglia express CSF-1R (Hume, 2015; Nandi et al., 2012). However, the signaling mechanisms discussed above have not been validated in microglia, and little is known regarding the mechanisms by which MiTF/TFE family of transcription factors regulates lysosomal biogenesis in these cells. Nevertheless, as discussed in sections 2.10.3 and 2.12.3, microglia do respond to M-CSF with increased lysosomal function. Interestingly, it has been shown that glial cells internalize exosomes of neuronal origin (Budnik et al., 2016). Therefore, although merely speculative, a mechanism by which microglia may tune their degradative capacity might involve the internalization of neuronal exosomes containing M-CSF.
2.9. Regulation of pH in late endosomes and lysosomes of microglia
In most cells, within 30 minutes following internalization, and as they progress through the endocytic pathway, ligands encounter an increasingly acidic environment ranging from pH 6 in SEs to a pH of 4.5–5 in LYs (Yamashiro and Maxfield, 1987).
Endosomal pH is regulated by a variety of ion transport processes. The main regulator of vesicular pH is the V-ATPase complex. The different subunits of this complex play a role in the regulation of membrane potential and vesicular pH as well as membrane function (Maxson and Grinstein, 2014). This complex has two domains, the V1 that carries out ATP hydrolysis and the V0 that pumps protons across the membrane. Transport of the protons into the lysosomal lumen generates an inside-positive membrane potential, which limits further proton transport and prevents full lysosomal acidification (Pillay et al., 2002). A number of pathways dissipate the electrical gradient and allow for full acidification of the compartments. The nature of these pathways is complex, but anion transport and specifically the influx of chloride ions (Cl−) constitute one important ion transport mechanism (Graves et al., 2008; Pillay et al., 2002; Stauber and Jentsch, 2013; Steinberg et al., 2010), although cation efflux has also been described as an important pathway (Steinberg et al., 2010). In the lysosomes of many cell types, the Cl− channel transporter ClC-7 is able to dissipate the voltage across the lysosomal membrane (Graves et al., 2008; Mindell, 2012). ClC-7 has been identified as the main regulator involved in LY pH regulation in microglia (Majumdar et al., 2011). ClC-7 forms a heterodimeric complex with another lysosomal membrane protein, Ostm1, and the formation of this complex is important for trafficking ClC-7 to LY (Lange et al., 2006). The neutralization of the lysosomal inside-positive membrane potential allows for further proton import into the lysosomal lumen, leading to full lysosomal acidification.
2.10. Internalization and degradation of Aβ by microglia
The amyloidogenic processing of the amyloid precursor protein (APP) occurs primarily in neurons (Haass et al., 2012). APP is transported both in neuronal axons and dendrites, and its lumenal fragments are degraded, at least in part, in LYs (Haass et al., 2012; Haass et al., 1992). Undegraded Aβ released by the neurons is taken up by microglia and other cells.
In non-polarized mammalian cells, approximately 10% of the total nascent APP reaches the PM, whereas the majority of APP at steady-state is localized in the Golgi apparatus and TGN (Haass et al., 2012). In the PM, a fraction of APP is cleaved in a non-amyloidogenic manner by α-secretase, producing a soluble fragment (αAPPs), which is released extracellularly (Haass et al., 2012). However, the majority of APP that reaches the PM is rapidly internalized by endocytosis. Following internalization, APP is processed in the amyloidogenic pathway and generation of Aβ occurs mainly in SEs and the ERC (Haass et al., 2012). BACE1 cleavage generates a soluble APP N-terminal fragment (βAPPs) and a C-terminal fragment (β-CTF) at the membrane of early endosomal compartments. Subsequently, the multimeric γ-secretase complex including presenilin (PS-1 or PS-2), nicastrin, anterior pharynx defective protein (APH-1) and presenilin enhancer protein (PEN-2) cleaves β-CTF within the transmembrane domain generating Aβ and the APP intracellular domain (AICD) (Haass et al., 2012). γ-secretase cleavage has been reported in different organelles, although Aβ accumulates primarily in LEs and MVBs (Takahashi et al., 2002).
Aβ is present in the brain in monomeric, oligomeric, protofibrillar and fibrillar forms (Haass and Selkoe, 2007). Aβ oligomers and fibrils accumulate both inside neurons (Gouras et al., 2010) and in the parenchymal extracellular space (Hardy and Selkoe, 2002; Meyer-Luehmann et al., 2003). However, the origin of the extracellular Aβ species is unclear. Presumably, Aβ can be released from cells by membrane trafficking (e.g., recycling from endosomes) (Cirrito et al., 2008) or perhaps by release from dying cells containing intracellular peptide.
In this section we discuss the mechanisms of internalization and degradation of the various Aβ species by microglia and the enzymatic processing taking place in acidic compartments. Figure 1 summarizes the proposed mechanisms for Aβ internalization in microglia.
2.10.1. Microglia uptake and degradation of sAβ species
Microglia internalize soluble Aβ (sAβ) by pinocytosis (Chung et al., 1999; Mandrekar et al., 2009), and it enters the endocytic pathway. Much of it is recycled undegraded, and some is delivered to LE/LY (Chung et al., 1999; Mandrekar et al., 2009). Acidic conditions facilitate formation of Aβ fibrils (Jiang et al., 2012; Stine et al., 2003). Therefore, some of the internalized pool of sAβ may rapidly polymerize and reach the LY as fibrillar Aβ (fAβ).
Endothelin-converting enzymes (ECEs) are type II membrane-bound zinc metalloproteases mainly involved in regulation of vasoconstriction (Turner and Murphy, 1996). ECE-1 is located extracellularly or in the lumen of organelles (Pacheco-Quinto et al., 2013). ECE-2, which is highly homologous to ECE-1, has a pH optimum of 5.0–5.5, suggesting that it is active in LE/LY (Pacheco-Quinto et al., 2013). ECEs have been observed in the endolysosomal system of neuronal cultures (Pacheco-Quinto and Eckman, 2013) and to lesser extent in astrocytes, microglia and macrophages of AD brains (Palmer et al., 2009). ECE-1 degrades both sAβ1–40 and sAβ1–42 in vitro (Eckman et al., 2001). ECE-1 cleaves at K16|L17, L17|V18 and F19|F20 within the hydrophobic domain of Aβ1–40/42 sequences (Eckman et al., 2001; Rogeberg et al., 2014) as well as at G33|L34 and L34|M35 of the C-terminal side of Aβ1–42 (Rogeberg et al., 2014). Due to the high degree of homology between ECE-1 and ECE-2, it is plausible that both enzymes process Aβ in a similar manner. Mice deficient in ECE-1 and −2 showed elevated fAβ1–40 and fAβ1–42 in their brains (Eckman et al., 2003) perhaps due to decreased sAβ degradation in neurons.
