Abstract
Background
Acute-phase cognitive therapy (CT) is an efficacious treatment for major depressive disorder (MDD), but responders experience varying post-acute outcomes (e.g., relapse vs. recovery). Responders’ symptom-change trajectories during response to acute-phase CT may predict longer term outcomes.
Method
We studied adult outpatients (N=220) with recurrent MDD who responded to CT but had residual symptoms. Responders with linear (steady improvement), log-linear (quicker improvement earlier and slower later), one-step (a single, relatively large, stable improvement between adjacent assessments), or undefined (not linear, log-linear, or one-step) symptom trajectories were assessed every 4 months for 32 additional months.
Results
Defined (linear, log-linear, one-step) versus undefined acute-phase trajectories predicted lower depressive symptoms (d=0.36), lower weekly probability of being in a major depressive episode (OR=0.46), higher weekly probabilities of remission (OR=1.93) and recovery (OR=2.35), less hopelessness (d=0.41), fewer dysfunctional attitudes (d=0.31), and better social adjustment (d=0.32) for 32 months after acute-phase CT. Differences among defined trajectory groups were nonsignificant.
Conclusions
Responding to acute-phase CT with a defined trajectory (orderly pattern) of symptom reduction predicts better longer term outcomes, but which defined trajectory (linear, log-linear, or one-step) appears unimportant. Frequent measurement of depressive symptoms to identify un/defined CT response trajectories may clarify need for continued clinical monitoring and treatment.
Keywords: major depressive disorder, cognitive therapy, trajectory, relapse, recovery
Introduction
Mood disorder researchers continue to search for predictors, moderators, and mechanisms of change that influence illness trajectories before, during, and after treatment (Oquendo et al., 2014; Shoham & Insel, 2011). Acute-phase cognitive therapy’s (CT; Beck et al., 1979) effect on major depressive disorder (MDD) is comparable to pharmacotherapy and superior to pill placebo (Cuijpers et al., 2013). Nonetheless, many acute-phase CT responders, and even more pharmacotherapy responders, experience relapse and recurrence after acute-phase treatments end (Biesheuvel-Leliefeld et al., 2015; Vittengl & Jarrett, 2014). Continuation treatments decrease relapse and residual symptoms among responders (Biesheuvel-Leliefeld et al., 2015; Jarrett, Minhajuddin, Gershenfeld, et al., 2013; Vittengl, Clark, Thase, & Jarrett, 2014) but may be more efficacious (and cost-effective) when provided to higher risk patients (Vittengl & Jarrett, 2014). The purpose of the current report is to test whether symptom-change trajectories during response to acute-phase CT predict longer term outcomes. In particular, we compared 32-month outcomes among responders who showed linear (small, steady decreases in symptoms), log-linear (larger decreases earlier and smaller later), one-step (a single, relatively large, stable drop in symptoms), or undefined (not clearly linear, log-linear, or one-step) trajectories during acute-phase CT (Vittengl et al., 2013). We analyzed responders judged to be at higher risk for relapse (due to substantial residual symptoms during the final weeks of CT), randomized to 8 months of continuation CT, fluoxetine, or pill placebo and followed 24 additional months (Jarrett, Minhajuddin, Gershenfeld, et al., 2013). We aimed to clarify higher risk CT responders’ prognoses or longer term outcomes based on their acute-phase response trajectories.
Psychotherapy dose-response curves (symptom levels plotted against treatment time or sessions; e.g., see Figure 1) are symptom-change trajectories that may vary by treatments, diagnoses, and individuals. The field has speculated about what different patterns of change suggest about treatment processes or mechanisms. For example, patients and clinicians may expect linear changes with steady, roughly equal decreases in symptoms across treatment sessions or time, although nonlinear patterns (e.g., log-linear, quadratic, cubic) also occur and should be studied (Hayes, Laurenceau, et al., 2007). Indeed, linear changes are common (Barkham et al., 1993; Percevic et al., 2006) and may reflect incremental learning, application, and generalization of skills learned in psychotherapy. In contrast, decelerating log-linear changes with more improvement earlier, and less later, may reflect initial restoration of hope (“remoralization”) followed by learning and practicing skills (“remediation” and “rehabilitation”; Howard et al., 1993; Lutz et al., 2002). In CT specifically, early sessions focus on quicker symptom reduction (e.g., via behavioral activation), whereas later sessions focus on relapse prevention (Beck et al., 1979). Finally, “insight” may produce abrupt drops in symptom scores (Caspar & Berger, 2007), including patients grasping key CT concepts such as “thoughts can be changed to improve mood” (Grosse Holtforth et al., 2007). Patients with rapid early response (e.g., Hayes, Feldman, et al., 2007) or “sudden gains” (e.g., Tang & DeRubeis, 1999) may demonstrate such processes and have been hypothesized to have better outcomes at the end of acute-phase treatment than do patients without instances of rapid improvement.
Figure 1.
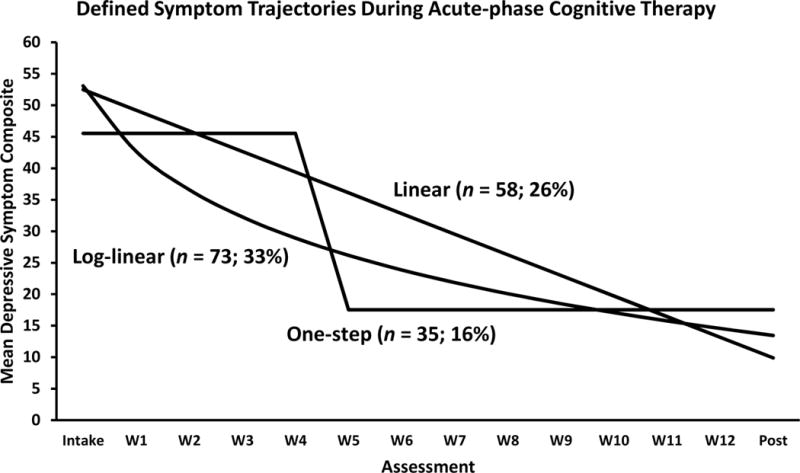
Lines represent mean symptom-change functions of 220 higher risk responders to acute-phase cognitive therapy (CT) fitting a defined (linear, log-linear, or one-step) trajectory (median fit correlation = .94, range .87–.99). The drop in symptom scores between W4 and W5 is the median timing for the 35 one-step patients. An additional 54 higher risk responders (not shown; 25%) did not have a significant fit to a linear, log-linear, or one-step pattern and were classified as following an undefined trajectory. Intake = first assessment before CT; W1-12 = assessment at CT Weeks 1–12; post = first assessment after CT.
Even though underlying mechanisms are unclear, differences in symptom change trajectories may contain valuable prognostic information. A recent study showed differences in longer term outcomes based on MDD patients’ initial symptom trajectories in primary care (Wardenaar et al., 2014). The first year of assessment clarified patients’ trajectories, and 2 subsequent years’ data allowed outcome comparisons among trajectory groups: Patients with a “chronic” trajectory showed small improvements (year 1) but maintained elevated symptoms (year 2); “early remission” patients showed relatively quick and large symptom reductions (especially during the first 9 months of year 1) and maintained their improvements (year 2); “remission + recurrence” patients showed improvements but then deterioration (both during year 1) and elevated symptoms thereafter (year 2); and “late remission” patients showed slower improvements than early remission patients (year 1) that were largely maintained (year 2). However, differences among trajectory groups were not significant during year 3. This study suggested that initial trajectories of symptom change in primary care predicted patients’ subsequent functioning and so informed our hypotheses regarding longer term outcomes of acute-phase CT responders.
