Abstract
Background and Purpose
Early markers for cerebral amyloid angiopathy (CAA) are largely unknown. We aimed to identify which MRI (performed at 7 tesla and 3 tesla) and cognitive markers are an early sign in (pre-)symptomatic subjects with Hereditary Cerebral Hemorrhage With Amyloidosis-Dutch type (HCHWA-D).
Methods
Twenty-seven DNA-proven Dutch type mutation carriers (15 symptomatic and 12 pre-symptomatic) (mean age of 45.9) and 33 controls (mean age of 45.6) were included. 7T and 3T MRI was performed, CAA and small vessel disease type MRI markers were estimated, and cognitive performance was assessed. Univariate general linear modeling analysis was used to assess the association between MRI markers and cognitive performance on the one hand, and on the other mutation status, adjusted for age, sex and education.
Results
In symptomatic patients, all established CAA MRI markers (microbleeds, intracerebral hemorrhages, subarachnoid hemorrhages, superficial siderosis, microinfarcts, volume of white matter hyperintensities (WMHs) and dilated perivascular spaces in centrum semiovale) were increased compared to controls (p<0.05). In pre-symptomatic subjects, the prevalence of microinfarcts and median volume of WMHs were increased in comparison to controls (p<0.05). Symptomatic patients performed worse on all cognitive domains, whereas pre-symptomatic subjects did not show differences in comparison with controls (p<0.05).
Conclusions
WMHs and microinfarcts are more prevalent among pre-symptomatic subjects and precede cognitive and neuropsychiatric symptoms and intracerebral hemorrhages.
Keywords: cerebral amyloid angiopathy, hemorrhage, magnetic resonance imaging, cognition
Introduction
A major problem in diagnosing sporadic cerebral amyloid angiopathy (sCAA) is the absence of reliable, non-invasive diagnostic tests. Hereditary cerebral hemorrhage with amyloidosis-Dutch type (HCHWA-D) is an autosomal dominant disease and the chemical composition and underlying pathology of the amyloid deposits is similar to that in sCAA.1 Clinically, the symptomatic stage of both sCAA and HCHWA-D is characterized by recurrent hemorrhagic strokes and cognitive impairment2 and common radiological manifestations are microbleeds, intracerebral hemorrhages (ICHs), superficial siderosis, convexity subarachnoid hemorrhages (SAHs)3, greater volumes of white matter hyperintensities (WMHs)4, 5 and microinfarcts.6 As sCAA and HCHWA-D are subtypes of small vessel disease (SVD), other SVD markers such as lacunar infarcts and dilated perivascular spaces (DPVS) might also be more prevalent in sCAA and HCHWA-D. Previous studies suggested that in patients with sCAA and HCHWA-D, cognitive impairment may also be an early disease marker, preceding stroke or any other brain lesion.2, 7 The goal of our study is to identify which markers are an early sign of HCHWA-D using the most sensitive MRI techniques at 3T and 7T, and to assess whether cognitive decline and/or neuropsychiatric abnormalities are an early sign of the disease.
Materials and Methods
HCHWA-D and control subjects were recruited via the HCHWA-D patient association in Katwijk (the Netherlands) and outpatient clinic of the Department of Neurology of the Leiden University Medical Center. Twenty-seven DNA-proven HCHWA-D mutation carriers and thirty-three controls were included (symptomatic (n=15) and pre-symptomatic (n=12) mutation carriers). Subjects were considered symptomatic when they had experienced signs of the disease reported to a general practitioner. At 7T T2*-weighted gradient-echo scans and fluid attenuated inversion recovery (FLAIR) sequences were performed. At 3T, FLAIR, T1-weighted and T2-weighted images were acquired. Microbleeds, ICHs, superficial siderosis and convexity SAHs were assessed at 7T T2*-weighted sequences. Cortical microinfarcts were scored as previously described.8 Since counting microinfarcts is a relatively new technique, these lesions were scored by two independent experienced raters and interrater reliability was calculated. At 3T, WMHs, DPVS in the basal ganglia (BG) and centrum semiovale (CSO) and lacunar infarcts were assessed. A battery of neuropsychological and neuropsychiatric tests was performed. Please see http://stroke.ahajournals.org for supplementary information.