The major LY proteases are the cathepsins (Nakanishi and Wu, 2009; Turk et al., 1999). Cathepsins B, L, and D are present in microglial LY (Banati et al., 1993; Majumdar et al., 2007; Nakanishi, 2003), with pH optima ranging from 3.5 for cathepsin D to 5.5 for cathepsin B (Mackay et al., 1997). Cathepsin B has been shown to cleave sAβ at the C-terminal sites G33|L34, G38|V39 and V40|I41 of the Aβ sequence in vitro (Mueller-Steiner et al., 2006), and cathepsin D cleaves at L17|V18, F19|F20, F20|A21 and L34|M35 (Hamazaki, 1996; Rogeberg et al., 2014; Sadik et al., 1999). Cathepsin D is also present in LEs (Cataldo et al., 1995) and might participate in the partial degradation of sAβ before the cargo ultimately reaches the LY. Moreover, cathepsin E, an endosomal aspartyl protease, is expressed in early endosomal compartments of microglia (Nishioku et al., 2002) and cleaves sAβ1–40 at the hydrophobic site F19|F20 (Mackay et al., 1997). It is therefore likely that cathepsin E partially processes sAβ1–42 following endocytosis.
2.10.2. Microglia degradation of fibrillar Aβ species
Small fibrils of Aβ1–42 are internalized in vitro by neonatal murine microglia, via RME mediated by scavenger receptors A class I and II (SRAs) and B-I (SRB-I) (Paresce et al., 1996). A complex composed of CD36 (SRB family), the integrin-associated protein CD47 and the integrin receptor α6β1 has also been associated with internalization of fAβ peptide sequences 25–35, 1–40 and 1–42 (Bamberger et al., 2003). Other receptors implicated in the uptake of fAβ1–42 include CD14 and TLRs 2 and 4 (Reed-Geaghan et al., 2009) as well as a heterotrimeric receptor complex composed of CD36, TLR4 and TLR6 (Stewart et al., 2010). However, in vivo studies have not always confirmed in vitro findings. In the CRND8 mouse model of AD harboring the human amyloid precursor protein gene with the Indiana and Swedish mutations (Chishti et al., 2001), microglia were efficient at internalizing protofibrillar Aβ but were unable to phagocytose congophillic fAβ, and microglial phagocytic activity was modulated by the chemokine receptor CX3CR1 (Liu et al., 2010). Furthermore, one study suggested that microglia have no involvement in the clearance of fAβ plaques (Grathwohl et al., 2009). After elimination of microglia in the APP/PS1 and APP23 models of AD, no changes were observed in Aβ plaque load, neuronal counts or neuronal morphology. In fact, a small reduction in total Aβ load was observed in the APP23 model upon microglia ablation. Similarly, ablation of microglia by inhibition of CSF-1R signaling in 5xFAD (Spangenberg et al., 2016) and 3xTg-AD (Dagher et al., 2015) mice models of AD prevented neuronal loss and improved cognition, respectively, without significantly altering Aβ pathology. A significant population of microglia is chronically activated in the AD brain, thus causing damage to neurons, while probably inefficiently degrading Aβ. Removal of these cells might partially rescue neuronal dysfunction while leaving Aβ pathology largely unaltered. In contrast, another study in the APP/PS1 mouse model of AD demonstrated that plaques surrounded by an increased number of microglia were smaller, suggesting that microglia were able to actively regulate plaque size (Bolmont et al., 2008). The role that microglia play in the regulation of Aβ deposition, clearance, and neuronal damage is largely dependent on their activation state.
Following internalization, fAβ is trafficked to LYs (Majumdar et al., 2007; Paresce et al., 1996) where it encounters degradative enzymes. Within non-stimulated microglia, however, fAβ shows considerable resistance to lysosomal proteolytic degradation (Chung et al., 1999; Majumdar et al., 2011; Majumdar et al., 2007; Paresce et al., 1997). In the LYs, cathepsin B cleaves at the C-terminal side of the fAβ peptides just as for sAβ (Mueller-Steiner et al., 2006), and deletion of the enzyme led to increased fAβ plaque load in the J20 mouse model of AD (Mueller-Steiner et al., 2006).
2.10.3. Insufficient lysosomal acidification leads to undegraded fAβ
The regulation of LY pH is crucial to the enzymatic activity. Surveillant microglia maintain their LY pH at about pH 6 but are able to alter it in response to physiological stimuli. In contrast, most other cells maintain their LY pH close to 5 or lower (Majumdar et al., 2011). The benefits of incomplete LY acidification are not well understood, but control of LY pH might be associated with the role of microglia in antigen processing and presentation (Majumdar et al., 2011).
The transcription of Ostm1 in surveillant microglia is greatly reduced when compared with other cell types. In the absence of sufficient Ostm1, most of the Cl− transporter ClC-7 (discussed in section 2.9) undergoes endoplasmic reticulum (ER) associated degradation (ERAD) and does not get to the LY (Majumdar et al., 2011). The higher LY pH of microglia results in decreased fAβ degradation compared to macrophages, which fully acidify their LYs (Majumdar et al., 2007).
Treatment of neonatal murine microglia with M-CSF increases the transcription of both ClC-7 and Ostm1, which is indicative of lysosomal biogenesis. Following this treatment, abundant ClC-7 is found in microglial LY, and the cells acidify their LY to pH 5 (Majumdar et al., 2011). Knockdown of either Ostm1 or ClC-7 reduces the ability of M-CSF to acidify microglial lysosomes, demonstrating the requirement for delivery of this complex to LY in order to achieve full acidification. Lysosomal acidification induced by M-CSF treatment led to increased degradation of fAβ by microglia at levels similar to macrophages (Majumdar et al., 2011; Majumdar et al., 2007).
