Abstract
Membrane lipids diffuse rapidly in the plane of the membrane but their ability to flip spontaneously across a membrane bilayer is hampered by a significant energy barrier. Thus spontaneous flip-flop of polar lipids across membranes is very slow, even though it must occur rapidly to support diverse aspects of cellular life. Here we discuss the mechanisms by which rapid flip-flop occurs, and what role lipid flipping plays in membrane homeostasis and cell growth. We focus on conceptual aspects, highlighting mechanistic insights from biochemical and in silico experiments, and the recent, ground-breaking identification of a number of lipid scramblases.
Keywords: flippase, floppase, membrane asymmetry, phosphatidylserine, photoreceptor, scramblase
1. Introduction
The defining feature of a biological membrane is its bilayer structure, a sandwich of two monolayers of phospholipids visible as a trilaminar track by thin-section electron microscopy. The two monolayers are coupled, a fact that is easily revealed by noting that the membrane bends if the number of lipids on one side exceeds that on the other. This is akin to the bending of a bimetallic strip in which the coupled metals have different coefficients of thermal expansion. Gorter and Grendel provided the first experimental evidence for bilayer organization in 1925 when they compared the area occupied by lipids extracted from red blood cells with the predicted area of the cell membrane using surface chemistry approaches [1] [2]. Sheetz and Singer proposed the bilayer couple hypothesis in 1974 [3].
Bilayer membranes form spontaneously when phospholipids are dispersed in water, a consequence of the hydrophobic effect discussed by Tanford [4]. The bilayers formed in this way are usually nested, one within the other, to form a multilamellar structure like the layered skin of an onion. These structures can be dispersed into individual unilamellar vesicles by sonication, or by extrusion through filters after freezing and thawing. The resulting vesicles can be quite small, <50 nanometer in diameter, in which case there are more lipids in the outer leaflet than in the inner leaflet. For large unilamellar vesicles, >150 nanometer in diameter, the number of lipids in each monolayer is almost identical. Phospholipids within an individual leaflet of the bilayer are very dynamic. They undergo a number of intramolecular motions that can be so great as to sometimes bring the methyl end of their acyl chains into the vicinity of the glycerol moiety. They also exhibit rapid rotational and lateral diffusion (Figure 1). Thus, a lipid spins around its own axis, normal to the plane of the membrane, with a characteristic time of ~1 nanosecond, and can diffuse laterally to occupy the position of a neighboring lipid (a displacement on the order of ~1 nanometer) within ~100 nanoseconds. In contrast to these fast movements, phospholipid exchange between the two leaflets of the bilayer occurs only slowly. Thus, reorientation of a phospholipid across the ~3 nanometer thickness of a membrane has a characteristic time of ~100 hours.
Figure 1. Phospholipid motions in a membrane.
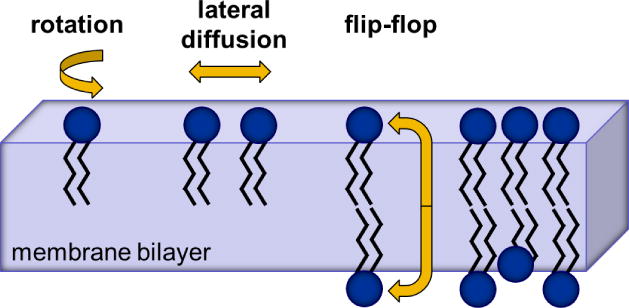
Phospholipid bilayers are two-dimensional fluids. Individual lipid molecules have a cross-sectional area of ~0.7 nm2. In each monolayer of the membrane bilayer they can rotate very rapidly around their head-to-tail axis with a characteristic time of 10−9 seconds, and diffuse laterally within the plane of a membrane leaflet with a translational diffusion coefficient of ~10−8 cm2 seconds−1, i.e. the time taken for a phospholipid to move ~1 nm to replace a neighboring phospholipid is ~100 nanoseconds. In contrast, spontaneous exchange of phospholipids between leaflets (flip-flop) is slow, taking typically ~100 hours. The energy barrier that must be overcome in order to move the phospholipid headgroup through the hydrophobic interior of the membrane is >20 kcal mol−1. Adapted from Mouritsen ‘Life - As a Matter of Fat’ [5].
The low frequency with which phospholipids flip spontaneously across pure lipid bilayers was first reported in 1971 by Kornberg and McConnell [6]. They reconstituted trace quantities of spin-labeled phospholipid analogues into synthetic vesicles, chemically reduced all labeled lipids in the outer leaflet with ascorbate to generate asymmetric vesicles in which the non-reduced lipid probes were located only in the luminal leaflet, and then monitored the translocation of the probes from the inner to the outer leaflet again using ascorbate. They reported a frequency of translocation of ~10−5 seconds−1 at 30°C. By carrying out the measurement at different temperatures they could deduce the activation energy E of the translocation process and also estimate the prefactor A in the Arrhenius rate equation (rate = A•exp (−E/kBT), where kB is the Boltzmann constant and T is the absolute temperature): E~20 kcal mol−1, A~109 seconds−1. The prefactor is typical for reactions that occur in liquids whereas the huge activation barrier, roughly equivalent to the energy derived from hydrolysis of 3 ATP molecules to ADP under standard conditions, is readily attributed to the fact that polar, ionic, or zwitterionic phospholipid headgroups have to traverse the highly hydrophobic interior of the bilayer as the lipid reorients from one leaflet to the other. An initially asymmetric bilayer is therefore relatively stable, with decay of its asymmetry occurring only very slowly over a time frame of 100 hours.
While transbilayer movement of polar lipids only rarely occurs in synthetic systems, fast flip-flop is crucial for cellular life. Thus, constitutive flip-flop of phospholipids is necessary to meet the demands of cell growth, while regulated flip-flop events are needed to sculpt the cellular responses to physiological challenges. Transbilayer translocation of lipids is also needed for membrane homeostasis, to control transbilayer lipid asymmetry in membranes, and to coordinate with protein machinery in generating intracellular transport vesicles. In almost all cases, these transport events are mediated or regulated by proteins without which flip-flop rates would be too slow to match physiological demands. Historically, the role of these proteins was defined through activity measurements that distinguished two types of translocation events: those that required ATP to move lipids vectorially across a membrane and those that were ATP-independent. Although these activity measurements were first reported in the late 1970s and 1980s, the identity of the proteins themselves remained mysterious. Only recently have members of both protein categories been identified and their activities verified by reconstitutions into phospholipid vesicles. The discovery of the molecular identity of the transporters has come hand in hand with fresh insights into the mechanisms of transbilayer translocation of lipids. This has been supplemented by informative in silico approaches using molecular dynamics methods to understand lipid translocation. Our objective in this review is to structure and highlight this explosion of new information.
2. Parameters influencing spontaneous lipid flip-flop
Although flip-flop of typical membrane lipids is slow, not all lipids flip-flop slowly. Lipids with a simple hydroxyl headgroup (ceramide, diacylglycerol, and cholesterol) have a very high spontaneous rate of flipping (t1/2 ~seconds/minutes), and glucosylceramide with an uncharged yet polar headgroup has a faster translocation rate than zwitterionic phosphatidylcholine (PC) or phosphatidylethanolamine (PE) (t1/2 ~10 hours, versus ~100 hours for PE) [7–11]. Anionic lipids such as phosphatidic acid (PA) or phosphatidylglycerol (PG) flip-flop slowly at neutral pH, but at pH~5 when they are fully protonated and uncharged they exchange between the leaflets of the bilayer very rapidly (t1/2 ~seconds/minutes) [12]. Indeed, elimination of headgroup polarity by synthetic chelators can promote the fast flipping of a variety of phospholipids [13, 14].
These points are readily illustrated by a simple visual experiment with giant unilamellar vesicles (GUVs) (Figure 2). The GUVs are placed under hyperosmotic conditions so that they become flaccid and adopt a relaxed prolate ellipsoid shape. On adding phospholipid, the GUVs undergo a characteristic shape change to accommodate the excess lipid in the outer leaflet of the membrane (Figure 2A). This happens because the leaflets are coupled and because the added phospholipid does not exchange into the inner leaflet on the time-scale of the experiment (several minutes). If the added lipid happens to be one that flip-flops rapidly, for example a lipid such as ceramide, the shape change eventually reverses as flip-flop normalizes the number of lipids in the two leaflets (Figure 2B).
Figure 2. Shape change in GUVs on expanding the outer monolayer of the membrane.
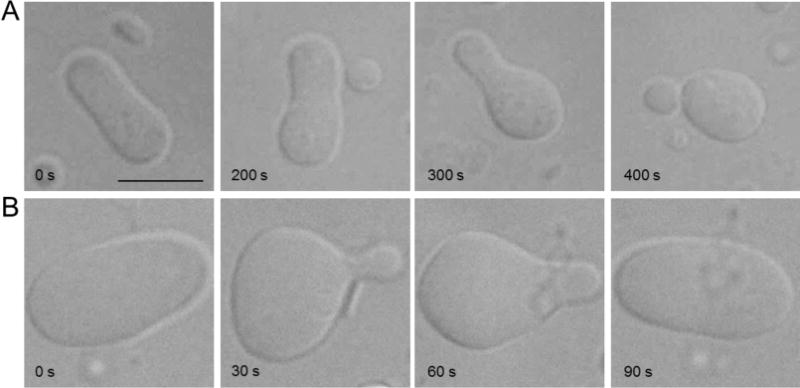
A, Lysophosphatidylcholine (16:0) was added to a prolate GUV and the sample was observed by differential interference contrast microscopy. A time-lapse sequence is shown, starting at the left and ending at the right. As the phospholipid does not exchange between the leaflets of the bilayer on the time-scale of this experiment (~6 minutes), and because the two leaflets of the membrane are coupled, the GUV undergoes a predicted shape change to minimize bilayer stress caused by the excess lipid in one leaflet [3]. B, The same experiment as in panel A, except that C6 ceramide (d18:1/6:0) was added to a prolate GUV. The shape change induced by excess ceramide in the outer leaflet is evident. However, as C6 ceramide flip-flops rapidly, the number of lipids in the two leaflets of the GUV membrane eventually normalizes to restore the original shape of the GUV. Images courtesy of Patricia Pipaluk Mia Mathiassen.
Studies of model membranes as well as molecular dynamics simulations (see for example [15, 16]) have shown that lipid flip-flop is also affected by the physical properties of the bilayer. An essential factor is lipid packing. Interestingly, molecular packing defects can enhance the flip-flop rate [17–19]. Such defects can occur at the border of coexisting liquid-ordered and liquid-disordered phases in the bilayer, and they become especially pronounced at the main phase transition temperature for membranes composed of a single lipid species [17, 19]. Thus, 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD)-labeled PE equilibrates extremely rapidly (t1/2 ~6 min) across a dipalmitoyl-PC bilayer at 41°C (the main phase transition temperature for DPPC) compared with an equilibration half-time of ~80 hours at 23°C in egg PC membranes [11]. At temperatures above the solid-liquid phase transition, flip-flop of short-chain phospholipids in human erythrocytes and PC membranes is reduced by cholesterol [19, 20]. Cholesterol suppresses packing defects by condensing the phospholipid arrangement and increasing the thickness of the hydrophobic core.
Perturbations of the bilayer structure can be triggered by imperfect matching between the transmembrane domains of proteins and the boundary lipid phase. This led to the proposal that the interplay between transmembrane domains and non-bilayer favoring lipids might be sufficient to allow fast flip-flop in the ER [21–23]. In line with these ideas, recent studies demonstrated that low-complexity synthetic transmembrane peptides are able to increase the rate of flipping of a variety of phospholipids in synthetic vesicles [24, 25]. However, a large number of peptide molecules were needed per vesicle to see this effect. For example, reconstitution of 100 peptides/vesicle corresponding to the transmembrane protein EDEM1 could improve the rate of NBD-PC flipping to 20 lipids per second [25]. However, this contrasts with the rapid flipping seen when vesicles contain only one or a few copies of an authentic scramblase (see below). In summary, the nature of the lipid headgroup and the structure of the membrane itself are key parameters that dictate the rate of spontaneous flip-flop.
3. Transbilayer lipid movement in biological membranes: an overview
To facilitate the energetically unfavorable movement of a lipid’s polar head group through the hydrophobic membrane interior, biological membranes are equipped with specific membrane proteins. These proteins are classified as flippases/floppases or scramblases, depending on whether they mediate ATP-dependent transport against a concentration gradient or ATP-independent bidirectional transport (Figure 3). Translocation mediated by scramblases can occur at rates faster than 10,000 seconds−1 (see e.g. [26, 27]), while based on the highest ATP hydrolysis rates reported so far, ATP-driven transporters would reach turnover numbers no greater than ~100 seconds−1 [28] ATP-driven lipid transporters belong to the family of P4-ATPases or ABC transporters. Scramblases come in two flavors: they are either constitutively active or regulated by physiological stimuli such as a rise in intracellular Ca2+. Here we preview these different categories of transporters before exploring each in detail in subsequent sections.
Figure 3. Lipid transporters and membrane lipid asymmetry.
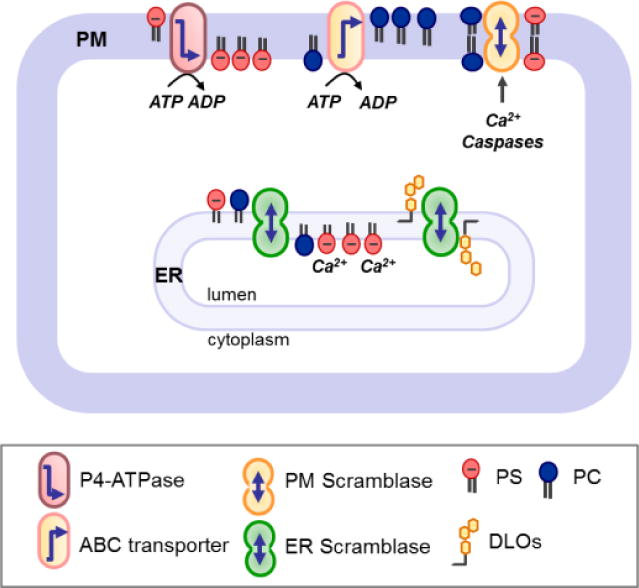
The endoplasmic reticulum (ER) harbors constitutive scramblases that facilitate rapid flip-flop of lipids and allow them to equilibrate between the two membrane leaflets independently of ATP. This system is unable to accumulate a given lipid in one leaflet. Thus, retentive mechanisms are required to trap lipids (e.g. PS) on the luminal side of the ER; also, for example, consumption of glycolipid biosynthetic intermediates such as DLOs on the luminal side of the ER drives scrambling from the cytoplasmic to the luminal leaflet. In the plasma membrane (PM) of eukaryotic cells, flip-flop of phospholipids is constrained by the absence/silencing of constitutive scramblases. Thus, ATP-dependent flippases (P4-ATPase family members) and floppases (ABC transporters) can maintain an asymmetric phospholipid distribution by moving specific lipids towards or away from the cytosolic leaflet. Cellular activation triggered by cytosolic calcium, caspases or other stimuli can collapse the lipid asymmetry by the transient activity of ATP-independent scramblases. Note that the term “flippase” is sometimes used to designate an enzyme that catalyses lipid flip-flop in both directions [33]. PC, phosphatidylcholine; PS, phosphatidylserine; DLOs, lipid-linked oligosaccharides.