The longer term outcomes of MDD patients with different acute-phase symptom trajectories in CT are largely unknown or restricted to broad contrasts. For example, patients with rapid early response, and the closely related phenomena of sudden gains, often have better outcomes at end of acute-phase CT (e.g., Aderka et al., 2012; Hayes, Feldman et al. 2007; Ilardi & Craighead, 1994) and pharmacotherapy (e.g., Henkel et al., 2009, Vermeiden et al., 2015; Vittengl, Clark, & Jarrett, 2005). However, longer term outcomes after sudden gains in CT for depression have been mixed (Vittengl et al., 2015a), perhaps because researchers have contrasted sudden-gainers with other patients altogether (e.g., without differentiating patients with other trajectories such as linear, log-linear, and undefined trajectories). The current analyses contrasted longer term outcomes across better differentiated acute-phase CT response trajectories.
Recently, Vittengl et al. (2013) estimated the frequency of linear, log-linear, and one-step trajectories among adult outpatients with recurrent MDD receiving acute-phase CT. Across 14 approximately weekly assessments, most patients showed a defined (more orderly) pattern of change in depressive symptoms. Specifically, 20% of patients showed linear (i.e., steady decreases in symptoms), 30% log-linear (i.e., larger decreases in symptoms earlier and smaller decreases later in acute-phase CT), and 16% one-step (i.e., a single, relatively large and stable, drop in symptoms between adjacent assessments) trajectories. The timing of drops in symptom scores in the one-step pattern varied from between weeks 1–2 to weeks 10–11 (median = weeks 3–4) in CT. Conversely, the remaining 34% had undefined (i.e., not clearly linear, log-linear, or one-step patterns) trajectories.1 Pre-treatment clinical (e.g., depression severity, age of MDD onset), demographic (e.g., age, gender), cognitive content, and social-interpersonal functioning measures did not predict which trajectories patients followed. Post-treatment, however, patients with undefined versus defined trajectories had lower probability of response to acute-phase CT (45% vs. 89%), greater depressive symptoms, poorer social-interpersonal functioning, and more depressive cognitive content. Patients with defined trajectories (linear, log-linear, one-step) varied little on these outcomes. Thus, in the shorter term, all defined trajectories signaled similar benefits to patients and were superior to an undefined symptom-change trajectory (Vittengl et al., 2013). The current analyses estimated longer term outcomes among a subset of these patients with higher risk response to acute-phase CT who participated in a randomized trial of continuation treatments (Jarrett, Minhajuddin, Gershenfeld, et al., 2013). Figure 1 shows the higher risk responders’ defined trajectories in acute-phase CT.
The prognostic value of acute-phase symptom trajectories would be greater if trajectories provided information incremental to depressive symptom levels before and at the end of acute-phase treatment. Greater pre-treatment symptoms have often predicted poorer outcomes in acute-phase CT (Hamilton & Dobson, 2002; Jarrett, Minhajuddin, Kangas, et al., 2013). Moreover, having higher residual symptoms at the end of acute-phase treatment is a robust predictor of poorer longer term outcomes (Jarrett et al., 2001; Fava, Ruini, & Belaise, 2007; Thase et al., 1992). For example, acute-phase treatment responders with higher residual symptoms are at increased risk for major depressive relapse (Jarrett, Vittengl, & Clark, 2008a), failure to achieve recovery (Fava et al., 2007), and concomitant deficits in psychosocial functioning (Zimmerman et al., 2007). Consequently, we also tested the acute-phase trajectories’ prediction of longer term outcomes after controlling acute-phase intake and residual symptom levels.
In particular, we tested the hypothesis that CT higher risk responders with defined (linear, log-linear, or one-step) versus undefined acute-phase trajectories would show better longer term outcomes, including lower probability of being in major depressive episodes, higher probability of remission and recovery, lower residual depressive symptoms, less depressive cognitive content, and better social-interpersonal functioning, on average, across 32 months of observation. We repeated hypothesis tests controlling responders’ symptom levels at the beginning and end of acute-phase CT to clarify trajectories’ incremental prediction of longitudinal outcomes. Finally, we explored differences among the defined acute-phase trajectories (linear, log-linear, and one-step), but did not test specific hypotheses because the defined groups had similar residual symptoms, cognitive content, and psychosocial functioning at the end of acute-phase CT (Vittengl et al., 2013).
Method
A two-site randomized clinical trial described in detail by Jarrett and Thase (2010) and Jarrett, Minhajuddin, Gershenfeld, et al. (2013) provided data. Below we summarize methods relevant to current analyses.
Participants
Participants were outpatients recruited through clinical referrals and newspaper, bulletin board, and Internet announcements who (a) provided written informed consent; (b) met criteria for recurrent MDD (American Psychiatric Association, 2000) on the Structured Clinical Interview for DSM-IV (First et al., 1996); (c) had a history of remission between depressive episodes, ≥ 1 depressive episode with complete inter-episode recovery, or antecedent dysthymic disorder; and (d) scored ≥14 on the 17-item HRSD.2 Individuals with (a) severe or poorly controlled concurrent medical disorders that could cause depression, (b) psychotic or organic mental disorders, bipolar disorder, active substance dependence, or primary obsessive-compulsive or eating disorders, (c) inability to complete questionnaires in English, (d) active suicide risk, (e) <18 or >70 years old, (f) history of non-response to ≥ 8 weeks of CT or 6 weeks of fluoxetine, or (g) pregnancy current or planned within 11 months post-intake were excluded.
Among 523 patients enrolled in acute-phase CT, 241 met a priori criteria for response (no major depressive episode and final HRSD ≤ 12) with higher risk for relapse (one or more of the last 7 weekly acute-phase HRSD scores ≥ 7) and so were randomized to 8 months of continuation CT (n = 86), fluoxetine (n = 86), or pill placebo with clinical management (n = 69). Among 241 randomized, 220 patients completed at least 39 of 42 symptom assessments allowing acute-phase trajectory identification (Vittengl et al., 2013), and their post-acute outcomes are now analyzed here.3 Figure 2 displays the CONSORT diagram for these analyses from the randomized clinical trial. These 220 patients were M = 42.8 (SD = 12.0) years old with M = 15.8 (SD = 2.8) years of education; 66.8% women; 85.0% white, 7.3% black, and 7.7% other races/ethnicities. Participants’ mean age of MDD onset was 20.6 (SD = 10.3) years and their current depressive episode had lasted M = 24.9 (median = 9; SD = 45.7) months. Including the episode at intake to the acute phase, patients had experienced a median of 4 (minimum 2) major depressive episodes.
Figure 2.