2.10.4. Decreased Aβ degradation with aging
Aging has been associated with impaired LY function in a wide variety of cell types and organisms (Cuervo and Dice, 2000; Martinez-Vicente et al., 2005; Terman and Brunk, 2004). During the course of aging, LY volume often increases, whereas LY stability and hydrolytic activity decrease (Terman and Brunk, 2004).
The progressive accumulation of undigested material (e.g., lipofuscin) by microglia, due to the accumulation of proteolysis-resistant material as well as, to some extent, phagocytosis of neurons (Nakanishi and Wu, 2009) might hinder LY function (Martinez-Vicente et al., 2005; Nixon, 2013; Terman and Brunk, 2004). Lipofuscin forms due to the progressive oxidation and polymerization of proteins and lipid residues and is considered a hallmark of aging (Terman and Brunk, 2004). With aging, postmitotic cells such as neurons and microglia accumulate large amounts of lipofuscin.
The increased amounts of Fe2+ contained in lipofuscin vesicles further promote reactive oxygen species (ROS) generation, perhaps causing LY membrane damage and permeabilization, leading to dysregulation of LY pH and altered enzymatic activity (Kurz et al., 2008; Nixon, 2013; Terman and Brunk, 2004).
Besides lipofuscin, other factors may contribute to the aging-related decline in LY proteolysis. A decrease in chaperones during aging might impair transport of enzymes to the LYs (Kaushik and Cuervo, 2015; Vilchez et al., 2014), and aging-related post-translational modifications and protein misfolding might alter the proteolytic susceptibility of proteins in part by altering normal chaperone function (Kaushik and Cuervo, 2015).
2.11. Microglia, apolipoprotein E, lipid metabolism and AD
Many studies have pointed to a disruption in lipid metabolism as an increased risk factor for AD (reviewed in (Di Paolo and Kim, 2011)), and lysosomal storage disorders have demonstrated the importance of proper cholesterol trafficking in the CNS (Vanier, 2010).
A major genetic risk factor for AD, associated with lipid metabolism, is the inherited polymorphism of the apolipoprotein E gene (APOE) (Corder et al., 1993). ApoE is the primary cholesterol carrier in the brain (Liu et al., 2013; Pitas et al., 1987). Its role is to maintain cholesterol homeostasis, an essential aspect of membrane and synapse integrity (Mahley and Rall, 2000). The three most common apoE isoforms are a result of changes in the coding sequence that alter the structure and function of the protein (Mahley et al., 2006). The lipidated ε2 and ε3 isoforms bind and clear Aβ from the brain better than the ε4 isoform and are associated with lower AD risk (Castellano et al., 2011; Liu et al., 2013). Cerebrospinal fluid (CSF) Aβ levels are lower in patients with Aβ plaque deposition in their brains (Fagan et al., 2005), and this is apoE ε4 dose-dependent (Sunderland et al., 2004), suggesting that apoE plays a role in the clearance of Aβ from the parenchyma to the CSF.
In the CNS, apoE is expressed primarily by astrocytes and microglia (Liu et al., 2013). In AD patient brains, apoE colocalizes with amyloid plaques (Namba et al., 1991) that are typically surrounded by a large number of reactive microglia (Solito and Sastre, 2012). The ATP-binding cassette transporter A1 (ABCA1), a related protein, ABCA7, and other related transporters transfer cholesterol to apoE-containing lipoproteins, reducing cholesterol levels and maintaining neuronal health (Sakae et al., 2016; Wahrle et al., 2004). Lipidated apoE acts as an Aβ chaperone within the cell and, in microglia, apoE ε3 promotes enzyme mediated (NEP and IDE) Aβ degradation more efficiently than ε4 (Jiang et al., 2008; Liu et al., 2013). The chaperone role of apoE in promoting Aβ degradation in microglia, however, is not definitive; altered cholesterol levels regulate Aβ delivery to lysosomes and degradation in microglia but do not seem to change the expression or activity of Aβ degrading enzymes (Lee et al., 2012).
Retinoid X receptor (RXR) and peroxisome proliferator-activated receptors (PPAR) are implicated in the apoE-related response to Aβ in AD as well as transcription and activation of TFEB. The transcription of APOE is activated by the dimerization of RXR with PPARγ (Chawla et al.; Corder et al., 1993; Schmechel et al., 1993). The activation of PPARγ and RXRs controls brain inflammation and alters the inflammatory state of microglia (Bernardo and Minghetti, 2006). In the APP/PS1 mouse model of AD, genistein (PPARγ agonist) and bexarotene (RXR agonist which crosses the blood-brain barrier) treatments lowered plaque load and Aβ levels due to increased apoE release from astrocytes, together with a reduction of microgliosis (Bonet-Costa et al., 2016). The decrease in Aβ by bexarotene treatment is dependent on ABCA1’s lipidation of apoE (Corona et al., 2016). Bexarotene also increases the expression of genes involved in microglial Aβ phagocytosis, including Trem2, Tyrobp, Apoe, and Mertk (Lefterov et al., 2015). Furthermore, RXRα also dimerizes with PPARα to form a transcriptional complex, and in primary astrocytes or BV-2 glial cells, treatment with gemfibrozil (a PPARα agonist), or retinoic acid upregulates TFEB transcription (Ghosh et al., 2015). Interestingly, in cultured human dermal fibroblasts, genistein also induces an upregulation in TFEB expression (Moskot et al., 2014). Thus, the reduction in mouse brain Aβ levels seen upon treatment with PPAR agonists may also be mediated in part by enhanced lysosomal biogenesis.
ApoE and ABCA1 transcription is also responsive to the ligand-activated liver X receptors (LXRα and LXRβ). The enhancement in Aβ clearance seen following treatment of APP23 AD mice with LXR ligands , together with the role played by ABCA1 in modulating the inflammatory response in the brain (Karasinska et al., 2013), suggests that both cholesterol transport and glial cell-mediated neuroinflammation and degeneration might be potential AD therapeutic targets. The receptors and their agonists discussed in this section are an active area of research in AD treatment.
2.12. Potential mechanisms to increase degradation of Aβ by microglia
A number of genome-wide association studies (GWAS) have revealed an association between genes relating to immune response and late-onset AD (Karch et al., 2014). Genetic epidemiology studies pointed out a genetic overlap between immune-mediated disease and AD (Yokoyama et al., 2016). These data indicate a central role played by the immune system and specifically by microglia in the pathogenesis of AD. Therefore, targeting of microglia and specifically their degradative capacity constitutes a promising therapeutic approach in the treatment of AD. In this section we discuss the role of microglia in mediating the effects of certain AD treatments, and we also discuss ways to increase Aβ degradation by enhancing microglial degradative capacity.
