Abstract
Gamma HPV197 was the most frequently identified HPV when human skin cancer specimens were analyzed by deep sequencing. To gain insight into the biological activities of HPV197, we investigated the cellular interactomes of HPV197 E6 and E7. HPV197 E6 protein interacts with a broad spectrum of cellular LXXLL domain proteins, including UBE3A and MAML1. HPV197 E6 also binds and inhibits the TP53 tumor suppressor and interacts with the CCR4-NOT ubiquitin ligase and deadenylation complex. Despite lacking a canonical retinoblastoma (RB1) tumor suppressor binding site, HPV197 E7 binds RB1 and activates E2F transcription. Hence, HPV197 E6 and E7 proteins interact with a similar set of cellular proteins as E6 and E7 proteins encoded by HPVs that have been linked to human carcinogenesis and/or have transforming activities in vitro.
INTRODUCTION
Papillomaviruses are small, non-enveloped viruses that contain approximately 8 kilobase double stranded circular DNA genomes and have been isolated from squamous epithelia of many vertebrate species. More than 200 human papillomavirus types (HPVs) have been identified. The most extensively studied HPVs are members of the alpha genus, which preferentially infect mucosal epithelia. Clinically, they are organized into “high-risk” and “low-risk” groups. High-risk HPVs cause approximately 5% of all human cancers. These include almost all cases of cervical carcinoma, a leading cause of female cancer death as well as other anogenital tract and an increasing fraction of oral cancers that affect both males and females (Schiffman et al., 2007).
High-risk HPV associated tumors express the E6 and E7 open reading frames and HPV-positive cervical cancer lines are addicted to E6/E7 expression. High-risk HPV E6 and E7 are small proteins of approximately 150 and 100 amino acid residues that exhibit oncogenic activities in cell-based and transgenic animal systems (Moody and Laimins, 2010). They lack intrinsic enzymatic activities and are not known to directly bind specific DNA sequences but exert their biological activity by binding to host cell proteins, thereby functionally reprogramming key cellular signaling circuits. High-risk alpha HPV E6 proteins interact with the cellular E3 ubiquitin ligase, UBE3A (E6AP), and target the associated TP53 tumor suppressor for proteasomal degradation (Huibregtse et al., 1993; Scheffner et al., 1993; Scheffner et al., 1990; Werness et al., 1990). High-risk HPV E6 proteins also contain a C-terminal binding site for cellular PDZ (post synaptic density protein-PSD95, Drosophila disc large tumor suppressor-Dlg1, and zonula occludens-1 protein-zo-1) domain proteins and target some of them for degradation (Glaunsinger et al., 2000; Nakagawa and Huibregtse, 2000; Thomas et al., 2002). In addition, they activate TERT (telomerase) expression and activity (Gewin and Galloway, 2001; Kiyono et al., 1998; Klingelhutz et al., 1996; Veldman et al., 2001). High-risk HPV E7 proteins bind the retinoblastoma tumor suppressor, RB1, and the related RBL1 (p107) and RBL2 (p130) proteins through a canonical LXCXE (L, Leucine; C, Cysteine; E, Glutamic acid; X, any amino acid) motif and target them for degradation.
Beta genus HPVs infect mostly cutaneous epithelia and cause warts. Some beta HPV-associated warts can progress to squamous cell carcinomas particularly in patients that suffer from a rare genetic disorder, Epidermodysplasia verruciformis (EV), or in the long-term, systemically immunosuppressed organ transplant patients. Beta HPV-associated cancers often arise at sun-exposed areas of the body, implicating UV exposure as a co-factor for cancer progression (Bouwes Bavinck et al., 2001; Euvrard et al., 1993; Orth et al., 1978; Pfister, 2003). In contrast to high-risk alpha HPV-associated cancers, beta HPV sequences are not maintained in every cancer cell. Hence, beta HPVs contribute to induction, but are not necessary for tumor maintenance (Weissenborn et al., 2005). This may explain the relative paucity of molecular studies on beta HPVs even though they were linked to non-melanoma skin cancers in EV patients several years before alpha HPV sequences were detected in cervical cancers (Dürst et al., 1983; Orth et al., 1978). The E7 proteins of HPV5 and 8, the prototypical beta HPVs associated with cancers in EV patients, bind RB1 weakly (Schmitt et al., 1994). Similarly, HPV5 and 8 E6 proteins only weakly interact with UBE3A, do not detectably bind TP53 (Rozenblatt-Rosen et al., 2012; White et al., 2012a), and they lack C-terminal PDZ binding sequences. These E6 proteins, however, inhibit the NOTCH and TGFß tumor suppressor pathways through association with MAML1 and SMAD3 proteins, respectively (Brimer et al., 2012; Mendoza et al., 2006; Meyers et al., 2013; Rozenblatt-Rosen et al., 2012; Tan et al., 2012; White et al., 2012a). HPV5 E6, E7 and E2 score as oncogenic when expressed in basal epithelial cells of transgenic mice particularly in combination with UV (Marcuzzi et al., 2009; Pfefferle et al., 2008; Schaper et al., 2005), further linking these viruses to initiation of non-melanoma skin cancers at least in EV and immune suppressed patients (McLaughlin-Drubin, 2015).
Although there is serological evidence for gamma HPVs in skin cancers (Waterboer et al., 2008), these viruses have not been definitively linked to any skin pathology (Farzan et al., 2013). A study published in 2015, however, identified HPV197, a member of species 24 of the gamma genus, as the most frequently detected HPV in skin cancers. Unlike most studies where HPVs are detected by polymerase chain reaction (PCR) using consensus primers, these authors used deep sequencing. HPV197 was detected in 34 of the 91 skin cancer specimens but none of the normal skin specimens that they analyzed (Arroyo Muhr et al., 2015). The authors also demonstrated that HPV197 sequences are not recognized by the consensus HPV PCR primers, explaining why HPV197 sequences have not previously been detected in skin lesions and cancers. As the authors were careful in pointing out, additional studies are necessary to investigate a potential etiologic link between HPV197 infections and skin carcinogenesis (Arroyo Muhr et al., 2015).
To study the biological activities of the HPV197 E6 and E7 proteins, we investigated their cellular interactomes. Since gamma HPVs have been detected in both cutaneous and mucosal epithelia (Fatahzadeh et al., 2013) we compared the HPV197 E6 and E7 interactomes to the previously published interactomes of the corresponding proteins of mucosal alpha and cutaneous beta HPVs (Rozenblatt-Rosen et al., 2012; White et al., 2012a). Here we report that the HPV197 E6 protein shares many cellular interactors with beta genus species 2 (beta 2) HPV E6 proteins. This includes the ability to bind and inhibit the TP53 tumor suppressor, as well as the capacity to associate with the CCR4-NOT (CNOT) ubiquitin ligase and deadenylation complex. Additionally, HPV197 E6 shares the capacity to bind UBE3A and proteasome subunits with alpha HPV E6 proteins. Even though HPV197 E7 lacks a canonical LXCXE motif, it can associate with RB1 and activate E2F responsive promoters and like most papillomavirus E7 proteins, HPV197 E7 also binds the UBR4 and KCMF1 ubiquitin ligases.
MATERIALS AND METHODS
Plasmids, Antibodies and Cell lines
Codon-optimized versions of HPV197 E6 and E7 were custom synthesized by Genscript, Piscataway, NJ. (Table S1). Amino (N) and carboxyl (C) terminally FLAG/HA epitope tagged as well as untagged versions of these proteins, were generated and cloned into pCMV-BamNeo (Baker et al., 1990) based expression vectors for proteomic analyses. The pC-p53 SN3 (Baker et al., 1990) and pCMV-Rb plasmids (Muller et al., 1994) were obtained from Phil Hinds (Tufts University School of Medicine, Boston, MA) , pGL3 6×E2F (Lukas et al., 1997) from Christian Helin (University of Copenhagen), and pRGC-luc, which contains 17 tandem repeats of the consensus p53 DNA binding sequence (Kern et al., 1992) was obtained from Moshe Oren (Weizmann Institute of Science). The pNCMV 16E6no* vector has been previously described (Spangle et al., 2012) and pCMV C16E7 was derived from MSCV-C 16E7 (Rozenblatt-Rosen et al., 2012) by cloning the epitope tagged HPV16 E7 fragment into the pCMV-BamNeo backbone. The EGFP-C1 plasmid used to monitor transfection was obtained from Clontech.
The following primary antibodies were used: Actin (MAB1501; Millipore), FLAG (F3165; Sigma), GFP (ab290; Abcam), MAML (12166; Cell Signalling), RB1 (Ab-5, OP66; Millipore), TP53 (ab2433; Abcam), UBE3A/E6AP (25509; Santa Cruz). Secondary anti-mouse or anti-rabbit antibodies conjugated to horseradish peroxidase were from GE Healthcare.
U-2 OS (ATCC® HTB-96™), Saos-2 (ATCC® HTB-85™) human osteosarcoma and the HCT116 (ATCC® CCL-247™) human colon carcinoma cell lines were obtained from ATCC and cultured according to their recommendations.