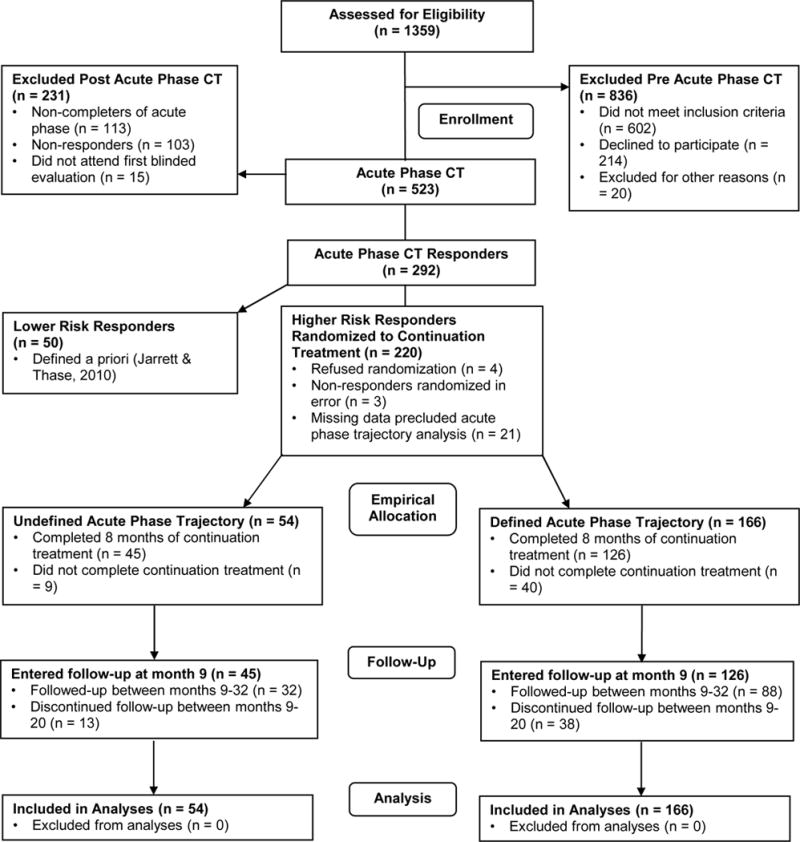
CONSORT diagram for current analysis of trajectories of higher risk responders from the randomized clinical trial. CT = cognitive therapy.
Acute Phase
Patients discontinued psychotropic medications before, and were not prescribed any during, the acute phase. Patients received a 12-week acute-phase CT protocol (Beck et al., 1979), with 2 additional weeks allowed for rescheduling. Patients received 2 CT sessions weekly for 4 weeks. Patients whose HRSD scores had decreased by ≥ 40% then received 8 weekly sessions (16 total sessions), whereas patients with < 40% symptom improvement received 8 twice-weekly before 4 weekly sessions (20 total sessions). The higher CT “dose” for patients with less early improvement increased their chance of response and eligibility for the continuation phase. The assessment schedule for depressive symptom severity (weekly during the 12-week CT protocol) was the same for patients receiving 16 or 20 sessions. Cognitive therapists (N = 16) had completed ≥ 1 year of CT training, submitted session videotapes for review, participated in weekly group supervision, and maintained Cognitive Therapy Scale (Young & Beck, 1980) scores ≥ 40, demonstrating competence.
Continuation and Follow-up Phases
The continuation-phase CT protocol included 10 sessions (4 biweekly then 6 monthly) of approximately 60 minutes each (Jarrett, 1989; Jarrett, Vittengl, & Clark, 2008b). Patients’ continuation-phase CT and acute-phase therapists were the same, with few exceptions (e.g., due to a therapist’s maternity leave). Experienced pharmacotherapists provided the double-blinded fluoxetine and placebo clinical-management protocol (Fawcett et al., 1987) on the same schedule as continuation-phase CT (10 sessions). Clinical management excluded specific CT methods. Patients received 10 mg/day for 2 weeks, 20 mg/day for 2 more weeks, and thereafter 40 mg/day of fluoxetine or identical placebo capsules. Pharmacotherapists could decrease doses or withdraw medication to reduce side effects, but most patients (73%) achieved 40mg/day of fluoxetine or placebo. After the continuation phase, patients entered a 24-month follow-up without protocol treatment. Patients meeting MDD criteria were referred out for treatment.
Measures
The current analyses focused on measures taken 0, 4, 8, 12, 16, 20, 24, 28, and 32 months after acute-phase CT.
Major depressive disorder status
The Longitudinal Interval Follow-Up Evaluation (LIFE; Keller et al., 1987) is a semi-structured retrospective interview providing weekly psychiatric status ratings (PSR) of MDD on a scale of 1 = no symptoms, 2 = one or two mild symptoms, 3 = obvious symptoms with moderate impairment, 4 = major symptoms and impairment but does not meet full MDD criteria, 5 = meets full MDD criteria, 6 = meets MDD criteria with severe impairment and/or psychosis. The LIFE also yielded weekly ratings (present or absent) for receipt of potentially mood-altering treatment outside the experimental protocol, which was rare (about 15% of patients received any; Jarrett et al., in press). Reliability for the LIFE’s many indices is generally > .70 (Keller et al. 1987), and MDD PSR ratings showed high retest reliability (median lag-1 correlation = .87) in the current sample. Using a priori criteria (Jarrett & Thase, 2010), for each of the 139 weeks of follow-up, we scored whether patients were experiencing a major depressive episode (PSR ≥ 5 for both current and prior week, starting at week 2), remission (PSR ≤ 2 for current and 5 prior consecutive weeks, starting at week 6), and recovery (PSR ≤ 2 for current and 34 prior consecutive weeks, starting at week 35) after acute-phase CT.
Depressive symptom severity
Measures included the patient-completed 21-item Beck Depression Inventory (BDI; Beck et al., 1961) and 30-item Inventory for Depressive Symptomatology self-report (IDS-SR; Rush et al., 1996), and the clinician-administered 17-item HRSD. These measures reflect the same depressive symptom construct during CT for MDD (Vittengl, Clark, Kraft, & Jarrett, 2005; Vittengl et al., 2013). In particular, the amount of pre-post change during CT was large for each measure and fell in a narrow range (d = 1.7–1.9); visual analyses suggested that the average rate and shape of change was very similar across measures; and factor analyses revealed that symptom levels varied primarily by time in treatment and not by measure (Vittengl et al., 2013), replicating findings in an earlier clinical trial (Vittengl et al., 2005). Consequently, we combined the three measures into a robust composite by standardizing the measures’ total scores based on their distributions (M and SD) at acute-phase intake and averaging them. Higher composite scores indicated more severe depressive symptomatology. Pooling data across assessments and treating the three scales as items, the depressive symptom composite showed high alpha internal consistency reliability (.95) in the current sample.
Cognitive content
Patients completed the 40-item Dysfunctional Attitudes Scale (DAS; Form A; Weissman, 1979) and 20-item Beck Hopelessness Scale (BHS; Beck et al., 1974; Beck & Steer, 1988). Higher DAS scores mark stronger and more pervasive depression-relevant thoughts and feelings (e.g. related to self-concept, happiness, perfectionism) and differentiate depressed persons from non-depressed controls (Otto et al., 2007; Nelson et al., 1992). Higher BHS scores indicate greater hopelessness and correlate with depression severity (Beck et al., 1975) and suicidality (Beck et al., 1985). Pooling data across assessments, the DAS (.95) and BHS (.92) showed high internal consistency in the current sample.