Constitutively active phospholipid scramblases
Biological membranes are not made from scratch but rather assembled organically by a process in which lipids and proteins are synthesized and integrated into preexisting membranes. Biogenic membranes, such as the endoplasmic reticulum (ER) and bacterial cytoplasmic membrane (bCM), are capable of such self-synthesis. Lipid synthesis is asymmetric such that newly formed lipids are deposited in the cytoplasmic leaflet of the ER or bCM [29]. For bilayer formation, new lipids must be moved to the opposite leaflet on a physiologically relevant time frame. This is accomplished by an ATP-independent, constitutively active phospholipid scramblase that has been extensively characterized but whose molecular identity is surprisingly yet unknown (see below, Section 4.1). Nevertheless, two other constitutively active scramblases have been identified: MprF, a bifunctional bacterial protein that functions as a resistance factor to mitigate the cytolytic effects of cationic antimicrobial peptides and, quite unexpectedly, G protein-coupled receptors (GPCRs). We discuss these topics in Sections 4.2 and 4.3.
Constitutively active glycolipid scramblases for glycoconjugate biosynthesis
Both the ER and bCM host a variety of glycosylation pathways, for example protein N-glycosylation, O-mannosylation, C-mannosylation and GPI anchoring in eukaryotes and cell wall and cell envelope synthesis in bacteria. Each of these pathways requires the critical participation of glycolipids that carry the building blocks for glycoconjugate assembly. Glycolipid synthesis invariably begins on the cytoplasmic face of the ER or bCM, but the final product is used on the luminal/periplasmic side necessitating transbilayer lipid movement. The scramblases needed for moving these lipids across the membrane are not known, except in the case of cell wall and cell envelope synthesis where scramblase candidates have been identified. These topics are discussed in Sections 5.1–5.3.
Control of transbilayer lipid asymmetry by ATP-driven lipid transporters and regulated scramblases
The plasma membrane is strongly asymmetric with respect to the transbilayer distribution of lipids. This has been known since the 1970s through the work of Bretscher, as well as van Deenen and colleagues [30, 31]. For example, choline-containing lipids and glycolipids are predominantly located at the exoplasmic leaflet whereas phosphoinositides and aminophospholipids are located in the cytoplasmic leaflet. This asymmetric arrangement of lipids is a consequence of multiple factors, including biophysical membrane properties that limit the ability of a lipid to cross the bilayer spontaneously, retentive mechanisms that trap lipids in one leaflet of the bilayer, as well as the presence of plasma membrane-localized ATP-dependent lipid transporters (presented in Section 6) that selectively catalyze the inward (flip) and outward (flop) vectorial movement of lipids. The function of the transporters at a minimum is to correct any loss of asymmetry that results from spontaneous flip-flop and/or deposition of new lipids through membrane trafficking and non-vesicular pathways. The extent to which they are responsible for generating lipid asymmetry in the first place is not yet clear [32]. Plasma membrane lipid asymmetry is lost in very specific physiological contexts, notably in cells undergoing apoptosis and in activated blood platelets. This is accomplished by regulated lipid scrambling, triggered by caspases (in apoptotic cells) and Ca2+ (in activated platelets). Proteins required for these scrambling events have very recently been identified and are discussed in Section 7.
4. Constitutive scrambling of phospholipids
4.1. Phospholipid scrambling in biogenic membranes: growth of the membrane bilayer
Biogenic membranes such as the ER and bacterial cytoplasmic membrane are ‘self-synthesizing’ membranes capable of synthesizing and integrating their protein and lipid components. Much is known about how proteins are assembled into these membranes, but integration of membrane lipids remains poorly understood. The key issue is the problem of lipid scrambling: phospholipids are synthesized on the cytoplasmic face of biogenic membranes but must be scrambled across the bilayer to populate the opposing leaflet. This is necessary for uniform expansion of the bilayer and must occur rapidly, on a timescale relevant to cell growth. For a bacterial cell that doubles every 30 minutes, ~5000 phospholipids must be scrambled every second. Reports published in the late 1970s [34] and late 1980s [35, 36] demonstrated phospholipid scrambling in ER microsomes and bacterial cytoplasmic membrane vesicles, and more recent publications describe the reconstitution of this activity in lipid vesicles [37, 38]. The ER scrambling activity displays a relatively low specificity as non-natural structural isomers of glycerophospholipids (for example lipids with an sn-2,3-diacylglycerol moiety instead of the -1,2-diacylglycerol found in eukaryotes [39] as well as ceramide-based lipids such as sphingomyelin and glucosylceramide are translocated equally well within the limited time-resolution of the activity assays [10, 11]. However, some lipids such as the isoprenoid-based glycolipids involved in the biosynthesis of protein N-glycans are not translocated by the phospholipid scramblase [40]. The reconstitution studies show unambiguously that not all proteins can scramble lipids. For example, detergent extracts of biogenic membranes (crude preparation of membrane proteins) can be fractionated by velocity gradient centrifugation, and only certain fractions show activity when reconstituted into vesicles. Thus, proteins that sediment operationally at ~4S have phospholipid scramblase activity whereas those that sediment more rapidly do not [38, 41]. Protein modification studies suggest that there are at least two proteins that contribute to overall scramblase activity in the ER [42] but their identifications await further investigations.
4.2. A bacterial defense mechanism requires phospholipid scrambling: the role of MprF
The bacterial cytoplasmic membrane is a target for membrane disrupting peptides (bacteriocins and defensins) that are attracted to the negative surface charge of the membrane created by PG. To protect against the action of these so-called cationic antimicrobial peptides a number of bacteria modulate their surface charge by modifying PG with lysine or alanine. The polytopic membrane protein MprF catalyzes this modification by transferring an amino acid from the corresponding aminoacyl-tRNAs to PG [43]. The reaction occurs on the cytoplasmic face of the membrane and MprF is proposed to then translocate the aminoacyl-PG to the external surface. The two functions of MprF are separable and can be assigned to different domains of the protein [44]. The synthase domain was recently crystallized [45], while the enigmatic scramblase domain (referred to as flippase in the literature and in Figure 4) remains to be studied in detail. MprF is a large polytopic membrane protein [46] and its scramblase function is associated with the N-terminal 6–8 transmembrane segments that contain charged residues as well as a proline that are critical for scramblase function. While there seems to be little doubt that MprF is indeed a dual function protein, only its synthase function is well defined; its proposed lipid scrambling function remains to be tested using purified protein. We refer to MprF as a scramblase as no obvious metabolic energy source is associated with its transport function. However, this is a point that awaits clarification through reconstitution studies with purified protein.
Figure 4. MprF-mediated bacterial CAMP resistance.
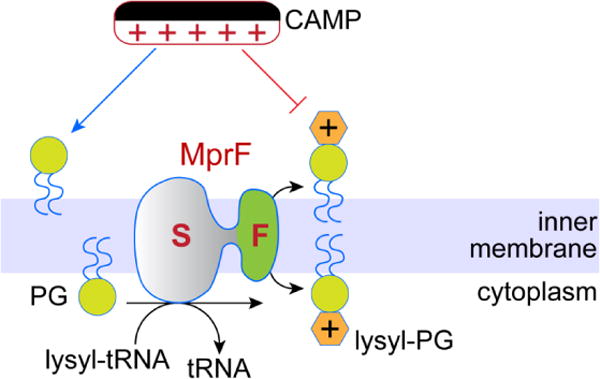
MprF is a bifunctional protein. Its synthase domain (S) transfers lysine from lysyl-tRNA to phosphatidylglycerol (PG) to synthesize lysyl-PG, whereas its flippase domain (F) transfers lysyl-PG across the inner membrane to the exoplasmic/periplasmic side. Negatively charged PG attracts cationic anti-microbial peptides (CAMPs), whereas lysyl-PG being neutral does not.
4.3. Rhodopsin-mediated phospholipid scrambling
The outer segments of photoreceptor cells in the retina are stacked with discs that contain the light-sensing protein rhodopsin (Figure 5). It has been known since the early 1990s that disc membranes are able to scramble phospholipids: thus, when labeled phospholipids are added to isolated discs, they equilibrate across the disc membrane on a time-scale of a few minutes [47–49]. Recent studies identified rhodopsin as the scramblase responsible for this phenomenon [27, 50, 51]. When reconstituted into lipid vesicles, rhodopsin is capable of scrambling all common phospholipids extremely rapidly, at a rate >10,000 phospholipids per rhodopsin per second. Rhodopsin’s scramblase activity was found to be constitutive, independent of its light-sensing function. Thus, both the apo-protein opsin and retinal-containing rhodopsin are able to scramble lipids.
Figure 5. Lipid transporters in photoreceptor discs.
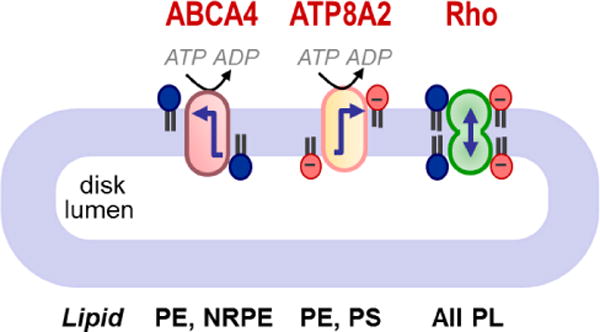
ABCA4 is an ABC transporter specific for PE and N-retinylidene-PE (NRPE); ATP8A2 is a P4-ATPase specific for PS and PE – however, it is not clear whether it is active in discs (see text); rhodopsin (Rho) is a scramblase that translocates common phospholipids (PL) in an ATP-independent manner. Arrows show the direction of lipid transport. ABCA4 is unusual amongst mammalian ABC transporters because it is the only one reported thus far that functions as an importer or flippase. Figure redrawn from [51].
The necessity for constitutive lipid scrambling in discs is not fully understood, but may be related to the function of the ABC transporter ABCA4 which is found in disc membranes [51]. ABCA4 is proposed to function as importer in moving N-retinylidene PE from the luminal side to the cytoplasmic face of discs. N-retinylidene PE transport helps to reduce synthesis of the bis-retinoid A2E, a risk factor associated with age-related macular degeneration, but a by-product is that it causes lipid accumulation on the cytoplasmic side of discs at the expense of the luminal side. Rhodopsin’s scramblase activity would correct this transbilayer lipid imbalance, which would otherwise distort the disc membrane. Furthermore, by reducing bilayer stress, rhodopsin-mediated lipid scrambling would permit ABCA4 to function. Discs also possess a P-type ATPase, ATP8A2, and its potential function in discs is discussed in Section 8.3.
5. Constitutive scrambling for glycoconjugate biosynthesis
5.1. Scrambling of dolichol-based glycolipids: key steps in protein glycosylation in the ER
The majority of proteins that enter the secretory pathway are N-glycosylated. Glycosylation occurs when the protein emerges into the ER lumen and is recognized by oligosaccharyltransferase (OST), as illustrated in Figure 6. The glycolipid Glc3Man9GlcNAc2-PP-dolichol (G3M9-DLO) provides the oligosaccharide that is needed for OST-mediated glycosylation. G3M9-DLO is synthesized in the ER through a multi-step pathway in which sugars are added sequentially to dolichyl phosphate, an isoprenoid lipid with a very long hydrocarbon chain [52]. Sugar addition occurs in two stages and on different sides of the ER [52, 53]. The first 7 steps of G3M9-DLO synthesis convert dolichyl-P to Man5GlcNAc2-PP-dolichol (M5-DLO) on the cytoplasmic face of the ER. Then, M5-DLO is flipped into the ER lumen and extended in 7 further steps to G3M9-DLO. The sugar donors for these luminal reactions are mannose-P-dolichol (MPD) and glucose-P-dolichol (GPD) that are synthesized on the cytoplasmic face of the ER and must be flipped to the luminal side. Although flipping of these glycolipids is ATP-independent and bidirectional, their consumption on the luminal side of the ER promotes a flow of lipid from the cytoplasmic to the luminal side.
Figure 6. Glycolipid scrambling is necessary for protein N-glycosylation in the ER.
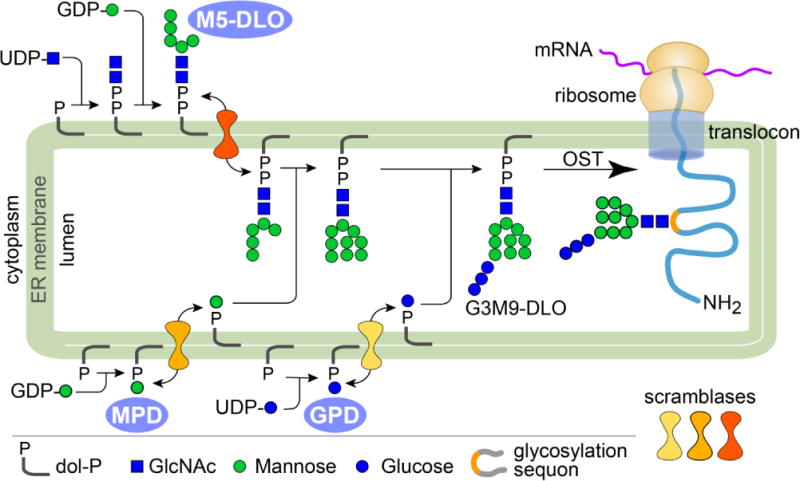
G3M9-DLO, the oligosaccharide donor for protein N-glycosylation, is synthesized in the ER in a multi-step, topologically split pathway. The first 7 steps convert dolichyl-P (dol-P) to M5-DLO on the cytoplasmic face of the ER. Then, M5-DLO is flipped into the ER lumen and extended in 7 further steps to G3M9-DLO. The sugar donors for these luminal reactions are MPD and GPD that are synthesized on the cytoplasmic face of the ER and must be flipped to the luminal side. In addition to its role in N-glycan biosynthesis, MPD is required in the ER lumen for GPI anchor biosynthesis, O-mannosylation, and C-mannosylation. Oligosaccharyltransferase (OST) transfers the oligosaccharide from G3M9-DLO to Asn residues within glycosylation sequences in translocating proteins as they emerge from the translocon into the ER lumen. The dolichyl-PP product of the OST reaction is recycled. The multistep synthesis and transfer of the oligosaccharide require at least 40 gene products.