Affinity purification/mass spectrometry analyses
For each sample, four 15 cm tissue culture plates were seeded with 8 ×106 HCT116 human colon cancer cells each. At 24 hours after plating, each plate was transfected with 30 μg of expression plasmid. At 48 hours post transfection, cells were harvested in phosphate buffered saline (PBS) and lysed in a total of 4 ml of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5 % NP40 supplemented with one complete EDTA-free protease inhibitor cocktail tablet (Roche) per 50 ml lysis buffer) at 4°C for one hour followed by centrifugation at 19,400 × g at for 20 minutes at 4°C. Supernatants were cleared by spinning through 0.45 μm spin filters (Millipore #UFC4 0HV 00). The cleared supernatants were then incubated with a total of 30 μl of a 50 % slurry of anti HA agarose beads (Sigma #A2095) in lysis buffer for 4 to 16 hours at 4°C with constant agitation. Beads were washed several times with ice cold lysis buffer, followed by several washes with PBS to remove any traces of detergent. Bound proteins were eluted 3 times each with 50 μl of 250 μg/ml HA peptide (Sigma #I2149) in PBS. Eluates were combined and proteins precipitated in 20 % trichloroacetic acid (TCA) on ice for 25 minutes followed by centrifugation at 20,000 × g for 25 minutes at 4°C. Pellets were washed with 500 μl ice cold 10% TCA followed by 3 washes with 1 ml each of ice cold acetone to remove precipitated HA peptide. Pellets were air dried and submitted to the Taplin Proteomics Facility at Harvard Medical School. Pellets were dissolved in 40 μl 50 mM ammonium bicarbonate containing 6 ng/μl modified sequencing-grade trypsin (Promega, Madison, WI) and digested at 37°C overnight. Peptides were extracted by removing the ammonium bicarbonate solution, followed by one wash with 50% acetonitrile and 1% formic acid. The extracts were then vacuum-dried for one hour and reconstituted in 5 to 10 μl of HPLC solvent A (2.5% acetonitrile, 0.1% formic acid). A nano-scale reverse-phase HPLC capillary column was created by packing 2.6 μm C18 spherical silica beads into a fused silica capillary (100 μm inner diameter; ~30 cm length) with a flame-drawn tip (2). After equilibrating the column, each sample was loaded via a Famos auto sampler (LC Packings, San Francisco CA) onto the column. Peptides were eluted with increasing concentrations of solvent B (97.5% acetonitrile, 0.1% formic acid). As peptides eluted, they were subjected to electrospray ionization and then entered into an LTQ Orbitrap Velos Pro ion-trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Peptides were detected, isolated, and fragmented to produce a tandem mass spectrum of specific fragment ions for each peptide. Peptide sequences (and hence protein identity) were determined by matching protein databases with the acquired fragmentation pattern by the software program, Sequest (Thermo Fisher Scientific, Waltham, MA) (Eng et al., 1994). All databases include a reversed version of all the sequences and the data was filtered to between a one and two percent peptide false discovery rate.
Co-precipitation/western blot experiments
HCT116 human colon cancer cells were transfected with the corresponding HPV197 E6 or E7 expression plasmids. At 48 hours post-transfection, cells were lysed in EBC (50mM Tris-HCl pH 8.0, 150mM NaCl, 0.5% NP-40, and 0.5mM EDTA), supplemented with protease inhibitors (Roche). Anti-HA agarose (Sigma #A2095) was used for immunoprecipitations followed by SDS PAGE and western blot analysis on PVDF membranes. After incubation with the appropriate primary and secondary antibodies, protein bands were visualized by enhanced chemiluminescence, and images were acquired on a Syngene ChemiXX6 imager equipped with Genesys software version 1.5.5.0. Signals were quantified with Genetools software version 4.03.05.0
TP53 and RB1 destabilization experiments
Saos-2 human osteosarcoma cells lacking detectable TP53 or RB1 were transfected with 3 μg of TP53 or RB expression plasmids and varying amounts of pCMV-197E6 or pCMV-197E7, respectively. HPV16 E6 and HPV16 E7 expression plasmids served as positive controls for TP53 and RB1 destabilization, respectively, and EGFP-C1 was co-transfected to monitor transfection efficiency. Cells were harvested at 48 hours post-transfection and lysed in EBC as described above, and 100 μg protein containing aliquots were subjected to SDS PAGE and western blot analysis. Incubation with primary and secondary antibodies as well as detection, visualization and quantification of proteins, was performed as described above.
Reporter assays
For TP53 reporter assay, Saos-2 cells were transfected in triplicate with the p53 responsive pRGC-luc reporter, pC-p53 SN3 and varying amounts of pCMV197 E6. For E2F reporter assays, U-2 OS cells were transfected in triplicate using PEI with the E2F pGL3-E2F6 reporter and varying amounts of pCMV 197E7. HPV16 E6 (TP53) or HPV16 E7 (E2F) expression plasmids were used as positive controls, and the pGL4.53[luc2/PGK] Renilla luciferase expression plasmid (Promega) was co-transfected to allow monitoring of transfection efficiency. Cells were harvested at 48 hours post-transfection in Passive Lysis Buffer (Promega) and analyzed with the Dual Luciferase Reporter Assay kit (Promega) according to the manufacturer's instructions.
RESULTS
HPV197 E6 and E7 proteins share sequence similarity and overall domain structures with the corresponding mucosal and cutaneous HPV proteins
Papillomavirus E6 proteins consist of two pairs of CXXC (C, Cysteine; X, any amino acid) sequence motifs, which have been shown to bind zinc ions (Zanier et al., 2013). Sequence alignments of HPV197 E6, with the cutaneous beta 1 HPV8, beta 2 HPV38 and the high-risk alpha 9 HPV16 E6 proteins shows conservation of the overall domain structure (Figure 1A). Papillomavirus E7 proteins consist of an N terminal intrinsically disordered domain followed by a pair of CXXC motifs that form a zinc binding domain (Liu et al., 2006; Ohlenschlager et al., 2006). The N-terminal portions of many papillomavirus E7 proteins share sequence similarity to a small portion of the conserved region (CR) 1 at the extreme N terminus and the entire CR2 of the adenovirus E1A proteins. CR2 contains an LXCXE motif, the core binding site for the retinoblastoma tumor suppressor RB1 and the related RBL1 (p107) and RBL2 (p130) proteins, as well as an adjacent acidic domain that contains a casein kinase II phosphorylation site (Roman and Munger, 2013). HPV197 E7 shares extensive sequence similarity with HPV16 and HPV8 E7 within the CR1 homology domain and the acidic domain in the CR2 homology domain, but like other gamma HPV E7s, it lacks a canonical LXCXE RB1 binding site (Figure 1B).
Figure 1.
Sequence alignment of HPV197 E6 (A) and E7 (B) with the corresponding beta 1 HPV8, beta 2 HPV38 and alpha 9 HPV16 proteins. Identical and chemically similar amino acid residues are shown by black and gray boxes, respectively. E7 sequences previously shown to be similar to Adenovirus E1A conserved region (CR) 1 and 2 are shown. The positions of the paired CXXC motifs that form zinc binding sites, the XTXL C terminal PDZ binding site in HPV16 E6 and the LXCXE canonical RB binding site in E7 are also indicated.
HPV197 E6 and E7 interact with a similar set of cellular proteins as known alpha and beta HPV E6 proteins
Previous large scale proteomic interaction screens with HPV proteins were performed using cells with stable ectopic expression of the corresponding viral proteins (Rozenblatt-Rosen et al., 2012; White et al., 2012a; White et al., 2012b). Since papillomavirus E6 and E7 proteins have short half-lives and are encoded by rare codons (Grossman et al., 1989; Smotkin and Wettstein, 1987; Zhao et al., 2003), they are often expressed at very low levels, making affinity purification-based proteomic studies cumbersome. Hence, we performed these proteomic studies after transient transfection of codon optimized N- and carboxyl C terminally HA/FLAG epitope tagged versions of HPV197 E6 and E7 into HCT116 human colon cancer cells. HCT116 cells were chosen because they are readily transfectable, of epithelial origin, express wild type TP53 and RB1 proteins and are not known to express exogenous viruses. We then compared and contrasted our list of candidate HPV197 E6 and E7 associated cellular proteins with cellular interactors reported in two previous proteomic analyses that were performed in normal human fibroblasts (IMR90) or immortalized human foreskin keratinocytes with stable, low-level expression of high-risk (HPV16/18) and low-risk (HPV6/11) alpha and beta 1 (HPV5/8) E6 and E7 proteins (Rozenblatt-Rosen et al., 2012; White et al., 2012a; White et al., 2012b) (Tables 1 and 2).
Table 1.
