Abstract
Background
Organophosphate compounds are commonly used in residential furniture, electronics, and baby products as flame retardants and are also used in other consumer products as plasticizers. Although the levels of exposure biomarkers are generally higher among children and decrease with age, relatively little is known about the individual characteristics associated with higher levels of exposure. Here, we investigate urinary metabolites of several organophosphate flame retardants (PFRs) in a cohort of pregnant women to evaluate patterns of exposure.
Methods
Pregnant North Carolina women (n=349) provided information on their individual characteristics (e.g. age and body mass index (BMI)) as a part of the Pregnancy Infection and Nutrition Study (2002–2005). Women also provided second trimester urine samples in which six PFR metabolites were measured using mass spectrometry methods.
Results
PFR metabolites were detected in every urine sample, with BDCIPP, DHPH, ip-PPP and BCIPHIPP detected in >80% of samples. Geometric mean concentrations were higher than what has been reported previously for similarly-timed cohorts. Women with higher pre-pregnancy BMI tended to have higher levels of urinary metabolites. For example, those classified as obese at the start of pregnancy had ip-PPP levels that were 1.52 times as high as normal weight range women (95% confidence interval: 1.23, 1.89). Women without previous children also tended to have higher urinary levels of DPHP, but lower levels of ip-PPP. In addition, we saw strong evidence of seasonal trends in metabolite concentrations (e.g. higher DPHP, BDCIPP, and BCIPHIPP in summer, and evidence of increasing ip-PPP between 2002 and 2005).
Conclusions
Our results indicate ubiquitous exposure to PFRs among NC women in the early 2000s. Additionally, our work suggests that individual characteristics are related to exposure and that temporal variation, both seasonal and annual, may exist.
Keywords: organophosphate flame retardants (PFRs), pregnancy, exposure
1. Introduction
Flame retardant chemicals have been added to a variety of household products to meet flammability standards for decades. Until the mid-2000s, polybrominated diphenyl ethers (PBDEs) accounted for a large proportion of flame retardants used in household products including polyurethane foam and electronics; however, regulatory action and concern over the persistence, bioaccumulation, and toxicity of PBDEs led to an increased use of alternative flame retardants (Stapleton et al. 2012b; van der Veen and de Boer 2012). Organophosphate flame retardants (PFRs) are now among the most commonly used PBDE alternatives in industries that manufacture residential furniture, electronics (e.g. TVs) and baby products (e.g. nursing pillows). They are commonly added to flame retardant mixtures, such as Firemaster® 550 (FM550), and to other consumer products as plasticizers (Ballesteros-Gomez et al. 2014; Fang et al. 2013; Patisaul et al. 2013; Stapleton et al. 2008; Stapleton et al. 2009; Stapleton et al. 2011).
PFRs have been detected with high frequency in recent studies of home, office, and automobile dust, demonstrating that they leach from products and suggesting ubiquitous exposure [e.g. (Brandsma et al. 2013; Brommer and Harrad 2015; Cao et al. 2014; Carignan et al. 2013; Cristale et al. 2016; Hoffman et al. 2015b; Stapleton et al. 2008; Stapleton et al. 2009; Stapleton et al. 2011)]. Additionally, an accumulating body of research indicates that the vast majority of U.S. adults (>90%) have detectable levels of PFR metabolites in their urine, and similar detection frequencies have been reported in Canadian, European, Asian and Australian populations (e.g. (Butt et al. 2014 and 2016; Cequier et al. 2015; Dodson et al. 2014; Hoffman et al. 2014; Hoffman et al. 2015a; Hoffman et al. 2015b; Kosarac et al. 2016; Meeker et al. 2013a; Van den Eede et al. 2015; Su et al. 2015)). Although data suggest that metabolite levels vary by age, with younger individuals shown to have higher exposures (e.g. Butt et al. 2014 and 2016; Hoffman et al. 2015b; Van den Eede et al. 2015), the individual characteristic and behaviors associated with higher levels of exposure are not well understood.
In our present work we investigate the levels of exposure in a large pregnancy cohort, and additionally assess factors associated with higher levels of PFR metabolites in urine samples. We focus on widely used PFRs and six metabolites (Figure 1). Identifying factors contributing to higher levels of exposure to these compounds is particularly important because certain PFRs can disrupt normal endocrine function (Liu et al. 2012; Wang et al. 2013; Meeker et al. 2013a) and 2013b), are carcinogenic (Faust and August 2011; Gold et al. 1978), neurotoxic (Dishaw et al. 2011), reproductive toxicants (Meeker et al. 2013a and 2013b; Liu et al. 2013; Farhat et al. 2013), and potentially adipogenic (Patisaul et al. 2013; Pillai et al. 2014). In addition, recent data suggests that PFRs may have similar or greater toxicity than their PBDE predecessors, particularly with respect to neurodevelopmental outcomes (Behl et al. 2015; Behl. et al 2016).
Figure 1.
Chemical structures of urinary PFR metabolites monitored. TPHP metabolite = DPHP; Isopropyl-phenyl diphenyl phosphate metabolite = ip-PPP; Tertbutyl-phenyl diphenyl phosphate metabolite= tb-PPP; TDCIPP metabolite = BDCIPP; and tris(1-chloro-2-isopropyl) phosphate (TCIPP metabolites) = BCIPP and BCIPHIPP.