The scramblases that move M5-DLO, MPD, and GPD across the ER membrane have not been identified. Genetic approaches assigned M5-DLO and MPD scramblase activity to the ER membrane proteins Rft1 and MPDU1, respectively, but disappointingly neither assignment held up in biochemical tests. For example, reconstitution-based assays were used to show that Rft1 could be separated from M5-DLO flippase activity by a number of chromatographic methods [54], and the absence of MPDU1 did not prevent the transbilayer movement of a MPD analog into the ER of cells where the plasma membrane had been permeabilized by a pore-forming toxin to allow access of the analog to the cytosol [55]. Thus, most likely Rft1 and MPDU1 are accessory proteins that assist the actual scramblases, or are proteins somehow involved in maintaining the lipid substrate in transport-competent form [52, 54, 56, 57]. One explanation for the failure of genetic approaches to identify the ER glycolipid scramblases is incomplete coverage in the screens reported to date: this is not surprising as, e.g., the first two enzymes of the DLO biosynthetic pathway were not revealed in the original screen that identified most other DLO glycosyltransferases. An alternative explanation is that the scramblases are dual function proteins whose transport functions are obscured by another function.
Although the identities of the three ‘glycosylation scramblases’ are not known, their activities have been measured in ER microsomes as well as in vesicles reconstituted with ER membrane proteins [40, 41, 54, 58–61]. These studies reveal that the M5-DLO and MPD scramblase activities can be resolved by conventional chromatography (for example, ion exchange on DE-52 and lectin affinity with Con A-Sepharose), that they are distinct from the ER activity that flips glycerophospholipids, and that they have exquisite specificities. Thus, Manβ-P-dolichol, the natural isomer of MPD, is scrambled >100-fold more rapidly than non-natural Manα-P-dolichol in vesicles reconstituted with ER membrane proteins from yeast or rat liver. Likewise, ‘biosynthetic’ M5-DLO is scrambled more rapidly than a structural isomer corresponding to ‘processed’ M5-DLO.
5.2. Glycolipid scrambling and bacterial cell wall assembly
The bacterial cell wall consists primarily of peptidoglycan, a sugar polymer cross-linked by short peptides. The cell wall provides protection against osmotic stresses and its synthesis is a target of many antibiotics. A key intermediate in cell wall biosynthesis is Lipid II, which provides the glycopeptide building blocks for cell wall assembly (Figure 7). In Lipid II, the glycopeptide is linked via a diphosphate bridge to an undecaprenol lipid. Lipid II is synthesized on the cytoplasmic face of the bacterial inner membrane and then translocated to the periplasmic face for cell wall construction. The identity of the scramblase required for Lipid II translocation is controversial [62, 63]: at least three candidates have been proposed, FtsW [64, 65], MurJ [66, 67], and AmJ [68]. FtsW belongs to the SEDS (shape, elongation, division, and sporulation) family whereas MurJ is a member of the multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) exporter superfamily. AmJ is functionally redundant with MurJ, yet the two proteins share no sequence similarity. Reconstitution based assays using purified protein show unambiguously that FtsW can scramble a fluorescent derivative of Lipid II, whereas MurJ cannot. However, cell-based assays show that MurJ is required for Lipid II flipping whereas depletion of SEDS proteins, including FtsW, has no effect on Lipid II translocation. Both approaches have their limitations [69]. For example, the lack of activity of the purified MurJ protein may simply be the result of its lability during purification and reconstitution. Also, the role of MurJ revealed in vivo may be indirect: while the accumulation of Lipid II in MurJ deficient cells is consistent with MurJ being a Lipid II transporter, this phenotype in itself does not provide proof that transport is directly due to MurJ. Conversely, while FtsW shows Lipid II scramblase activity on reconstitution into vesicles, FtsW-deficient cells unexpectedly accumulate downstream products of the Lipid II biosynthetic pathway. Recent work described a Bacillus subtilis strain lacking all 10 members of the MOP superfamily that are known to be expressed in these cells. The decuple mutant was viable and grew at rates similar to that of wild-type cells, but turned out to express the protein AmJ, which is unrelated to the MOP superfamily and necessary for viability in the absence of MOP family members. AmJ may provide essential Lipid II transport activity when MOP superfamily members, including MurJ, are not available. These data only fuel the controversy surrounding the true identity of the Lipid II scramblase. Much more work will be needed to clarify this issue and it may turn out that these proteins act redundantly at some level. An excellent recent review elaborates on these points, making the case for and against each of the flippase candidates [62].
Figure 7. Bacterial cell wall (peptidoglycan) assembly.
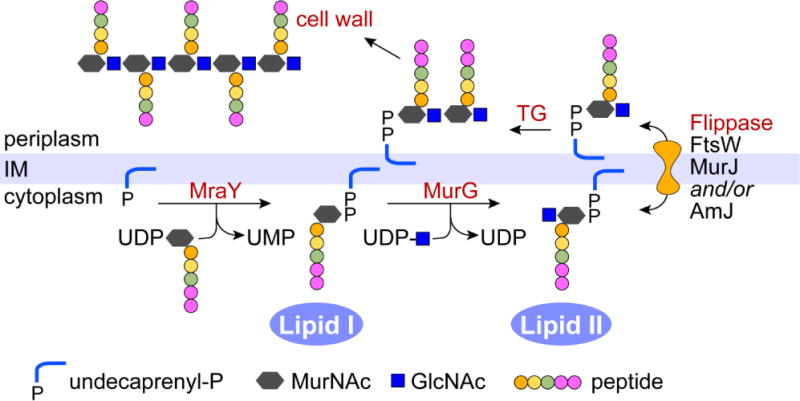
The peptidoglycan building block is assembled on the lipid undecaprenyl phosphate on the cytoplasmic side of the bacterial inner membrane (IM). The enzyme MraY uses UDP-N-acetylmuramic acid-L-Ala-γ-D-Glu-A2pm-D-Ala-D-Ala (UDP-MurNAc-pentapeptide) to synthesize Lipid I. MurG then catalyzes the transfer of GlcNAc from UDP-GlcNAc to Lipid I to generate Lipid II. Lipid II is flipped across the inner membrane (depicted here as a bidirectional process, although this is not fully established) where transglycosylases (TG) polymerize the GlcNAc-MurNac-pentapeptide units into glycan chains attached to undecaprenol by a pyrophosphate linkage. These chains are crosslinked to pre-existing peptidoglycan by transpeptidases while the terminal D-Ala residues in each unit are removed by carboxypeptidases. Figure redrawn from Ref. [62].
5.3. Synthesis of the bacterial cell envelope
The cell envelope of Gram-negative bacteria is dominated by lipopolysaccharide (LPS), a complex glycolipid composed minimally of Lipid A (endotoxin) capped by a core oligosaccharide. In many bacteria, LPS is extended by O-antigen, a polysaccharide that consists of repeating units of 2–6 sugar residues. The composition and length of O-antigen repeats can be quite diverse and are typical of individual bacterial strains, enabling strains to be serotyped. The O-antigen repeat unit is synthesized as a glycolipid, undecaprenol-PP-(O-antigen unit), on the cytoplasmic side of the bacterial inner membrane and must be translocated to the periplasmic side to be polymerized into undecaprenol-PP-(O-antigen unit)n, followed by transfer of the O-antigen polymer to Lipid A-core oligosaccharide. Translocation of the undecaprenol-PP-(O-antigen unit) is likely to be facilitated by Wzx proteins [70]. These proteins are polytopic membrane proteins (12 transmembrane spans) encoded by genes that are found within gene clusters associated with O-antigen biosynthesis, and they are closely related to the multidrug and toxin extrusion (MATE) family of inner membrane efflux proteins. Homology to the MATE family suggests that Wzx proteins may use an ion gradient to power the movement of the undecaprenol-PP-(O-antigen unit) [70]. However, in the only example where the lipid translocation role of a Wzx protein was explicitly examined using everted bacterial membrane vesicles, no energy requirement was found [71]. Thus, it remains to be determined whether the Wzx proteins are directly responsible for lipid movement, and if so, whether they function as translocases or scramblases.
6. Control of transbilayer lipid asymmetry by ATP-driven lipid transporters
ATP-driven transporters are primary active pumps responsible for a net transfer of specific lipids to one side of a membrane. Current genetic and biochemical evidence indicates that these energy-dependent proteins are primarily members of the P4 subfamily of P-type ATPases (P4-ATPases) and the ATP-binding cassette (ABC) family of transporters. Several excellent reviews have recently treated different aspects of both families of pumps [72–75]. Here we highlight the recent progress in the characterization of their lipid specificities.
6.1. P4-ATPases – inward lipid translocases with different substrate specificities
P4-ATPases catalyze the translocation of phospholipids from the exoplasmic to the cytosolic leaflet of membranes. They belong to the family of P-type ATPases, whose members include the ion transporting Na+/K+-ATPase and the Ca2+-ATPase. These proteins are multispan transmembrane pumps that couple ATP hydrolysis to the transport of substrates against their concentration gradient. In doing so, they cycle between a number of conformational states, auto-phosphorylating and dephosphorylating a conserved aspartate residue within a conserved signature sequence (hence the designation ‘P-type’). The lipid-translocating sub-family of P-type ATPases is found exclusively in eukaryotes and classified as type IV, therefore these transporters are referred to as P4-ATPases. Most of these pumps are known to associate with an accessory subunit known as Cdc50 proteins resulting in a heterodimeric complex. This association is required for both proper localization and activity of the pump [76–78] but seems not to affect its substrate specificity [79]. The number of CDC50 isoforms appears to differ between organisms. While only one isoform has been described in Caenorhabditis elegans [80], three isoforms are present in yeast, humans, and the unicellular parasite Leishmania, and up to five exist in plants. Some P4-ATPases interact specifically with only one β-subunit isoform [81–84] while others are more promiscuous and can interact with several isoforms [79, 85–87]. The molecular basis for this difference is unknown.
Although the activity of translocases was measured in the 1980s in pioneering work done with red blood cells [88, 89], their molecular identities were only revealed more recently in Baker’s yeast (Saccharomyces cerevisiae). In total, S. cerevisiae harbors five members of this family, namely Neo1p (Neomycin resistant 1), Drs2p (Deficient for Ribosomal Subunit 2), Dnf1p (Drs2p/Neo1p family), Dnf2p, and Dnf3p. Studies on four of these P4-ATPases revealed striking differences in their cellular locations and lipid specificities. Drs2p and Dnf3p are mainly confined to intracellular membranes of the late secretory and endocytic pathways and primarily transport the aminophospholipids phosphatidylserine (PS) and PE [90–92]. By contrast, Dnf1p and Dnf2p are characterized as plasma membrane translocases with relatively broad phospholipid specificities, including PC, lysophospholipids and synthetic alkylphospholipids [93–95] [96, 97].
In mammals, three subunits of the CDC50 family (CDC50A, B and C) and at least 14 P4-ATPases, designated ATP8A1 through ATP11C, have been identified. Among them, ATP8A1, ATP8A2, ATP8B3, ATP11A, and ATP11C have, so far, been connected to aminophospholipid translocation. ATP8A1 is dependent on PS and PE for ATPase activity [98, 99], is able to translocate fluorescent PS upon expression in yeast [100], and regulates PS asymmetry in the late secretory pathway [101]. Likewise, ATP8A2, a P4-ATPase highly expressed in the brain, testes, and retina, exhibits PS-dependent ATPase activity and the ability to translocate fluorescent PS, and to some extend PE, in proteoliposomes [28, 84, 102]. In the spermatozoa of mice, ATP8B3 is necessary for PS asymmetry and fertilization [103]. ATP11C, a P4-ATPase important for B cell and erythrocyte development, was found to play a crucial role in PS translocation [104, 105]. During apoptosis, ATP11C undergoes caspase-mediated cleavage and is consequently inactivated, thereby contributing to PS exposure on the cell surface [106, 107]. ATP11C is crucial for PS flipping in CHO-K1 cells and a lack of the functional ATP11C protein is responsible for the defect in PS uptake in UPS-1 cells [108].
Notably, not all mammalian P4- ATPases are aminophospholipid specific translocators. ATP8B1, initially characterized as a translocase for aminophospholipids [109] and cardiolipin [110], was found to translocate PC rather than PS upon overexpression in cell lines with low endogenous phospholipid translocase activity [111]. In the same setup, ATP8B2 and ATP10A were shown to specifically transport PC [111, 112]. Using heterologous expression in yeast, ATP8B5 from mouse testes was found to transport PC and PE [92]. Moreover, previous studies on mammalian cells uncovered a role of the human P4-ATPase subunit CDC50A (TMEM30a) in the uptake of the inflammatory lipid PAF (platelet-activating factor, a short-chain PC) and synthetic alkylphospholipids, implying the presence of yet unidentified P4-ATPase(s) that in complex with CDC50A facilitate(s) the uptake of alkylphospholipids [113].
Recent studies in the plant Arabidopsis thaliana further substantiate the notion that members of the P4-ATPase family differ in their substrate specificities. The Arabidopsis genome encodes 12 P4-ATPases termed ALA1 to ALA12 (for AminophosphoLipid ATPase) [114]. The lipid specificity of some of these plant ALA proteins has been characterized. ALA2 flips PS across the lipid bilayer in the endosomal system. Golgi-localized ALA3 facilitates transport of PS, PE and PC but not of the lysoPC analogue miltefosine [79, 86]. ALA10 localizes to the plasma membrane and internalizes a broad range of exogenous phospholipids, including lysoPC [115].
Strikingly, many P4-ATPases localizing to subcellular membranes seem to display a narrow substrate specificity only recognizing one (typically PS) or few lipid species as substrate. By contrast, several P4-ATPases at the plasma membrane of eukaryotic cells transport a broad range of exogenous phospholipids, including lysolipids and derivatives, suggesting functions for these P4-ATPases in lipid scavenging [94, 95, 115]. In fact, the cellular processes known to be directly or indirectly affected by P4-ATPases have expanded over the last years to include the regulation of membrane traffic, cytoskeletal dynamics, cell division, lipid metabolism, and lipid signaling (reviewed in [73, 75, 116]).
6.2. ABC transporters – a family of exporters and importers
ABC transporters comprise a superfamily of membrane proteins that actively transport chemically diverse substrates across biological membranes. They typically consist of four core domains: two transmembrane domains surrounding a single central cavity, presumably along the pathway of substrate transport, and two ATP-binding cassettes (ABCs), which supply the energy for substrate transport through the binding and hydrolysis of ATP [117, 118]. In eukaryotes, ABC transporters exist as either full transporters, in which all four domains are present within a single polypeptide chain, or half-transporters, in which an ABC and transmembrane domain reside within a polypeptide chain that assembles as homo- or heterodimers. In prokaryotes, the domains can exist as individual subunits or together in various combinations to generate an active transporter.