HPV197 E6 associated cellular proteins previously identified in association with high-risk (HPV16, HPV18), low risk (HPV6, HPV11) and beta HPV (HPV5, HPV8) E6 proteins
| HPV5 NE61 |
HPV8 NE61 |
HPV6 NE61 |
HPV11 NE61 |
HPV16 NE61 |
HPV18 NE61 |
HPV197 CE6 Unique/Total/Coverage |
HPV197 NE6 Unique/Total/Coverage |
|---|---|---|---|---|---|---|---|
| ANKRD13A | ND | ND | |||||
| COL6A2 | ND | ND | |||||
| GALNT6 | ND | ND | |||||
| HERC2 | 8/9/3.10 % | 2/2/0.79 % | |||||
| KPNB1 | 18/20/26.48 % | 10/10/15.98 % | |||||
| MMP14 | ND | ND | |||||
| PDZRN3 | ND | ND | |||||
| PFKP | 23/26/32.40 % | 13/13/23.47 % | |||||
| PPP2R1A | 12/15/26.99 % | 9/11/17.83 % | |||||
| PPP2R2A | 10/13/31.54 % | 7/7/20.13 % | |||||
| PSMA1 | 4/4/14.83 % | ND | |||||
| PSMA4 | PSMA4 | 2/2/6.51 % | ND | ||||
| PSMA5 | ND | ND | |||||
| PSMA6 | PSMA6 | 3/3/15.45 % | ND | ||||
| PSMB1 | 1/1/8.30 % | 1/1/7.47 % | |||||
| PSMB2 | ND | ND | |||||
| PSMB4 | 5/6/29.17 % | 2/2/14.77 % | |||||
| PSMC1 | PSMC1 | 35/91/62.05 % | 28/57/64.55 % | ||||
| PSMC2 | PSMC2 | 32/69/55.66 % | 29/52/54.04 | ||||
| PSMC3 | PSMC3 | 39/90/65.15 % | 32/47/60.83 % | ||||
| PSMC4 | PSMC4 | 31/63/58.37 % | 25/41/61.24 % | ||||
| PSMC5 | PSMC5 | 34/82/54.68 % | 30/47/53.20 % | ||||
| PSMC6 | PSMC6 | 24/44/53.98 % | 22/32/53.73 % | ||||
| PSMD1 | PSMD1 | 52/106/54.35 % | 41/70/44.60 % | ||||
| PSMD3 | PSMD3 | 42/88/64.04 % | 38/68/59.93 % | ||||
| PSMD4 | PSMD4 | 11/19/28.38 % | 8/11/26.26 % | ||||
| PSMD6 | 31/55/66.84 % | 22/32/61.44 % | |||||
| PSMD7 | PSMD7 | 16/37/45.37 % | 12/24/42.28 % | ||||
| PSMD8 | PSMD8 | 13/25/37.43 % | 9/13/23.71 % | ||||
| PSMD11 | PSMD11 | 30/57/ 64.45 % | 26/46/57.35 % | ||||
| PSMD12 | PSMD12 | 30/51/48.03 % | 27/36/46.93 % | ||||
| PSMD13 | PSMD13 | PSMD13 | 23/36/53.46 % | 19/26/46.81 % | |||
| PSMD14 | PSMD14 | 19/34/57.42 % | 16/22/61.94 % | ||||
| RPL36AL | ND | ND | |||||
| RTCD1 | ND | ND | |||||
| SMU1 | ND | ND | |||||
| SNTB2 | 1/1/1.67 % | 1/1/1.67 % | |||||
| SNX27 | 1/1/2.59 % | ND | |||||
| SRBD1 | ND | ND | |||||
| SURF4 | SURF4 | ND | ND | ||||
| TOMM2 | ND | ND | |||||
| TP53 | 10/14/24.94 % | 6/6/16.03 % | |||||
| TRIM24 | ND | ND | |||||
| UBE3A | UBE3A | UBE3A | UBE3A | 31/32/39.66 % | 15/17/21.37 % | ||
| UTRN | 2/2/0.90 % | 2/2/1.05 % | |||||
| ZFR | ND | ND | |||||
| CCDC165 | ND | ND | |||||
| CD2BP2 | ND | ND | |||||
| CLUH | ND | ND | |||||
| COL6A1 | COL6A1 | 4/4/5.74 % | ND | ||||
| CREBBP | CREBBP | ND | ND | ||||
| CTTNBP2NL | ND | ND | |||||
| EP300 | EP300 | ND | ND | ||||
| FASN | 84/125/42.73 % | 66/83/35.96 % | |||||
| GFPT1 | 33/38/47.07 % | 22/23/35.19 % | |||||
| GJA1 | ND | ND | |||||
| HDGFRP2 | ND | ND | |||||
| INF2 | ND | ND | |||||
| IWS1 | ND | ND | |||||
| KANK2 | 7/7/10.34 % | 8/8/10.81 % | |||||
| LRPPRC | 7/8/7.68 % | 2/2/2.08 % | |||||
| MAML1 | 19/21/34.84 % | 6/7/14.27 % | |||||
| MAP3K11 | ND | ND | |||||
| MBOAT7 | MBOAT7 | ND | ND | ||||
| MCM3 | 30/34/54.70 % | 18/19/31.44 % | |||||
| MCM5 | 15/16/22.75 % | 9/9/16.76 | |||||
| MMS19 | 2/2/3.69 % | 3/3/5.15 % | |||||
| MTA2 | 2/2/3.69 % | 2/3/5.39 % | |||||
| NUP153 | 8/9/9.63 % | 1/1/0.75 % | |||||
| PDLIM7 | 1/1/2.41 % | 4/4/15.32 % | |||||
| PDZD11 | ND | ND | |||||
| PFKM | 31/53/40.13 % | 28/41/39.62 % | |||||
| PFN2 | 2/2/15.71 % | 1/1/4.26 % | |||||
| PPM1B | ND | ND | |||||
| PXN | 19/28/37.56 % | 14/18/35.87 % | |||||
| SHC1 | ND | ND | |||||
| SIK3 | 8/9/7.68 % | 6/6/6.41 % | |||||
| SMAD3 | ND | ND | |||||
| SOGA1 | ND | ND | |||||
| SQRDL | SQRDL | 6/6/16.67 % | 1/1/3.33 % | ||||
| SRPR | 2/2/4.39 % | ND | |||||
| SRPRB | SRPRB | 2/2/11.81 % | 2/4/11.81 % | ||||
| STARD13 | ND | ND | |||||
| TNC | ND | ND | |||||
| TNS3 | ND | ND | |||||
| TRAFD1 | 2/3/5.15 % | 1/1/2.06 % | |||||
| TRAM1 | ND | ND | |||||
| VKORC1 | VKORC1 | ND | ND | ||||
| VPS11 | ND | ND | |||||
| ZHX2 | ND | ND | |||||
| ATP2A2 | ATP2A2 | 8/8/21.98 % | 6/6/14.11 % | ||||
| CCT6A | CCT6A | 11/11/33.15 % | 8/8/26.74 % | ||||
| CNP | CNP | 7/9/14.96 % | ND | ||||
| JAK1 | JAK1 | ND | ND | ||||
| LOX | LOX | ND | ND | ||||
| PLEKHA5 | PLEKHA5 | ND | ND | ||||
| RNH1 | RNH1 | 6/7/14.53 % | 4/4/11.28 % | ||||
| RPL13A | RPL13A | 1/1/12.32 % | ND | ||||
| RPS27 | RPS27 | 1/1/28.57 % | 1/1/28.57 % | ||||
| SEPT9 | SEPT9 | 5/5/8.87 % | 1/1/2.56 % | ||||
| SRRM2 | SRRM2 | 7/7/3.74 % | 9/9/4.11 % | ||||
| UBR5 | UBR5 | 8/8/4.89 % | 1/1/0.43 % |
ND Not Detected
Data from (Rozenblatt-Rosen et al., 2012; White et al., 2012a). Data were only included if an interaction was detected in at least 2 independent experiments.
Table 2.
HPV197 E7 associated proteins previously identified in association with beta 1 HPV (HPV5, HPV8), high-risk alpha 9 (HPV16), alpha 7 (HPV18), low-risk alpha 10 (HPV6, HPV11) E7 proteins
| HPV5 CE71 |
HPV8 CE71 |
HPV6 CE71 |
HPV11 CE71 |
HPV16 CE71 |
HPV18 CE71 |
HPV197 CE7 Unique/Total/Coverage |
HPV197 NE7 Unique/Total/Coverage |
|---|---|---|---|---|---|---|---|
| AAK1 | AAK1 | ND | ND | ||||
| ABHD10 | ABHD10 | 2/3/10.46 % | 2/2/9.15 % | ||||
| ACOT9 | 7/8/20.05 % | 5/5/13.21 % | |||||
| AP2A1 | AP2A1 | 20/22/24.26 % | 23/27/27.94 % | ||||
| AP2A2 | 9/9/24.60 % | 13/13/29.29 % | |||||
| AP2B1 | 16/23/29.99 % | 25/30/47.49 % | |||||
| AP2M1 | AP2M1 | 10/12/28.05 % | 14/16/31.72 % | ||||
| AP2S1 | 1/1/6.34 % | 5/5/35.92 % | |||||
| ARHGAP35 | 4/4/5.07 % | 1/1/1.40 % | |||||
| C9orf167 (TOR4A) | 8/8/21.04 % | 4/5/11.35 % | |||||
| CCNA2 | ND | ND | |||||
| CCND3 | ND | ND | |||||
| CCNE1 | ND | ND | |||||
| CDK2 | ND | ND | |||||
| CDK4 | ND | ND | |||||
| CDK5 | 5/6/22.95 % | ND | |||||
| CDKN1A | ND | ND | |||||
| CEP170 | 20/20/22.66 % | 30/35/33.27 % | |||||
| COPE | COPE | ND | ND | ||||
| E2F1 | ND | ND | |||||
| E2F4 | E2F4 | ND | ND | ||||
| E2F5 | ND | ND | |||||
| FOXK1 | 6/6/12.96 % | 9/15/16.92 % | |||||
| GAS6 | ND | ND | |||||
| JAK1 | JAK1 | 5/5/7.45 % | 2/3/3.99 % | ||||
| KLHL25 | ND | ND | |||||
| LRRC16B | ND | ND | |||||
| MGEA5 | 1/1/1.42 % | ND | |||||
| MRPS12 | MRPS12 | ND | ND | ||||
| PCLO | ND | ND | |||||
| PCNA | 12/13/50.96 % | 8/10/36.40 % | |||||
| PSMC2 | 19/24/38.34 % | 18/27/44.34 % | |||||
| RBL2 | RBL2 | RBL2 | ND | ND | |||
| RPL21 | 3/3/20.62 % | 2/2/16.25 % | |||||
| RPS27 | ND | 1/1/28.57 % | |||||
| RPS27L | 2/2/15.48 % | 1/1/15.48 % | |||||
| SFRP1 | ND | ND | |||||
| SLAIN2 | 3/3/12.74 % | 2/4/7.92 % | |||||
| SRP9 | ND | ND | |||||
| SRRM2 | ND | 4/4/1.78 % | |||||
| TCEB1 | TCEB1 | 2/2/26.79 % | 1/1/17.86 % | ||||
| TCEB2 | 3/3/22.73 % | 3/3/23.73 % | |||||
| TFDP1 | TFDP1 | TFDP1 | TFDP1 | ND | ND | ||
| TFDP2 | ND | ND | |||||
| UBE2A | ND | ND | |||||
| ZER1 | ND | ND | |||||
| PTRF | 9/9/27.44 % | 7/7/23.08 % | |||||
| WWC2 | 4/5/5.12 % | 2/2/2.27 % | |||||
| CNP | CNP | CNP | CNP | 8/10/17.34 % | 5/5/12.35 % | ||
| COPA | COPA | COPA | COPA | COPA | 14/16/16.67 % | 14/14/16.83 % | |
| KCMF1 | KCMF1 | KCMF1 | KCMF1 | KCMF1 | KCMF1 | 2/2/9.97 % | ND |
| LOX | LOX | LOX | ND | ND | |||
| MAP4 | MAP4 | 5/5/6.68 % | 2/2/2.52 % | ||||
| PDLIM7 | PDLIM7 | 1/1/2.41 % | 2/2/5.03 % | ||||
| PTPN14 | PTPN14 | PTPN14 | PTPN14 | 1/1/1.01 % | ND | ||
| PTPN21 | PTPN21 | PTPN21 | PTPN21 | ND | 1/2/1.19 % | ||
| RB1 | RB1 | RB1 | RB1 | RB1 | RB1 | 17/18/22.09 % | 5/5/9.27 % |
| RBL1 | RBL1 | RBL1 | RBL1 | RBL1 | ND | ND | |
| SARS2 | SARS2 | SARS2 | SARS2 | ND | 1/1/2.32 % | ||
| SQRDL | SQRDL | SQRDL | SQRDL | SQRDL | SQRDL | 9/11/31.11 % | 4/4/12.22 % |
| UBR4 | UBR4 | UBR4 | UBR4 | UBR4 | UBR4 | 116/146/27.09 % | 24/30/7.81 % |
| VKORC1 | VKORC1 | VKORC1 | ND | ND |
ND Not determined
Data from (Rozenblatt-Rosen et al., 2012; White et al., 2012b). Data were only included if an interaction was detected in at least 2 independent experiments.