2. Methods
2.1 Study Population
The Pregnancy Infection and Nutrition (PIN) Study enrolled a cohort of central North Carolina women in early pregnancy and conducted follow-up through delivery (PIN 2012). PIN women were recruited from the University of North Carolina prenatal care clinic, and delivered their infants at University of North Carolina hospitals between 2001 and 2006 (n = 2009; PIN phase 3). This analysis is part of a larger project investigating the impacts of exposure to environmental chemicals on children’s growth. This sample is limited to 349 mothers recruited during the final four years of the cohort study, whose children had growth measurements collected at multiple time points (infants born 2002–2005). Self-administered questionnaires, telephone interviews, and home visits were used to collect pregnancy and postpartum health and lifestyle information throughout pregnancy and after the child’s birth (PIN 2012). All study protocols were approved by the institutional review board at the University of North Carolina at Chapel Hill and all mothers provided informed consent prior to completing any study activities.
2.2 Urine Collection and Analysis
During the late-second or early-third trimester, PIN women collected a spot urine sample in a standard urine collection cup. The time and date of collection was recorded, and urine samples were aliquoted into polyethylene storage tubes and frozen at −80° C until analysis.
Urine samples were extracted using enzyme deconjugation and solid phase extraction (SPE) techniques as previously described (Van den Eede et al. 2013) but adapted for 5 ml of urine (Butt et al. 2016). In brief, samples were thawed, 5 ml of urine were aliquoted into a clean glass test tube, the internal standard mixture was spiked (10 ng of d10-BDCIPP, 8.8 ng of d10-DPHP; 25 ng of d12-TCEP) and samples vortexed. After pH adjustment with sodium acetate (1.75 ml of 1 M sodium acetate, pH 5), the enzyme solution was added (250 µl of 1000 units/ml µ-glucuronidase, 33 units/ml sulfatase in 0.2 M sodium acetate buffer), and the samples were vortexed and incubated overnight in a 37°C water bath. Samples were extracted and cleaned using SPE with a StrataX-AW (60 mg, 3 ml) column, and were reconstituted in 500 ul of 1:1 water:methanol, as previously described (Butt et al. 2016). Internal standard recovery was quantified by spiking with 13C2-DPHP.
Extracts were analyzed using electrospray ionization (ESI) liquid chromatography tandem mass spectrometry (LC-MS/MS) with a Phenomenex Luna C18 column on an Agilent 1100 series LC and an Agilent 6410B tandem mass spectrometer as previously described (Butt et al. 2014 and 2016). Data were acquired under multiple reaction monitoring conditions using optimized parameters. Analyte responses were normalized to internal standard responses. BCIPP and BDCIPP were normalized using d10-BDCIPP, DPHP, ip-PPP and tb-PPP were normalized using d10-DPHP and BCIPHIPP was normalized using d12-TCEP. The mean recovery of the mass-labelled standards in the urine samples (n=349) was 97% (standard error (SE) = 2.1%) for d10-DPHP, 98% (SE=3.0%) for d10-BDCIPP and 34% (SE=1.0%) for d12-TCEP. The low d12-TCEP recovery is partially due to quantification inaccuracies resulting from matrix suppression since the d12-TCEP recovery was 55–73% in the blank samples (clean water only). Analyte values were blank corrected using the mean laboratory blank levels. Method detection limits (MDLs) were calculated as three times the standard deviation of the laboratory blanks, normalized to the average urine volume (3 ml). Sample were assessed in three batches and MDLs were calculated separately for each batch (MDLs: 136–333 pg/ml for BCIPP, 127–243 pg/ml for DPHP, 60–197 pg/ml for BDCIPP, 37–177 pg/ml for ip-PPP, 213–846 pg/ml for tb-PPP and 3–33 pg/ml for BCIPHIPP.
Specific gravity (SG) was measured in each urine sample prior to analysis using a digital handheld refractometer (Atago). Relative method accuracy was assessed by measuring PFR metabolites in SRM 3673 (n=3). Specific gravity-normalized concentrations were 1.56 ng/ml (SE=0.09) for BDCIPP, 0.44 ng/ml (0.02) for BCIPHIPP, 0.65 ng/ml (0.08) for DPHP and 6.0 ng/ml (0.30) for ip-PPP. These values are similar to those previously reported by our lab for SRM 3673 with the exception of ip-PPP, which was 0.6-times those of the current study (Hammel et al. 2016). BCPP and tb-PPP were not detected in the SRM.
To investigate the impacts of differences in urine dilution on results, we conducted analyses of urinary metabolites using raw PFR metabolite measures as well as using SG-corrected concentrations (Boeniger et al. 1993). Corrected and uncorrected concentrations were very highly correlated [Spearman correlations (rs) >0.82 for all metabolites] and results were very similar using both methods. Here we present only the results obtained with the specific gravity corrected concentrations to facilitate comparison with previous studies.