Psychosocial functioning
Patients completed the 56-item Social Adjustment Scale— Self-Report (SAS-SR; Weissman & Bothwell, 1976) and the 127-item Inventory of Interpersonal Problems (IIP; Horowitz et al., 1988). Higher total SAS-SR scores indicate poorer performance in social-role functions (e.g., work, leisure, parental, marital, friend) and differentiate depressed from non-depressed persons (Weissman et al., 2001). Higher total IIP scores mark problematic behaviors, thoughts, and feelings in significant social relationship, and IIP scores decrease as patients improve in psychotherapy (Horowitz et al., 1988). Pooling data across assessments, the SAS-SR (.89) and IIP (.98) showed high internal consistency in the current sample.
Prior Analyses of Acute-Phase Trajectories
Vittengl et al. (2013) differentiated individual patients’ trajectories during acute-phase CT on the depressive symptom-severity composite described above. Each patient’s series of 14 weekly symptom scores was fit to linear (assessment number 1–14), log-linear (log assessment), and one-step (the single, clearest change-point between adjacent assessments) functions in time-series regression analyses. Linear, log-linear, and one-step trajectories were hypothesized based on theories of change in psychotherapy (e.g., Barkham et al., 1993; Grosse Holtforth et al., 2007; Hayes, Laurenceau, et al., 2007; Howard et al., 1993; Lutz et al., 2002; Percevic et al., 2006), making this model-fitting approach preferable to exploratory growth mixture modeling. Patients were classified based on the trajectory (linear, log-linear, one-step) with the strongest, statistically significant correlation. Patients without a statistically significant fit were classified as following an undefined trajectory. Among the 220 CT responders analyzed in this report, Figure 1 shows the defined trajectory groups’ average symptom-change patterns. Vittengl et al. (2013) detailed acute-phase analyses, whereas the current analyses focus on trajectory groups’ post-acute outcomes.
Prior Analyses of Post-Acute Phase Outcomes
Comparisons among the continuation-treatment arms are addressed in other papers and are only summarized here. Continuation CT (18%) and fluoxetine (18%) reduced relapse during the experimental phase compared to pill placebo (33%), but arms did not differ in relapse/recurrence over 32 months (Jarrett, Minhajuddin, Gershenfeld, et al., 2013). Continuation CT and fluoxetine also reduced depressive symptoms (mean PSR) compared to placebo (by 0.21–0.25 SD) during the experimental phase, but not during follow-up (Vittengl et al., 2014). The three arms did not differ in time to remission or recovery (Vittengl et al., 2014) or in mean DAS or BHS scores (Vittengl et al., 2015b) over 32 months. Predictors of poorer longer term outcomes (e.g., relapse, recurrence, absence of stable remission and recovery) included greater residual depression (including emotional, cognitive, and social aspects), lower positive emotionality and behavioral activation, younger age, and earlier MDD onset (Vittengl et al., 2015c), as well as a priori higher versus lower risk response to acute-phase CT (Jarrett et al., 2016).
Current Analyses of Post-Acute Phase Outcomes
We compared trajectory groups’ outcomes using a series of repeated-measures multilevel models. Multilevel models support intent-to-treat analyses and can provide unbiased hypothesis tests when some data are missing (Schafer & Graham, 2002). Linear models of continuous outcomes (depressive symptom severity, cognitive content, psychosocial functioning) assumed a normal distribution and identity link function. Logistic models of dichotomous outcomes (major depressive episodes, remission, recovery) assumed a binary distribution and logit link function. We predicted each outcome from the a priori time period (8-month continuation phase and years 1 and 2 of follow-up), trajectory group, and the time × trajectory interaction. Models controlled continuation-treatment condition, the time × continuation-treatment interaction, and receipt of non-protocol treatment. Models included first-order autoregressive error structures. Consistent with randomization, trajectory and continuation-treatment groups were not correlated significantly, χ2 (6) = 2.92, p = .82. Trajectory × continuation-treatment interactions were not hypothesized, not significant (ps > .05), and excluded from final models.
Results
Do Responders with Defined versus Undefined Acute-Phase Trajectories have Better Longitudinal Outcomes?
Yes. We tested longitudinal differences between higher risk responders with undefined versus defined symptom trajectories during acute-phase CT in a series of multilevel models (see Table 1). As hypothesized, defined (vs. undefined) acute-phase symptom trajectory predicted lower mean PSRs of MDD (d = 0.36, p < .01). Moreover, patients with defined (vs. undefined) acute-phase symptom trajectories showed a significantly lower weekly probability of being in a major depressive episode (2.8 vs. 6.0%; OR = 0.46, p < .01) and higher weekly probabilities of remission (72.7 vs. 58.0%; OR = 1.93, p < .01) and recovery (48.4 vs. 28.5%; OR = 2.35, p < .01) for 32 months after acute-phase CT.
Table 1.
Prediction of Longitudinal Outcomes 0–32 Months after Acute-Phase Cognitive Therapy from Defined versus Undefined Trajectories
| Outcome Variables | |||||||
|---|---|---|---|---|---|---|---|
| PSR | DEP | DAS | BHS | IIP | SAS-SR | ||
| Predictor | F-test df | F-tests from multilevel models | |||||
| Time period | 2, 250–294 | 8.86** | 1.05 | 1.01 | 1.95 | 0.23 | 1.31 |
| Un/defined trajectory | 1, 166–184 | 21.40** | 11.53** | 5.43* | 11.14** | 0.60 | 6.19* |
| Period × trajectory | 2, 250–294 | 0.60 | 0.10 | 0.16 | 0.78 | 0.23 | 0.60 |
| Group means estimated in multilevel models | |||||||
| Defined trajectory group | Mean | 1.80 | 15.98 | 105.97 | 4.52 | 1.01 | 1.81 |
| SE | 0.04 | 0.54 | 2.26 | 0.28 | 0.04 | 0.02 | |
| Undefined trajectory group | Mean | 2.20 | 19.63 | 116.30 | 6.38 | 1.07 | 1.93 |
| SE | 0.08 | 0.93 | 3.84 | 0.48 | 0.06 | 0.04 | |
Note. N = 220. PSR = Psychiatric Status Rating of major depressive disorder. DEP = residual depressive symptom composite score. DAS = Dysfunctional Attitudes Scale. BHS = Beck Hopelessness Scale. IIP = Inventory of Interpersonal Problems. SAS-SR = Social Adjustment Scale—Self-report. PSR assessed weekly for 139 weeks after acute-phase CT. DEP, DAS, BHS, IIP, SOC assessed every 4 months for 32 months after acute-phase CT. Time period is months 0–8, 9–20, and 21–32 (continuation phase and years 1 and 2 of follow-up, respectively). Un/defined trajectory refers to more versus less orderly patterns of response in acute-phase CT. Analyses control receipt of non-protocol treatment, continuation-treatment group, and the interaction of continuation-treatment group with time period.
p < .05.
p < .01.
Also as hypothesized, defined (vs. undefined) trajectory predicted lower average residual depressive symptoms (d = 0.34, p < .01), lower depressive cognitive content on the BHS (d = 0.41, p < .01) and DAS (d = 0.31, p = .02), and better social functioning on the SAS-SR (d = 0.32, p = .01) for 32 months after acute-phase CT (see Table 1). However, defined-trajectory patients had only non-significantly better functioning on the IIP (d = 0.11, p = .44). Trajectory group × time interactions were not significant, suggesting that differences between trajectory groups were relatively consistent for 32 months. Figure 3 depicts the mean differences between the defined and undefined trajectory groups.