HPV197 E6 does not detectably associate with EP300/CREBBP
Since HCT116 cells do not express full length EP300 (Bryan et al., 2002), we did not expect to detect any EP300 peptides, but we also did not find any evidence for CREBBP binding to HPV197 E6. CREBBP peptides have been readily detected in previous proteomic analyses with HPV5 and HPV8 E6 (Rozenblatt-Rosen et al., 2012; White et al., 2012a) and we previously detected an excess of 100 individual CREBBP peptides in a proteomic analysis of HPV8 E6 associated cellular proteins that was performed by transient transfection of HCT116 cells as described here for HPV197 E6 (Grace and Munger, unpublished).
HPV197 E6 binds a variety of cellular LXXLL domain containing proteins
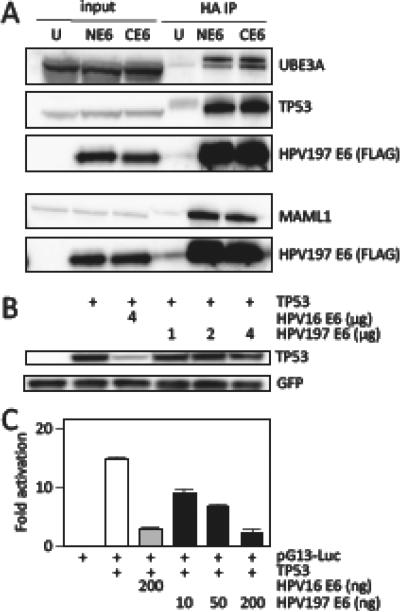
Many cellular proteins that can interact with papillomavirus E6 proteins contain LXXLL amino acid sequence motifs (L, Leucine; X, any amino acid) (Bohl et al., 2000; Chen et al., 1998). LXXLL motifs were initially identified as a common feature of transcriptional cofactors associating with nuclear hormone receptors (Plevin et al., 2005), but they have subsequently been noted in many other cellular proteins. Structural studies with HPV16 and the bovine papillomavirus 1 (BPV1) E6 proteins have shown that the LXXLL peptide motif binds into a deep groove that is formed by N terminal, as well as C terminal, E6 residues (Zanier et al., 2013). Interestingly, different papillomavirus E6 proteins appear to preferentially associate with specific LXXLL proteins. The specificity arises by interactions with the “X” amino acids of the LXXLL motif, as well as residues adjacent to the LXXLL (Zanier et al., 2013). Alpha HPV E6 proteins efficiently bind UBE3A and weakly PXN (paxillin) and MAML1, beta HPV E6 proteins bind strongly to MAML1 and weakly to PXN and UBE3A, whereas the bovine papillomavirus type 1 (BPV1) E6 protein efficiently associates with PXN and MAML1 and less efficiently with UBE3A (Vande Pol, 2015). Our experiments provided evidence for an association of HPV197 E6 with UBE3A, as well as with MAML1 and PXN, each at high peptide counts and protein coverage (Table 3). The interactions of HPV197 E6 with MAML1 and UBE3A was confirmed by immunoprecipitation/western blot experiments (Figure 2A).
Table 3.
HPV197 E6 associated cellular LXXLL domain proteins reported to interact with other papillomavirus E6 proteins (see text for references)
| Complex | Protein | HPV197 CE6 Unique/Total/Coverage | HPV197 NE6 Unique/Total/Coverage |
|---|---|---|---|
| TP53 | UBE3A | 31/32/39.66 % | 15/17/21.37 % |
| TP53 | 10/14/24.94 % | 6/6/16.03 % | |
| UBE2L3 | 3/3/29.87 % | 2/2/24.03 % | |
| NOTCH | MAML1 | 19/21/34.84 % | 6/7/14.27 % |
| NOTCH1 | 19/22/9.71 % | 8/8/3.80 % | |
| NOTCH2 | 30/40/16.92% | 23/30/13.72 % | |
| NOTCH3 | 9/11/5.34 % | 9/10/6.16 % | |
| other | PXN | 19/28/37.56 % | 14/18/35.87 % |
| other | TADA3 | 12/19/35.88 % | 6/6/19.91 % |
| other | IRF3 | 10/13/29.51 % | 6/7/19.44 % |
| other | TGFB1I1 (HIC5) | 3/3/10.63 % | 1/1/3.47 % |
| other | AP1G1 | ND | 3/3/5.72 % |
| other | RCN2 (E6BP) | 5/6/33.12 % | 5/5/37.22 % |
ND Not Detected
Figure 2.
Binding of HPV197 E6 to the LXXLL domain proteins UBE3A and MAML1 proteins and to TP53. (A) Immunoprecipitation/western blot experiments documenting interaction of ectopically expressed amino (N) and carboxyl (C) terminally HA/FLAG epitope tagged HPV197 E6 proteins with endogenously expressed MAML1, UBE3A and TP53 in HCT116 cells. Input lanes contain 4% of protein extracts used for HA immunoprecipitation (HA IP). HPV197 E6 levels were assessed by immunoblotting with a FLAG antibody. The results of a single IP are shown. UBE3A and TP53 binding was analyzed separately from MAML1 binding. (B) TP53 destabilization assays performed by transient expression of the indicated proteins in Saos-2 osteosarcoma cells. TP53 steady state levels were determined by western blotting. A GFP blot is shown to normalize TP53 levels by transfection efficiency. HPV16 E6 was used as a positive control for TP53 destabilization. (C) TP53 dependent transcriptional reporter assays. The corresponding plasmids were transfected into Saos-2 osteosarcoma cells and firefly luciferase activity was determined. Values were normalized to co-transfected renilla luciferase expression and represent averages and standard deviations from an experiment performed in triplicate. HPV16 E6 was used as a positive control for repression of TP53 transcriptional activity. Similar results were obtained in three independent experiments.
HPV197 E6 preferentially interacts with slower migrating forms of UBE3A. These may represent specific UBE3A isoforms and/or post-translationally modified versions. Similar slower migrating UBE3A forms have also been noted by other investigators (Martinez-Noel et al., 2012) but their exact biochemical nature is unknown. We also detected a few peptides of the Ubiquitin Conjugating Enzyme E2 L3 (UBE2L3) that was previously shown to contribute to UBE3A-mediated TP53 ubiquitylation by HPV16 E6 (Kumar et al., 1997) (Table 3). Similarly, in addition to MAML1, we also detected peptides corresponding to NOTCH1, NOTCH2 and NOTCH3. Of note, all of the detected peptides mapped to C-terminal NOTCH sequences, consistent with HPV197 E6 binding to a nuclear transcription factor complex that contains MAML bound to activated, cleaved NOTCH, as was previously shown for HPV8 E6 (Meyers et al., 2013).