2.3 Statistical Analysis
Preliminary analyses indicated that urinary PFR metabolite levels were not normally distributed and were positively skewed (i.e. skewed right). Accordingly, we used non-parametric analyses or log10-transformed metabolite concentrations in statistical analyses. We calculated descriptive statistics for each PFR metabolite and conducted additional analyses for those that were detected in >70% of urine samples. For these metabolites, samples with concentrations below the method limit of detection (MDL) were replaced with the MDL/2 prior to adjustment for specific gravity (see below). Spearman correlations (rs) were used to assess relationships between urinary PFR metabolites. We used linear regression models with log10-transformed metabolite levels as the outcome to assess maternal predictors of PFR levels. Predictive analyses were conducted only for metabolites detected in at least 80% of the samples. Beta coefficients from these models were exponentiated (10β) for interpretation and represent the multiplicative change relative to the reference category for categorical variables, and the multiplicative change for a one unit increase for continuous variables. In addition to univariate models, we conducted multivariate regression analyses including all variables of interest [age (≤25, 26–30, 31–35 and ≥36), race (white and non-white), education (≤15and ≥16, pre-pregnancy BMI (underweight, normal range, overweight and obese), parity(0 and≥1), gestational duration at the time of sample collection (24–26, 27–28 and 29–30), date of sample collection (continuous measure scaled to years) and season of collection (December–February, March–May, June–August, September–November)] to obtain the independent effect of each variable. Unadjusted and adjusted models produced very similar results. Here, we present adjusted analyses; however, unadjusted results are shown in Supplemental Table 1.
All statistical analyses were conducted in SAS (version 9.4; Cary, NC) and statistical significance was set at alpha=0.05.
3. Results and Discussion
Women averaged 29.6 years of age at the time of enrollment and were highly educated, with nearly 70% having a college education (Table 1). Women included in the present study (Table 1) were more likely to be white, have higher educational attainment, and be older than mothers in the larger PIN cohort (Daniels et al. 2010; PIN 2012). Nearly half of the participants were primiparous (47.6%); and the majority (55.6%) had a BMI within the normal range at the start of their pregnancy. Urine samples were collected between 24 and 30 weeks gestation, and the average collection time was gestational week 27.
Table 1.
Selected characteristics of 349 pregnant North Carolina women (2002–2005).
| N | % | ||
|---|---|---|---|
| Total | 349 | 100.0 | |
| Age | |||
| ≤25 | 76 | 21.8 | |
| 26–30 | 126 | 36.1 | |
| 31–35 | 107 | 30.7 | |
| ≥36 | 40 | 11.5 | |
| Race | |||
| white | 278 | 79.7 | |
| non-white | 71 | 20.3 | |
| Education (years) | |||
| ≤15 | 106 | 30.4 | |
| ≥16 | 243 | 69.6 | |
| Parity | |||
| 0 | 166 | 47.6 | |
| ≥1 | 183 | 52.4 | |
| Pre-pregnancy BMI | |||
| Underweight | 46 | 13.2 | |
| Normal range | 194 | 55.6 | |
| Overweight | 42 | 12.0 | |
| Obese | 67 | 19.2 | |
|
Gestational weeks at urine sample collection |
|||
| 24–26 weeks | 72 | 20.6 | |
| 27–28 weeks | 139 | 39.8 | |
| 29–30 weeks | 138 | 39.5 | |
|
Season of urine sample collection |
|||
| Winter (Dec – Feb) | 81 | 23.2 | |
| Spring (Mar – May) | 90 | 25.8 | |
| Summer (Jun – Aug) | 94 | 26.9 | |
| Fall (Sep – Nov) | 84 | 24.1 | |
3.1 PRF Metabolite Levels
BDCIPP, DPHP, ip-PPP and BCIPHIPP were detected frequently in urine samples (92.8%, 83.7%, 99.4% and 98.3%, respectively), and concentrations varied considerably between women. Among these compounds, concentration ranged from non-detectable to approximately 100 ng/mL for all analytes (Table 2). BCIPP and tb-PPP were detected less frequently (48.7% and 2.0% detect, respectively). Correlations between DPHP and other PFR metabolites were generally small, but statistically significant (rs from 0.11 for ip-PPP to 0.31 for BDCIPP, with p<0.05 for both). BDCIPP was additionally correlated with BCIPHIPP (rs=0.21, p=0.001) but not ip-PPP, and BCIPHIPP was not correlated with ip-PPP among women in our cohort. The full correlation matrix is shown in Supplemental Table 2. While some overlap in patterns of use or exposure pathways for the parent PFRs is probable, the small magnitude of correlation suggests potential for different sources of exposure or differences in their toxicokinetics (or pharmacokinetics).
Table 2.
