Abstract
Background
Family history of diabetes is one of the major risk factors for diabetes but significant variability in this association remains unexplained, suggesting the presence of important effect modifiers.
Purpose
To our knowledge, no previous work has examined whether psychological factors moderate the degree to which family history of diabetes increases diabetes risk.
Methods
We investigated the relationships among parental history of diabetes, affective states (positive affect, negative affect, and depressed affect), and diabetes in 978 adults from the MIDUS 2 national sample.
Results
As expected, parental history of diabetes was associated with an almost threefold increase in diabetes risk. We found a significant interaction between positive affect and parental history of diabetes on diabetes (p=.009): higher positive affect was associated with a statistically significant lower relative risk for diabetes in participants who reported having a parental history of diabetes (RR=.66 per unit increase in positive affect; 95% CI: .47; .93), but it did not influence diabetes risk for participants who reported no parental history of diabetes (p=.34). This pattern persisted after adjusting for an extensive set of health and sociodemographic covariates and was independent of negative and depressed affect.
Conclusions
These results suggest that psychological well-being may protect individuals at increased risk from developing diabetes. Understanding such interactions between non-modifiable risk factors and modifiable psychological resources is important for delineating biopsychosocial pathways to diabetes and informing theory-based, patient-centered interventions to prevent the development of diabetes.
Keywords: diabetes, positive affect, family history of diabetes
Introduction
Diabetes is a significant problem in the United States and presents multiple challenges to public health, accounting for substantial morbidity and premature mortality. According to the National Diabetes Statistics Report, 9.3% of the U.S. population (29.1 million people) have diabetes and 32% are at increased risk for developing type 2 diabetes due to insulin resistance (1). Among adults aged 65 and older, 26% have diabetes, making diabetes a common threat to older adults’ well-being and independence. Further, the economic burden associated with diabetes in the U.S. is staggering: the total cost of diagnosed diabetes in 2012 was $245 billion and care for people with diabetes accounted for more than 1 in 5 healthcare dollars (2).
Type 2 diabetes is a multifactorial disease that arises from the interplay of genetic, sociodemographic, and psychological vulnerabilities. Family history of diabetes is a key risk factor that represents genomic information and the complex interplay between genes, shared environments and behaviors, and epigenetic effects (3). It predicts a range of metabolic abnormalities and a two-to six-fold higher risk for type 2 diabetes (4–7). While the clustering of obesity and physical inactivity in families may account for some of the risk associated with family history of diabetes, only a small percent of the variance has been explained by lifestyle, anthropomorphic, and genetic risk factors (8). Importantly, the relationship between family history of diabetes and risk for diabetes appears to be modifiable: physical inactivity and obesity, the main targets of diabetes prevention efforts, modify the effects of family history of diabetes (4, 9–12).
An emerging line of research into the pathophysiology of type 2 diabetes has identified depression as a key risk factor for developing diabetes as well as for poor glycemic control, complications, and functioning among those diagnosed with diabetes (13–16) (17–19). Depressed adults have 37% to 60% increased risk of developing diabetes than adults without depression (16, 20). Depressive symptoms have also been linked to metabolic abnormalities preceding the development of diabetes, and a recent meta-analysis of 18 studies documented a small but significant cross-sectional association between depression and insulin resistance (15). Even though the directionality of the relationship between depression and glucose metabolism has been called in question (21), behavioral and physiological mechanisms have been proposed as explanations of the increased risk of diabetes among depressed adults. For example, depressed individuals show abnormalities in the hypothalamic-pituitary-adrenal axis, particularly in regulation of cortisol, which is in turn related to dysregulated glucose metabolism (22). Importantly, depression is associated with obesity-promoting behaviors (23) (24) and it is well-established that the epidemic of type 2 diabetes has been fueled by the recent dramatic rise in obesity levels.
While past research has predominantly focused on the relationship between poor psychological functioning and diabetes (21, 25, 26), negative emotions are only part of one’s emotional landscape. There is growing interest in positive affect, or the feelings that reflect a pleasurable engagement with the environment (27). Positive affect has been characterized as a dimensional attribute that refers to the general tendency to experience joy, enthusiasm, interest, and energy (28). There is evidence that positive affect is not simply the opposite side of depressive symptoms and negative affect, but rather is a separate construct that is independently associated with lower morbidity and increased longevity, particularly in cases where behavioral factors play a role in disease trajectory (29). Emerging research on how positive affect is instantiated in glucoregulation has shown that positive affect and well-being predict better glycemic control and lower risk for diabetes over time (30–34) as well as lower risk of mortality among people with diabetes (35). Importantly, the associations between positive affect and glucoregulation persist despite adjusting for concurrent depressive symptoms and negative affect, suggesting that positive affect could provide benefits independent from possible costs associated with poor psychological functioning.