Our proteomic experiments with HPV197 E6 also provide evidence for association with several additional cellular LXXLL domain proteins that were identified as E6 associated proteins in other studies. These include the transcriptional cofactor Transcriptional Adaptor 3 (TADA3) (Kumar et al., 2002), the Interferon Regulatory Factor 3 (IRF3) (Ronco et al., 1998), Transforming Growth Factor Beta 1 Induced Transcript 1 (TGFB1I1; HIC5) (Bryant et al., 2014), Adaptor Related Protein Complex 1 Gamma 1 Subunit (AP1G1) (Tong et al., 1998), and Reticulocalbin 2 (RCN2; ERC-55, E6BP) (Chen et al., 1995) (Table 3).
HPV197 E6 associates with and inhibits the TP53 tumor suppressor
Papillomavirus E6 proteins also interact with cellular proteins that do not contain LXXLL domains. High-risk alpha HPV E6 proteins contain a C terminal PDZ binding motif XTXL/V (T, Threonine; L, Leucine; V, Valine; X, any amino acid) (Glaunsinger et al., 2000; Nakagawa and Huibregtse, 2000). Since HPV197 lacks such a sequence (Figure 1A) it was not surprising that none of the known high-risk alpha HPV E6 binding cellular PDZ proteins were detected. In addition, HPV16 E6 associates with the TP53 tumor suppressor and HPV8 E6 with the SMAD3 protein, which do not contain LXXLL or PDZ sequences (Mendoza et al., 2006; Werness et al., 1990). A recent structural analysis of HPV16 E6, in complex with UBE3A and TP53, has revealed that there are separate binding sites for the two proteins (Martinez-Zapien et al., 2016). HPV197 E6 can bind TP53 but no SMAD3 peptides were detected (Table 1). TP53 binding by HPV197 E6 was confirmed by immunoprecipitation/western blot experiments (Figure 2A). Despite association with UBE3A, there was no evidence for TP53 destabilization by HPV197 E6 (Figure 2B). However, HPV197 E6 blunted p53 transcriptional activity in reporter assays suggesting that the association of HPV197 with TP53 may have biological consequences (Figure 2C). These results suggest that HPV197 E6 can interfere with TP53 tumor suppressor activity, although the mechanism does not involve TP53 degradation.
HPV197 E6 binds the CCR4-NOT complex and other beta 2 HPV E6 interactors
An earlier comparative proteomic analysis of E6 associated cellular proteins performed by White and colleagues provided evidence that E6 proteins of beta 2 HPVs, HPV17 and HPV38, interacted with components of the CCR4-NOT complex (White et al., 2012a). Our proteomic analysis of HPV197 E6 provided evidence for HPV197 E6 interaction with all known core components of this complex (Table 4). Given that CCR4-NOT complex interaction was specifically observed for members of the beta 2 HPV17 and HPV38 E6 proteins, we next investigated whether HPV197 E6 could also interact with other previously reported high confidence interactors of the beta 2 HPV17 and HPV38 E6 proteins (White et al., 2012a). As shown in Table 5, HPV197 E6 shares the ability to interact with a considerable number of these beta 2 HPV E6 selective interactors. In contrast, there was much less overlap with previously identified beta 4 HPV E6 selective cellular interactors (White et al., 2012a) (Table 6).
Table 4.
Association of HPV197 E6 with CCR4-NOT complex components
| Beta 2 HPV E61 | HPV197 CE6 Unique/Total/Coverage | HPV197 NE6 Unique/Total/Coverage | |
|---|---|---|---|
| CNOT1 | HCIP | 100/137/43.98 % | 76/93/39.10 % |
| CNOT2 | HCIP | 22/37/54.26 % | 14/18/47.04 % |
| CNOT3 | HCIP | 16/21/27.49 % | 9/10/14.48 % |
| CNOT4 | HCIP | 16/29/32.35 % | 13/18/27.48 % |
| CNOT6 | HCIP | 6/6/24.78 % | 4/4/14.90 % |
| CNOT6L | HCIP | 9/10/19.46 % | 8/9/16.76 % |
| CNOT7 | HCIP | 9/13/49.12 % | 6/9/31.23 % |
| CNOT8 | - | 7/7/38.70 % | 5/5/20.55 % |
| RQCD1 (CNOT9) | HCIP | 11/14/37.79 % | 10/13/37.12 % |
| CNOT10 | HCIP | 18/19/30.38 % | 10/10/20.43 % |
| C2ORF29 (CNOT11) | HCIP | 11/13/30.78 % | 7/8/20.59 % |
data from (White et al., 2012a)
Table 5.
Association of HPV197 E6 with previously identified beta 2 HPV E6 cellular interactors
| Beta 2 HPV E61 | HPV197 CE6 Unique/Total/Coverage | HPV197 NE6 Unique/Total/Coverage |
|---|---|---|
| TNKS1BP12 | 48/46/36.03 % | 35/42/26.95 % |
| TJAP13 | ND | ND |
| TANC13 | 1/1/0.54 % | ND |
| TANC2 | 27/29/20.50 % | 5/5/4.32 % |
| AMBRA1 | 21/23/27.73 % | 5/6/6.70 % |
| C21ORF2 | 2/2/9.77 % | 1/1/4.30 % |
| MX24 | ND | ND |
| NEK13 | 12/12/13.35 % | ND |
| UHMK1 | ND | ND |
| MTA25 | 2/2/3.89 % | 2/3/5.39 % |
| UBR4 | 19/19/6.52 % | 17/19/5.77°% |
| KCMF1 | 2/2/13.65 % | ND |
| UBA5 | ND | ND |
| UFM16 | ND | ND |
| JMJD1C3 | 21/27/12.09 % | 9/11/6.38 % |
Data from (White et al., 2012a)
also alpha 9 HPV16, alpha 7 HPV45, and alpha 10 HPV6 E6
HPV17 E6 only
also alpha 4 HPV57, alpha 7 HPV18, alpha 9 HPV52, beta 1 HPV20, and beta 4 HPV92 E6
also alpha 9 HPV16, beta 1 HPV20, HPV26, and beta 4 HPV92 E6
HPV17 E6 only, also alpha 4 HPV57 E6
ND Not Detected
Table 6.
Association of HPV197 E6 with previously identified beta 4 HPV92 E6 cellular interactors
| HPV92 E61 | HPV197 CE6 Unique/Total/Coverage | HPV197 NE6 Unique/Total/Coverage |
|---|---|---|
| ARNT | ND | ND |
| BANF12 | ND | 3/3/42.70 % |
| HIF1A | ND | ND |
| DUSP3 | ND | ND |
| AJUBA (JUB)3 | 14/16/30.67 % | 7/8/19.33 % |
| AAMP4 | 4/4/11.06 % | 2/2/5.53 % |
| SLC12A8 | ND | ND |
| AZI1 | 3/3/4.06 % | 2/2/3.14 % |
| CEP152 | ND | ND |
| CEP63 | ND | ND |
| THUMPD3 | 3/3/9.47 % | 2/3/6.51 % |
| CASP1 | ND | ND |
| CEP44 (KIAA1712) | ND | ND |
| PRKCQ | ND | ND |
| CAMSAP3 (KIAA1543) | 15/16/18.98 % | 6/6/6.81 % |
| VDR | ND | ND |
Data from (White et al., 2012a)
also detected with beta 2 HPV38 E6
also detected with beta 1 HPV25 E6
Also detected with alpha 7 HPV45 E6
ND Not Detected
HPV197 E7 can interact with the retinoblastoma tumor suppressor and other known papillomavirus E7 interactors
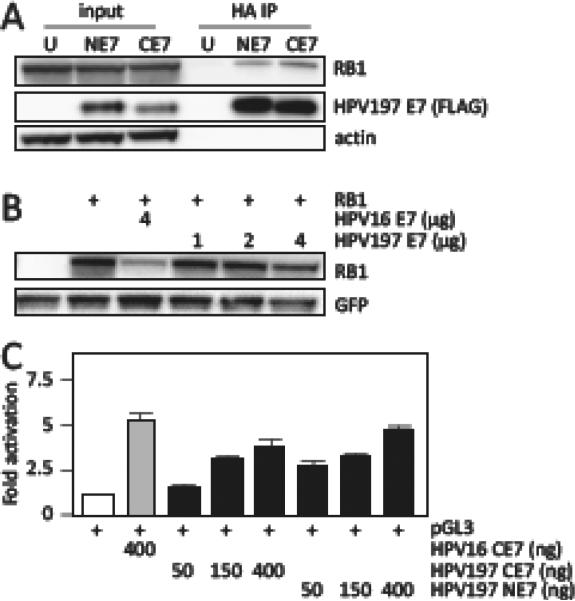
Despite lacking a canonical LXCXE-based RB1 binding site (Figure 1B), we detected evidence for RB1 binding by HPV197 E7 (Table 2). Strikingly, and in contrast to LXCXE containing HPV E7 proteins, there was no evidence for association with the RB-related RBL1 (p107) and RBL2 (p130) proteins (Table 2). RB1 binding by HPV197 E7 was validated by immunoprecipitation/western blot experiments (Figure 3A). RB1 binding and destabilization of some non-LXCXE containing papillomavirus E7 proteins has been previously reported (Wang et al., 2010) but there was no evidence for RB1 destabilization by HPV197 E7 (Figure 3B). E2F reporter assays, however, show that HPV197 E7 can activate E2F-dependent transcription. Since no peptides mapping to E2F and DP transcription factors were detected in our experiments with HPV197 E7 (Table 2), E2F activation by HPV197 E7 presumably represents a consequence of RB1 association (Figure 3C).
Figure 3.