Statistics
Mann-Whitney U-testing was used to assess differences in age between groups, univariate general linear modeling (GLM) analysis was used to assess differences in blood pressure measurements, adjusted for age and sex, and chi-square tests were used to assess differences in sex, educational level and percentage cardiovascular risk factors between groups. For assessment of microinfarcts, interobserver variability was calculated. Univariate GLM analysis was used to assess the association between MRI markers and cognitive performance on the one hand, and on the other mutation status, adjusted for age, sex and education. Please see http://stroke.ahajournals.org.
Results
The characteristics of the pre-symptomatic mutation carriers and symptomatic mutation carriers versus controls are shown in supplementary table I. All symptomatic patients clinically experienced one or multiple strokes as first symptomatic sign of the disease. None of the symptomatic patients experienced objective cognitive impairment as first sign of the disease. None of the pre-symptomatic mutation carriers reported cognitive complaints or showed objective cognitive impairment.
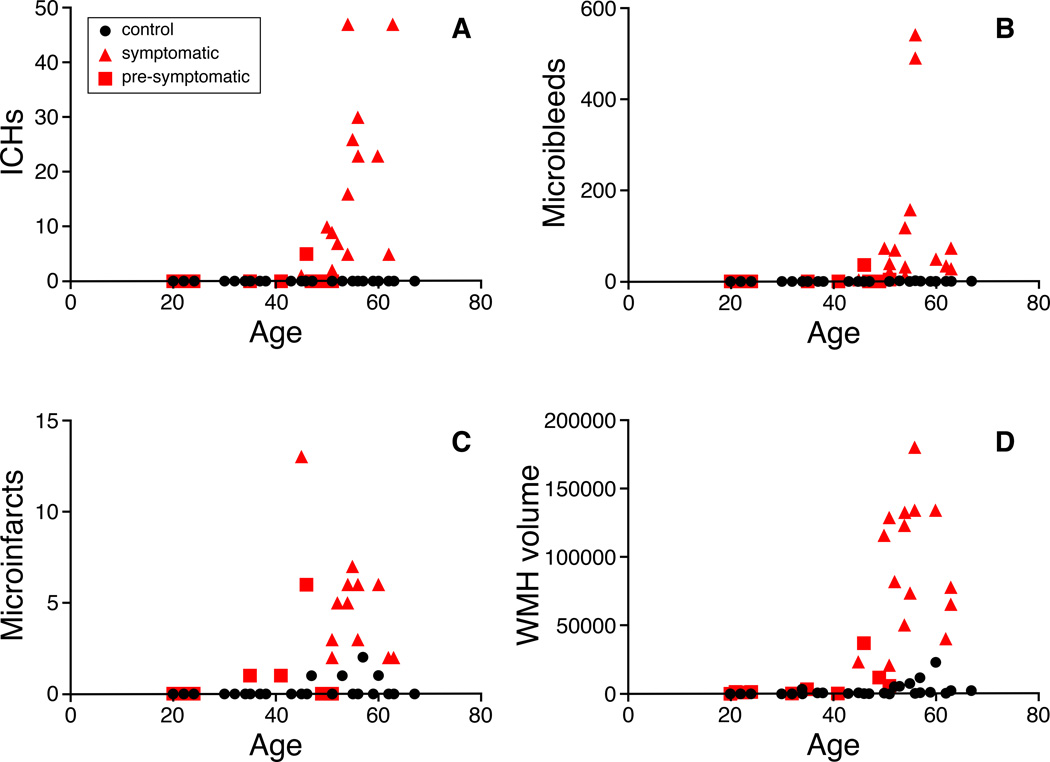
The κ value for interobserver agreement was almost perfect for detecting presence of cortical microinfarcts, κ = 0.88 (p<0.001). The prevalence and median count of microbleeds, ICHs, convexity SAHs, superficial siderosis, microinfarcts, median volume of WMHs and DPVS-CSO is significantly higher in the symptomatic mutation carriers than in controls, adjusted for age and sex (p<0.05) (table 1). In pre-symptomatic mutation carriers, the prevalence of microinfarcts (p=0.044) and median volume of WMHs (p=0.000) is significantly increased compared to controls adjusted for age and sex. As illustrated in Figure 1 our cross-sectional data suggest that patients start developing changes on MRI at about five years prior to developing their first symptoms (at age 45).
Table 1.