Figure 3.
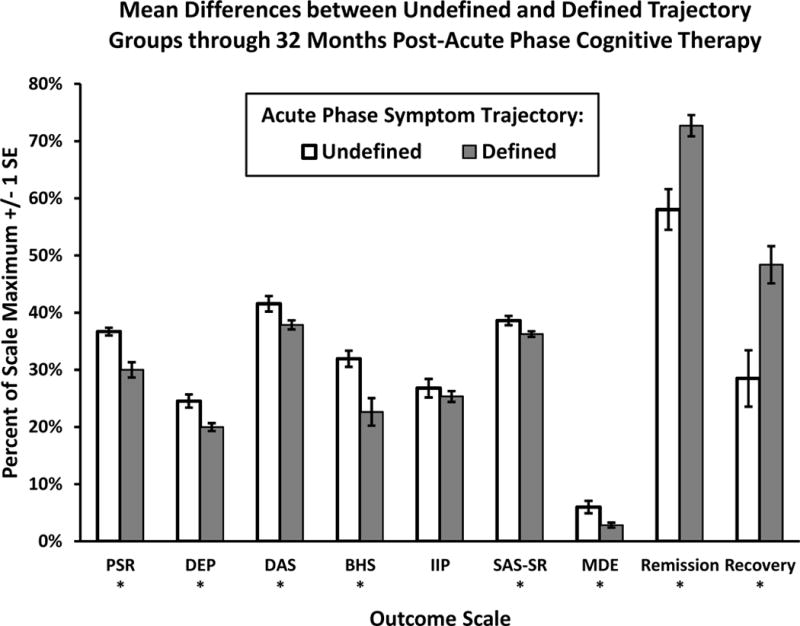
Patients with defined (vs. undefined) symptom change trajectories during acute-phase cognitive therapy showed better longitudinal outcomes. PSR = psychiatric status ratings of major depressive disorder. DEP = residual depressive symptom severity composite (3 SD above the mean at acute-phase intake was used as the scale maximum). DAS = Dysfunctional Attitudes Scale. BHS = Beck Hopelessness Scale. IIP = Inventory of Interpersonal Problems. SAS-SR = Social Adjustment Scale—Self-report. Weekly probability of being in a major depressive episode (MDE), remission, and recovery estimated using PSR.
* p < .05, two-tailed, difference between groups.
Do Defined versus Undefined Acute-Phase Trajectories Predict Better Longitudinal Outcomes after Controlling Intake and Residual Depressive Symptoms?
Yes. We controlled these variables because higher risk acute-phase CT responders with defined (vs. undefined) acute-phase trajectories had more severe depressive symptoms at acute-phase intake (defined M = 49.38, undefined M = 45.94; d = 0.40, p = .01) and lower residual symptoms at the end of the acute phase (defined M = 16.87, undefined M = 19.95; d = 0.44, p < .01). After adding intake and residual symptoms as covariates to the multilevel models, the defined (vs. undefined) trajectory group continued to show significantly lower PSRs of MDD (d = 0.28, p < .01), lower weekly probability of a being in a major depressive episode (OR = 0.43, p < .01) and higher weekly probabilities of remission (OR = 1.71, p < .01) and recovery (OR = 2.16, p < .01) for 32 months after acute-phase CT. In addition, the defined trajectory group continued to have lower BHS (d = 0.26, p = .03) and DAS (d = 0.27, p = .04) scores than the undefined group. However, the difference between defined and undefined trajectory groups was reduced to a non-significant level on the SAS-SR (d = 0.09, p = .44). In sum, incremental to intake and residual symptoms, a defined (vs. undefined) acute-phase trajectory predicted several important longer term outcomes.4
Do Longitudinal Outcomes vary among Responders with Different Defined Acute-Phase Trajectories?
Not significantly. We contrasted the defined trajectories (linear, log-linear, one-step) within the multilevel models used for hypothesis tests. Because these analyses were exploratory (we did not hypothesize differences) and involved 72 contrasts (pairwise differences among 3 trajectory groups on the 6 variables in Table 1 in 4 time frames, overall and at the end of the continuation phase and follow-up years 1 and 2) we used a more conservative alpha level of p < .01. We detected no differences between the linear, log-linear, and one-step trajectory groups.
Discussion
The current results expand the literature on predictors of longer term outcomes after response to acute-phase CT for recurrent depression, among patients judged to be at higher risk for relapse due to residual symptoms during the final weeks of CT. We found that MDD patients with defined (vs. undefined; i.e., more orderly vs. less orderly) acute-phase CT response trajectories showed better longer term outcomes. Responders with defined trajectories had less depressive symptomatology (including lower weekly probability of being in a major depressive episode and higher probabilities of remission and recovery), less depressive cognitive content (hopelessness and dysfunctional attitudes), and better social adjustment for 32 months after acute-phase CT. Responders with defined acute-phase trajectories also showed less depressive symptomatology (including lower probability of being in a major depressive episode and higher probabilities of remission and recovery) and less depressive cognitive content after controlling symptom severity both before and immediately after acute-phase CT. Thus, patients’ trajectories to response in acute-phase CT provided incremental prognostic information.
Our results underscore the value of frequent measurement during antidepressant treatments and, if replicable, may inform use of continuation-phase cognitive therapy or fluoxetine. Measurement-based care of depression involves repeated assessment of symptoms and other target domains (e.g., psychosocial functioning) to gauge treatment progress and improve clinical decisions (Scott & Lewis, 2015). Understanding MDD patients’ change trajectories requires frequent (e.g., each week or CT session) assessment using a validated measure of depressive symptoms (e.g., the BDI, HRSD, or IDS-SR). Although this statement appears straightforward, applying it in routine clinical practice requires resources and time to teach clinicians, health systems, and patients why and how to measure symptoms to identify the un/defined trajectories, as well as providing technological infrastructures, contingencies, and incentives to support such usage. Material costs of frequent assessment might be reduced by using freely available measures (e.g., Beidas et al., 2015) and/or by implementing measures on secured computers and mobile electronic devices (e.g., Epstein & Bequette, 2013). If the longitudinal superiority of defined (vs. undefined) acute-phase trajectories to response replicates, an important next step will be to develop empirical guidelines (e.g., algorithms, continuing education forums, electronic tools) to allow clinicians and health systems to respond productively to observed symptom trajectories.
To our knowledge, the current analyses provide the first longitudinal comparisons among patients with multiple trajectories during acute-phase CT for MDD. Earlier, we found that at the end of acute-phase CT, patients with defined (vs. undefined) trajectories showed lower depressive symptoms, less depressive cognitive content, and better social-interpersonal functioning, whereas patients with any of the three defined trajectories showed few differences (Vittengl et al., 2013). The current analyses revealed that higher risk responders to acute-phase CT with defined (vs. undefined) acute-phase trajectories continued to show a wide range of advantages for 32 months after acute-phase CT. Our results for patients with defined (vs. undefined) acute-phase CT trajectories are broadly parallel with findings of less relapse among antidepressant pharmacotherapy patients showing “specific” (presumably due to true drug effects) versus “non-specific” (perhaps due to placebo or non-treatment effects) initial symptom patterns (McGrath et al., 2000; Nierenberg et al., 2004).