Binding of HPV197 E7 to the RB1 tumor suppressor. (A) Immunoprecipitation/western blot experiments documenting interaction of ectopically expressed amino (NE7) and carboxyl (CE7) terminally HA/FLAG epitope tagged HPV197 E7 proteins with endogenously expressed RB1 in HCT116 cells. Untransfected (U) cells were used as controls. Input lanes contain 3.3 % of protein extracts used for HA immunoprecipitation (HA IP). HPV197 E7 levels were assessed by immunoblotting with a FLAG antibody. (B) RB1 destabilization assays performed by transient expression of the indicated proteins in Saos-2 osteosarcoma cells. RB1 steady state levels were determined by western blotting. A GFP blot is shown to normalize RB1 levels by transfection efficiency. HPV16 E7 was used as a positive control for RB1 destabilization. (C) E2F dependent transcriptional reporter assays. The corresponding plasmids were transfected into U-2 OS osteosarcoma cells and firefly luciferase activity was determined. Values were normalized to co-transfected renilla luciferase expression and represent averages and standard deviations from an experiment performed in triplicate. HPV16 E7 was used as a positive control for induction of E2F transcriptional activity. Similar results were obtained in three independent experiments.
Like most E7 proteins, HPV197 E7 also associated with the UBR4 (p600) and KCMF1 ubiquitin ligases, as well as with the non-receptor tyrosine phosphatases PTPN14 and PTPN21, albeit the latter were detected at extremely low peptide counts (Table 2).
DISCUSSION
Since gamma HPVs are largely unstudied at a molecular level, we set out to determine the cellular interactomes of the HPV197 E6 and E7 proteins. Such studies can provide important insights regarding the specific cellular signaling pathways that are targeted (White and Howley, 2013). Here we report HPV197 E6 and E7 cellular interactors that have been previously discovered in proteomic studies with alpha and beta papillomavirus E6 and E7 proteins.
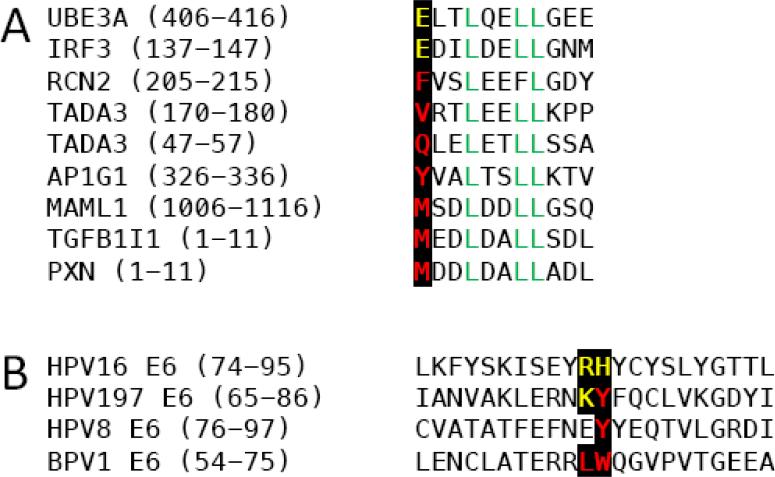
Previous investigations revealed that specific papillomavirus E6 proteins showed marked selectivity for LXXLL domain protein binding (Vande Pol, 2015). Strikingly, however, HPV197 E6 was found associated with a wide range of cellular LXXLL domain proteins (Table 3). While it may be argued that this could represent a consequence of high level, ectopic HPV197 E6 expression, similar proteomic experiments performed by transient expression in HCT116 cells, showed preferential binding of HPV16 E6 to UBE3A and HPV8 E6 to MAML1 (M. Grace and K. Munger, unpublished), as has been observed in previous experiments where the E6 proteins were expressed at lower levels (Rozenblatt-Rosen et al., 2012; White et al., 2012a). The crystal structures of BPV1 E6 bound to PXN and HPV16 E6 in complex with UBE3A derived LXXLL containing peptides, respectively, provided important insights regarding the selectivity of LXXLL peptide binding (Zanier et al., 2013). The UBE3A LXXLL motif contains a negatively charged residue (glutamic acid-E) at the −3 position, whereas the PXN LXXLL motif contains a hydrophobic residue (methionine-M) at this position (Figure 4A). In HPV16 E6, the positively charged arginine (R) 77, histidine (H) 78 and R128 residues form an electrostatic interaction with the negatively charged glutamic acid (E) residue in the UBE3A sequence. In the BPV1 E6 protein the HPV16 E6 R77 and H78 are substituted by leucine (L64) and tryptophan (W65), respectively, which form a hydrophobic patch that mediate interaction with the methionine (M) residue in the PXN sequence (Zanier et al., 2013). Similar to PXN, MAML1 has a methionine residue at the −3 position (Figure 4A) and would be predicted to preferentially bind to E6 proteins with hydrophobic amino acid residues at these positions. Consistent with this model, the MAML binding HPV8 E6 protein contains a tyrosine (Y) at the H78 position. The HPV16 E6 R77 residue is substituted by a glutamic acid (E) (Figure 4B). Although not hydrophobic, the negatively charged E will electrostatically repel the UBE3A LXXLL or others with negatively charged residues at −3. Interestingly, the HPV197 E6 protein contains a positively charged lysine (K) followed by a hydrophobic tyrosine (Y) residue at these positions, which may allow HPV197 to interact with LXXLL proteins regardless of the chemical nature of the −3 residue as we have observed in our study (Figure 4B).
Figure 4.
(A) Sequence alignments of LXXLL motifs contained in cellular proteins associated with HPV197 E6. Negatively charged and hydrophobic amino acid residues at the −3 position and the LXXLL sequence are highlighted. (B) Alignment of sequences corresponding to the alpha helix 2 and beta sheet 4 of the BPV1 and HPV16 E6 sequences and the corresponding HPV8 and HPV197 E6 sequences. Basic residues shown to be critical for binding of LXXLL proteins with an acidic residue at −3 and hydrophobic residues critical for LXXLL protein binding with hydrophobic amino acid residues at −3 are highlighted.
HPV197 E6 was found in complex with TP53 (Figure 2A) and reporter assays showed that HPV197 E6 efficiently inhibited TP53 mediated transcriptional activity (Figure 2C). It is interesting to note that a number of beta genus HPV E6 proteins, specifically the beta 2 HPV17 and HPV38, as well as the beta 4 HPV92 E6, were reported to bind and inhibit some aspects of TP53 mediated induction of target genes following DNA damage, while expression of the beta 1 HPV8 E6 protein neither bound TP53 nor modified the TP53 response in the same experimental format (White et al., 2014). However, in contrast to what was observed with the beta 2 HPV38 and HPV17 E6 (White et al., 2014), we did not observe TP53 stabilization in human keratinocytes with stable HPV197 E6 expression (data not shown).
Both N and C terminally tagged HPV197 E6 proteins bound to a large number of proteasome subunits (Table 1 - PSM). While it cannot be ruled out that this may represent an artifact of high level ectopic HPV197 E6 expression, proteasome subunit association was previously reported for alpha HPV E6 proteins, where the interactomes were investigated in cells with low level E6 expression and, in some cases, by tandem affinity purification (Rozenblatt-Rosen et al., 2012; Vos et al., 2009; White et al., 2012a). Since proteasome subunit association may be mediated by the UBE3A protein (White et al., 2012a), and given that HPV197 E6 binds to UBE3A, it may be speculated that HPV197 E6 binds to proteasome-bound UBE3A. Similarly, the HECT And RLD Domain Containing E3 Ubiquitin Protein Ligase 2 (HERC2) (Table 2) may bind to HPV197 E6 through UBE3A (Galligan et al., 2015).
Unlike the beta 1 HPV5 and HPV8 or the alpha HPV16 E6 proteins, HPV197 E6 does not detectably interact with CREBBP. Similar to HPV197, the beta 2 HPV17 and HPV38 E6 proteins do not interact with CREBBP (White et al., 2012a). Hence, the HPV197 E6 cellular interactome appears to be more similar to those of beta 2 HPV E6s than beta 1 HPV E6 proteins. Consistent with this notion, HPV197 E6 can bind to many of the beta 2 HPV E6 selective cellular interactors that have been previously reported including the CCR4-NOT transcription factor complex (Tables 4 and 5). The CCR4-NOT complex was first identified in yeast and contains both deadenylase and ubiquitin ligase activity. This complex importantly regulates many aspects of mRNA metabolism in the nucleus as well as in the cytoplasm, including transcriptional initiation and elongation, nuclear RNA processing and export as well as co-translational quality control. In addition some studies have implicated this complex in the regulation of histone modifications, particularly trimethylation of histone H3 at lysine 4 (H3K4me) (Collart, 2016; Miller and Reese, 2012).
HPV38 is the best studied beta 2 HPV, and it is frequently detected in cutaneous lesions and tumors (Feltkamp et al., 2003). The HPV38 E6 and E7 proteins have oncogenic activities in cell based and genetically engineered mouse models (Caldeira et al., 2003; Dong et al., 2005; Gabet et al., 2008; Viarisio et al., 2013; Viarisio et al., 2011) and it will be interesting to determine whether HPV197 E6 has oncogenic activities in standard transformation assays.