Detection frequency, geometric mean and distribution information (ng/mL) for urinary PFR metabolites (N=349). Sample were assessed in three batches and MDLs were calculated separately for each batch (MDL ranges: 136–333 pg/ml for BCIPP, 127–243 pg/ml for DPHP, 60–197 pg/ml for BDCIPP, 37–177 pg/ml for ip-PPP, 213–846 pg/ml for tb-PPP and 3–33 pg/ml for BCIPHIPP.
| Metabolite | % Detect | GMa | 25th %ile | 50th %ile | 75th %ile | Maximum |
|---|---|---|---|---|---|---|
| BCIPP | 48.7 | -- | -- | 0.7 | 1.1 | 6.1 |
| BDCIPP | 92.8 | 1.8 | 0.8 | 1.9 | 3.6 | 140 |
| DPHP | 83.7 | 1.4 | 0.8 | 1.3 | 2.7 | 112 |
| ip-PPP | 99.4 | 6.8 | 4.2 | 7.1 | 10.9 | 69 |
| tb-PPP | 2.0 | -- | -- | -- | -- | 8.6 |
| BCIPHIPP | 98.3 | 0.5 | 0.2 | 0.4 | 0.8 | 98 |
GM: geometric mean
Although PRFs are commonly considered replacements for the PentaBDE mixture, which was phased out in the U.S. at the approximate time of our sample collection, our results indicate that exposures were ubiquitous in the early 2000s, suggesting that PFR use was already common. This finding is consistent with previous work identifying PFRs in National Institute of Standards and Technology Standards Reference Material dust samples collected in the 1990s (e.g. SRM 2585; Van den Eede et al. 2011). Urinary DPHP and BDCIPP levels in the present study were similar to those measured in a 2011–2012 cohort of pregnant women from North Carolina (Hoffman et al. 2014). This was surprising since the present cohort was sampled approximately 6–8 years earlier than the 2011–2012 cohort, and PFR chemical usage is thought to have been increased since the phase-out of Penta BDE(Stapleton et al. 2012b; van der Veen and de Boer 2012). In addition, DPHP and BDCIPP levels in the present cohort are approximately 1 order of magnitude greater than those from a Massachusetts cohort studied during a similar time frame (2002–2007) (Meeker et al. 2013a); however, the Massachusetts cohort was exclusively male which may explain observed differences. Data have previously shown that women have higher levels of DPHP than men (e.g. Hoffman et al. 2015). TPHP was recently detected in nail polish, which has been offered as a possible explanation for this pattern (Mendelsohn et al. 2016); however, we are unaware whether TPHP was used in nail polish in the early 2000s. In addition BDCIPP levels were also higher in our cohort compared to the men in Meeker et. al. 2013a. Metabolic differences could also explain this pattern. Data from Hays et al. (2015) demonstrate that urine flaw rates differ between males and females (higher in males over 12 years of age), a factor which may impact urinary PFR metabolite concentrations. The ip-PPP levels were approximately 3–6 times higher than recent cohorts from New Jersey and California (Butt et al. 2014 and 2016). Interestingly, the women in the PIN cohort were also found to have higher levels of PBDEs in their breast milk than women in other similarly timed U.S. cohorts (Daniels et al. 2010).
3.2 Predictors of PFR levels
We found little evidence of association between PFR metabolites and maternal age at the start of pregnancy after adjusting for other factors. Only associations between maternal age and BCIPHPP remained suggestive of an inverse association after adjustment; though confidence intervals were imprecise (Table 3). Although past research has shown that metabolite concentrations decrease with age (Van den Eede et al. 2015 and Hoffman et al. 2015b), the age range of participants in our cohort was relatively narrow, potential limiting our ability to detect real difference occurring with age. Women experiencing their first pregnancy had lower levels of ip-PPP (10β=0.83; 95% CI: 0.71, 0.97; p=0.02), but significantly higher levels of urinary DPHP (10β=1.27; 95% CI: 1.04–1.55; p=0.02). Information on differences in consumer patterns between primiparous women and those with previous children could be helpful in identifying drivers of these associations.
Table 3.
Multiplicative change (10β) in PFR metabolite concentration by maternal characteristic (simultaneously adjusted for all included factors).
| Predictor | BDCIPP | DPHP | ip-PPP | BCIPHIPP | |||||
|---|---|---|---|---|---|---|---|---|---|
| 10β (95% CI) | p | 10β (95% CI) | p | 10β (95% CI) | p | 10β (95% CI) | p | ||
| Age | |||||||||
| ≤25 | Reference | -- | Reference | -- | Reference | -- | Reference | -- | |
| 26–30 | 1.06 (0.78, 1.45) | 0.71 | 1.12 (0.83, 1.52) | 0.44 | 0.99 (0.79, 1.26) | 0.96 | 0.92 (0.63, 1.34) | 0.66 | |
| 31–35 | 0.93 (0.66, 1.30) | 0.66 | 0.97 (0.70, 1.35) | 0.87 | 0.84 (0.65, 1.09) | 0.20 | 0.74 (0.49, 1.12) | 0.15 | |