Among defined trajectories, theorists suggest that different patterns of symptom decreases during psychotherapy signal operation of varying change mechanisms (e.g., Howard et al., 1993; Grosse Holtforth et al., 2007; Laurenceau et al., 2007). Of course, the importance of hypothesized change mechanisms would be greater if their benefits were durable. Here we found no longer term differences among defined (linear, log-linear, one-step) trajectory groups for 32 months after acute-phase CT, consistent with few differences at the end of acute-phase CT (Vittengl et al., 2013). Previous evidence supporting the superiority of a single trajectory group against all others during follow-up (e.g., patients with vs. without sudden gains; Aderka et al., 2012; Vittengl et al., 2015a) may be limited by combining several trajectories (e.g., linear, log-linear, undefined) in the “all others” group. These data underscore the importance of longitudinal studies of persons with mood disorders. We encourage future researchers to differentiate acute-phase trajectories more fully when comparing longer term outcomes among patients with MDD.
Cognitive therapists may want to consider these issues when discussing expectations for treatment process and outcomes with patients. Acknowledging that patients often have different patterns of change during cognitive therapy, and that several patterns likely mark durable improvement, is potentially useful. Such discussions could help justify why depressive symptom measures should be completed regularly and how clinicians use this information. For example, prolonged flat (non-response) or fluctuating (undefined) symptom levels may signal need to identify barriers and opportunities for change. However, the duration or number of assessments required to identify response trajectories that predict favorable longitudinal outcomes requires empirical clarification. Moreover, the similarity in longitudinal outcomes among defined trajectories (linear, log-linear, one-step) highlights the possibility that additional variables (e.g., the amount of pre-post change or slope of change) might clarify prognoses within this subset of responders. Finally, additional research is needed to understand client, therapist, and environmental variables that interact to facilitate or limit improvement during CT (e.g., Kazdin, 2009). Study of symptom-change trajectories linked with coincident measures of therapy process and social-interpersonal functioning (e.g., in cross-lagged time-series analyses) is one possible method to clarify such interactions.
Although we limited our analyses to longitudinal outcomes of higher risk responders with three hypothesized symptom-change trajectories in acute-phase CT (linear, log-linear, one-step), other patterns are possible and may be important to consider in future research. For example, a defined pattern not studied here is cubic (i.e., a depressive symptom “spike” preceded and followed by improvement), hypothesized to reflect affective arousal as schema are explored and challenged, and predictive of better outcomes at the end of exposure-based CT (Hayes, Feldman, et al., 2007). On the other hand, sudden gains are often followed by reversals of those gains (e.g., Tang & DeRubeis, 1999; Vittengl, Clark, & Jarrett, 2005), and such up-and-down variability has predicted poorer outcomes (Lutz et al., 2013; Thompson et al., 1995), similar to the current findings for the undefined trajectory group. Both depression spikes and sudden gains followed by losses may have placed patients into the current undefined trajectory category because their changes were not clearly linear, log-linear, or one-step. Additional research is required to determine whether the varying results reflect sampling error across studies, differences in operational definitions of patterns of symptom change, and/or influences of unknown moderators (e.g., specific types of treatment or diagnostic subtypes).
Qualities of our sample, procedure, and analyses limit our conclusions. Patients had carefully diagnosed recurrent MDD, received acute-phase CT from experienced and closely supervised therapists in a research protocol, and responded to CT with higher risk for relapse (Jarrett & Thase, 2010). The extent to which the current findings generalize to other patient populations and treatments is unknown. Moreover, patients consented to randomization to continuation CT, fluoxetine, or pill placebo with clinical management, all of which may have improved outcomes relative to the natural course of depression (Jarrett, Minhajuddin, Gershenfeld, et al., 2013; Vittengl et al., 2014). Although we found no interactions of trajectory group with continuation-treatment arm, better longer term outcomes with continuation treatment may lead to over- or under-estimation of the importance of trajectory groups relative to patients who stop treatment at the end of acute-phase CT. Finally, investigations using different measures (e.g., only patient- or clinician-reports instead of our multi-method composite measure of depressive symptoms) and methods (e.g., exploratory growth mixture modeling instead of our theory-driven trajectory-fitting analyses) might classify patients’ trajectories differently and change conclusions about patients’ longer term outcomes.
Nevertheless, our finding that MDD patients’ trajectories of response during acute-phase CT predict their depression status and functioning over the next 32 months is consistent with research on treatment of depression in primary care (Wardenaar et al., 2014). We found that higher risk responders with defined (vs, undefined) acute-phase trajectories, which might be characterized as more versus less orderly patterns of symptom improvement, respectively, had better longitudinal outcomes (e.g., less depression, better functioning). In contrast, whether patients’ improvement in acute-phase CT was sudden, gradual, or decelerating (i.e., our one-step, linear, and log-linear trajectories, respectively) appeared unimportant. If these results replicate, then differentiating patients’ response trajectories during acute-phase CT may inform the need for continuation treatment and clinical monitoring.
Highlights.
Responders to cognitive therapy for depression had varying symptom trajectories.
Defined (orderly) symptom trajectories predicted better outcomes post-treatment.
Response trajectories predicted outcomes incrementally to residual symptoms.
Undefined response trajectories may signal need for additional treatment.
Acknowledgments
Financial Support: This report was supported by Grants Number K24 MH001571, R01 MH58397, R01 MH69619 (to Robin B. Jarrett, Ph.D.) and R01 MH58356 and R01 MH69618 (to Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health. We also appreciate the careful review by members of the trial’s Data Safety and Monitoring Board. We are indebted to our research teams and our colleagues at The University of Texas Southwestern Medical Center, the University of Pittsburgh (where Dr. Thase was located during patient accrual), and the University of Pennsylvania (Dr. Thase’s current affiliation). We appreciate the participation of colleagues, previously named, and study participants without whom such research could not have been completed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Symptom changes of patients in the undefined trajectory group were explored using growth mixture modeling and plots of mean symptom scores across time (Vittengl et al., 2013). Some patients appeared to show roughly linear or log-linear improvements during CT. However, compared to the defined trajectory groups (mean R2 = .87), the fit of these post-hoc groups to linear and log-linear functions, respectively, was poor (mean R2 = .45) due to fluctuations in weekly symptom intensity and was not statistically significant. The remaining patients in the undefined group (about 8%) showed no appreciable improvement, and because all were non-responders, are not included in current analyses. In sum, responders in the undefined group had less orderly change in symptom scores during CT.
Two patients erroneously entered CT with HRSD = 13 at one of two diagnostic visits. During CT, one of these patients responded and one dropped out. As recommended by the Data Safety and Monitoring Board, the two patients are analyzed here as they were treated during data collection.
The 220 patients analyzed versus 21 not analyzed here did not differ significantly on the clinical and demographic characteristics described following, or in depressive symptom levels before or at the end of acute-phase CT, ps > .08.
We also explored whether undefined versus defined trajectories predicted longitudinal outcomes incremental to the number of elevated acute-phase HRSD scores. We tallied the number of HRSD scores of 7 or greater during the 14 assessments during acute-phase CT for each patient and add this covariate to the analyses. The results were substantively unchanged. Patients in the defined (vs. undefined) trajectory groups continued to show significantly lower residual symptoms, lower relapse and higher remission and recovery probabilities, and less depressive cognitive content.