Humans have almost 50 different ABC transporters that are grouped into seven sub-families (A–G). The members of these subfamilies are not exclusively expressed at the plasma membrane but also localizes to intracellular organelles such as peroxisomes (ABCD1–3) [119], lamellar bodies (ABCA3, ABCA12) [120–122], lysosomes (ABCA2, ABCA5) [123, 124], and endosomes (ABCG1, ABCG2) [125, 126]. Several members of these subfamilies have been implicated in the transport of lipids or lipid-related compounds. Their substrate specificities have mostly been inferred from disease-associated phenotypes, analysis of knock-out mice, and cell-based studies. Progress in purification and reconstitution of some members has provided directed evidence for their capability to directly catalyze phospholipid transport. Purified ABCB1 reconstituted into proteoliposomes flips a variety of short-chain fluorescent phospholipids and sphingolipids [127], including simple glycolipids, but is unable to restore transport of PC into the bile of Mdr2 (Abcb4)-knockout mice [128]. Thus, direct evidence for the transport of endogenous lipids is still lacking. The glutathione dependent multidrug transporter ABCC1 transports fluorescent PC after reconstitution in proteoliposomes [129] and may help to maintain the outward orientation of natural choline phospholipids in the plasma membrane [130–132]. Reconstituted ABCA1 actively transports fluorescent PC, PS, and SM with a preference for PC, whereas ABCA7 preferentially transports fluorescent PS [133]. In yeast, Yor1p and Pdr5 have been implicated in the transport of PE [93, 134] while Ybt1 translocates PC across the vacuolar membrane as part of choline recycling [135]. Thus, similar to P4-ATPases discussed in the previous section, individual ABC transporters differ in their substrate specificities. Notably, several plant ABC transporters have been implicated in the transport of cuticular lipids comprising C16 and C18 hydroxy and epoxy fatty acids [136–140]. Although it has been possible to demonstrate lipid transport by purified ABC transporters after reconstitution into phospholipid vesicles, such experiments remain challenging since the amplitude of transport is very small. This is because even the smallest movement of phospholipids from the outer leaflet to the inner leaflet of the vesicles exerts bilayer stress (the bilayer couple hypothesis [3] that prevents further transport. One example where this is not a problem is the case of PglK, a bacterial ABC transporter that transports a specific lipid that is not a component of the bulk membrane. This example is discussed below.
Apart from outwardly-directed transporters, eukaryotes also express ABC transporters that transport lipids towards the cytosolic leaflet of cellular membranes. Such an inward-directed lipid translocase activity has been demonstrated for the mammalian ABC transporter ABCA4 flipping the Schiff base adduct of retinal and PE known as N-retinylidene-PE from the lumen to the cytosolic leaflet of photoreceptor disc membranes and proteoliposomes [133, 141]. Notably, ABCC7 expression has been correlated with an increased uptake of the signaling lipids sphingosine-1-phosphate and lysophosphatidic acid, implying that other mammalian members might act as inward lipid transporters as well [142]. In the yeast Candida albicans, a subfamily member (Cdr3p) has been identified that exhibits an inward-directed phospholipid translocase activity. Some yeast ABC transporters (Aus1p and Pdr11p in S. cerevisiae; Aus1p in Candida glabrata) facilitate exogenous sterol uptake [143–148]. Three Arabidopsis proteins, TGD1-3, are proposed to form an ABC transporter complex in the inner envelope of the chloroplast that imports PA into the inside of the chloroplast for the synthesis of thylakoid lipids [149, 150].
Finally, ABC transporters are also widely expressed in prokaryotes, and some bacterial ABC transporters function as lipid translocases. MsbA is one of these proteins being an essential inner membrane transporter in Gram-negative Escherichia coli genetically linked to the export of the Lipid A core of lipopolysaccharides to the bacterial outer membrane. Depletion of cellular MsbA or the presence of a conditionally inactivating mutation in this protein results in loss of lipid A and phospholipid transport from the cytoplasmic to the outer membrane in bacterial cells, suggesting a general lipid translocase function for MsbA [151, 152]. Reconstitution studies using purified MsbA showed that it can transport a variety of fluorescent glycerophospholipid and even simple glycosphingolipids, albeit with low activity [153], while other data suggest that this is not likely to be the case [22, 154]. Another ABC protein, PglK, is required for the translocation of isoprenoid-linked oligosaccharides [155–157]. This function was recently confirmed by reconstituting PglK-mediated flipping of isoprenoid-linked oligosaccharide intermediates from the outer leaflet to the lumen of proteoliposomes [158]; comments in [159] and [160].
7. Loss of plasma membrane asymmetry and surface exposure of PS: role of regulated scramblases
It has long been recognized that PS, normally sequestered in the cytoplasmic leaflet of the plasma membrane by inward ATP-driven transporters, becomes exposed at the cell surface in response to specific stimuli [161]. Thus blood cells, especially platelets, expose PS when activated and this is important in providing a nucleating platform for the maturation of coagulation factors and the production of blood clots. Likewise, cells undergoing programmed cell death expose PS and this helps to target them for removal through the phagocytic action of macrophages. In a specific and quite dramatic example, PS is exposed at the tips of photoreceptor cells in the retina in a diurnal manner and this contributes a signal for the consumption of the tips by neighboring retinal pigment epithelial cells [162]. Recent discoveries have implicated membrane proteins belonging to the TMEM16 and Xkr families in PS exposure [163–165]. The former are specifically associated with Ca2+-dependent PS exposure related to blood clotting whereas the latter play a role in apoptosis.
Many cells expose PS when treated with a Ca2+ ionophore in the presence of extracellular Ca2+. It is generally assumed that ionophore treatment prompts an increase in intracellular Ca2+ that, in turn, activates a plasma membrane scramblase. The predicted high rate of scrambling (>10,000 phospholipids per scramblase per second) counters the slow corrective effects of P-type ATPases (1–100 phospholipids per ATPase per second) in restoring PS asymmetry, and thus PS can be detected at the cell surface by fluorescent probes such as fluorescein-labeled Annexin V [165]. Scramblase action is not specific to PS; all common phospholipids are translocated between the two leaflets of the membrane, resulting in loss of lipid asymmetry of which a byproduct is PS exposure. Mouse Ba/F3 cells expose PS in response to ionophore even under low Ca2+ conditions [165]. A subline of these cells was selected by repeated fluorescence-activated cell sorting and shown to be highly sensitive to Ca2+ ionophore-elicited PS exposure. To identify the protein(s) responsible for hyperactive scrambling in these cells, a cDNA library was generated, fractionated into clones >2.5 kb and 1.0–2.5 kb, and introduced into the parental Ba/F3 cell line. Further rounds of selection revealed the identities of the genes responsible for enhanced PS exposure. The use of cDNA clones >2.5 kb identified TMEM16F [165], whereas clones of 1.0–2.5kb identified Xkr8 [163]. Both proteins belong to evolutionarily conserved protein families and subsequent work has implicated other members of each family in scrambling [163, 164, 166].
Scott syndrome is a bleeding disorder caused by the inability of activated blood platelets to expose PS, and this disease has figured prominently in the search for the Ca2+-activated scramblase [161]. Scott syndrome patients express a severely truncated non-functional TMEM16F protein, a result of a mutation in the TMEM16F gene that causes a frame shift leading to premature termination [165]. In contrast, the Ba/F3 subline that is hyperactive in PS exposure expresses TMEM16F with a constitutively activating D409G mutation. While these correlations make it clear that TMEM16F plays a critical role in lipid scrambling, it is possible that the protein functions indirectly. Indeed, as TMEM16 belongs to a family of ion channels, one hypothesis is that it is involved in providing Ca2+ in a localized way to support the activity of the ‘real scramblase’. This idea is unlikely to be correct as direct evidence that TMEM16 proteins have Ca2+-activated scramblase activity has come from reconstitution studies. Purified fungal TMEM16 homologues (afTMEm16 and nhTMEM16) were reconstituted into large unilamellar vesicles and shown to scramble phospholipids [167, 168]. Scrambling was sensitive to Ca2+ as the rate of scrambling in the presence of Ca2+ was at least an order of magnitude faster than in its absence. Some TMEM16 proteins do not scramble lipids and this provides an opportunity to understand what makes a TMEM16 protein a scramblase. Analysis of chimeras between the inactive TMEM16A protein, a Ca2+-activated chloride channel with no scramblase activity, and TMEM16F identified a sequence in 16F that, when transplanted into 16A, allowed the chimeric protein to promote Ca2+-dependent PS exposure in cells [169]. While a definitive conclusion will only be possible after reconstitution and testing of purified protein, these results nevertheless strongly suggest that TMEM16F is indeed a scramblase.
The other protein family implicated in lipid scrambling is less well understood. Of the 8 members of the mouse Xkr family, three are needed to facilitate PS exposure in cells undergoing programmed cell death [166]. It is unclear whether these proteins are scramblases themselves, but as they are polytopic membrane proteins located at the plasma membrane they are well positioned to either carry out or regulate scrambling. Xkr proteins need to be activated in order to play a role in PS exposure, and, unlike the Ca2+ trigger needed for TMEM16 proteins, the trigger here appears to be caspase-mediated cleavage of the C-terminal tail.
8. Lipid transporters and membrane lipid asymmetry
Differential distribution of phospholipids across the bilayer is a feature that defines the many different membranes in a eukaryotic cell. A remarkable assortment of selective and non-selective lipid transporters with overlapping sub-cellular distributions and substrate specificities appears to help in controlling the lipid arrangement in the various membrane systems (Figure 3). Thus, their activities need to be differentially regulated. In addition, lipid arrangement also depends on the membrane lipid composition and retentive mechanisms that trap lipids on one side of the bilayer. Here we will highlight three examples illustrating how cells might combine these principles to control the texture of their membranes.
8.1. Eukaryotic plasma membranes: lipid asymmetry based on constrained flip-flop
In the plasma membrane, the late Golgi, and endosomal compartments ATP-driven vectorial transporters are responsible for a net transfer of specific lipids to one side of a membrane and thereby regulate their transbilayer lipid arrangement. A prime example is the specific transport of PS and PE towards the cytosolic leaflet of these membranes by members of the P4-ATPase family. This selective transport is held responsible for generating and maintaining an asymmetric lipid distribution with PS and PE largely confined to the cytosolic leaflet. Some P4-ATPases display a PC translocase activity implying that certain cell types might restrict PC to the cytoplasmic leaflet as well. The clearance of PC and other phospholipids from the cell surface would lead to an enrichment of sphingolipids in the exoplasmic leaflet. As sphingolipids have saturated acyl chains and therefore pack at a higher density than glycerophospholipids, their enrichment in the exoplasmic leaflet of the plasma membrane would support the barrier function of this organelle.
Sphingolipids are primarily synthesized in the luminal leaflet of Golgi and delivered to the cell surface by vesicular transport, which explains their asymmetric distribution across the plasma membrane [170]. However, it is still unclear what permits the accumulation of the majority of PC in the outer plasma membrane leaflet of most mammalian cells. One possibility is that ABC proteins move phospholipids unspecifically to the outer leaflet while P4-ATPases restore specific lipids, e.g. PS, PE, to the inner leaflet [171] [172] [127] [129] [173]. Alternatively, it was proposed on theoretical grounds that inward transport of aminophospholipids, together with passive fluxes, would be sufficient to accumulate choline containing lipids in the outer leaflet [174] [175]. Discrimination between these models awaits evaluation of the substrate specificity and transport efficiency of the different ATP-dependent lipid transporters and the characterization of their regulation in the living cell. Despite these questions, it is clear that the plasma membrane does not support constitutive, protein-mediated lipid scrambling. Non-selective ATP-independent scramblases are either retained in the ER or silenced during their trafficking to the plasma membrane, possibly by the increasing sterol content from the ER (around 5 mol%) to the plasma membrane (more than 40 mol% sterol), leading to a thicker, more organized bilayer that is not compatible with scrambling. It is interesting to speculate that scramblases destined for the plasma membrane actually contribute to lipid scrambling in the ER while they are in transit through the ER [27].
8.2. Early secretory organelles: lipid asymmetry in presence of scramblases
In early secretory organelles, scramblases are important for the proper assembly of the membrane. They allow lipids to equilibrate rapidly between the two bilayer leaflets, and thereby in principle promote a symmetric lipid distribution across the bilayer. However, studies with a genetically-encoded fluorescent PS sensor indicate that PS is not detectable at the ER membrane, consistent with it residing in the luminal leaflet of the ER, an asymmetric arrangement opposite to that of the plasma membrane [176]. In line with this notion, phospholipid monolayers around lipid droplets, which are thought to arise from the cytoplasmic leaflet of the ER, contain hardly any PS [177]. This suggests the presence of retentive mechanisms that trap PS on the luminal side of the ER. Such retentive mechanisms could include calcium-mediated interactions of PS with luminal proteins [178]. In addition, recent work uncovered lipid-transfer proteins that mediate non-vesicular transport of PS from the cytosolic leaflet of the ER to the cytosolic surface of the plasma membrane, in exchange for phosphatidylinositol-4-phosphate [179–181]. Such lipid-exchange cycles might contribute in the control of PS asymmetry in early secretory organelles and the plasma membrane.
8.3. Photoreceptor discs: lipid asymmetry based on asymmetric charge distribution
Photoreceptor disc membranes are another example of a subcellular membrane with an asymmetric lipid arrangement. These membranes are asymmetric with respect to PS (65–80% in the cytoplasmic leaflet), and roughly symmetric in their transbilayer distribution of PC and PE [47–49]. This asymmetry was predicted by the transbilayer coupling model of Hubbell [182], i.e., the combination of phospholipid scrambling and the asymmetric charge distribution (positive on the cytoplasmic face of discs), created by the large number of oriented rhodopsin molecules in the disc membrane, explains why negatively charged PS is mainly located on the cytoplasmic side at steady state whereas zwitterionic PC and PE are symmetrically distributed across the disc membranes. Interestingly, the transbilayer asymmetry of PS changes reversibly in response to light, presumably because of associated changes in transbilayer charge asymmetry [49]. Given the transbilayer coupling model [182] it is not necessary to invoke the activity of the aminophospholipid specific transporter ATP8A2 to explain PS asymmetry. Indeed the function of ATP8A2 in discs is enigmatic as its activity would needlessly deplete ATP. We speculate that Atp8a2 may be silent in disc membranes because of unrelieved auto-inhibition, or because of regulation by phosphorylation as shown for other lipid-translocating P-type ATPases [183, 184]. Atp8a2 may instead play a functional role in the secretory pathway [101, 185] by regulating protein traffic to the disc membrane.
9. General mechanisms of handling lipids by lipid transporters
How would proteins facilitate lipid flip-flop? A simple look at the Arrhenius rate equation presented in section 1, suggests that if the prefactor stays the same then the energy barrier must decrease from ~20 kcal mol−1 to ~7 kcal mol−1 in order to accelerate the rate of flipping to that measured for known scramblases. While it is also possible that the prefactor may change, if the frequency with which lipids access the flippase is somehow enhanced, it is typical to consider mechanisms that result in reduction of the energy barrier or those that short-circut the bilayer by fusing the two leaflets. Thus, the usual depiction of phospholipid movement across a bilayer shows a path where the two leaflets of the bilayer are connected. Evidence for such a path is provided in studies of pore-forming amphipathic peptides, such as magainin, which are predicted to form toroidal pores in the membrane [186, 187]. Such pores would necessarily connect the two leaflets leading to lipid mixing/scrambling. In silico studies promote the importance of water [188] (Figure 8). Thus, the presence of a transbilayer region involving water, or at least polar molecules, would promote lipid movement as the lipid headgroup would be able to interact with this region specifically, thereby minimizing the energy cost of transfer.
Figure 8. In silico analysis of pore-mediated lipid flip-flop.