HPV197 E7 shares extensive amino acid sequence similarity in the N-terminal CR1 homology region and the C-terminal metal binding domain with alpha and beta HPV E7 proteins but it lacks the LXCXE canonical RB binding site (Figure 1B). Nonetheless, we found that it can bind RB1 (Figure 3A). This has been previously noted for the canine papillomavirus type 2 (CPV2) and the gamma 1 HPV4 E7 proteins, which also lack LXCXE motifs (Wang et al., 2010). The CPV1 and HPV4 E7 proteins associate with RB1 through C terminal sequences (Wang et al., 2010). Interestingly, the high-risk alpha HPV16 E7 protein has also been reported to contain a second, low affinity binding site in the C terminus (Liu et al., 2006; Patrick et al., 1994; Todorovic et al., 2012) and one may speculate that this domain is evolutionarily conserved with other papillomaviruses, including CPV1 and gamma papillomavirus E7s. CPV E7 was able to cause RB1 destabilization (Wang et al., 2010), but this activity does not appear to be conserved with HPV197 E7 (Figure 3B). Moreover, our studies revealed that HPV197 E7 preferentially interacts with RB1 and there was no evidence for interaction with the related RBL1 and RBL2 proteins (Table 2). Similarly, and in contrast to LXCXE containing papillomavirus E7 proteins, there was no evidence for association of HPV197 E7 with CDK/Cyclin complexes or E2F/DP transcription factors (Table 2). Hence, it is likely that the observed activation of E2F transcription is due to RB1 association.
In summary, our proteomic analyses revealed that the HPV197 E6 and E7 proteins interact with important cellular targets of mucosal and/or cutaneous HPV E6 and E7 proteins including UBE3A, MAML1 and TP53 as well as RB1 and UBR4. Some of these are key mediators of the oncogenic activities of high-risk alpha and beta HPV E6 and E7 proteins. Thus, it will be interesting to determine if the E6 and/or E7 proteins of HPV197, and potentially other gamma HPVs, have oncogenic activities and if they contribute to some aspect of human carcinogenesis or whether they truly are biologically innocuous components of the skin virome.
Supplementary Material
Highlights.
HPV197 is a gamma genus HPV that has been detected in human skin cancers
We investigated HPV197 E6 and E7 cellular interactomes
HPV197 E6 and E7 bind important cellular targets of cancer associated HPV E6 and E7
ACKNOWLEDGMENTS
We thank Joakim Dillner and Carolina Eklund (Karolinska Institutet, Stockholm, Sweden) for providing the HPV197 genomic clones, Ross Tomaino and Steve Gygi (Taplin Mass Spectrometry Facility, Harvard Medical School, Boston, MA) for expert advice and guidance with the affinity purification/mass spectrometry experiments, Elizabeth White (Harvard Medical School, Boston MA) and Scott Vande Pol (University of Virginia School of Medicine, Charlottesville VA) for stimulating discussions and advice and Elizabeth White and members of the Munger Lab, in particular Katherine R. Mattaini and Mallory E. Harden, for helpful suggestions and comments on this manuscript. Supported by PHS grant CA066980 (KM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arroyo Muhr LS, Hultin E, Bzhalava D, Eklund C, Lagheden C, Ekstrom J, Johansson H, Forslund O, Dillner J. Human papillomavirus type 197 is commonly present in skin tumors. Int J Cancer. 2015;136:2546–2555. doi: 10.1002/ijc.29325. [DOI] [PubMed] [Google Scholar]
- Baker SJ, Markowitz S, Fearon ER, Willson JKV, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bohl J, Das K, Dasgupta B, Vande Pol SB. Competitive binding to a charged leucine motif represses transformation by a papillomavirus E6 oncoprotein. Virology. 2000;271:163–170. doi: 10.1006/viro.2000.0316. [DOI] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Feltkamp M, Struijk L, ter Schegget J. Human papillomavirus infection and skin cancer risk in organ transplant recipients. The journal of investigative dermatology. Symposium proceedings / the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research. 2001;6:207–211. doi: 10.1046/j.0022-202x.2001.00048.x. [DOI] [PubMed] [Google Scholar]
- Brimer N, Lyons C, Wallberg AE, Vande Pol SB. Cutaneous papillomavirus E6 oncoproteins associate with MAML1 to repress transactivation and NOTCH signaling. Oncogene. 2012;31:4639–4646. doi: 10.1038/onc.2011.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan EJ, Jokubaitis VJ, Chamberlain NL, Baxter SW, Dawson E, Choong DY, Campbell IG. Mutation analysis of EP300 in colon, breast and ovarian carcinomas. Int J Cancer. 2002;102:137–141. doi: 10.1002/ijc.10682. [DOI] [PubMed] [Google Scholar]
- Bryant D, Tristram A, Liloglou T, Hibbitts S, Fiander A, Powell N. Quantitative measurement of Human Papillomavirus type 16 L1/L2 DNA methylation correlates with cervical disease grade. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2014;59:24–29. doi: 10.1016/j.jcv.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Caldeira S, Zehbe I, Accardi R, Malanchi I, Dong W, Giarre M, de Villiers EM, Filotico R, Boukamp P, Tommasino M. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol. 2003;77:2195–2206. doi: 10.1128/JVI.77.3.2195-2206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Hong Y, Rustamzadeh E, Baleja JD, Androphy EJ. Identification of an alpha helical motif sufficient for association with papillomavirus E6. J Biol Chem. 1998;273:13537–13544. doi: 10.1074/jbc.273.22.13537. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Reid CE, Band V, Androphy EJ. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995;269:529–531. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- Collart MA. The Ccr4-Not complex is a key regulator of eukaryotic gene expression. Wiley interdisciplinary reviews. RNA. 2016;7:438–454. doi: 10.1002/wrna.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Kloz U, Accardi R, Caldeira S, Tong WM, Wang ZQ, Jansen L, Durst M, Sylla BS, Gissmann L, Tommasino M. Skin hyperproliferation and susceptibility to chemical carcinogenesis in transgenic mice expressing E6 and E7 of human papillomavirus type 38. J Virol. 2005;79:14899–14908. doi: 10.1128/JVI.79.23.14899-14908.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proceedings of the National Academy of Sciences USA. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Euvrard S, Chardonnet Y, Pouteil-Noble C, Kanitakis J, Chignol MC, Thivolet J, Touraine JL. Association of skin malignancies with various and multiple carcinogenic and noncarcinogenic human papillomaviruses in renal transplant recipients. Cancer. 1993;72:2198–2206. doi: 10.1002/1097-0142(19931001)72:7<2198::aid-cncr2820720722>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Waterboer T, Gui J, Nelson HH, Li Z, Michael KM, Perry AE, Spencer SK, Demidenko E, Green AC, Pawlita M, Karagas MR. Cutaneous alpha, beta and gamma human papillomaviruses in relation to squamous cell carcinoma of the skin: a population-based study. Int J Cancer. 2013;133:1713–1720. doi: 10.1002/ijc.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatahzadeh M, Schlecht NF, Chen Z, Bottalico D, McKinney S, Ostoloza J, Dunne A, Burk RD. Oral human papillomavirus detection in older adults who have human immunodeficiency virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 doi: 10.1016/j.oooo.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltkamp MC, Broer R, di Summa FM, Struijk L, van der Meijden E, Verlaan BP, Westendorp RG, ter Schegget J, Spaan WJ, Bouwes Bavinck JN. Seroreactivity to epidermodysplasia verruciformis-related human papillomavirus types is associated with nonmelanoma skin cancer. Cancer Res. 2003;63:2695–2700. [PubMed] [Google Scholar]
- Gabet AS, Accardi R, Bellopede A, Popp S, Boukamp P, Sylla BS, Londono-Vallejo JA, Tommasino M. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J. 2008;22:622–632. doi: 10.1096/fj.07-8389com. [DOI] [PubMed] [Google Scholar]
- Galligan JT, Martinez-Noel G, Arndt V, Hayes S, Chittenden TW, Harper JW, Howley PM. Proteomic analysis and identification of cellular interactors of the giant ubiquitin ligase HERC2. J Proteome Res. 2015;14:953–966. doi: 10.1021/pr501005v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewin L, Galloway DA. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J Virol. 2001;75:7198–7201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19:5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SR, Mora R, Laimins LA. Intracellular localization and DNA-binding properties of human papillomavirus type 18 E6 protein expressed with a baculovirus vector. J Virol. 1989;63:366–374. doi: 10.1128/jvi.63.1.366-374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Molecular and Cellular Biology. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern SE, Pietenpol JA, Thiagalingam S, Seymour A, Kinzler KW, Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992;256:827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhao Y, Meng G, Zeng M, Srinivasan S, Delmolino LM, Gao Q, Dimri G, Weber GF, Wazer DE, Band H, Band V. Human papillomavirus oncoprotein E6 inactivates the transcriptional coactivator human ADA3. Mol Cell Biol. 2002;22:5801–5812. doi: 10.1128/MCB.22.16.5801-5812.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kao WH, Howley PM. Physical interaction between specific E2 and Hect E3 enzymes determines functional cooperativity. J Biol Chem. 1997;272:13548–13554. doi: 10.1074/jbc.272.21.13548. [DOI] [PubMed] [Google Scholar]
- Liu X, Clements A, Zhao K, Marmorstein R. Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J Biol Chem. 2006;281:578–586. doi: 10.1074/jbc.M508455200. [DOI] [PubMed] [Google Scholar]