Conflict of Interest Statement
Dr. Vittengl is a paid reviewer for UpToDate. Dr. Clark has no financial interest or conflict of interest in the research. During the past 3 years Dr. Thase has consulted with and/or served on advisory boards for Advir, Alkermes, Allergan, AstraZeneca, Avenir, Bristol-Myers Squibb Company, Cerecor, Cerenex, Eli Lilly and Company, Forest Laboratories, Janssen Pharmaceutica, Johnson & Johnson, Lundbeck, MedAvante, Merck, Moksha8, Naurex, Neuronetics, Novartis, Otsuka, Nestlé (formerly Pamlab), Pfizer Pharmaceuticals, Roche, Shire, Sunovion, Takeda, and Teva. During this time, he has received grant support from Alkermes, Assurerx, AstraZeneca, Avenir, Eli Lilly and Company, Forest Laboratories, Janssen/Johnson & Johnson, Otsuka, and Roche, as well as funding from the Agency for Healthcare Research and Quality and the NIMH. He has equity holdings for MedAvante, Inc. and has received royalties from American Psychiatric Publishing, Inc. (APPI), Guilford Publications, Herald House, and W.W. Norton & Company, Inc. Two books currently promoted by the APPI specifically pertain to cognitive therapy. Dr. Thase also discloses that his spouse is an employee of Peloton Advantage, which does business with several pharmaceutical companies that market medications used to treat depression. Dr. Jarrett’s medical center collects the payments from the cognitive therapy she provides to patients. Dr. Jarrett is a paid consultant to the NIH, NIMH, and UpToDate.
Contributor Information
Jeffrey R. Vittengl, Department of Psychology, Truman State University.
Lee Anna Clark, Department of Psychology, University of Notre Dame.
Michael E. Thase, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania
Robin B. Jarrett, Department of Psychiatry, The University of Texas Southwestern Medical Center.
References
- Aderka IM, Nickerson A, Bøe HJ, Hofmann SG. Sudden gains during psychological treatments of anxiety and depression: A meta-analysis. Journal of Consulting and Clinical Psychology. 2012;80:93–101. doi: 10.1037/a0026455. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Barkham M, Stiles WB, Shapiro DA. The shape of change in psychotherapy: Longitudinal assessment of personal problems. Journal of Consulting and Clinical Psychology. 1993;61:667–677. doi: 10.1037/0022-006X.61.4.667. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Hopelessness Scale. San Antonio, TX: The Psychological Corporation; 1988. [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Hopelessness and suicidal behavior: An overview. JAMA: Journal of the American Medical Association. 1975;234:1146–1149. doi: 10.1001/jama.234.11.1146. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: A 10-year prospective study of patients hospitalized with suicidal ideation. American Journal of Psychiatry. 1985;142:559–563. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The Hopelessness Scale. Journal of Consulting & Clinical Psychology. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Beidas RS, Stewart RE, Walsh L, Lucas S, Downey MM, Jackson K, Mandell DS. Free, brief, and validated: Standardized instruments for low-resource mental health settings. Cognitive and Behavioral Practice. 2015;22:5–19. doi: 10.1016/j.cbpra.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesheuvel-Leliefeld KM, Kok GD, Bockting CH, Cuijpers P, Hollon SD, van Marwijk HJ, Smit F. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: Meta-analysis and meta-regression. Journal of Affective Disorders. 2015;174:400–410. doi: 10.1016/j.jad.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Caspar F, Berger T. Insight and Cognitive Psychology. In: Castonguay LG, Hill C, Castonguay LG, Hill C, editors. Insight in psychotherapy. Washington, DC, US: American Psychological Association; 2007. pp. 375–399. [DOI] [Google Scholar]
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. The Canadian Journal of Psychiatry. 2013;58:376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- Epstein J, Bequette AW. Smart phone applications in clinical practice. Journal of Mental Health Counseling. 2013;35:283–295. [Google Scholar]
- Fava GA, Ruini C, Belaise C. The concept of recovery in major depression. Psychological Medicine. 2007;37:307–317. doi: 10.1017/S0033291706008981. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management–imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacology Bulletin. 1987;23:309–324. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- Grosse Holtforth M, Castonguay LG, Boswell JF, Wilson LA, Kakouros AA, Borkovec TD. Insight in Cognitive-Behavioral Therapy. In: Castonguay LG, Hill C, Castonguay LG, Hill C, editors. Insight in psychotherapy. Washington, DC, US: American Psychological Association; 2007. pp. 57–80. [DOI] [Google Scholar]
- Grosse Holtforth M, Hayes AM, Sutter M, Wilm K, Schmied E, Laurenceau J, Caspar F. Fostering cognitive-emotional processing in the treatment of depression: A preliminary investigation in exposure-based cognitive therapy. Psychotherapy and Psychosomatics. 2012;81:259–260. doi: 10.1159/000336813. [DOI] [PubMed] [Google Scholar]
- Hamilton KE, Dobson KS. Cognitive therapy of depression: Pretreatment patient predictors of outcome. Clinical Psychology Review. 2002;22:875–894. doi: 10.1016/S0272-7358(02)00106-X. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AM, Feldman GC, Beevers CG, Laurenceau J, Cardaciotto L, Lewis-Smith J. Discontinuities and cognitive changes in an exposure-based cognitive therapy for depression. Journal of Consulting and Clinical Psychology. 2007;75:409–421. doi: 10.1037/0022-006X.75.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AM, Laurenceau J, Feldman G, Strauss JL, Cardaciotto L. Change is not always linear: The study of nonlinear and discontinuous patterns of change in psychotherapy. Clinical Psychology Review. 2007;27:715–723. doi: 10.1016/j.cpr.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel V, Seemüller F, Obermeier M, Adli M, Bauer M, Mundt C, Riedel M. Does early improvement triggered by antidepressants predict response/remission?—Analysis of data from a naturalistic study on a large sample of inpatients with major depression. Journal of Affective Disorders. 2009;115:439–449. doi: 10.1016/j.jad.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Horowitz LM, Rosenberg SE, Baer BA, Ureño G, Villaseñor VS. Inventory of interpersonal problems: psychometric properties and clinical applications. Journal of Consulting and Clinical Psychology. 1988;56:885–892. doi: 10.1037//0022-006x.56.6.885. [DOI] [PubMed] [Google Scholar]
- Howard KI, Lueger RJ, Maling MS, Martinovich Z. A phase model of psychotherapy outcome: Causal mediation of change. Journal of Consulting and Clinical Psychology. 1993;61:678–685. doi: 10.1037/0022-006X.61.4.678. [DOI] [PubMed] [Google Scholar]
- Ilardi SS, Craighead W. The role of nonspecific factors in cognitive-behavior therapy for depression. Clinical Psychology: Science and Practice. 1994;1:138–156. [Google Scholar]