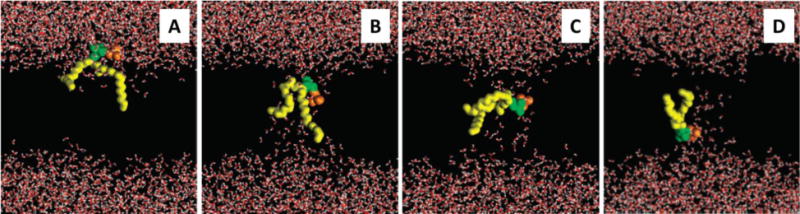
Molecular dynamics simulation showing that the appearance of a water pore facilitates the spontaneous migration of lipids across a phospholipid membrane. (A) 0 picoseconds, (C) 118.9 nanoseconds, (D) 122.4 nanoseconds, (E) 152.7 nanoseconds. Lipids (except for the flip-flopped one) are not shown; water is shown in red and white, acyl chains of the flip-flopped lipid are shown in yellow, and its choline and phosphate groups are shown in orange and green, respectively. Adapted and reprinted by permission from [189], American Chemical Society, copyright 2007.
A lipid transporter might provide such a pathway via a hydrophilic membrane-facing groove. The translocation mechanism is imagined to resemble the swiping of a card through a card reader (Figure 9). In this “card reader” model, the polar headgroup of the phospholipid (the magnetic strip on the card) is protected during passage across the hydrophobic interior of the membrane until it emerges on the other side; the acyl chains of the lipid remain in the hydrophobic milieu of the membrane during this process. Thus, the groove of the flippase/card-reader provides a low energy path for the lipid headgroup by sequestering it from the unfavorable hydrophobic environment of the membrane interior. Evidence for such potential grooves has been provided for several lipid transporters. For example, structural homology modeling and molecular dynamics simulations on the mammalian P4-ATPase ATP8A2 provided an indication for a groove formed by the transmembrane segments M1, M2, M4, and M6, which likely contains water-filled pockets [190]. The cytoplasmic end of this groove coincides with a bound PE molecule present in several crystal structures of the sarcoplasmic reticulum Ca2+-ATPase [191], in line with a transport pathway from the exoplasmic side to a cytoplasmically facing exit site situated approximately at the location of the PE molecule in SERCA. Based on mutational studies on the yeast P4-ATPases Dnf1p and Drs2p, a transport pathway along potential grooves formed by the transmembrane segments M1, M3, and M4, or alternatively M3, M4, and M5 has been proposed [192]. Furthermore, current models for ABC-type translocases propose an alternating opening of lateral entry and exit pathways for the transported phospholipid substrate to the central cavity formed by the transmembrane helices. Coarse-grained modelling has revealed lipid binding to the ABC transporter MsbA within the grooves formed between transmembrane helices [193]. Similarly, scramblases appear to display such potential grooves. The crystal structure of a Ca2+-bound TMEM16 scramblase (nhTMEM16) was recently reported [168]. The structure is that of a dimer, the native state in which TMEM16 proteins are isolated from cells. Each monomer has a remarkable groove in its transmembrane domain on the side of the protein opposite to that of the dimer interface. The groove is ~1 nm wide and of polar character, and could thereby accommodate the headgroup of a transiting phospholipid. While considerable work needs to be done to verify that this is indeed the translocation pathway, this structural feature of nhTMEM16 provides the first hint of how a scramblase might function, reinforcing models that have been discussed for many years.
Figure 9. Model for substrate flipping by TMEM16 and ATP8A2.
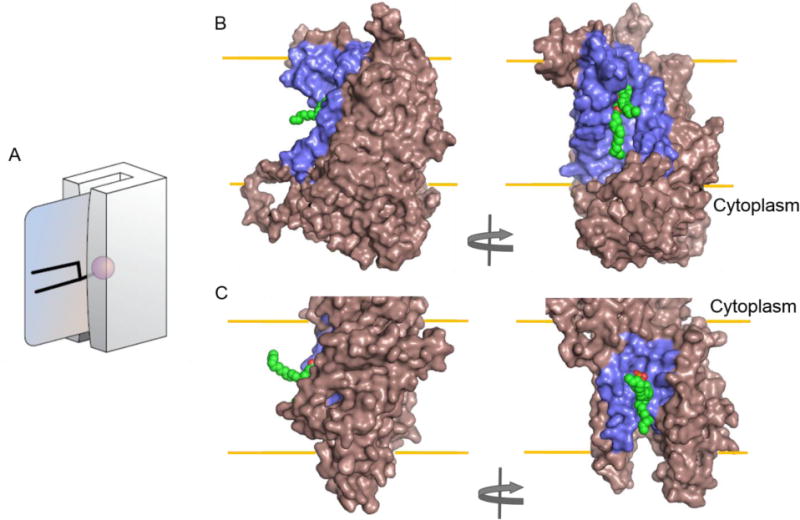
(A) Credit card swiping model. The magnetic strip on the card (= polar headgroup of the phospholipid being transported) is protected from the lipid environment (by passage through the groove in the card reader) as it transits the hydrophobic interior of the membrane. See text for details and for a discussion. Adapted from a figure drawn by Adam Steinberg and published in Ref.[194]. Panel (B) and (C) show alternate views of the proposed conduit groove in nhTMEM16 (PDBID 4WIS) and homology modeled bovine ATP8a2 (Uniprot ID: C7EXK4, cytoplasmic domains are omitted), respectively. Both proteins are shown in surface representation with dark salmon color. The grooves are highlighted in blue with a hypothetical lipid placed inside shown as green CPK representation. The ATP8A2 homology model was generated based on the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase I structure (PDBID: 3B9B) and in vacuo energy minimized using GROMOS96 implementation in Swiss-Pdb Viewer [195]. All models were generated with Pymol (DeLano Scientific, San Carlos, California).
Apart from utilizing a hydrophilic membrane facing groove, some scramblases might perturb the lipid bilayer thereby facilitating lipid flip-flop. Such a mechanism might apply to rhodopsin-mediated lipid scrambling. Although it was originally suggested that a possible lipid translocation pathway might be provided by the central water-lined core of the protein [50], this was ruled out since access to the core is occluded by a loop (the E2-loop) on the exoplasmic side, and since the core itself is blocked by retinal in rhodopsin and metarhodopsin II, both of which are active as scramblases [27]. A suitable environment for lipid translocation could be provided instead by specific residues on the surface of the rhodopsin transmembrane (TM) helical bundle or by membrane packing defects that are specifically generated in the vicinity of rhodopsin. As other rhodopsin-like GPCRs, e.g. the A2A adenosine receptor, β1-, and β2-adrenergic receptors also scramble lipids [27, 50]; a structural element common amongst these proteins likely contributes to the scramblase activity. GPCRs contain an intracellular amphipathic helix 8 that immediately follows the last transmembrane helix. As this helix runs parallel to the membrane and often contains palmitoylated cysteine, it has the potential to disturb bilayer structure on one side of the membrane; propagation of this disturbance across the bilayer may contribute to bidirectional scrambling.
10. Concluding remarks and future perspectives
As is clear from this overview, phospholipid translocation across cellular membranes is facilitated by a remarkable assortment of lipid translocases and scramblases. Some progress has been made on the in vitro reconstitution of purified P4-ATPases, ABC transporters, and scramblases, thereby demonstrating that these proteins indeed participate directly in lipid translocation. Yet, analysis of natural lipid translocation by these enzymes remains challenging due to the hydrophobic nature of the lipid substrate. Nevertheless, such studies are important to determine their transport kinetic properties and substrate specificities, and thus to understand their physiological role and interplay in the various cellular membrane systems. Additional biophysical approaches will be required to test the impact of the lipid composition – especially cholesterol content – on lipid translocation by translocases and scramblases. The answer to this question cannot be easily obtained by experiments on living cells, nor can it be obtained via ensemble reconstitution experiments because of the compositional heterogeneity of reconstitutions [196]. The key lies in single vesicle experiments where the lipid content is precisely known. At the same time, further biophysical studies on model membrane systems are needed to unravel how the physical properties of the bilayer itself dictate the rate of spontaneous flip-flop. Techniques such as small-angle neutron scattering and sum-frequency vibrational spectroscopy that do not require lipids labeled with bulky reporter groups are promising but have yielded contradictory results in a few instances. For example, using sum-frequency vibrational spectroscopy, fast flip-flop rates (t1/2 <10 min) were measured for DPPC and DMPC in supported fluid bilayers attached directly to silica [197–199]. In comparison, flip-flop was predictably slow in bilayers formed on polymer supports [200], suggesting that the roughness of the silica surface induces imperfections within the bilayer which promote fast flipping [201]. Clarification of these issues is warranted in future studies.
Promising steps have also been taken to dissect the inner workings of lipid-translocating proteins. This includes the improvement of protein expression and purification approaches and crystallization attempts [202, 203]. Yet, the lipid-translocating proteins identified so far belong to large protein superfamilies, and many family members remain to be characterized. At the same time, there is now the probability that individual proteins have multiple functions depending on their cellular context, as has been demonstrated for the G protein-coupled receptor rhodopsin. Dissecting how all these enzymes are coordinately regulated within the cell will be a challenge for years to come. Notably, mitochondria and chloroplasts derive many of their membrane lipids from the ER through a non-vesicular pathway that exploits regions of close membrane contact between the two organelles [204, 205]: ER-derived lipids arriving at the surface of these organelles must flip across the outer and inner membranes to gain access to the organelle interior [206–208]. Identification of the mitochondrial and plastid translocases/scramblase(s) will be important in future studies. Similarly, the phospho- and glycolipid scramblases of biogenic membranes have not yet been identified. Although some bacterial proteins such as MprF appear to be able to scramble phospholipids, none of these proteins are needed for viability, and, as a group, they are unlikely to shoulder the burden of scrambling phospholipids to sustain growth. It has been almost a decade since the last substantial progress was made on lipid flipping in biogenic membranes. The spate of recent discoveries in the scramblase field may provide new impetus to go after these fundamentally important transporters.
Acknowledgments
The authors are particularly grateful to the members of their research teams for stimulating discussions and comments on the manuscript; we would like to warmly thank Ida Louise Jørgensen for her commitment to improving and eliminating errors in the manuscript and Kalpana Pandey for generating Figure 9. We thank Natascha Ruiz for comments on the section relating to peptidoglycan synthesis. TGP acknowledges support from the Research Centre ‘bioSYNergy’ funded by the University of Copenhagen’s Excellence Programme, the Danish National Research Foundation through the PUMPKIN Center of Excellence (DNRF85), and the Danish Council for Independent Research |Natural Sciences (DFF1323-00297). AKM acknowledges support from the National Institutes of Health (grants GM106717, EY024207 and NS093457).