- Lukas J, Herzinger T, Hansen K, Moroni MC, Resnitzky D, Helin K, Reed SI, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- Marcuzzi GP, Hufbauer M, Kasper HU, Weissenborn SJ, Smola S, Pfister H. Spontaneous tumour development in human papillomavirus type 8 E6 transgenic mice and rapid induction by UV-light exposure and wounding. J Gen Virol. 2009;90:2855–2864. doi: 10.1099/vir.0.012872-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Noel G, Galligan JT, Sowa ME, Arndt V, Overton TM, Harper JW, Howley PM. Identification and proteomic analysis of distinct UBE3A/E6AP protein complexes. Mol Cell Biol. 2012;32:3095–3106. doi: 10.1128/MCB.00201-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Zapien D, Ruiz FX, Poirson J, Mitschler A, Ramirez J, Forster A, Cousido-Siah A, Masson M, Vande Pol S, Podjarny A, Trave G, Zanier K. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature. 2016;529:541–545. doi: 10.1038/nature16481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME. Human papillomaviruses and non-melanoma skin cancer. Semin Oncol. 2015;42:284–290. doi: 10.1053/j.seminoncol.2014.12.032. [DOI] [PubMed] [Google Scholar]
- Mendoza JA, Jacob Y, Cassonnet P, Favre M. Human papillomavirus type 5 E6 oncoprotein represses the transforming growth factor beta signaling pathway by binding to SMAD3. J Virol. 2006;80:12420–12424. doi: 10.1128/JVI.02576-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JM, Spangle JM, Munger K. The human papillomavirus type 8 E6 protein interferes with NOTCH activation during keratinocyte differentiation. J Virol. 2013;87:4762–4767. doi: 10.1128/JVI.02527-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Reese JC. Ccr4-Not complex: the control freak of eukaryotic cells. Crit Rev Biochem Mol Biol. 2012;47:315–333. doi: 10.3109/10409238.2012.667214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- Muller H, Lukas J, Schneider A, Warthoe P, Bartek J, Eilers M, Strauss M. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc Natl Acad Sci U S A. 1994;91:2945–2949. doi: 10.1073/pnas.91.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlenschlager O, Seiboth T, Zengerling H, Briese L, Marchanka A, Ramachandran R, Baum M, Korbas M, Meyer-Klaucke W, Durst M, Gorlach M. Solution structure of the partially folded high-risk human papilloma virus 45 oncoprotein E7. Oncogene. 2006 doi: 10.1038/sj.onc.1209584. [DOI] [PubMed] [Google Scholar]
- Orth G, Jablonska S, Favre M, Croissant O, Jarzabek-Chorzelska M, Rzesa G. Characterization of two types of human papillomaviruses in lesions of epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1978;75:1537–1541. doi: 10.1073/pnas.75.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick DR, Oliff A, Heimbrook DC. Identification of a novel retinoblastoma gene product binding site on human papillomavirus type 16 E7 protein. Journal of Biological Chemistry. 1994;269:6842–6850. [PubMed] [Google Scholar]
- Pfefferle R, Marcuzzi GP, Akgul B, Kasper HU, Schulze F, Haase I, Wickenhauser C, Pfister H. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J Invest Dermatol. 2008;128:2310–2315. doi: 10.1038/jid.2008.73. [DOI] [PubMed] [Google Scholar]
- Pfister H. Human papillomavirus and skin cancer. Journal of the National Cancer Institute. 2003:52–56. doi: 10.1093/oxfordjournals.jncimonographs.a003483. Chapter 8, Monographs. [DOI] [PubMed] [Google Scholar]
- Plevin MJ, Mills MM, Ikura M. The LxxLL motif: a multifunctional binding sequence in transcriptional regulation. Trends in biochemical sciences. 2005;30:66–69. doi: 10.1016/j.tibs.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Roman A, Munger K. The papillomavirus E7 proteins. Virology. 2013;445:138–168. doi: 10.1016/j.virol.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Deo RC, Padi M, Adelmant G, Calderwood MA, Rolland T, Grace M, Dricot A, Askenazi M, Tavares M, Pevzner SJ, Abderazzaq F, Byrdsong D, Carvunis AR, Chen AA, Cheng J, Correll M, Duarte M, Fan C, Feltkamp MC, Ficarro SB, Franchi R, Garg BK, Gulbahce N, Hao T, Holthaus AM, James R, Korkhin A, Litovchick L, Mar JC, Pak TR, Rabello S, Rubio R, Shen Y, Singh S, Spangle JM, Tasan M, Wanamaker S, Webber JT, Roecklein-Canfield J, Johannsen E, Barabasi AL, Beroukhim R, Kieff E, Cusick ME, Hill DE, Munger K, Marto JA, Quackenbush J, Roth FP, DeCaprio JA, Vidal M. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature. 2012;487:491–495. doi: 10.1038/nature11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper ID, Marcuzzi GP, Weissenborn SJ, Kasper HU, Dries V, Smyth N, Fuchs P, Pfister H. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005;65:1394–1400. doi: 10.1158/0008-5472.CAN-04-3263. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP Complex Functions as a Ubiquitin-Protein Ligase in the Ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Harry JB, Rapp B, Wettstein FO, Iftner T. Comparison of the properties of the E6 and E7 genes of low- and high-risk cutaneous papillomaviruses reveals strongly transforming and high Rb-binding activity for the E7 protein of the low-risk human papillomavirus type 1. J Virol. 1994;68:7051–7059. doi: 10.1128/jvi.68.11.7051-7059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D, Wettstein FO. The major human papillomavirus protein in cervical cancers is a cytoplasmic phosphoprotein. J Virol. 1987;61:1686–1689. doi: 10.1128/jvi.61.5.1686-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangle JM, Ghosh-Choudhury N, Munger K. Activation of cap-dependent translation by mucosal human papillomavirus E6 proteins is dependent on the integrity of the LXXLL binding motif. J Virol. 2012;86:7466–7472. doi: 10.1128/JVI.00487-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MJ, White EA, Sowa ME, Harper JW, Aster JC, Howley PM. Cutaneous beta-human papillomavirus E6 proteins bind Mastermind-like coactivators and repress Notch signaling. Proc Natl Acad Sci U S A. 2012;109:E1473–1480. doi: 10.1073/pnas.1205991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21:5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- Todorovic B, Hung K, Massimi P, Avvakumov N, Dick FA, Shaw GS, Banks L, Mymryk JS. Conserved region 3 of human papillomavirus 16 E7 contributes to deregulation of the retinoblastoma tumor suppressor. J Virol. 2012;86:13313–13323. doi: 10.1128/JVI.01637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Boll W, Kirchhausen T, Howley PM. Interaction of the bovine papillomavirus E6 protein with the clathrin adaptor complex AP-1. J Virol. 1998;72:476–482. doi: 10.1128/jvi.72.1.476-482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Pol S. Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems. PLoS Pathog. 2015;11:e1005138. doi: 10.1371/journal.ppat.1005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75:4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viarisio D, Decker KM, Aengeneyndt B, Flechtenmacher C, Gissmann L, Tommasino M. Human papillomavirus type 38 E6 and E7 act as tumour promoters during chemically induced skin carcinogenesis. J Gen Virol. 2013;94:749–752. doi: 10.1099/vir.0.048991-0. [DOI] [PubMed] [Google Scholar]
- Viarisio D, Mueller-Decker K, Kloz U, Aengeneyndt B, Kopp-Schneider A, Grone HJ, Gheit T, Flechtenmacher C, Gissmann L, Tommasino M. E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog. 2011;7:e1002125. doi: 10.1371/journal.ppat.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos RM, Altreuter J, White EA, Howley PM. The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. J Virol. 2009;83:8885–8892. doi: 10.1128/JVI.00605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou D, Prabhu A, Schlegel R, Yuan H. The canine papillomavirus and gamma HPV E7 proteins use an alternative domain to bind and destabilize the retinoblastoma protein. PLoS Pathog. 2010;6:e1001089. doi: 10.1371/journal.ppat.1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterboer T, Abeni D, Sampogna F, Rother A, Masini C, Sehr P, Michael KM, Pawlita M. Serological association of beta and gamma human papillomaviruses with squamous cell carcinoma of the skin. Br J Dermatol. 2008;159:457–459. doi: 10.1111/j.1365-2133.2008.08621.x. [DOI] [PubMed] [Google Scholar]
- Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, Majewski S, Pfister H, Wieland U. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol. 2005;125:93–97. doi: 10.1111/j.0022-202X.2005.23733.x. [DOI] [PubMed] [Google Scholar]
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- White EA, Howley PM. Proteomic approaches to the study of papillomavirus-host interactions. Virology. 2013;435:57–69. doi: 10.1016/j.virol.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Kramer RE, Tan MJ, Hayes SD, Harper JW, Howley PM. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J Virol. 2012a;86:13174–13186. doi: 10.1128/JVI.02172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Sowa ME, Tan MJ, Jeudy S, Hayes SD, Santha S, Munger K, Harper JW, Howley PM. Systematic identification of interactions between host cell proteins and E7 oncoproteins from diverse human papillomaviruses. Proc Natl Acad Sci U S A. 2012b;109:E260–267. doi: 10.1073/pnas.1116776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Walther J, Javanbakht H, Howley PM. Genus Beta Human Papillomavirus E6 Proteins Vary in Their Effects on the Transactivation of p53 Target Genes. J Virol. 2014;88:8201–8212. doi: 10.1128/JVI.01197-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanier K, Charbonnier S, Sidi AO, McEwen AG, Ferrario MG, Poussin-Courmontagne P, Cura V, Brimer N, Babah KO, Ansari T, Muller I, Stote RH, Cavarelli J, Vande Pol S, Trave G. Structural basis for hijacking of cellular LxxLL motifs by papillomavirus E6 oncoproteins. Science. 2013;339:694–698. doi: 10.1126/science.1229934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao KN, Liu WJ, Frazer IH. Codon usage bias and A+T content variation in human papillomavirus genomes. Virus Res. 2003;98:95–104. doi: 10.1016/j.virusres.2003.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.