2.12.1. Anti-inflammatory therapies
The role of inflammation during the pathogenesis of AD is controversial. Based on an inflammation theory of AD, the main focus of research has been on finding suitable antiinflammatory agents that cross the blood-brain barrier (Trepanier and Milgram, 2010). Prostaglandin H synthase (COX) inhibitors constitute the major anti-inflammatory agents tested on AD patients, and most non-steroidal anti-inflammatory drugs (NSAIDS) possess potent antiinflammatory activity based on their COX inhibition. In addition, some NSAIDs act as PPARγ agonists, inhibiting interleukin-6 (IL6) and RNF-alpha in microglia and monocytes (Combs et al., 1999) and preventing microglial activation (Combs et al., 2000). 3xTg-AD mice treated with a COX-1 selective inhibitor showed reduced glial activation and increased brain expression of anti-inflammatory markers along with learning and memory improvements and reduced Aβ deposits (Choi et al., 2013). Unfortunately, clinical trials have failed to show beneficial effects of NSAIDs in AD patients (Heneka et al., 2015). Recent data showed that prostaglandin signaling mediates a beneficial immune modulatory response via prostaglandin EP4 receptors. However, prostaglandin signaling also induces a toxic response via EP2 and EP3 receptors. This dichotomous response argues against NSAIDs as appropriate therapeutic agents. Targeting downstream of COX might avoid this detrimental response (Johansson et al., 2015). This topic has been reviewed in detail (Woodling and Andreasson, 2016).
Aspirin-triggered lipoxin A4 (ATL) treatment has been suggested to promote the production of anti-inflammatory molecules while decreasing pro-inflammatory factors. Compared to NSAID treatment, ATL induces high responses with lower drug doses, thus constituting a therapeutic candidate for the treatment of AD. ATL treatment in the Tg2576 mouse model of AD led to upregulation of arginase-1 and YM1 alternative activation markers (anti-inflammatory) and downregulation of iNOS expression (Medeiros et al., 2013). Interestingly, YM1 showed colocalization with CD45-positive microglia and 6E10-positive Aβ deposits. This alternative microglia activation was associated with enhanced phagocytic phenotype, fAβ plaque clearance, synapse recovery and improvement in cognitive performance tests (Dunn et al., 2015; Medeiros et al., 2013). However the mechanism at the cellular level needs further investigation.
Despite the promising results of anti-inflammatory treatments on animal models, drugs to block the inflammatory signaling mechanisms on which microglia rely to modulate their phagocytic and proteolytic functions might magnify the progression of AD (Lucin and Wyss-Coray, 2009; Wyss-Coray and Mucke, 2002). This apparently paradoxical effect in microglia might be related to the plasticity of the cells responding to different environmental signals. Aging might cause an imbalance of the inflammatory response, and this might contribute to or aggravate AD pathology. Controlling the subtle shift from classical, pro-inflammatory, to alternatively activated microglia may be a key factor to consider when designing efficient anti-inflammatory therapies.
2.12.2. Immunization therapies
In human AD brains and in transgenic mouse models of AD, microglia associate with fAβ deposits (reviewed in (Wyss-Coray and Rogers, 2012)). However, as discussed in previous sections, non-activated microglia do not degrade fAβ efficiently due to insufficient lysosomal acidification (Majumdar et al., 2011; Majumdar et al., 2007). Immunotherapy studies in mouse models of AD showed that both active and passive immunization against the Aβ peptide were successful at resolving Aβ pathology, but clinical trials involving AD patients have not been successful so far (extensively reviewed in (Wisniewski and Goni, 2015)).
In a seminal study, Schenk and collaborators showed that active immunization against Aβ in PDAPP mice led to a striking reduction in brain Aβ pathology. Areas typically occupied by plaques appeared decorated with Aβ-immunoreactive, MHC-II positive microglia and/or monocyte-derived cells. The study suggested that these cells phagocytose Aβ via Fc receptors (Schenk et al., 1999) and set the ground for clinical trials involving AD patients (reviewed in (Boche et al., 2010; Wisniewski and Goni, 2015)).
Similarly, peripherally administered antibodies (passive immunotherapy) against Aβ penetrate the parenchyma and trigger microglia to clear plaques through Fc receptor-mediated phagocytosis, leading to degradation (Bard et al., 2000; Koenigsknecht-Talboo et al., 2008; Wilcock et al., 2004). Interestingly, PDAPP mice showed increased expression of Fc receptors and CD45 inflammation marker in microglia 1 and 2 months after injection of anti-Aβ antibodies, respectively (Wilcock et al., 2004).
In line with animal studies, several post-mortem studies in patients of the AN1792 vaccine trial of active immunization against Aβ revealed neocortical areas with reduced plaque load. These areas contained small punctate immunostained Aβ with a distribution matching that of CD68 positive microglia (Ferrer et al., 2004; Masliah et al., 2005; Nicoll et al., 2006; Nicoll et al., 2003), suggesting both microglial activation and phagocytosis of Aβ. Patient data indicated that Aβ mobilization started within 4 months of immunization, but extensive plaque removal required several years (Holmes et al., 2008; Nicoll et al., 2006). This suggests continuous but extremely slow plaque uptake and degradation by microglia in vivo.
2.12.3. Enhanced microglial lysosomal function as a therapeutic target
Factors that promote microglial lysosome biogenesis might enhance lysosomal function, thus allowing for enhanced degradative capacity. As discussed in section 2.8, M-CSF has been reported to induce lysosomal biogenesis in several cell types.