| ≥36 | 1.04 (0.70, 1.55) | 0.84 | 0.99 (0.68, 1.46) | 0.97 | 1.10 (0.81, 1.49) | 0.53 | 0.67 (0.41, 1.09) | 0.11 | |
| Race | |||||||||
| white | Reference | -- | Reference | -- | Reference | -- | Reference | -- | |
| non-white | 1.09 (0.84, 1.42) | 0.52 | 0.91 (0.70, 1.17) | 0.45 | 0.88 (0.72, 1.07) | 0.21 | 0.89 (0.64, 1.22) | 0.46 | |
| Education (years) | |||||||||
| ≤15 | 1.03 (0.77, 1.38) | 0.83 | 1.07 (0.81, 1.41) | 0.65 | 1.25 (1.01, 1.56) | 0.04 | 0.79 (0.56, 1.13) | 0.20 | |
| ≥16 | Reference | -- | Reference | -- | Reference | -- | Reference | -- | |
| Parity | |||||||||
| 0 | 0.93 (0.75, 1.14) | 0.46 | 1.27 (1.04, 1.55) | 0.02 | 0.83 (0.71, 0.97) | 0.02 | 1.22 (0.95, 1.57) | 0.12 | |
| ≥1 | Reference | -- | Reference | -- | Reference | -- | Reference | -- | |
| Pre-pregnancy BMI | |||||||||
| Underweight | 1.01 (0.74, 1.37) | 0.95 | 1.12 (0.83, 1.51) | 0.45 | 1.09 (0.86, 1.37) | 0.48 | 0.73 (0.50, 1.07) | 0.10 | |
| Normal range | Reference | -- | Reference | -- | Reference | -- | Reference | -- | |
| Overweight | 1.09 (0.79, 1.50) | 0.59 | 1.21 (0.89, 1.65) | 0.22 | 1.36 (1.07, 1.73) | 0.01 | 0.9 (0.61, 1.33) | 0.60 | |
| Obese | 1.17 (0.88, 1.57) | 0.28 | 1.22 (0.92, 1.61) | 0.17 | 1.52 (1.23, 1.89) | 0.0002 | 1.14 (0.80, 1.62) | 0.46 | |
| Gestational weeks at urine sample collection | |||||||||
| 24–26 weeks | Reference | -- | Reference | -- | Reference | -- | Reference | -- | |
| 27–28 weeks | 0.84 (0.64, 1.11) | 0.22 | 0.86 (0.66, 1.13) | 0.28 | 0.89 (0.72, 1.10) | 0.30 | 0.87 (0.62, 1.23) | 0.43 | |
| 29–30 weeks | 0.85 (0.65, 1.13) | 0.26 | 0.85 (0.65, 1.12) | 0.25 | 1.07 (0.87, 1.32) | 0.52 | 1.10 (0.78, 1.54) | 0.60 | |
| Date (years) | 1.08 (0.96, 1.21) | 0.20 | 0.96 (0.84, 1.08) | 0.54 | 1.18 (1.08, 1.28) | 0.0003 | 1.08 (0.92, 1.23) | 0.33 | |
| Season of Sample Collection | |||||||||
| Winter (Dec – Feb) | Reference | -- | Reference | -- | Reference | -- | Reference | -- | |
| Spring (Mar – May) | 2.73 (2.05, 3.65) | <.0001 | 1.05 (0.80, 1.39) | 0.73 | 0.67 (0.54, 0.83) | 0.0003 | 1.43 (1.01, 2.03) | 0.04 | |
| Summer (Jun – Aug) | 3.97 (2.96, 5.32) | <.0001 | 1.62 (1.22, 2.15) | 0.0008 | 0.75 (0.60, 0.93) | 0.01 | 1.96 (1.37, 2.79) | 0.0002 | |
| Fall (Sep – Nov) | 1.73 (1.29, 2.32) | 0.0003 | 1.15 (0.86, 1.53) | 0.34 | 1.01 (0.81, 1.27) | 0.90 | 1.40 (0.98, 2.01) | 0.06 | |
Neither race nor education were strongly associated with urinary levels. However, the cohort was primarily white and well-educated women, reducing our power to fully investigate patterns by these demographics. Still, we did observe higher levels of ip-PPP among women with less education (25% higher for women with less than a 4 year college degree; 10β=1.25; 95% CI: 1.01, 1.56; p=0.04). Educational attainment, a marker of socioeconomic position (SEP), has previously been associated with biomarkers of PBDE exposure in children (e.g. Stapleton et al. 2012a).
Compared to women with a normal pre-pregnancy BMI, those who were overweight or obese prior to pregnancy had higher levels of urinary BDCIPP, DPHP and ip-PPP. For example, obese women had urinary ip-PPP levels 1.52 times those of women with pre-pregnancy BMIs in the normal range (95% CI: 1.23, 1.89; p=0.0002). Our previous research indicates that rats exposed to FM550 in early-life gain weight more readily, suggesting that FM550’s components may be obesogenic (Patisaul et al. 2013). Additional work with FM550 suggests that the obesogenic potential may be driven by PFRs present in FM550 (e.g. TPHP and ip-TPHP), which are ligands for the peroxisome proliferator-activated receptor gamma which is a critical nuclear receptor in adipocyte differentiation and lipid storage (Belcher et al. 2014; Fang et al. 2015; Pillai et al. 2014). However, it is also possible that PFR metabolism or excretion is intrinsically associated with BMI. For example, previous work from Hays et al. (2015) indicated that failing to account for urine dilution could induce correlation between categories of BMI and urinary BPA metabolites. Although we have conducted analyses both with and without correction for dilution (i.e. specific gravity) and observed similar associations with both methods, other factors linking BMI and PFR metabolism or excretion could be at play. Alternatively, differences in behavior and activity patterns that are associated with BMI may explain differences in PFR exposure. Based on our study design we are unable to distinguish which factors may be causal in associations between urinary metabolites and BMI; however, this is an important consideration for future research.