MRI markers in pre-symptomatic and symptomatic mutation carriers versus controls.
| MRI markers | Controls (N=33) | Pre-symptomatic carriers (N=12) | Symptomatic carriers (N=15) | |||
|---|---|---|---|---|---|---|
| Prevalence | Median (25th–75th percentile) |
Prevalence | Median (25th–75th percentile) |
Prevalence | Median (25th–75th percentile) |
|
| Microbleeds | 6.7% (2/30) | 0 (0 – 0) | 18.2% (2/11) | 0 (0 – 0) | 100% (15/15)** | 49 (28 – 118)** |
| ICHs | 0% (0/30) | 0 (0 – 0) | 9.1% (1/11) | 0 (0 – 0) | 100% (14/14)** | 13 (5 – 27)** |
| Convexity SAHs | 0% (0/30) | 0 (0 – 0) | 18.2% (2/11) | 0 (0 – 0) | 50% (7/14)** | 0.5 (0 – 2.3)* |
| Siderosis (%) | 0 % (0/30) | - | 9.1% (1/11) | - | 93.3% (14/15)** | - |
| Cortical microinfarcts (mean, range) | 13.8% (4/29) | 0.2 (0 – 2) | 30.0% (3/10)* | 0.8 (0 – 6) | 100% (12/12)** | 5 (2 – 13)** |
| WMHs volume (cm3) | - | 0.6 (0.2 – 2.2) | - | 1.4 (0.4 – 6.0)** | - | 82.4 (50.7 – 133.0)** |
| DPVS-CSO | - | 3 (2 – 3) | - | 3 (2 – 4) | - | 4 (3 – 4)* |
| DPVS-BG | - | 2 (2 – 2) | - | 2 (2 – 2) | - | 2 (2 – 2) |
| Lacunar infarcts | 32.3% (10/31) | 0 (0 – 1) | 27.3% (3/11) | 0 (0 – 1) | 13.3% (2/15)* | 0 (0 – 0) |
=p<0.05 and
=p<0.001; controls vs pre-symptomatic carriers and controls vs symptomatic carriers
Figure 1.
Graphs of A) ICHs, B) microbleeds, C) microinfarcts and D) WMH volume against age in the three different groups.
All cognitive tests were performed significantly worse by symptomatic mutation carriers than controls, adjusted for age, sex and education (p<0.05). They also showed a higher score on the HADS anxiety and depression scale (p<0.05). Pre-symptomatic mutation carriers did not show any significant differences compared with controls on the cognitive and neuropsychiatric tests (table 2).
Table 2.
Cognitive and neuropsychiatric markers in pre-symptomatic and symptomatic mutation carriers versus controls.
| Cognitive domain | Cognitive tests | Controls (N=33) (SD; range) |
Pre-symptomatic mutation carriers (N=12) (SD; range) |
Symptomatic mutation carriers (N=15) (SD; range) |
|---|---|---|---|---|
| Global cognitive function | MMSE (mean points) | 29.4 (0.7; 27 – 30) | 29.8 (0.6; 28 – 30) | 26.7 (3.8; 16 – 30)** |
| Memory | WMS (mean Memory quotient) | 124.9 (10.8; 105 – 143) | 121.6 (11.5; 103 – 137) | 110.5 (24.9; 72 – 143)* |
| HVLT immediate recall | 28.2 (4.3; 16 – 35) | 28.0 (2.8; 24 – 32) | 21.4 (7.5; 7 – 31)* | |
| HVLT delayed recall | 10.4 (2.3; 6 – 19) | 9.8 (1.7; 7 – 12) | 6.8 (3.7; 0 – 12)** | |
| Psychomotor speed | TMT A (mean sec) | 30.3 (10.7; 15 – 53) | 26.6 (8.8; 16 – 44) | 54.8 (21.7; 29 – 96)** |
| Executive function | TMT B (mean sec) | 59.9 (22.5; 30 – 105) | 54.5 (13.4; 39 – 85) | 161.2 (127.1; 67 – 540)** |
| DSST (mean nr correct symbols) | 77.1 (15.5; 46 – 110) | 84.0 (11.8; 62 – 101) | 47.3 (17.3; 18 – 79)** | |
| Clock drawing (median points) | 3.0 (0.2; 2 – 3) | 3.0 (0.5; 2 – 3) | 2.0 (0.6; 1 – 3)** | |
| Language | Letter fluency (mean nr of correct words) | 33.9 (9.1; 13 – 50) | 32.9 (11.8; 19 – 54) | 23.5 (7.9; 12 – 38)* |
| Category fluency (mean nr of correct words) | 22.2 (5.6; 14 – 40) | 23.0 (6.0; 14 – 32) | 15.3 (5.5; 3 – 23) * | |
| BNT (mean nr of correct items) | 28.6 (1.0; 27 – 30) | 28.3 (1.1; 26 – 30) | 23.0 (6.6; 4 – 29)** | |
| Apathy | Apathy scale of Starkstein (mean points) | 9.4 (3.7; 2 – 15) | 8.9 (5.2; 2 – 20) | 8.9 (4.9; 2 – 18) |