M-CSF treatment of an AD mouse model presenting AD-like pathology and associated cognitive impairment reversed the Aβ load and improved performance in cognitive tests (Boissonneault et al., 2009). In addition, M-CSF treatment in young animals without neurological alterations or evident Aβ plaque load prevented AD-like pathology (Boissonneault et al., 2009). Treatment with M-CSF increased the number of microglia associated with amyloid deposits and enhanced fAβ phagocytosis by the cells. Moreover, internalized Aβ highly co-localized with LE/LY markers in the microglia (Boissonneault et al., 2009). These studies in mouse models are consistent with in vitro studies showing that M-CSF activation of primary neonatal murine microglia leads to normal lysosomal acidification and enhanced fAβ degradation (Majumdar et al., 2011). In these studies, and similar to microglial activation seen with immunization therapies, M-CSF treatment increased microglial expression of inflammatory surface markers CD45 and CD11b as well as MHC-II (Majumdar et al., 2011). Therefore, microglial activation by immunization or other mechanisms might prove beneficial in enhancing Aβ degradation. Interestingly, healthy individuals and individuals with mild cognitive impairment (MCI) present lower M-CSF levels when compared to AD patients (Laske et al., 2010; Ray et al., 2007). Additionally, in mouse brains, not only microglia but also an important fraction of neuronal populations express the CSF-1R receptor (Chitu et al., 2015). Therefore, M-CSF treatment may also enhance lysosomal function in neurons.
Treatments that induce neuroinflammation would not be appropriate, but it is possible that the cellular mechanisms by which M-CSF leads to enhanced degradation of fAβ could be activated in other ways. For example, activation of TFEB or other transcription factors might lead to overall enhanced synthesis of lysosomal proteins, including Ostm1 and ClC-7. In general, direct activation of transcription factors is considered to be a challenging pharmacological target. However, TFEB is regulated by mTORC pathways, and pharmacological manipulation of these signaling pathways has been considered (Richardson et al., 2015; Xiao et al., 2015).
3. Conclusions and future directions
Endocytosis by microglia and subsequent incorporation of substrates into the endocytic pathway play critical roles in immune cell response, synapse pruning, scavenging of cell debris and in the degradation of protein aggregates. The effect of aging and AD on the microglial endocytic pathway is likely to have a dramatic impact on brain homeostasis.
The accumulation of undigested material in lysosomes increases with age as the cells and their respective organelles slow their metabolic turnover. A classic hallmark of AD, the accumulation of Aβ plaques surrounded by activated, proinflammatory microglia, may be related to impaired microglial phagocytosis and lysosomal degradation. With aging, the rate of degradation of Aβ may be reduced (Hickman et al., 2008; Norden and Godbout, 2013). Probably due to their role as antigen-presenting cells (discussed in section 2.10.3), the lysosomes of non-inflammatory surveillant microglia are not fully acidified. This may be one of the main reasons why deposits of fAβ are not efficiently degraded.
Harnessing the brain’s immune system for Aβ clearance and prevention of plaque accumulation through microglia appears promising. A specific immune response by microglia to Aβ that leads to increased phagocytosis followed by efficient degradation in acidic compartments would be ideal. Increased numbers of microglia with more acidic lysosomes may be required not only for plaque internalization but also for enhanced degradation of pre-fAβ, which may be a crucial target for disease prevention. In addition, enhancing lysosomal function may be a way to increase Aβ clearance without a concurrent inflammatory response that is damaging to the neurons.
Taken together, microglial endocytic pathways and their alterations caused by aging and AD are of vital importance in the understanding and prevention of disease. In that context, further research into the role of microglia and their ability to degrade Aβ is crucial.
Acknowledgments
This project was supported by National Center for Research Resources [award number S10RR031855] and National Institutes of Health grants [R37-DK27083 and R01-HL093324]. S.S.D. is supported by the Swedish Research Council (VR) International Postdoctoral Grant DNR. 637-2013-503.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors declared no potential conflict of interests regarding the publication of this manuscript.
References
- Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD, Bu G. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) J Biol Chem. 2015;290:26043–26050. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Rothe G, Valet G, Kreutzberg GW. Detection of lysosomal cysteine proteinases in microglia: flow cytometric measurement and histochemical localization of cathepsin B and L. Glia. 1993;7:183–191. doi: 10.1002/glia.440070208. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol. 2010;120:369–384. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonet-Costa V, Herranz-Perez V, Blanco-Gandia M, Mas-Bargues C, Ingles M, Garcia-Tarraga P, Rodriguez-Arias M, Minarro J, Borras C, Garcia-Verdugo JM, Vina J. Clearing Amyloid-beta through PPARgamma/ApoE Activation by Genistein is a Treatment of Experimental Alzheimer’s Disease. J Alzheimers Dis. 2016;51:701–711. doi: 10.3233/JAD-151020. [DOI] [PubMed] [Google Scholar]
- Booth PL, Thomas WE. Evidence for motility and pinocytosis in ramified microglia in tissue culture. Brain Res. 1991;548:163–171. doi: 10.1016/0006-8993(91)91118-k. [DOI] [PubMed] [Google Scholar]
- Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151:1353–1368. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronisz A, Sharma SM, Hu R, Godlewski J, Tzivion G, Mansky KC, Ostrowski MC. Microphthalmia-associated transcription factor interactions with 14-3-3 modulate differentiation of committed myeloid precursors. Mol Biol Cell. 2006;17:3897–3906. doi: 10.1091/mbc.E06-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni C, Bollman B, Licastro D, Xie M, Mikesell R, Schmidt R, Yuede CM, Galimberti D, Olivecrona G, Klein RS, Cross AH, Otero K, Piccio L. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 2015;129:429–447. doi: 10.1007/s00401-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HA, Bronisz A, Cabrera J, Hildreth BE, 3rd, Cuitino M, Fu Q, Ahmad A, Toribio RE, Ostrowski MC, Sharma SM. Failure to Target RANKL Signaling Through p38-MAPK Results in Defective Osteoclastogenesis in the Microphthalmia Cloudy-Eyed Mutant. J Cell Physiol. 2016;231:630–640. doi: 10.1002/jcp.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Barnett JL, Berman SA, Li J, Quarless S, Bursztajn S, Lippa C, Nixon RA. Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron. 1995;14:671–680. doi: 10.1016/0896-6273(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Chitu V, Gokhan S, Gulinello M, Branch CA, Patil M, Basu R, Stoddart C, Mehler MF, Stanley ER. Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP) Neurobiol Dis. 2015;74:219–228. doi: 10.1016/j.nbd.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitu V, Gokhan S, Nandi S, Mehler MF, Stanley ER. Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System. Trends Neurosci. 2016;39:378–393. doi: 10.1016/j.tins.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Caracciolo L, Minami SS, Niikura T, Matsuoka Y, Turner RS, Mattson MP, Bosetti F. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J Neurochem. 2013;124:59–68. doi: 10.1111/jnc.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer’s amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: Inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPAR gamma agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corona AW, Kodoma N, Casali BT, Landreth GE. ABCA1 is Necessary for Bexarotene-Mediated Clearance of Soluble Amyloid Beta from the Hippocampus of APP/PS1 Mice. J Neuroimmune Pharmacol. 2016;11:61–72. doi: 10.1007/s11481-015-9627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrotte M, Chasserot-Golaz S, Huang P, Du G, Ktistakis NT, Frohman MA, Vitale N, Bader MF, Grant NJ. Dynamics and function of phospholipase D and phosphatidic acid during phagocytosis. Traffic. 2006;7:365–377. doi: 10.1111/j.1600-0854.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. When lysosomes get old. Exp Gerontol. 2000;35:119–131. doi: 10.1016/s0531-5565(00)00075-9. [DOI] [PubMed] [Google Scholar]
- Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, West BL, Green KN. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation. 2015;12:139. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn HC, Ager RR, Baglietto-Vargas D, Cheng D, Kitazawa M, Cribbs DH, Medeiros R. Restoration of lipoxin A4 signaling reduces Alzheimer’s disease-like pathology in the 3xTg-AD mouse model. J Alzheimers Dis. 2015;43:893–903. doi: 10.3233/JAD-141335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, Maxfield FR. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J Cell Biol. 1992;117:301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Reed DK, Eckman CB. Degradation of the Alzheimer’s amyloid beta peptide by endothelin-converting enzyme. J Biol Chem. 2001;276:24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Csernansky CA, Morris JC, Holtzman DM. The search for antecedent biomarkers of Alzheimer’s disease. J Alzheimers Dis. 2005;8:347–358. doi: 10.3233/jad-2005-8404. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Settembre C, Shimazu J, Lacombe J, Kato S, Rawlings DJ, Ballabio A, Karsenty G. A RANKL-PKCbeta-TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev. 2013;27:955–969. doi: 10.1101/gad.213827.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagorska A, Rothlin CV, Nimmerjahn A, Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev. 2014;262:193–215. doi: 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M. The phagosome proteome: Insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Immunological Genome C. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Jana M, Modi K, Gonzalez FJ, Sims KB, Berry-Kravis E, Pahan K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: implications for lysosomal storage disorders. J Biol Chem. 2015;290:10309–10324. doi: 10.1074/jbc.M114.610659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol. 2015;7:a020537. doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Phagocytosis: An Immunobiologic Process. Immunity. 2016;44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol. 2010;119:523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, Aguzzi A, Staufenbiel M, Mathews PM, Wolburg H, Heppner FL, Jucker M. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AR, Curran PK, Smith CL, Mindell JA. The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008;453:788–792. doi: 10.1038/nature06907. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, Becher B. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes C, Lee CY, Wilkinson BL, Jiang Q, Koenigsknecht-Talboo JL, Varnum B, Landreth GE. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J Neuroimmune Pharmacol. 2008;3:130–140. doi: 10.1007/s11481-007-9090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J, Alzheimer Genetic Analysis G. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hamazaki H. Cathepsin D is involved in the clearance of Alzheimer’s beta-amyloid protein. FEBS Lett. 1996;396:139–142. doi: 10.1016/0014-5793(96)01087-3. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Ginhoux F. Ontogeny of Tissue-Resident Macrophages. Front Immunol. 2015;6:486. doi: 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Holowka D, Sil D, Torigoe C, Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol Rev. 2007;217:269–279. doi: 10.1111/j.1600-065X.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA. The Many Alternative Faces of Macrophage Activation. Front Immunol. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34:2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem. 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- Janeway CJ, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th. New York: Garland Science; 2001. [Google Scholar]
- Jaworowski A, Wilson NJ, Christy E, Byrne R, Hamilton JA. Roles of the mitogen-activated protein kinase family in macrophage responses to colony stimulating factor-1 addition and withdrawal. J Biol Chem. 1999;274:15127–15133. doi: 10.1074/jbc.274.21.15127. [DOI] [PubMed] [Google Scholar]
- Jiang D, Rauda I, Han S, Chen S, Zhou F. Aggregation pathways of the amyloid beta(1–42) peptide depend on its colloidal stability and ordered beta-sheet stacking. Langmuir. 2012;28:12711–12721. doi: 10.1021/la3021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, Brown HD, Mhatre SD, Loui T, Andreasson KI. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J Clin Invest. 2015;125:350–364. doi: 10.1172/JCI77487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasinska JM, de Haan W, Franciosi S, Ruddle P, Fan J, Kruit JK, Stukas S, Lutjohann D, Gutmann DH, Wellington CL, Hayden MR. ABCA1 influences neuroinflammation and neuronal death. Neurobiol Dis. 2013;54:445–455. doi: 10.1016/j.nbd.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Karch CM, Cruchaga C, Goate AM. Alzheimer’s disease genetics: from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Owen D, Harrison SC. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb Perspect Biol. 2014;6:a016725. doi: 10.1101/cshperspect.a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Duncan ID. Selective reduction in microglia density and function in the white matter of colony-stimulating factor-1-deficient mice. J Neurosci Res. 2009;87:2686–2695. doi: 10.1002/jnr.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol. 2008;129:389–406. doi: 10.1007/s00418-008-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 2006;440:220–223. doi: 10.1038/nature04535. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Hoffmann N, Maetzler W, Straten G, Eschweiler GW, Leyhe T. Macrophage colony-stimulating factor (M-CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2010;7:409–414. doi: 10.2174/156720510791383813. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Lee CY, Tse W, Smith JD, Landreth GE. Apolipoprotein E promotes beta-amyloid trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem. 2012;287:2032–2044. doi: 10.1074/jbc.M111.295451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterov I, Schug J, Mounier A, Nam KN, Fitz NF, Koldamova R. RNA-sequencing reveals transcriptional up-regulation of Trem2 in response to bexarotene treatment. Neurobiol Dis. 2015;82:132–140. doi: 10.1016/j.nbd.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon SK. Clathrin uncoating: Auxilin comes to life. Curr Biol. 2001;11:R49–R52. doi: 10.1016/s0960-9822(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Lew ED, Oh J, Burrola PG, Lax I, Zagorska A, Traves PG, Schlessinger J, Lemke G. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife. 2014;3:23. doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WH. Pinocytosis. Bulletin of the Johns Hopkins Hospital. 1931;49:17–27. [Google Scholar]
- Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- Lin SX, Mallet WG, Huang AY, Maxfield FR. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol Biol Cell. 2004;15:721–733. doi: 10.1091/mbc.E03-07-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-beta phagocytosis. J Neurosci. 2010;30:17091–17101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubke T, Lobel P, Sleat DE. Proteomics of the lysosome. Biochim Biophys Acta. 2009;1793:625–635. doi: 10.1016/j.bbamcr.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Hackmann Y, Dieckmann NM, Griffiths GM. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb Perspect Biol. 2014;6:a016840. doi: 10.1101/cshperspect.a016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P, Mali RS, Munugalavadla V, Krishnan S, Ramdas B, Sims E, Martin H, Ghosh J, Li S, Chan RJ, Krystal G, Craig AW, Takemoto C, Kapur R. The PI3K pathway drives the maturation of mast cells via microphthalmia transcription factor. Blood. 2011;118:3459–3469. doi: 10.1182/blood-2011-04-351809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, Branstetter D, Smith J, Paxton RJ, Cerretti DP, Bonham L, Hill GR, Hume DA. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- Mackay EA, Ehrhard A, Moniatte M, Guenet C, Tardif C, Tarnus C, Sorokine O, Heintzelmann B, Nay C, Remy JM, Higaki J, Van Dorsselaer A, Wagner J, Danzin C, Mamont P. A possible role for cathepsins D, E, and B in the processing of beta-amyloid precursor protein in Alzheimer’s disease. Eur J Biochem. 1997;244:414–425. doi: 10.1111/j.1432-1033.1997.00414.x. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Weisgraber KH. Putting cholesterol in its place: apoE and reverse cholesterol transport. J Clin Invest. 2006;116:1226–1229. doi: 10.1172/JCI28632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Capetillo-Zarate E, Cruz D, Gouras GK, Maxfield FR. Degradation of Alzheimer’s amyloid fibrils by microglia requires delivery of ClC-7 to lysosomes. Mol Biol Cell. 2011;22:1664–1676. doi: 10.1091/mbc.E10-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18:1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Diab HI, Li H, Puertollano R. Novel roles for the MiTF/TFE family of transcription factors in organelle biogenesis, nutrient sensing, and energy homeostasis. Cell Mol Life Sci. 2014;71:2483–2497. doi: 10.1007/s00018-014-1565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Puertollano R. Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J Cell Biol. 2013;200:475–491. doi: 10.1083/jcb.201209135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Maxson ME, Grinstein S. The vacuolar-type H(+)-ATPase at a glance - more than a proton pump. J Cell Sci. 2014;127:4987–4993. doi: 10.1242/jcs.158550. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Meadows NA, Sharma SM, Faulkner GJ, Ostrowski MC, Hume DA, Cassady AI. The expression of Clcn7 and Ostm1 in osteoclasts is coregulated by microphthalmia transcription factor. J Biol Chem. 2007;282:1891–1904. doi: 10.1074/jbc.M608572200. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, LaFerla FM. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am J Pathol. 2013;182:1780–1789. doi: 10.1016/j.ajpath.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Kaksonen M. Endocytic accessory factors and regulation of clathrin-mediated endocytosis. Cold Spring Harb Perspect Biol. 2014;6:a016733. doi: 10.1101/cshperspect.a016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Stalder M, Herzig MC, Kaeser SA, Kohler E, Pfeifer M, Boncristiano S, Mathews PM, Mercken M, Abramowski D, Staufenbiel M, Jucker M. Extracellular amyloid formation and associated pathology in neural grafts. Nat Neurosci. 2003;6:370–377. doi: 10.1038/nn1022. [DOI] [PubMed] [Google Scholar]
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Moskot M, Montefusco S, Jakobkiewicz-Banecka J, Mozolewski P, Wegrzyn A, Di Bernardo D, Wegrzyn G, Medina DL, Ballabio A, Gabig-Ciminska M. The phytoestrogen genistein modulates lysosomal metabolism and transcription factor EB (TFEB) activation. J Biol Chem. 2014;289:17054–17069. doi: 10.1074/jbc.M114.555300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyckova G, Weilbaecher KN, Horstmann M, Rieman DJ, Fisher DZ, Fisher DE. Linking osteopetrosis and pycnodysostosis: regulation of cathepsin K expression by the microphthalmia transcription factor family. Proc Natl Acad Sci U S A. 2001;98:5798–5803. doi: 10.1073/pnas.091479298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Nakamichi Y, Udagawa N, Takahashi N. IL-34 and CSF-1: similarities and differences. J Bone Miner Metab. 2013;31:486–495. doi: 10.1007/s00774-013-0476-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi H. Microglial functions and proteases. Mol Neurobiol. 2003;27:163–176. doi: 10.1385/MN:27:2:163. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Wu Z. Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res. 2009;201:1–7. doi: 10.1016/j.bbr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, Stanley ER. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nishioku T, Hashimoto K, Yamashita K, Liou SY, Kagamiishi Y, Maegawa H, Katsube N, Peters C, von Figura K, Saftig P, Katunuma N, Yamamoto K, Nakanishi H. Involvement of cathepsin E in exogenous antigen processing in primary cultured murine microglia. J Biol Chem. 2002;277:4816–4822. doi: 10.1074/jbc.M108382200. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2×4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- Pacheco-Quinto J, Eckman EA. Endothelin-converting enzymes degrade intracellular beta-amyloid produced within the endosomal/lysosomal pathway and autophagosomes. J Biol Chem. 2013;288:5606–5615. doi: 10.1074/jbc.M112.422964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Quinto J, Herdt A, Eckman CB, Eckman EA. Endothelin-converting enzymes and related metalloproteases in Alzheimer’s disease. J Alzheimers Dis 33 Suppl. 2013;1:S101–S110. doi: 10.3233/JAD-2012-129043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JC, Baig S, Kehoe PG, Love S. Endothelin-converting enzyme-2 is increased in Alzheimer’s disease and up-regulated by Abeta. Am J Pathol. 