The week of gestation during which the urine sample was collected tended to be inversely associated with BDCIPP and DPHP; however, associations were imprecisely estimated and not statistically significant. If real, differences in kidney function and metabolism during pregnancy may explain these patterns. These results are an important consideration for epidemiologic studies investigating the consequences of prenatal exposure to PFRs with a single urine sample during pregnancy and suggest that gestational timing of sample collection may be an important factor driving measured concentrations.
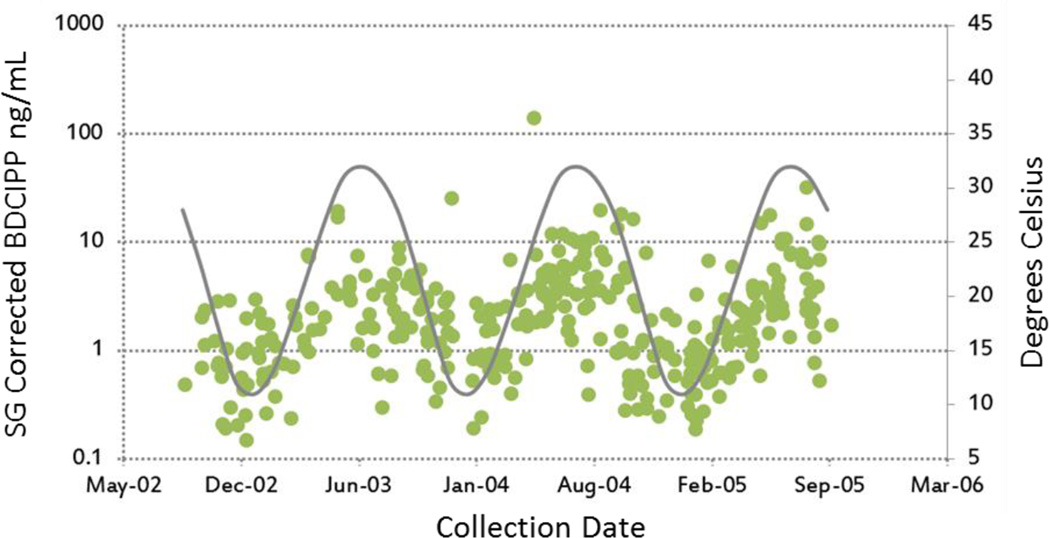
Season of collection was the strongest predictor of urinary PFR metabolite concentrations. Compared to samples collected in the winter, samples collected in the summer had significantly higher concentrations of BDCIPP, DHPH and BCIPHIPP. For example, BDCIPP concentrations in samples collected in the summer were approximately 3 times higher in the summer (10β = 3.97; 95% CI: 2.96, 5.32; p<0.0001). Figure 2 depicts individual concentration measures as well as the average month temperature in our study area. This pattern suggests that behavior or exposure varies with temperature. Past research has shown that indoor dust samples collected in China had lower PFR concentrations in the summer months (Cao et al. 2014). This could mean that PFRs are partition into air more readily in warmer summer months, potentially increasing inhalation exposure which is increasingly recognized as an important exposure route for PFRs (e.g. Xu et al. 2016). However, although we did not directly evaluate indoor temperatures, they are likely to be relatively stable over time in North Carolina (central heating and air conditioning are exceedingly common in the study area) which suggests that exposure in other environments (e.g. outdoors or in vehicles) could be a substantial contributor to total the body burden. For example, PFRs are commonly detected in dust samples collected in cars (e.g. Harrada, 2016). It is possible that exposure inside cars in the summer is higher due to higher temperatures. In contrast, concentrations of ip-PPP were lower in the spring and summer than in the winter. Cumulatively, these results indicate that season of collection could be an important confounder in future epidemiology studies using spot urine samples as a proxy for longer-term PFR exposure. Despite a relatively narrow time frame of sample collection, we also observed statistical evidence of increases in urinary ip-PPP concentration; levels of ip-PPP increased by 18% per year (10β = 1.18; 95% CI: 1.08, 1.28; p=0.0003).
Figure 2.
Individual Urinary BDCIPP (ng/mL) concentrations plotted by date of sample collection (green dots), overlaid with the average month temperature in Chapel Hill, North Carolina (Degrees Celsius; grey line).
Our results represent the first large-scale assessment of individual factors related to the levels of urinary PFR metabolites during pregnancy. These results should be interpreted in the context of several limitations. First, our cohort was relatively homogeneous, with the majority of women reporting white race and having high educational attainment, potentially limiting the generalizability of these results to other populations. However, the cohort’s homogeneity may also be an asset for planned future research investigating health impacts of exposure, as it could reduce the potential for confounding. Another limitation is that these results rely on a single spot urine sample. Although we have previously shown that measures of BDCIPP and DPHP in urine to be relatively stable over the course of pregnancy (Hoffman et al. 2014), and moderately to highly stable over a week (Hoffman et al. 2015b), we were unable to evaluate the potential for differences over time in this cohort. In addition, previous research has shown that urinary metabolite concentrations may vary throughout the day (Cequier et al. 2015; Hoffman et al. 2015b). While we were not able to evaluate diurnal variation because the vast majority of urine samples were collected in the same time window (>95% of samples were collected between 0700 and 1200 hours), the temporal standardization served to control such variation for this comparison. Because samples were mainly collected in the early morning, they are likely to represent exposure over the previous night, which we expect many women would have spent in their homes.