| Anxiety | HADS subscale anxiety (mean points) | 3.5 (2.7; 0 – 10) | 4.0 (2.8; 0 – 9) | 5.5 (3.8; 0 – 13)* |
| Depression | HADS subscale depression (mean points) | 1.9 (2.3; 0 – 9) | 1.8 (3.0; 0 – 10) | 4.1 (3.2; 0 – 11) * |
=p<0.05 and
=p<0.001; controls vs pre-symptomatic carriers and controls vs symptomatic carriers
Discussion
Of all sCAA markers, WMHs and cortical microinfarcts (ischemic manifestations of CAA) are more prevalent among pre-symptomatic subjects and precede cognitive and neuropsychiatric symptoms and intracerebral hemorrhages, whereas other sCAA MRI related markers are only more prevalent in symptomatic patients. Microinfarcts are a new finding likely related to the fact that 7T MRI was not available in previous studies. Although it has been suggested that in sCAA amyloid-β deposition alone could cause cognitive impairment7 and that in HCHWA-D mutation carriers cognitive deterioration can precede the first clinical stroke2, we showed that cognitive abnormalities are not present in pre-symptomatic subjects with HCHWA-D. The generalizability of these findings to sCAA remains to be established as it occurs in older individuals in which there is a closer association with amyloid plaques and the clinical features of Alzheimer's disease. Using the most sensitive MRI techniques for all lesions, we found several abnormal MRI characteristics in the pre-symptomatic phase but no cognitive deficits suggesting that HCHWA-D starts with abnormalities in the brain caused by amyloid-β deposition which then causes cognitive deficits.
Supplementary Material
Acknowledgments
Sources of funding
This work was supported by NIH (R01 NS070834).
Footnotes
Disclosures: None.
References
- 1.Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, Roos RA, Frosch MP, Greenberg SM. The cerebral beta-amyloid angiopathies: Hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornebroek M, van Buchem MA, Haan J, Brand R, Lanser JB, de Bruine FT, et al. Hereditary cerebral hemorrhage with amyloidosis-dutch type: Better correlation of cognitive deterioration with advancing age than with number of focal lesions or white matter hyperintensities. Alzheimer Dis. Assoc. Disord. 1996;10:224–231. doi: 10.1097/00002093-199601040-00008. [DOI] [PubMed] [Google Scholar]
- 3.Linn J, Herms J, Dichgans M, Bruckmann H, Fesl G, Freilinger T, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am. J. Neuroradiol. 2008;29:184–186. doi: 10.3174/ajnr.A0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YW, Gurol ME, Rosand J, Viswanathan A, Rakich SM, Groover TR, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haan J, Algra PR, Roos RA. Hereditary cerebral hemorrhage with amyloidosis-dutch type. Clinical and computed tomographic analysis of 24 cases. Arch. Neurol. 1990;47:649–653. doi: 10.1001/archneur.1990.00530060059018. [DOI] [PubMed] [Google Scholar]
- 6.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatry. 2012;83:124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Yang C, Wang L. Cerebral amyloid angiopathy in aged chinese: A clinico-neuropathological study. Acta Neuropathol. 2003;106:89–91. doi: 10.1007/s00401-003-0706-1. [DOI] [PubMed] [Google Scholar]
- 8.van Veluw SJ, Biessels GJ, Luijten PR, Zwanenburg JJ. Assessing cortical cerebral microinfarcts on high resolution mr images. J. Vis. Exp. 2015 doi: 10.3791/53125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.