2009;175:262–270. doi: 10.2353/ajpath.2009.081054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer’s disease amyloid beta-protein by microglial cells. J Biol Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Pillay CS, Elliott E, Dennison C. Endolysosomal proteolysis and its regulation. Biochem J. 2002;363:417–429. doi: 10.1042/0264-6021:3630417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175:2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- Prada I, Furlan R, Matteoli M, Verderio C. Classical and unconventional pathways of vesicular release in microglia. Glia. 2013;61:1003–1017. doi: 10.1002/glia.22497. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Galvan V, Lin AL, Oddo S. How longevity research can lead to therapies for Alzheimer’s disease: The rapamycin story. Exp Gerontol. 2015;68:51–58. doi: 10.1016/j.exger.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal. 2012;5:ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogeberg M, Furlund CB, Moe MK, Fladby T. Identification of peptide products from enzymatic degradation of amyloid beta. Biochimie. 2014;105:216–220. doi: 10.1016/j.biochi.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Sadik G, Kaji H, Takeda K, Yamagata F, Kameoka Y, Hashimoto K, Miyanaga K, Shinoda T. In vitro processing of amyloid precursor protein by cathepsin D. Int J Biochem Cell Biol. 1999;31:1327–1337. doi: 10.1016/s1357-2725(99)00053-9. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sakae N, Liu CC, Shinohara M, Frisch-Daiello J, Ma L, Yamazaki Y, Tachibana M, Younkin L, Kurti A, Carrasquillo MM, Zou F, Sevlever D, Bisceglio G, Gan M, Fol R, Knight P, Wang M, Han X, Fryer JD, Fitzgerald ML, Ohyagi Y, Younkin SG, Bu G, Kanekiyo T. ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer’s Neuronal Pathology. J Neurosci. 2016;36:3848–3859. doi: 10.1523/JNEUROSCI.3757-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M, Schroder B, Saftig P. Lysosomal membrane proteins and their central role in physiology. Traffic. 2013;14:739–748. doi: 10.1111/tra.12056. [DOI] [PubMed] [Google Scholar]
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Klisch TJ, Wollenberg AC, Di Bernardo D, Chan L, Irazoqui JE, Ballabio A. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013a;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013b;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskova Z, Tremblay ME. Microglia and synapse: interactions in health and neurodegeneration. Neural Plast. 2013;2013:425845. doi: 10.1155/2013/425845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito E, Sastre M. Microglia function in Alzheimer’s disease. Front Pharmacol. 2012;3:14. doi: 10.3389/fphar.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, Green KN. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Brain. 2016;139:1265–1281. doi: 10.1093/brain/aww016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber T, Jentsch TJ. Chloride in vesicular trafficking and function. Annu Rev Physiol. 2013;75:453–477. doi: 10.1146/annurev-physiol-030212-183702. [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Huynh KK, Brodovitch A, Jabs S, Stauber T, Jentsch TJ, Grinstein S. A cation counterflux supports lysosomal acidification. J Cell Biol. 2010;189:1171–1186. doi: 10.1083/jcb.200911083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Brodie SE, Cohn ZA. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976;68:665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Soares H, Kimmel L, Friedman D, Bergeson J, Csako G, Levy JA, Bartko JJ, Cohen RM. Cerebrospinal fluid beta-amyloid1-42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu HX, Greengard P, Gouras GK. Intraneuronal Alzheimer A beta 42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Matsuwaki T, Yamanouchi K, Nishihara M. Increased lysosomal biogenesis in activated microglia and exacerbated neuronal damage after traumatic brain injury in progranulin-deficient mice. Neuroscience. 2013;250:8–19. doi: 10.1016/j.neuroscience.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Lipofuscin. Int J Biochem Cell Biol. 2004;36:1400–1404. doi: 10.1016/j.biocel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier CH, Milgram NW. Neuroinflammation in Alzheimer’s disease: are NSAIDs and selective COX-2 inhibitors the next line of therapy? J Alzheimers Dis. 2010;21:1089–1099. doi: 10.3233/jad-2010-090667. [DOI] [PubMed] [Google Scholar]
- Turk B, Dolenc I, Lenarcic B, Krizaj I, Turk V, Bieth JG, Bjork I. Acidic pH as a physiological regulator of human cathepsin L activity. Eur J Biochem. 1999;259:926–932. doi: 10.1046/j.1432-1327.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Murphy LJ. Molecular pharmacology of endothelin converting enzymes. Biochem Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Saez I, Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun. 2014;5:5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher KN, Motyckova G, Huber WE, Takemoto CM, Hemesath TJ, Xu Y, Hershey CL, Dowland NR, Wells AG, Fisher DE. Linkage of M-CSF signaling to Mitf, TFE3, and the osteoclast defect in Mitf(mi/mi) mice. Mol Cell. 2001;8:749–758. doi: 10.1016/s1097-2765(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J Cell Biol. 2000;151:1207–1220. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman MJ, Gordon MN, Morgan D. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goni F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodling NS, Andreasson KI. Untangling the Web: Toxic and Protective Effects of Neuroinflammation and PGE2 Signaling in Alzheimer’s Disease. ACS Chem Neurosci. 2016;7:454–463. doi: 10.1021/acschemneuro.6b00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich P, Glebov K, Kemmerling N, Tien NT, Neumann H, Walter J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and gamma-secretase-dependent intramembranous cleavage. J Biol Chem. 2013;288:33027–33036. doi: 10.1074/jbc.M113.517540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A, Gonzales E, Tripoli DL, Czerniewski L, Ballabio A, Cirrito JR, Diwan A, Lee JM. Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Abeta Generation and Amyloid Plaque Pathogenesis. J Neurosci. 2015;35:12137–12151. doi: 10.1523/JNEUROSCI.0705-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Maxfield FR. Kinetics of endosome acidification in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987;105:2713–2721. doi: 10.1083/jcb.105.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yokoyama JS, Wang Y, Schork AJ, Thompson WK, Karch CM, Cruchaga C, McEvoy LK, Witoelar A, Chen CH, Holland D, Brewer JB, Franke A, Dillon WP, Wilson DM, Mukherjee P, Hess CP, Miller Z, Bonham LW, Shen J, Rabinovici GD, Rosen HJ, Miller BL, Hyman BT, Schellenberg GD, Karlsen TH, Andreassen OA, Dale AM, Desikan RS Alzheimer’s Disease Neuroimaging, I. Association Between Genetic Traits for Immune-Mediated Diseases and Alzheimer Disease. JAMA Neurol. 2016;73:691–697. doi: 10.1001/jamaneurol.2016.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]