4. Conclusions
Although PFRs are commonly thought to be a replacement for the Penta-BDE mixture, which was phased out of used as flame retardants in the U.S. in the mid-2000s, our results indicate that exposure to PFRs was wide-spread by 2002. We observed strong seasonal trends in metabolite level suggesting season of collection may be an important factor to consider in future epidemiologic investigations. In addition, our results suggest that levels vary by BMI, parity, and education. Additional data are needed to identify the mechanisms explaining observed associations and to determine whether the levels of exposure that we observed are associated with any adverse help impacts among pregnant women of their children.
Supplementary Material
Highlights.
PFR metabolites were detected in all urine samples provided by pregnant women.
Geometric mean concentrations were higher than for similarly-timed cohorts.
Women with higher pre-pregnancy BMI had higher levels of urinary metabolites.
PFR metabolite concentrations in urine vary seasonally.
Acknowledgments
This research was supported in part by grants from the National Institute of Environmental Health Sciences (R21 ES023904and P30ES10126) and the U.S. Environmental Protection Agency (RD832736). The work of KH was funded in part by a training grant from the National Institute of Environmental Health Sciences (T32 ES007018)
Abbreviations
- BCIPHIPP
1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate
- BMI
body mass index
- BCIPP
bis(1-chloro-2-propyl) phosphate
- BDCIPP
bis(1,3-dichloro-2-propyl) phosphate
- CI
confidence interval
- DPHP
diphenyl phosphate
- FM550
Firemaster® 550
- GM
geometric mean
- ip-PPP
isopropyl-phenyl phenyl phosphate
- MDL
method limit of detection
- PFRs
organophosphate flame retardants
- PBDEs
polybrominated diphenyl ethers
- PIN
Pregnancy Infection and Nutrition Study
- tb-PPP
tert-butyl phenyl phenyl phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Conflict Disclosure: The authors have no actual or potential conflicting relationships relevant to this article to disclose.
References
- Ballesteros-Gomez A, Brandsma SH, de Boer J, Leonards PE. Analysis of two alternative organophosphorus flame retardants in electronic and plastic consumer products: Resorcinol bis-(diphenylphosphate) (PBDPP) and bisphenol a bis (diphenylphosphate) (BPA-BDPP) Chemosphere. 2014;116:10–14. doi: 10.1016/j.chemosphere.2013.12.099. [DOI] [PubMed] [Google Scholar]
- Behl M, Rice JR, Smith MV, Co CA, Bridge M, Hsieh JH, Freedman JH, Boyd WA. Comparative Toxicity of Organophosphate Flame Retardants and Polybrominated Diphenyl Ethers to C. elegans. Toxicol Sci. 2016 doi: 10.1093/toxsci/kfw162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Hsieh JH, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES, 3rd, Jarema KA, Padilla S, Tice RR. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol Teratol. 2015;52(Pt B):181–193. doi: 10.1016/j.ntt.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Lett. 2014;228:93–102. doi: 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Brandsma SH, Sellstrom U, de Wit CA, de Boer J, Leonards PE. Dust measurement of two organophosphorus flame retardants, resorcinol bis(diphenylphosphate) (RBDPP) and bisphenol a bis(diphenylphosphate) (BPA-BDPP), used as alternatives for BDE-209. Environ Sci Technol. 2013;47:14434–14441. doi: 10.1021/es404123q. [DOI] [PubMed] [Google Scholar]
- Brommer S, Harrad S. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from uk cars, classrooms, living rooms, and offices. Environ Int. 2015;83:202–207. doi: 10.1016/j.envint.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol. 2014;48:10432–10438. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int. 2016 doi: 10.1016/j.envint.2016.06.029. pii: S0160-4120(16)30247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Xu F, Covaci A, Wu M, Yu G, Wang B, et al. Differences in the seasonal variation of brominated and phosphorus flame retardants in office dust. Environ Int. 2014;65:100–106. doi: 10.1016/j.envint.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, et al. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters — a biomonitoring study of mother-child pairs. Environ Int. 2015;75:159–165. doi: 10.1016/j.envint.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Cristale J, Hurtado A, Gomez-Canela C, Lacorte S. Occurrence and sources of brominated and organophosphorus flame retardants in dust from different indoor environments in Barcelona, Spain. Environ Res. 2016;149:66–76. doi: 10.1016/j.envres.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Pan IJ, Jones R, Anderson S, Patterson DG, Jr, Needham LL, et al. Individual characteristics associated with PBDE levels in U.S. human milk samples. Environ Health Perspect. 2010;118:155–160. doi: 10.1289/ehp.0900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, et al. Is the pentabde replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in pc12 cells. Toxicology and applied pharmacology. 2011;256:281–289. doi: 10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: Levels in California adults and recommendations for future studies. Environ Sci Technol. 2014;48:13625–13633. doi: 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Webster TF, Gooden D, Cooper EM, McClean MD, Carignan C, et al. Investigating a novel flame retardant known as V6: Measurements in baby products, house dust, and car dust. Environ Sci Technol. 2013;47:4449–4454. doi: 10.1021/es400032v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ML, Webster TF, Ferguson PL, Stapleton HM. Characterizing the peroxisome proliferator-activated receptor (ppar gamma) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environmental Health Perspectives. 