Abbreviations
- ABC transporter
ATP-binding cassette transporter
- bCM
bacterial cytoplasmic membrane
- DLO
dolichol-linked oligosaccharide
- ER
endoplasmic reticulum
- GPCR
G protein-coupled receptor
- GPD
glucose-P-dolichol
- GPI
glycosylphosphatidylinositol
- LPS
lipopolysaccharide
- MPD
mannose-P-dolichol
- NBD
7-nitrobenz-2-oxa-1,3-diazol-4-yl
- OST
oligosaccharyltransferase
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorter E, Grendel F. ON BIMOLECULAR LAYERS OF LIPOIDS ON THE CHROMOCYTES OF THE BLOOD. The Journal of Experimental Medicine. 1925;41:439–43. doi: 10.1084/jem.41.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zwaal RFA, Demel RA, Roelofsen B, van Deenen LLM. The lipid bilayer concept of cell membranes. Trends in Biochemical Sciences. 1976;1:112–4. [Google Scholar]
- 3.Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71:4457–61. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanford C. The hydrophobic effect: formation of micelles and biological membranes. New York: John Wiley & Sons; 1973. [Google Scholar]
- 5.Mouritsen OG. Life - As a Matter of Fat. Berlin Heidelberg: GmbH & Co K.: Springer-Verlag; 2005. [Google Scholar]
- 6.Kornberg RD, McConnell HM. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971;10:1111–20. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- 7.Ganong BR, Bell RM. Transmembrane movement of phosphatidylglycerol and diacylglycerol sulfhydryl analogs. Biochemistry. 1984;23:4977–83. doi: 10.1021/bi00316a023. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Montero I, Rodriguez N, Cribier S, Pohl A, Velez M, Devaux PF. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280:25811–9. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- 9.Bai J, Pagano RE. Measurement of Spontaneous Transfer and Transbilayer Movement of BODIPY-Labeled Lipids in Lipid Vesicles. Biochemistry. 1997;36:8840–8. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- 10.Buton X, Hervé P, Kubelt J, Tannert A, Burger KNJ, Fellmann P, et al. Transbilayer Movement of Monohexosylsphingolipids in Endoplasmic Reticulum and Golgi Membranes†. Biochemistry. 2002;41:13106–15. doi: 10.1021/bi020385t. [DOI] [PubMed] [Google Scholar]
- 11.Chalat M, Menon I, Turan Z, Menon AK. Reconstitution of Glucosylceramide Flip-Flop across Endoplasmic Reticulum: IMPLICATIONS FOR MECHANISM OF GLYCOSPHINGOLIPID BIOSYNTHESIS. The Journal of Biological Chemistry. 2012;287:15523–32. doi: 10.1074/jbc.M112.343038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullis PR, Hope MJ, Bally MB, Madden TD, Mayer LD, Fenske DB. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 1997;1331:187–211. doi: 10.1016/s0304-4157(97)00006-3. [DOI] [PubMed] [Google Scholar]
- 13.Boon JM, Smith BD. Facilitated phosphatidylcholine flip-flop across erythrocyte membranes using low molecular weight synthetic translocases. J Am Chem Soc. 2001;123:6221–6. doi: 10.1021/ja010160q. [DOI] [PubMed] [Google Scholar]
- 14.Boon JM, Smith BD. Synthetic membrane transporters. Current Opinion in Chemical Biology. 2002;6:749–56. doi: 10.1016/s1367-5931(02)00399-x. [DOI] [PubMed] [Google Scholar]
- 15.Ogushi F, Ishitsuka R, Kobayashi T, Sugita Y. Rapid flip-flop motions of diacylglycerol and ceramide in phospholipid bilayers. Chemical Physics Letters. 2012;522:96–102. [Google Scholar]
- 16.Bennett WFD, Tieleman DP. Molecular simulation of rapid translocation of cholesterol, diacylglycerol, and ceramide in model raft and nonraft membranes. Journal of Lipid Research. 2012;53:421–9. doi: 10.1194/jlr.M022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Kruijff B, Van Zoelen EJJ. Effect of the phase transition on the transbilayer movement of dimyristoyl phosphatidylcholine in unilamellar vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1978;511:105–15. doi: 10.1016/0005-2736(78)90068-8. [DOI] [PubMed] [Google Scholar]
- 18.Wimley WC, Thompson TE. Exchange and flip-flop of dimyristoyl phosphatidylcholine in liquid-crystalline, gel and two-component, two-phase large unilamellar vesicles. Biochemistry. 1990;29:1296–303. doi: 10.1021/bi00457a027. [DOI] [PubMed] [Google Scholar]
- 19.John K, Schreiber S, Kubelt J, Herrmann A, Müller P. Transbilayer movement of phospholipids at the main phase transition of lipid membranes: implications for rapid flip-flop in biological membranes. Biophysical Journal. 2002;83:3315–23. doi: 10.1016/S0006-3495(02)75332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrot G, Herve P, Zachowski A, Fellmann P, Devaux PF. Aminophospholipid translocase of human erythrocytes: phospholipid substrate specificity and effect of cholesterol. Biochemistry. 1989;28:3456–62. doi: 10.1021/bi00434a046. [DOI] [PubMed] [Google Scholar]
- 21.Kol MA, de Kroon AIPM, Killian JA, de Kruijff B. Transbilayer Movement of Phospholipids in Biogenic Membranes. Biochemistry. 2004;43:2673–81. doi: 10.1021/bi036200f. [DOI] [PubMed] [Google Scholar]
- 22.Kol MA, van DA, de Kroon AI, de KB. Translocation of phospholipids is facilitated by a subset of membrane-spanning proteins of the bacterial cytoplasmic membrane. J Biol Chem. 2003;278:24586–93. doi: 10.1074/jbc.M301875200. [DOI] [PubMed] [Google Scholar]
- 23.Kol MA, van Laak AN, Rijkers DT, Killian JA, de Kroon AI, de KB. Phospholipid flop induced by transmembrane peptides in model membranes is modulated by lipid composition. Biochemistry. 2003;42:231–7. doi: 10.1021/bi0268403. [DOI] [PubMed] [Google Scholar]
- 24.Langer M, Sah R, Veser A, Gütlich M, Langosch D. Structural Properties of Model Phosphatidylcholine Flippases. Chemistry & Biology. 2013;20:63–72. doi: 10.1016/j.chembiol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Nakao H, Ikeda K, Ishihama Y, Nakano M. Membrane-Spanning Sequences in Endoplasmic Reticulum Proteins Promote Phospholipid Flip-Flop. Biophysical Journal. 2016;110:2689–97. doi: 10.1016/j.bpj.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx U, Lassmann G, Holzhutter HG, Wustner D, Muller P, Hohlig A, et al. Rapid flip-flop of phospholipids in endoplasmic reticulum membranes studied by a stopped-flow approach. Biophys J. 2000;78:2628–40. doi: 10.1016/S0006-3495(00)76807-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goren MA, Morizumi T, Menon I, Joseph JS, Dittman JS, Cherezov V, et al. Constitutive phospholipid scramblase activity of a G protein-coupled receptor. Nat Commun. 2014;5 doi: 10.1038/ncomms6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman JA, Vestergaard AL, Molday RS, Vilsen B, Andersen JP. Critical role of a transmembrane lysine in aminophospholipid transport by mammalian photoreceptor P4-ATPase ATP8A2. Proc Natl Acad Sci U S A. 2012;109:1449–54. doi: 10.1073/pnas.1108862109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan N, Farine L, Pandey K, Menon AK, Bütikofer P. Lipid topogenesis - 35 years on. Biochemica et Biophysica (BBA) Molecular and Cell Biology of Lipids. 2016 doi: 10.1016/j.bbalip.2016.02.025. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bretscher MS. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972;236:11–2. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- 31.Verkleij AJ, Zwaal RFA, Roelofsen B, Comfurius P, Kastelijn D, van Deenen LLM. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973;323:178–93. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- 32.Mioka T, Fujimura-Kamada K, Tanaka K. Asymmetric distribution of phosphatidylserine is generated in the absence of phospholipid flippases in Saccharomyces cerevisiae. Microbiology Open. 2014;3:803–21. doi: 10.1002/mbo3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal S, Menon AK. Flipping lipids: why an whats the reason for? ACS Chem Biol. 2009 doi: 10.1021/cb900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman JE, Kennedy EP. Rapid transmembrane movement of newly synthesized phospholipids during membrane assembly. Proc Natl Acad Sci USA. 1977;74:1821–5. doi: 10.1073/pnas.74.5.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop WR, Bell RM. Assembly of the endoplasmic reticulum phospholipid bilayer: the phosphatidylcholine transporter. Cell. 1985;42:51–60. doi: 10.1016/s0092-8674(85)80100-8. [DOI] [PubMed] [Google Scholar]
- 36.Backer JM, Dawidowicz EA. Reconstitution of a phospholipid flippase from rat liver microsomes. Nature. 1987;327:341–3. doi: 10.1038/327341a0. [DOI] [PubMed] [Google Scholar]
- 37.Hrafnsdóttir S, Menon AK. Reconstitution and Partial Characterization of Phospholipid Flippase Activity from Detergent Extracts of the Bacillus subtilis Cell Membrane. Journal of Bacteriology. 2000;182:4198–206. doi: 10.1128/jb.182.15.4198-4206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menon AK, Watkins WE, Hrafnsdottir S. Specific proteins are required to translocate phosphatidylcholine bidirectionally across the endoplasmic reticulum. Curr Biol. 2000;10:241–52. doi: 10.1016/s0960-9822(00)00356-0. [DOI] [PubMed] [Google Scholar]
- 39.Vishwakarma RA, Vehring S, Mehta A, Sinha A, Pomorski T, Herrmann A, et al. New fluorescent probes reveal that flippase-mediated flip-flop of phosphatidylinositol across the endoplasmic reticulum membrane does not depend on the stereochemistry of the lipid. Org Biomol Chem. 2005;3:1275–83. doi: 10.1039/b500300h. [DOI] [PubMed] [Google Scholar]
- 40.Sanyal S, Menon AK. Specific transbilayer translocation of dolichol-linked oligosaccharides by an endoplasmic reticulum flippase. Proc Natl Acad Sci USA. 2009;106:767–72. doi: 10.1073/pnas.0810225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanyal S, Frank CG, Menon AK. Distinct flippases translocate glycerophospholipids and oligosaccharide diphosphate dolichols across the endoplasmic reticulum. Biochemistry. 2008;47:7937–46. doi: 10.1021/bi800723n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang Q-l, Gummadi SN, Menon AK. Chemical Modification Identifies Two Populations of Glycerophospholipid Flippase in Rat Liver ER†. Biochemistry. 2004;43:10710–8. doi: 10.1021/bi049063a. [DOI] [PubMed] [Google Scholar]
- 43.Ernst CM, Peschel A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Molecular Microbiology. 2011;80:290–9. doi: 10.1111/j.1365-2958.2011.07576.x. [DOI] [PubMed] [Google Scholar]
- 44.Ernst CM, Staubitz P, Mishra NN, Yang S-J, Hornig G, Kalbacher H, et al. The Bacterial Defensin Resistance Protein MprF Consists of Separable Domains for Lipid Lysinylation and Antimicrobial Peptide Repulsion. PLoS Pathogens. 2009;5:e1000660. doi: 10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hebecker S, Krausze J, Hasenkampf T, Schneider J, Groenewold M, Reichelt J, et al. Structures of two bacterial resistance factors mediating tRNA-dependent aminoacylation of phosphatidylglycerol with lysine or alanine. Proceedings of the National Academy of Sciences. 2015;112:10691–6. doi: 10.1073/pnas.1511167112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ernst CM, Kuhn S, Slavetinsky CJ, Krismer B, Heilbronner S, Gekeler C, et al. The Lipid-Modifying Multiple Peptide Resistance Factor Is an Oligomer Consisting of Distinct Interacting Synthase and Flippase Subunits. mBio. 2015:6. doi: 10.1128/mBio.02340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu G, Hubbell WL. Phospholipid asymmetry and transmembrane diffusion in photoreceptor disk membranes. Biochemistry. 1993;32:879–88. doi: 10.1021/bi00054a020. [DOI] [PubMed] [Google Scholar]
- 48.Hessel E, Herrmann A, Müller P, Schnetkamp PPM, Hofmann K-P. The transbilayer distribution of phospholipids in disc membranes is a dynamic equilibrium. European Journal of Biochemistry. 2000;267:1473–83. doi: 10.1046/j.1432-1327.2000.01147.x. [DOI] [PubMed] [Google Scholar]
- 49.Hessel E, Müller P, Herrmann A, Hofmann KP. Light-induced Reorganization of Phospholipids in Rod Disc Membranes. Journal of Biological Chemistry. 2001;276:2538–43. doi: 10.1074/jbc.M009061200. [DOI] [PubMed] [Google Scholar]
- 50.Menon I, Huber T, Sanyal S, Banerjee S, Barré P, Canis S, et al. Opsin is a phospholipid flippase. Current biology: CB. 2011;21:149–53. doi: 10.1016/j.cub.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ernst OP, Menon AK. Phospholipid scrambling by rhodopsin. Photochemical & Photobiological Sciences. 2015 doi: 10.1039/c5pp00195a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schenk B, Fernandez F, Waechter CJ. The ins(ide) and outs(ide) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology. 2001;11:61R–70R. doi: 10.1093/glycob/11.5.61r. [DOI] [PubMed] [Google Scholar]
- 53.Hirschberg CB, Snider MD. Topography of Glycosylation in The Rough Endoplasmic Reticulum and Golgi Apparatus. Annual Review of Biochemistry. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- 54.Frank CG, Sanyal S, Rush JS, Waechter CJ, Menon AK. Does Rft1 flip an N-glycan lipid precursor? Nature. 2008;454:E3–E4. doi: 10.1038/nature07165. [DOI] [PubMed] [Google Scholar]
- 55.Anand M, Rush JS, Ray S, Doucey M-A, Weik J, Ware FE, et al. Requirement of the Lec35 Gene for All Known Classes of Monosaccharide-P-Dolichol-dependent Glycosyl transferase Reactions in Mammals. Molecular Biology of the Cell. 2001;12:487–501. doi: 10.1091/mbc.12.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jelk J, Gao N, Serricchio M, Signorell A, Schmidt RS, Bangs JD, et al. Glycoprotein Biosynthesis in a Eukaryote Lacking the Membrane Protein Rft1. The Journal of Biological Chemistry. 2013;288:20616–23. doi: 10.1074/jbc.M113.479642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rush JS, Gao N, Lehrman MA, Matveev S, Waechter CJ. Suppression of Rft1 Expression Does Not Impair the Transbilayer Movement of Man(5)GlcNAc(2)-P-P-Dolichol in Sealed Microsomes from Yeast. The Journal of Biological Chemistry. 2009;284:19835–42. doi: 10.1074/jbc.M109.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rush JS, van Leyen K, Ouerfelli O, Wolucka B, Waechter CJ. Transbilayer movement of Glc-P-dolichol and its function as a glucosyl donor: protein-mediated transport of a water-soluble analog into sealed ER vesicles from pig brain. Glycobiology. 1998;8:1195–205. doi: 10.1093/glycob/8.12.1195. [DOI] [PubMed] [Google Scholar]
- 59.Rush JS, Waechter CJ. Transmembrane movement of a water-soluble analogue of mannosylphosphoryldolichol is mediated by an endoplasmic reticulum protein. The Journal of Cell Biology. 1995;130:529–36. doi: 10.1083/jcb.130.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanyal S, Menon AK. Stereoselective transbilayer translocation of mannosyl phosphoryl dolichol by an endoplasmic reticulum flippase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11289–94. doi: 10.1073/pnas.1002408107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rush JS, Waechter CJ. Functional Reconstitution into Proteoliposomes and Partial Purification of a Rat Liver ER Transport System for a Water-Soluble Analogue of Mannosylphosphoryldolichol. Biochemistry. 2004;43:7643–52. doi: 10.1021/bi036083o. [DOI] [PubMed] [Google Scholar]
- 62.Ruiz N. Lipid Flippases for Bacterial Peptidoglycan Biosynthesis. Lipid insights. 2015;8:21–31. doi: 10.4137/LPI.S31783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheffers D-J, Tol MB. LipidII: Just Another Brick in the Wall? PLoS Pathogens. 2015;11:e1005213. doi: 10.1371/journal.ppat.1005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohammadi T, Sijbrandi R, Lutters M, Verheul J, Martin NI, den Blaauwen T, et al. Specificity of the Transport of Lipid II by FtsW in Escherichia coli. The Journal of Biological Chemistry. 2014;289:14707–18. doi: 10.1074/jbc.M114.557371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammadi T, van Dam V, Sijbrandi R, Vernet T, Zapun A, Bouhss A, et al. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. The EMBO Journal. 2011;30:1425–32. doi: 10.1038/emboj.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruiz N. Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli. Proceedings of the National Academy of Sciences. 2008;105:15553–7. doi: 10.1073/pnas.0808352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sham L-T, Butler EK, Lebar MD, Kahne D, Bernhardt TG, Ruiz N. MurJ is the flippase of lipid-linked precursors for peptidoglycan biogenesis. Science. 2014;345:220–2. doi: 10.1126/science.1254522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meeske AJ, Sham L-T, Kimsey H, Koo B-M, Gross CA, Bernhardt TG, et al. MurJ and a novel lipid II flippase are required for cell wall biogenesis in Bacillus subtilis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6437–42. doi: 10.1073/pnas.1504967112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young KD. A flipping cell wall ferry. Science. 2014;345:139–40. doi: 10.1126/science.1256585. [DOI] [PubMed] [Google Scholar]