Family history of diabetes, and to a lesser degree affective states, have been independently linked to type 2 diabetes, but to our knowledge, no previous research has focused on their interplay. The overarching goals of our study were to investigate the independent and joint relationships among parental history of diabetes, affect, and glucoregulation in adults. Specifically, we expected that depressed and negative affect would amplify the influence of parental history of diabetes on diabetes risk, while positive affect would attenuate it. These hypotheses were informed by the differential susceptibility framework (36, 37) which suggests that some people, due to a genetic or behavioral vulnerability in their makeup, are disproportionally more likely to be affected by experiences, both positive and negative. Importantly, we investigated whether the associations among positive affect and diabetes were independent from the presence of negative affectivity, linked to the debate on whether positive affect and negative are polar extremes of a single continuum, or are largely orthogonal constructs (29, 38).
Methods
Sample
The National Survey of Midlife Development in the U.S. (MIDUS 1) began in 1995–96 as a national random digit dial sample of non-institutionalized, English-speaking adults living in the United States. A final sample of 7108 participants ages 25–74 completed telephone and mail surveys in MIDUS 1. Approximately 9–10 years later, 4963 (75% response rate adjusted for mortality) were successfully contacted to participate in another phone interview and self-administered questionnaire (MIDUS 2 Survey). Participants who completed both MIDUS 1 and MIDUS 2 Survey were invited to be part of the MIDUS 2 biomarker project. Biological data were collected from a subset of respondents (N = 1054) who agreed to travel to one of three General Clinical Research Centers (GCRCs) for an overnight visit. The response rate was 43% among those eligible (adjusted for those who could not be reached), a rate somewhat lower than other epidemiological studies involving a clinic visit (e.g., 57% in the Cardiovascular Health Study) (39). However, the MIDUS protocol is demanding in requiring extensive travel for many participants and two full days of assessments. The biological sample was comparable with overall MIDUS 2 sample on most sociodemographic and health characteristics, although the participants were significantly better educated and less likely to smoke than nonparticipants (40). The study was approved by the institutional review board at each GCRC and informed written consent was obtained from all participants.
Further details of the study design, recruitment, and retention are available at http://www.icpsr.umich.edu/icpsrweb/NACDA/. The current study used data from MIDUS 1 and the MIDUS 2 survey and Biomarker projects. MIDUS 2 survey assessments on average preceded biomarker assessments by 26 months (SD = 14.8). Of the 1054 participants who participated in the MIDUS 1 Survey as well as the MIDUS 2 Survey and Biomarker projects, 76 cases were excluded from the present analyses. Participants who reported diabetes at MIDUS 1 (N=23) were excluded to avoid confounding by the influence of long-standing diabetes on affective states. Although the MIDUS 2 sample was expanded to include African Americans from Milwaukee, WI, the lack of MIDUS 1 data on the diabetes status of these individuals precluded use of their data in the current analyses. Further excluded were participants who were missing diabetes data in MIDUS 1 (N=32) or on any covariate in the analysis (N=55), resulting in 978 participants with complete data.
Measures
Positive and negative affect were each assessed during the MIDUS 2 survey project with six items in the self-administered questionnaire and probed according to the following stem: “During the past 30 days, how much of the time did you feel:” Positive items included cheerful, in good spirits, extremely happy, calm and peaceful, satisfied, and full of life. Negative items included so sad nothing could cheer you up, nervous, restless or fidgety, hopeless, that everything was an effort, and worthless. Response options ranged from 1 (all of the time) to 5 (none of the time). Internal consistency reliability for positive affect and negative affect in the present sample was excellent (α= .84 and α= .92, respectively). The 30-day response frame for both affect measures assessed more generalized affect, rather than immediate and situationally-specific affect (41), and as such, reflected relatively stable, trait-like characteristics (29). Depressed affect was assessed with information from the phone interview and coded as “present” if a participant reported experiencing a period of at least two weeks of depressed mood most of the day, nearly every day, and at least four symptoms typically found to accompany depression, such as problems with eating, sleeping, energy, concentration, feelings of self-worth, and suicidal thoughts or actions (42).
Parental history of diabetes was assessed during the MIDUS 2 biomarker project and is a binary measure based on responses to questions about parental history of diabetes and was coded as 0 if neither parent had diabetes or 1 if either or both parents had diabetes. While the MIDUS 2 medical history forms included information about diabetes status for additional family members, reporting parental history is a frequently preferred index of familiar risk due to the difficulty in reporting diabetes status in more distant or younger relatives, and further, it avoids the bias inherent in trying to compare different family structures (e.g., number of siblings).