- Jarrett RB. Cognitive therapy for recurrent unipolar major depressive disorder: The continuation/maintenance phase. 1989. Unpublished treatment manual. [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase. Archives of General Psychiatry. 2001;58:381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher risk cognitive therapy responders: A randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry. 2013;70:1152–1160. doi: 10.1001/jamapsychiatry.2013.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Kangas JL, Friedman ES, Callan JA, Thase ME. Acute phase cognitive therapy for recurrent major depressive disorder: Who drops out and how much do patient skills influence response? Behaviour Research and Therapy. 2013;51:221–230. doi: 10.1016/j.brat.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Vittengl JR, Clark LA, Thase ME. Quantifying and qualifying the preventive effects of acute-phase cognitive therapy: Pathways to personalizing care. Journal of Consulting and Clinical Psychology. 2016;84:365–376. doi: 10.1037/ccp0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA. How much cognitive therapy, for which patients, will prevent depressive relapse? Journal of Affective Disorders. 2008a;111:185–192. doi: 10.1016/j.jad.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA. Preventing recurrent depression. In: Whisman MA, editor. Adapting Cognitive Therapy for Depression: Managing Complexity and Comorbidity. New York: Guilford Press; 2008b. pp. 132–156. [Google Scholar]
- Jarrett R, Thase M. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemporary Clinical Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Understanding how and why psychotherapy leads to change. Psychotherapy Research. 2009;19(4–5):418–428. doi: 10.1080/10503300802448899. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Laurenceau J, Hayes AM, Feldman GC. Some methodological and statistical issues in the study of change processes in psychotherapy. Clinical Psychology Review. 2007;27(6):682–695. doi: 10.1016/j.cpr.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W, Ehrlich T, Rubel J, Hallwachs N, Röttger M, Jorasz C, Tschitsaz-Stucki A. The ups and downs of psychotherapy: Sudden gains and sudden losses identified with session reports. Psychotherapy Research. 2013;23:14–24. doi: 10.1080/10503307.2012.693837. [DOI] [PubMed] [Google Scholar]
- Lutz W, Martinovich Z, Howard KI, Leon SC. Outcomes management, expected treatment response, and severity-adjusted provider profiling in outpatient psychotherapy. Journal of Clinical Psychology. 2002;58:1291–1304. doi: 10.1002/jclp.10070. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Stewart JW, Petkova E, Quitkin FM, Amsterdam JD, Fawcett J, Beasley CJ. Predictors of relapse during fluoxetine continuation or maintenance treatment of major depression. Journal of Clinical Psychiatry. 2000;61:518–524. doi: 10.4088/JCP.v61n0710. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Stern SL, Cicchetti DV. The Dysfunctional Attitude Scale: How well can it measure depressive thinking? Journal of Psychopathology and Behavioral Assessment. 1992;14:217–223. doi: 10.1007/BF00962629. [DOI] [Google Scholar]
- Nierenberg AA, Quitkin FM, Kremer C, Keller MB, Thase ME. Placebo-controlled continuation treatment with mirtazapine: Acute pattern of response predicts relapse. Neuropsychopharmacology. 2004;29:1012–1018. doi: 10.1038/sj.npp.1300405. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, McGrath P, Weissman MM. Biomarker studies and the future of personalized treatment for depression. Depression and Anxiety. 2014;31:902–905. doi: 10.1002/da.22300. [DOI] [PubMed] [Google Scholar]
- Otto MW, Teachman BA, Cohen LS, Soares CN, Vitonis AF, Harlow BL. Dysfunctional attitudes and episodes of major depression: Predictive validity and temporal stability in never-depressed, depressed, and recovered women. Journal of Abnormal Psychology. 2007;116:475–483. doi: 10.1037/0021-843X.116.3.475. [DOI] [PubMed] [Google Scholar]
- Percevic R, Lambert MJ, Kordy H. What is the predictive value of responses to psychotherapy for its future course? Empirical explorations and consequences for outcome monitoring. Psychotherapy Research. 2006;16:364–373. doi: 10.1080/10503300500485524. [DOI] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/S0033291700035558. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Scott K, Lewis CC. Using measurement-based care to enhance any treatment. Cognitive and Behavioral Practice. 2015;22:49–59. doi: 10.1016/j.cbpra.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham V, Insel TR. Rebooting for whom?: Portfolios, technology, and personalized intervention. Perspectives on Psychological Science. 2011;6:478–482. doi: 10.1177/1745691611418526. [DOI] [PubMed] [Google Scholar]
- Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, Friedman E. Relapse after cognitive behavior therapy of depression: Potential implications for longer courses of treatment. American Journal of Psychiatry. 1992;149:1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- Thompson MG, Thompson L, Gallagher-Thompson D. Linear and nonlinear changes in mood between psychotherapy sessions: Implications for treatment outcome and relapse risk. Psychotherapy Research. 1995;5:327–336. doi: 10.1080/10503309512331331436. [DOI] [Google Scholar]
- Vermeiden M, Kamperman AM, Vulink ME, van den Broek WW, Birkenhäger TK. Early improvement as a predictor of eventual antidepressant treatment response in severely depressed inpatients. Psychopharmacology. 2015;232:1347–1356. doi: 10.1007/s00213-014-3765-1. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Validity of Sudden Gains in Acute Phase Treatment of Depression. Journal of Consulting And Clinical Psychology. 2005;73:173–182. doi: 10.1037/0022-006X.73.1.173. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Kraft D, Jarrett RB. Multiple measures, methods, and moments: A factor-analytic investigation of change in depressive symptoms during acute-phase cognitive therapy for depression. Psychological Medicine. 2005;35:693–704. doi: 10.1017/S0033291704004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Nomothetic and idiographic symptom change trajectories in acute-phase cognitive therapy for recurrent depression. Journal of Consulting and Clinical Psychology. 2013;81:615–626. doi: 10.1037/a0032879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Stable remission and recovery after acute-phase cognitive therapy for recurrent major depressive disorder. Journal of Consulting and Clinical Psychology. 2014;82:1049–1059. doi: 10.1037/a0037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Detecting valid sudden gains during treatment of major depressive disorder: Cautions from a Monte Carlo analysis. Current Psychiatry Reviews. 2015a;11:19–31. doi: 10.2174/1573400510666140929195441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Improved cognitive content endures for two years among unstable responders to acute-phase cognitive therapy for recurrent major depressive disorder. Psychological Medicine. 2015b doi: 10.1017/S0033291715001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Predictors of longitudinal outcomes after unstable response to acute-phase cognitive therapy for major depressive disorder. Psychotherapy. 2015c;52:268–277. doi: 10.1037/pst0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardenaar KJ, Conradi H, de Jonge P. Data-driven course trajectories in primary care patients with major depressive disorder. Depression and Anxiety. 2014;31:778–786. doi: 10.1002/da.22228. [DOI] [PubMed] [Google Scholar]
- Weissman AN. The Dysfunctional Attitudes Scale: A validation study. Dissertation Abstracts International. 1979;40:1389B–1390B. [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Olfson M, Gameroff MJ, Feder A, Fuentes M. A comparison of three scales for assessing social functioning in primary care. American Journal of Psychiatry. 2001;158:460–466. doi: 10.1176/appi.ajp.158.3.460. [DOI] [PubMed] [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Philadelphia, PA: Center for Cognitive Therapy; 1980. [Google Scholar]
- Zimmerman M, Posternak MA, Chelminski I. Heterogeneity among depressed outpatients considered to be in remission. Comprehensive Psychiatry. 2007;48:113–117. doi: 10.1016/j.comppsych.2006.10.005. [DOI] [PubMed] [Google Scholar]