- 70.Islam ST, Lam JS. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Canadian Journal of Microbiology. 2014;60:697–716. doi: 10.1139/cjm-2014-0595. [DOI] [PubMed] [Google Scholar]
- 71.Rick PD, Barr K, Sankaran K, Kajimura J, Rush JS, Waechter CJ. Evidence That the wzxE Gene of Escherichia coli K-12 Encodes a Protein Involved in the Transbilayer Movement of a Trisaccharide-Lipid Intermediate in the Assembly of Enterobacterial Common Antigen. Journal of Biological Chemistry. 2003;278:16534–42. doi: 10.1074/jbc.M301750200. [DOI] [PubMed] [Google Scholar]
- 72.Coleman JA, Quazi F, Molday RS. Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport. Biochimica Et Biophysica Acta. 2013;1831:555–74. doi: 10.1016/j.bbalip.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hankins HM, Baldridge RD, Xu P, Graham TR. Role of Flippases, Scramblases and Transfer Proteins in Phosphatidylserine Subcellular Distribution. Traffic. 2015;16:35–47. doi: 10.1111/tra.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.López-Marqués RL, Poulsen LR, Bailly A, Geisler M, Pomorski TG, Palmgren MG. Structure and mechanism of ATP-dependent phospholipid transporters. Biochimica et Biophysica Acta (BBA) - General Subjects. 2015;1850:461–75. doi: 10.1016/j.bbagen.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Panatala R, Hennrich H, Holthuis JCM. Inner workings and biological impact of phospholipid flippases. Journal of Cell Science. 2015 doi: 10.1242/jcs.102715. [DOI] [PubMed] [Google Scholar]
- 76.Saito K, Fujimura-Kamada K, Furuta N, Kato U, Umeda M, Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:3418–32. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryde S, Hennrich H, Verhulst PM, Devaux PF, Lenoir G, Holthuis JC. CDC50 proteins are critical components of the human class-1 P4-ATPase transport machinery. J Biol Chem. 2010;285:40562–72. doi: 10.1074/jbc.M110.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lenoir G, Williamson P, Puts CF, Holthuis JC. Cdc50p Plays a Vital Role in the ATPase Reaction Cycle of the Putative Aminophospholipid Transporter DRS2P. J Biol Chem. 2009 doi: 10.1074/jbc.M109.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.López-Marqués RL, Poulsen LR, Hanisch S, Meffert K, Buch-Pedersen MJ, Jakobsen MK, et al. Intracellular targeting signals and lipid specificity determinants of the ALA/ALIS P4-ATPase complex reside in the catalytic ALA α-subunit. Mol Biol Cell. 2010;21:791–801. doi: 10.1091/mbc.E09-08-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen B, Jiang Y, Zeng S, Yan J, Li X, Zhang Y, et al. Endocytic sorting and recycling require membrane phosphatidylserine asymmetry maintained by TAT-1/CHAT-1. PLoSGenet. 2010;6:e1001235. doi: 10.1371/journal.pgen.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puts CF, Panatala R, Hennrich H, Tsareva A, Williamson P, Holthuis JCM. Mapping Functional Interactions in a Heterodimeric Phospholipid Pump. The Journal of Biological Chemistry. 2012;287:30529–40. doi: 10.1074/jbc.M112.371088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pérez-Victoria FJ, Sánchez-Cañete MP, Castanys S, Gamarro F. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J Biol Chem. 2006;281:23766–75. doi: 10.1074/jbc.M605214200. [DOI] [PubMed] [Google Scholar]
- 83.Kato U, Emoto K, Fredriksson C, Nakamura H, Ohta A, Kobayashi T, et al. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J Biol Chem. 2002;277:37855–62. doi: 10.1074/jbc.M205564200. [DOI] [PubMed] [Google Scholar]
- 84.Coleman JA, Molday RS. Critical role of the β-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2. J Biol Chem. 2011;286:17205–16. doi: 10.1074/jbc.M111.229419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Velden LM, Wichers CGK, van Breevoort AED, Coleman JA, Molday RS, Berger R, et al. Heteromeric interactions required for abundance and subcellular localization of human CDC50 proteins and class 1 P4-ATPases. J Biol Chem. 2010;285:40088–96. doi: 10.1074/jbc.M110.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poulsen LR, López-Marqués RL, McDowell SC, Okkeri J, Licht D, Schulz A, et al. The Arabidopsis P4-ATPase ALA3 localizes to the Golgi and requires a β-subunit to function in lipid translocation and secretory vesicle formation. Plant Cell. 2008;20:658–76. doi: 10.1105/tpc.107.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costa Sara R, Marek M, Axelsen Kristian B, Theorin L, Pomorski Thomas G, López-Marqués Rosa L. Role of post-translational modifications at the β-subunit ectodomain in complex association with a promiscuous plant P4-ATPase. Biochemical Journal. 2016;473:1605–15. doi: 10.1042/BCJ20160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci USA. 1984;81:3751–5. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daleke DL, Huestis WH. Erythrocyte morphology reflects the transbilayer distribution of incorporated phospholipids. The Journal of Cell Biology. 1989;108:1375–85. doi: 10.1083/jcb.108.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alder-Baerens N, Lisman Q, Luong L, Pomorski T, Holthuis JC. Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol Biol Cell. 2006;17:1632–42. doi: 10.1091/mbc.E05-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou X, Graham TR. Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc Natl Acad Sci USA. 2009;106:16586–91. doi: 10.1073/pnas.0904293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu P, Okkeri J, Hanisch S, Hu R-Y, Xu Q, Pomorski TG, et al. Identification of a novel mouse P4-ATPase family member highly expressed during spermatogenesis. J Cell Sci. 2009;122:2866–76. doi: 10.1242/jcs.047423. [DOI] [PubMed] [Google Scholar]
- 93.Pomorski T, Lombardi R, Riezman H, Devaux PF, van Meer G, Holthuis JCM. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–54. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riekhof WR, Voelker DR. Uptake and utilization of lyso-phosphatidylethanolamine by Saccharomyces cerevisiae. J Biol Chem. 2006;281:36588–96. doi: 10.1074/jbc.M608851200. [DOI] [PubMed] [Google Scholar]
- 95.Riekhof WR, Wu J, Gijón MA, Zarini S, Murphy RC, Voelker DR. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae: the role of P-type ATPases in transport and a broad specificity acyltransferase in acylation. J Biol Chem. 2007;282:36853–61. doi: 10.1074/jbc.M706718200. [DOI] [PubMed] [Google Scholar]
- 96.Riekhof WR, Wu J, Jones JL, Voelker DR. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2007;282:28344–52. doi: 10.1074/jbc.M705256200. [DOI] [PubMed] [Google Scholar]
- 97.Baldridge RD, Xu P, Graham TR. Type IV P-type ATPases Distinguish Mono- versus Diacyl Phosphatidylserine Using a Cytofacial Exit Gate in the Membrane Domain. J Biol Chem. 2013;288:19516–27. doi: 10.1074/jbc.M113.476911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, et al. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem. 2000;275:23378–86. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 99.Paterson JK, Renkema K, Burden L, Halleck MS, Schlegel RA, Williamson P, et al. Lipid specific activation of the murine P4-ATPase Atp8a1 (ATPase II) Biochemistry. 2006;45:5367–76. doi: 10.1021/bi052359b. [DOI] [PubMed] [Google Scholar]
- 100.Soupene E, Kemaladewi DU, Kuypers FA. ATP8A1 activity and phosphatidylserine transbilayer movement. J Receptor Ligand Channel Res. 2008;1:1–10. doi: 10.2147/jrlcr.s3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee S, Uchida Y, Wang J, Matsudaira T, Nakagawa T, Kishimoto T, et al. Transport through recycling endosomes requires EHD1 recruitment by a phosphatidylserine translocase. The EMBO Journal. 2015;34:669–88. doi: 10.15252/embj.201489703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coleman JA, Kwok MC, Molday RS. Localization, purification and functional reconstitution of the P4-ATPASE, ATP8A2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009 doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Beserra C, Garbers DL. A novel aminophospholipid transporter exclusively expressed in spermatozoa is required for membrane lipid asymmetry and normal fertilization. Dev Biol. 2004;267:203–15. doi: 10.1016/j.ydbio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Yabas M, Coupland LA, Cromer D, Winterberg M, Teoh NC, D’Rozario J, et al. Mice Deficient in the Putative Phospholipid Flippase ATP11C Exhibit Altered Erythrocyte Shape, Anemia, and Reduced Erythrocyte Life Span. The Journal of Biological Chemistry. 2014;289:19531–7. doi: 10.1074/jbc.C114.570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yabas M, Jing W, Shafik S, Bröer S, Enders A. ATP11C Facilitates Phospholipid Translocation across the Plasma Membrane of All Leukocytes. PLoS ONE. 2016;11:e0146774. doi: 10.1371/journal.pone.0146774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–8. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 107.Segawa K, Kurata S, Nagata S. Human Type IV P-type ATPases That Work as Plasma Membrane Phospholipid Flippases and Their Regulation by Caspase and Calcium. Journal of Biological Chemistry. 2016;291:762–72. doi: 10.1074/jbc.M115.690727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takada N, Takatsu H, Miyano R, Nakayama K, Shin H-W. ATP11C mutation is responsible for the defect in phosphatidylserine uptake in UPS-1 cells. Journal of Lipid Research. 2015;56:2151–7. doi: 10.1194/jlr.M062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, et al. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–78. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 110.Ray NB, Durairaj L, Chen BB, McVerry BJ, Ryan AJ, Donahoe M, et al. Dynamic regulation of cardiolipin by the lipid pump, ATP8b1, determines the severity of lung injury in experimental bacterial pneumonia. Nature medicine. 2010;16:1120–7. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takatsu H, Tanaka G, Segawa K, Suzuki J, Nagata S, Nakayama K, et al. Phospholipid Flippase Activities and Substrate Specificities of Human Type IV P-type ATPases Localized to the Plasma Membrane. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M114.593012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naito T, Takatsu H, Miyano R, Takada N, Nakayama K, Shin H-W. Phospholipid Flippase ATP10A Translocates Phosphatidylcholine and Is Involved in Plasma Membrane Dynamics. Journal of Biological Chemistry. 2015;290:15004–17. doi: 10.1074/jbc.M115.655191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen R, Brady E, McIntyre TM. Human TMEM30a Promotes Uptake of Antitumor and Bioactive Choline Phospholipids into Mammalian Cells. The Journal of Immunology. 2011;186:3215–25. doi: 10.4049/jimmunol.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- 115.Poulsen LR, Lopez-Marques RL, Pedas PR, McDowell SC, Brown E, Kunze R, et al. A phospholipid uptake system in the model plant Arabidopsis thaliana. Nat Commun. 2015;6 doi: 10.1038/ncomms8649. [DOI] [PubMed] [Google Scholar]
- 116.Lopez-Marques R, Theorin L, Palmgren M, Pomorski T. P4-ATPases: lipid flippases in cell membranes. Pflugers Arch - Eur J Physiol. 2014;466:1227–40. doi: 10.1007/s00424-013-1363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Higgins CF, Gottesman MM. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992;17:18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- 118.Linton KJ, Higgins CF. The Escherichia coli ATP-binding cassette (ABC) proteins. Molecular Microbiology. 1998;28:5–13. doi: 10.1046/j.1365-2958.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 119.Kemp S, Theodoulou FL, Wanders RJA. Mammalian peroxisomal ABC transporters: from endogenous substrates to pathology and clinical significance. British Journal of Pharmacology. 2011;164:1753–66. doi: 10.1111/j.1476-5381.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 Gene Mutations in Newborns with Fatal Surfactant Deficiency. New England Journal of Medicine. 2004;350:1296–303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 121.Sakai K, Akiyama M, Sugiyama-Nakagiri Y, McMillan JR, Sawamura D, Shimizu H. Localization of ABCA12 from Golgi apparatus to lamellar granules in human upper epidermal keratinocytes. Experimental Dermatology. 2007;16:920–6. doi: 10.1111/j.1600-0625.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 122.Whitsett JA, Wert SE, Weaver TE. Alveolar Surfactant Homeostasis and the Pathogenesis of Pulmonary Disease. Annual review of medicine. 2010;61:105–19. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ile KE, Davis W, Jr, Boyd JT, Soulika AM, Tew KD. Identification of a novel first exon of the human ABCA2 transporter gene encoding a unique N-terminus. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2004;1678:22–32. doi: 10.1016/j.bbaexp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 124.Kubo Y, Sekiya S, Ohigashi M, Takenaka C, Tamura K, Nada S, et al. ABCA5 Resides in Lysosomes, and ABCA5 Knockout Mice Develop Lysosomal Disease-Like Symptoms. Molecular and Cellular Biology. 2005;25:4138–49. doi: 10.1128/MCB.25.10.4138-4149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proceedings of the National Academy of Sciences. 2011;108:19719–24. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tarr PT, Edwards PA. ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. Journal of Lipid Research. 2008;49:169–82. doi: 10.1194/jlr.M700364-JLR200. [DOI] [PubMed] [Google Scholar]
- 127.Romsicki Y, Sharom FJ. Phospholipid flippase activity of the reconstituted P-glycoprotein multidrug transporter. Biochemistry. 2001;40:6937–47. doi: 10.1021/bi0024456. [DOI] [PubMed] [Google Scholar]
- 128.Smit JJM, Schinkel AH, Oude Elferink RPJ, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine MDR2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–62. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 129.Huang Z, Chang X, Riordan JR, Huang Y. Fluorescent modified phosphatidylcholine floppase activity of reconstituted multidrug resistance-associated protein MRP1. Biochim Biophys Acta. 2004;1660:155–63. doi: 10.1016/j.bbamem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 130.Kamp D, Haest CW. Evidence for a role of the multidrug resistance protein (MRP) in the outward translocation of NBD-phospholipids in the erythrocyte membrane. Biochimica Et Biophysica Acta. 1998;1372:91–101. doi: 10.1016/s0005-2736(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 131.Raggers RJ, van Helvoort A, Evers R, van Meer G. The human multidrug resistance protein MRP1 translocates sphingolipid analogs across the plasma membrane. J Cell Sci. 1999;112(Pt 3):415–22. doi: 10.1242/jcs.112.3.415. [DOI] [PubMed] [Google Scholar]
- 132.Dekkers DW, Comfurius P, van Gool RG, Bevers EM, Zwaal RF. Multidrug resistance protein 1 regulates lipid asymmetry in erythrocyte membranes. Biochemical Journal. 2000;350:531–5. [PMC free article] [PubMed] [Google Scholar]
- 133.Quazi F, Molday RS. Differential Phospholipid Substrates and Directional Transport by ATP Binding Cassette Proteins ABCA1, ABCA7, and ABCA4 and Disease-causing Mutants. J Biol Chem. 2013 doi: 10.1074/jbc.M113.508812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Decottignies A, Grant AM, Nichols JW, de Wet H, McIntosh DB, Goffeau A. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem. 1998;273:12612–22. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- 135.Gulshan K, Moye-Rowley WS. Vacuolar import of phosphatidylcholine requires the ATP-binding cassette transporter Ybt1. Traffic. 2011;12:1257–68. doi: 10.1111/j.1600-0854.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, et al. Plant Cuticular Lipid Export Requires an ABC Transporter. Science. 2004;306:702–4. doi: 10.1126/science.1102331. [DOI] [PubMed] [Google Scholar]
- 137.Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, et al. Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion†. The Plant Journal. 2007;52:485–98. doi: 10.1111/j.1365-313X.2007.03252.x. [DOI] [PubMed] [Google Scholar]