2015;123:166–172. doi: 10.1289/ehp.1408522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, et al. In ovo effects of two organophosphate flame retardants—TCPP and TDCPP—on pipping success, development, mrna expression, and thyroid hormone levels in chicken embryos. Toxicol Sci. 2013;134:92–102. doi: 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- Farhat A, Buick JK, Williams A, Yauk CL, O’Brien JM, Crump D, et al. Tris(1,3-dichloro-2-propyl) phosphate perturbs the expression of genes involved in immune response and lipid and steroid metabolism in chicken embryos. Toxicology and Applied Pharmacology. 2014;275:104–112. doi: 10.1016/j.taap.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Faust JB, August LM. Evidence on the carcinogenicity of tris(1,3-dichloro-2-propyl) phosphate. Sacramento, ca: Reproductive and cancer hazard assessment branch, office of environmental health hazard assessment, california environmental protection agency; 2011. [Google Scholar]
- Gold MD, Blum A, Ames BN. Another flame retardant, tris-(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science. 1978;200:785–787. doi: 10.1126/science.347576. [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM. Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ Sci Technol. 2016;50:4483–4491. doi: 10.1021/acs.est.6b00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrada S, Brommera S, Mueller JF. Concentrations of organophosphate flame retardants in dust from cars, homes, and offices: An international comparison. Emerging Contaminants. 2016;2(2):66–72. [Google Scholar]
- Hayes SM, Aylward LL, Blount BC. Variation in urine flow rates according to demographic characteristics and body mass index in NHANES; Potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect. 123(4):293–300. doi: 10.1289/ehp.1408944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int. 2014;63:169–172. doi: 10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Chen A, Limkakeng AT, Jr, Stapleton HM. High exposure to organophosphate flame retardants in infants: Associations with baby products. Environ Sci Technol. 2015a;49:14554–14559. doi: 10.1021/acs.est.5b03577. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: Hand wipes and house dust. Environ Health Perspect. 2015b;123:160–165. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosarac I, Kubwabo C, Foster WG. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) J Chromatogr B. 2016;1014:24–30. doi: 10.1016/j.jchromb.2016.01.035. [DOI] [PubMed] [Google Scholar]
- Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in h295r and mvln cell lines and in zebrafish. Aquat Toxicol. 2012;114–115:173–181. doi: 10.1016/j.aquatox.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of tdcpp or tpp on gene transcriptions and hormones of hpg axis, and their consequences on reproduction in adult zebrafish (danio rerio) Aquat Toxicol. 2013;134–135:104–111. doi: 10.1016/j.aquatox.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocrine Disruptors. 2013a doi: 10.4161/endo.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect. 2010;118(3):318–323. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo AM, Congleton J, Webster TF, Stapleton HM. Nail Polish as a Source of Exposure to Triphenyl Phosphate. Environ Int. 2016;86 doi: 10.1016/j.envint.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: An exploratory assessment. J Biochem Mol Toxicol. 2013;27:124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, et al. Ligand binding and activation of ppargamma by Firemaster® 550: Effects on adipogenesis and osteogenesis in vitro. Environ Health Perspect. 2014;122:1225–1232. doi: 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIN. Pin — pregnancy, infection, and nutrition study. 2012 Available: http://www.cpc.unc.edu/projects/pin. [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, et al. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43:7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45:5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a north carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012a;120:1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, et al. Novel and high volume use flame retardants in us couches reflective of the 2005 pentaBDE phase out. Environ Sci Technol. 2012b;46:13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Letcher RJ, Yu H. Determination of organophosphate diesters in urine samples by a high-sensitivity method based on ultra high pressure liquid chromatography-triple quadrupole-mass spectrometry. J Chromatogr A. 2015;1426:154–160. doi: 10.1016/j.chroma.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int. 37:454–461. doi: 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Neels H, Jorens PG, Covaci A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J Chromatogr A. 2013;1303:48–53. doi: 10.1016/j.chroma.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, et al. Age as a determinant of phosphate flame retardant exposure of the australian population and identification of novel urinary PFR metabolites. Environ Int. 2015;74:1–8. doi: 10.1016/j.envint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Van der Veen I, de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, et al. Exposure of zebrafish embryos/larvae to tdcpp alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol. 2013;126:207–213. doi: 10.1016/j.aquatox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Xu F, Giovanoulis G, van Waes S, Padilla-Sanchez JA, Papadopoulou E, et al. Comprehensive Study of Human External Exposure to Organophosphate Flame Retardants via Air, Dust, and Hand Wipes: The Importance of Sampling and Assessment Strategy. Environ Sci Technol. 2016;50:7752–7760. doi: 10.1021/acs.est.6b00246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.