- 138.Luo B, Xue X-Y, Hu W-L, Wang L-J, Chen X-Y. An ABC Transporter Gene of Arabidopsis thaliana, AtWBC11, is Involved in Cuticle Development and Prevention of Organ Fusion. Plant and Cell Physiology. 2007;48:1790–802. doi: 10.1093/pcp/pcm152. [DOI] [PubMed] [Google Scholar]
- 139.Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Höfer R, et al. The Arabidopsis DESPERADO/AtWBC11 Transporter Is Required for Cutin and Wax Secretion. Plant Physiology. 2007;145:1345–60. doi: 10.1104/pp.107.105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ukitsu H, Kuromori T, Toyooka K, Goto Y, Matsuoka K, Sakuradani E, et al. Cytological and Biochemical Analysis of COF1, an Arabidopsis Mutant of an ABC Transporter Gene. Plant and Cell Physiology. 2007;48:1524–33. doi: 10.1093/pcp/pcm139. [DOI] [PubMed] [Google Scholar]
- 141.Quazi F, Lenevich S, Molday RS. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun. 2012;3:925. doi: 10.1038/ncomms1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA, et al. Cystic Fibrosis Transmembrane Regulator Regulates Uptake of Sphingoid Base Phosphates and Lysophosphatidic Acid: MODULATION OF CELLULAR ACTIVITY OF SPHINGOSINE 1-PHOSPHATE. Journal of Biological Chemistry. 2001;276:35258–64. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 143.Lorenz RT, Parks LW. Involvement of heme components in sterol metabolism of Saccharomyces cerevisiae. Lipids. 1991;26:598–603. doi: 10.1007/BF02536423. [DOI] [PubMed] [Google Scholar]
- 144.Wilcox LJ, Balderes DA, Wharton B, Tinkelenberg AH, Rao G, Sturley SL. Transcriptional Profiling Identifies Two Members of the ATP-binding Cassette Transporter Superfamily Required for Sterol Uptake in Yeast. Journal of Biological Chemistry. 2002;277:32466–72. doi: 10.1074/jbc.M204707200. [DOI] [PubMed] [Google Scholar]
- 145.Li Y, Prinz WA. ATP-binding Cassette (ABC) Transporters Mediate Nonvesicular, Raft-modulated Sterol Movement from the Plasma Membrane to the Endoplasmic Reticulum. Journal of Biological Chemistry. 2004;279:45226–34. doi: 10.1074/jbc.M407600200. [DOI] [PubMed] [Google Scholar]
- 146.Reiner S, Micolod D, Zellnig G, Schneiter R. A Genomewide Screen Reveals a Role of Mitochondria in Anaerobic Uptake of Sterols in Yeast. Molecular Biology of the Cell. 2006;17:90–103. doi: 10.1091/mbc.E05-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagi M, Tanabe K, Ueno K, Nakayama H, Aoyama T, Chibana H, et al. The Candida glabrata sterol scavenging mechanism, mediated by the ATP-binding cassette transporter Aus1p, is regulated by iron limitation. Molecular Microbiology. 2013;88:371–81. doi: 10.1111/mmi.12189. [DOI] [PubMed] [Google Scholar]
- 148.Marek M, Silvestro D, Fredslund MD, Andersen TG, Pomorski TG. Serum albumin promotes ATP-binding cassette transporter-dependent sterol uptake in yeast. FEMS Yeast Research. 2014;14:1223–33. doi: 10.1111/1567-1364.12219. [DOI] [PubMed] [Google Scholar]
- 149.Wang Z, Anderson NS, Benning C. The Phosphatidic Acid Binding Site of the Arabidopsis Trigalactosyldiacylglycerol 4 (TGD4) Protein Required for Lipid Import into Chloroplasts. The Journal of Biological Chemistry. 2013;288:4763–71. doi: 10.1074/jbc.M112.438986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roston RL, Gao J, Murcha MW, Whelan J, Benning C. TGD1, -2, and -3 Proteins Involved in Lipid Trafficking Form ATP-binding Cassette (ABC) Transporter with Multiple Substrate-binding Proteins. The Journal of Biological Chemistry. 2012;287:21406–15. doi: 10.1074/jbc.M112.370213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou Z, White KA, Polissi A, Georgopoulos C, Raetz CRH. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J Biol Chem. 1998;273:12466–75. doi: 10.1074/jbc.273.20.12466. [DOI] [PubMed] [Google Scholar]
- 152.Doerrler WT, Reedy MC, Raetz CR. An Escherichia coli mutant defective in lipid export. J Biol Chem. 2001;276:11461–4. doi: 10.1074/jbc.C100091200. [DOI] [PubMed] [Google Scholar]
- 153.Eckford PD, Sharom FJ. The reconstituted Escherichia coli MsbA protein displays lipid flippase activity. Biochemical Journal. 2010;429:195–203. doi: 10.1042/BJ20100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tefsen B, Geurtsen J, Beckers F, Tommassen J, de Cock H. Lipopolysaccharide Transport to the Bacterial Outer Membrane in Spheroplasts. Journal of Biological Chemistry. 2005;280:4504–9. doi: 10.1074/jbc.M409259200. [DOI] [PubMed] [Google Scholar]
- 155.Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, et al. N-Linked Glycosylation in Campylobacter jejuni and Its Functional Transfer into E. coli. Science. 2002;298:1790–3. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 156.Young NM, Brisson J-R, Kelly J, Watson DC, Tessier L, Lanthier PH, et al. Structure of the N-Linked Glycan Present on Multiple Glycoproteins in the Gram-negative Bacterium, Campylobacter jejuni. Journal of Biological Chemistry. 2002;277:42530–9. doi: 10.1074/jbc.M206114200. [DOI] [PubMed] [Google Scholar]
- 157.Alaimo C, Catrein I, Morf L, Marolda CL, Callewaert N, Valvano MA, et al. Two distinct but interchangeable mechanisms for flipping of lipid-linked oligosaccharides. EMBO J. 2006;25:967–76. doi: 10.1038/sj.emboj.7601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perez C, Gerber S, Boilevin J, Bucher M, Darbre T, Aebi M, et al. Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature. 2015;524:433–8. doi: 10.1038/nature14953. [DOI] [PubMed] [Google Scholar]
- 159.Verchere A, Menon AK. Structural biology: Lipid gymnastics. Nature. 2015;524:420–2. doi: 10.1038/nature15202. [DOI] [PubMed] [Google Scholar]
- 160.Lehrman MA. Flipping a Lipid-Linked Oligosaccharide? You Must Whip It! Trends in Biochemical Sciences. 2015;40:715–7. doi: 10.1016/j.tibs.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bevers EM, Williamson PL. Phospholipid scramblase: An update. FEBS Letters. 2010;584:2724–30. doi: 10.1016/j.febslet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 162.Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proceedings of the National Academy of Sciences. 2012;109:8145–8. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-Related Protein 8 and CED-8 Promote Phosphatidylserine Exposure in Apoptotic Cells. Science. 2013;341:403–6. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- 164.Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013;288:13305–16. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–8. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 166.Suzuki J, Imanishi E, Nagata S. Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M114.583419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Malvezzi M, Chalat MN, Janjusevic R, Picollo A, Terashima H, Menon AK, et al. Ca(2+)-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nature communications. 2013;4:2367. doi: 10.1038/ncomms3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–12. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 169.Yu K, Whitlock JM, Lee K, Ortlund EA, Yuan Cui Y, Hartzell HC. Identification of a lipid scrambling domain in ANO6/TMEM16F. eLife. 2015;4:e06901. doi: 10.7554/eLife.06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kälin N, Fernandes J, Hrafnsdóttir S, van Meer G. Natural phosphatidylcholine is actively translocated across the plasma membrane to the surface of mammalian cells. J Biol Chem. 2004;279:33228–36. doi: 10.1074/jbc.M401751200. [DOI] [PubMed] [Google Scholar]
- 172.Dekkers DWC, Comfurius P, Schroit AJ, Bevers EM, Zwaal RFA. Transbilayer movement of NBD-labeled phospholipids in red blood cell membranes: outward-directed transport by the multidrug resistance protein 1 (MRP1) Biochemistry. 1998;37:14833–7. doi: 10.1021/bi981011w. [DOI] [PubMed] [Google Scholar]
- 173.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, et al. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–17. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 174.Heinrich R, Brumen M, Jaeger A, Müller P, Herrmann A. Modelling of Phospholipid Translocation in the Erythrocyte Membrane: A Combined Kinetic and Thermodynamic Approach. Journal of Theoretical Biology. 1997;185:295–312. doi: 10.1006/jtbi.1996.0325. [DOI] [PubMed] [Google Scholar]
- 175.Frickenhaus S, Heinrich R. Kinetic and thermodynamic aspects of lipid translocation in biological membranes. Biophysical Journal. 1999;76:1293–309. doi: 10.1016/S0006-3495(99)77292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, et al. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. The Journal of Cell Biology. 2011;194:257–75. doi: 10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The Surface of Lipid Droplets Is a Phospholipid Monolayer with a Unique Fatty Acid Composition. Journal of Biological Chemistry. 2002;277:44507–12. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 178.Holthuis JCM, Menon AK. Lipid landscapes and pipelines in membrane homeostasis. Nature. 2014;510:48–57. doi: 10.1038/nature13474. [DOI] [PubMed] [Google Scholar]
- 179.Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, et al. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER–plasma membrane contacts. Science. 2015;349:428–32. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, et al. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–61. doi: 10.1038/nature12430. [DOI] [PubMed] [Google Scholar]
- 181.Moser von Filseck J, Čopič A, Delfosse V, Vanni S, Jackson CL, Bourguet W, et al. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349:432–6. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]
- 182.Hubbell WL. Transbilayer coupling mechanism for the formation of lipid asymmetry in biological membranes. Application to the photoreceptor disc membrane. Biophysical Journal. 1990;57:99–108. doi: 10.1016/S0006-3495(90)82510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou X, Sebastian TT, Graham TR. Auto-inhibition of Drs2p, a Yeast Phospholipid Flippase, by Its Carboxyl-terminal Tail. The Journal of Biological Chemistry. 2013;288:31807–15. doi: 10.1074/jbc.M113.481986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roelants FM, Baltz AG, Trott AE, Fereres S, Thorner J. A protein kinase network regulates the function of aminophospholipid flippases. Proc Natl Acad Sci USA. 2010;107:34–9. doi: 10.1073/pnas.0912497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Coleman JA, Zhu X, Djajadi HR, Molday LL, Smith RS, Libby RT, et al. Phospholipid flippase ATP8A2 is required for normal visual and auditory function and photoreceptor and spiral ganglion cell survival. Journal of Cell Science. 2014;127:1138–49. doi: 10.1242/jcs.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Matsuzaki K, Murase O, Fujii N, Miyajima K. An Antimicrobial Peptide, Magainin 2, Induced Rapid Flip-Flop of Phospholipids Coupled with Pore Formation and Peptide Translocation. Biochemistry. 1996;35:11361–8. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 187.Illya G, Deserno M. Coarse-Grained Simulation Studies of Peptide-Induced Pore Formation. Biophysical Journal. 2008;95:4163–73. doi: 10.1529/biophysj.108.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gurtovenko AA, Anwar J, Vattulainen I. Defect-Mediated Trafficking across Cell Membranes: Insights from in Silico Modeling. Chemical Reviews. 2010;110:6077–103. doi: 10.1021/cr1000783. [DOI] [PubMed] [Google Scholar]
- 189.Gurtovenko AA, Vattulainen I. Lipid transmembrane asymmetry and intrinsic membrane potential: two sides of the same coin. J Am Chem Soc. 2007;129:5358–9. doi: 10.1021/ja070949m. [DOI] [PubMed] [Google Scholar]
- 190.Vestergaard AL, Coleman JA, Lemmin T, Mikkelsen SA, Molday LL, Vilsen B, et al. Critical roles of isoleucine-364 and adjacent residues in a hydrophobic gate control of phospholipid transport by the mammalian P4-ATPase ATP8A2. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1334–E43. doi: 10.1073/pnas.1321165111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Obara K, Miyashita N, Xu C, Toyoshima I, Sugita Y, Inesi G, et al. Structural role of countertransport revealed in Ca2+ pump crystal structure in the absence of Ca2+ Proc Natl Acad Sci USA. 2005;102:14489–96. doi: 10.1073/pnas.0506222102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Baldridge RD, Graham TR. Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases. Proc Natl Acad Sci USA. 2012;109:E290–E8. doi: 10.1073/pnas.1115725109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ward AB, Guvench O, Hills RD., Jr Coarse grain lipid-protein molecular interactions and diffusion with MsbA flippase. Proteins. 2012;80:2178–90. doi: 10.1002/prot.24108. [DOI] [PubMed] [Google Scholar]
- 194.Pomorski T, Menon AK. Lipid flippases and their biological functions. Cell MolLife Sci. 2006;63:2908–21. doi: 10.1007/s00018-006-6167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. ELECTROPHORESIS. 1997;18:2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 196.Larsen J, Hatzakis NS, Stamou D. Observation of Inhomogeneity in the Lipid Composition of Individual Nanoscale Liposomes. Journal of the American Chemical Society. 2011;133:10685–7. doi: 10.1021/ja203984j. [DOI] [PubMed] [Google Scholar]
- 197.Liu J, Conboy JC. 1,2-Diacyl-Phosphatidylcholine Flip-Flop Measured Directly by Sum-Frequency Vibrational Spectroscopy. Biophysical Journal. 2005;89:2522–32. doi: 10.1529/biophysj.105.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Anglin TC, Conboy JC. Lateral Pressure Dependence of the Phospholipid Transmembrane Diffusion Rate in Planar-Supported Lipid Bilayers. Biophysical Journal. 2008;95:186–93. doi: 10.1529/biophysj.107.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Anglin TC, Cooper MP, Li H, Chandler K, Conboy JC. Free Energy and Entropy of Activation for Phospholipid Flip-Flop in Planar Supported Lipid Bilayers. The Journal of Physical Chemistry B. 2010;114:1903–14. doi: 10.1021/jp909134g. [DOI] [PubMed] [Google Scholar]
- 200.Kiessling V, Crane JM, Tamm LK. Transbilayer Effects of Raft-Like Lipid Domains in Asymmetric Planar Bilayers Measured by Single Molecule Tracking. Biophysical Journal. 2006;91:3313–26. doi: 10.1529/biophysj.106.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khan A, Ducker WA, Mao M. Flip-Flop in Adsorbed Bilayers. The Journal of Physical Chemistry B. 2006;110:23365–72. doi: 10.1021/jp063498l. [DOI] [PubMed] [Google Scholar]
- 202.Jacquot A, Montigny C, Hennrich H, Barry R, le Maire M, Jaxel C, et al. Phosphatidylserine stimulation of Drs2p.Cdc50p lipid translocase dephosphorylation is controlled by phosphatidylinositol-4-phosphate. J Biol Chem. 2012;287:13249–61. doi: 10.1074/jbc.M111.313916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Groen A, Romero MR, Kunne C, Hoosdally SJ, Dixon PH, Wooding C, et al. Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology. 2011;141:1927–37. doi: 10.1053/j.gastro.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 204.Helle SCJ, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B. Organization and function of membrane contact sites. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2013;1833:2526–41. doi: 10.1016/j.bbamcr.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 205.Wang Z, Benning C. Chloroplast lipid synthesis and lipid trafficking through ER–plastid membrane contact sites. Biochemical Society Transactions. 2012;40:457–63. doi: 10.1042/BST20110752. [DOI] [PubMed] [Google Scholar]
- 206.Janssen MJFW, Koorengevel MC, de Kruijff B, de Kroon AIPM. Transbilayer movement of phosphatidylcholine in the mitochondrial outer membrane of Saccharomyces cerevisiae is rapid and bidirectional. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1999;1421:64–76. doi: 10.1016/s0005-2736(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 207.Gallet PF, Zachowski A, Julien R, Fellmann P, Devaux PF, Maftah A. Transbilayer movement and distribution of spin-labelled phospholipids in the inner mitochondrial membrane. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1999;1418:61–70. doi: 10.1016/s0005-2736(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 208.Rajasekharan A, Gummadi SN. Flip-Flop of Phospholipids in Proteoliposomes Reconstituted from Detergent Extract of Chloroplast Membranes: Kinetics and Phospholipid Specificity. PLoS ONE. 2011;6:e28401. doi: 10.1371/journal.pone.0028401. [DOI] [PMC free article] [PubMed] [Google Scholar]