HbA1c and fasting glucose samples were obtained during an overnight stay in a General Clinical Research Center (GCRC) during MIDUS 2 biomarker project. Criteria from the American Diabetes Association were used to define diabetes in our participants (HbA1c above 6.5%, fasting glucose above 126 mg/dl, or taking medications that lower glucose levels such as Metformin) (43). Fasting glucose was measured via an enzymatic assay photometrically on an automated analyzer (Roche Modular Analytics P). The HbA1c assay was a colorimetric total-hemoglobin determination combined with an immunoturbidometric HbA1c assay, carried out using a Cobas Integra Systems instrument (Roche Diagnostics) (44).
Demographic covariates included age (in years), gender (male or female), and race/ethnicity (White or Minority). Socioeconomic status (SES) variables were created for three time periods: childhood and two adult periods (MIDUS 1 and MIDUS 2, approximately ten years apart). The childhood SES disadvantage score was computed by summing values on 3 indicators: financial level growing up (2 - worse off than others, 1 - about the same as others, 0 - better off than others), highest level of parental education (2 - less than high school, 1 - high school/GED, 0 - some college or higher), and childhood welfare status (2 - ever on welfare, 0 – never on welfare). Information on childhood SES was collected retrospectively at the MIDUS 1 exam. MIDUS 1 and 2 adult SES disadvantage scores were computed by summing values on 5 indicators at each time point: education level (2 - high school/GED or less, 1 - some college/associate arts degree, 0 - bachelor’s degree or higher), family-size adjusted income to poverty ratio (2 - less than 300%, 1–300 -599%, 0 – 600% or more), current financial situation (2 – worst possible, 1 - average, 0 - best possible), availability of money to meet basic needs (2 - not enough, 1 - just enough, 0 - more than enough), and difficulty level of paying bills (2 - very or somewhat difficult, 1 - not very difficult, 0 - not at all difficult). A cumulative disadvantage score was created by summing the childhood and two adult disadvantage scores. The health covariates included body mass index (BMI), frequency of drinking alcohol in past month (0–5, ranging from never drinking to drinking every day), being a current smoker (yes or no), MET/minutes of exercise, and presence of chronic conditions excluding diabetes (yes or no). Given the emerging literature on links between antidepressant and corticosteroid medications and diabetes risk (45, 46), two binary covariates were added to control for use of these medications (yes or no).
Statistical Analyses
First, descriptive statistics were generated. Means, standard deviations, and ranges for all continuous variables and proportions for categorical variables were examined. Modified Poisson regression with robust estimates of error was used to estimate relative risks (RR) and 95% confidence intervals (CI) in successively built models. Our outcome, diabetes, was not rare, so we used modified Poisson regression instead of logistic regression (47). All two-way interaction terms were created by multiplying the two variables comprising the interaction. Model 1 tested the main effects of parental history of diabetes and affect measures, net of sociodemographic covariates (age, race, gender, cumulative SES). Models 1a–c included the individual measures of affect, while Model 1d included all measures of affect. Models 2a–d added the focal interactions between parental history of diabetes and affect and potential mediators such as health behaviors (smoking, alcohol use, regular exercise, medications usage, chronic conditions, and BMI), as well as interactions between the focal predictors and age, gender, BMI and exercise. These additional interactions were included in the models to remove potential confounding by differential effects of important factors (such as exercise) on diabetes risk in individuals with and without parental history of diabetes and with varying levels of positive affect. Models 2a–c included the interactions between parental history of diabetes and individual affective states, while Model 2d included interactions between parental history of diabetes and all affective states. All analytical models controlled for the time lag between affect and diabetes assessments.
Results
Descriptive statistics for all measures are presented in Table 1. On average, participants in the current study were aged 55.5 years, 93% were white, and 55% were women. Diabetes was present in 123 participants (13%) and 242 reported a parental history of diabetes (25%). Zero-order correlations among the affect measures revealed significant associations: depressed affect was positively related to negative affect (r=.43) and negatively related to positive affect (r=−.35). Positive and negative affect were inversely related (r=−.64).
Table 1.
Means (and SDs) or Proportions for All Measures (N=978)
| Variables | % | M (SD) | Range |
|---|---|---|---|
| Key Predictors | |||
| Parental Diabetes | 25 | 0–1 | |
| Positive Affect | 3.46 (.7) | 1–5 | |
| Negative Affect | 1.47 (.5) | 1–5 | |
| Depressed Affect | 9 | 0–1 | |
| Outcome | |||
| Diabetes | 13 | 0–1 | |
| Demographic and Health Covariates | |||
| Race (1=White) | 93 | 0–1 | |
| Age | 55.31 (11.8) | 34–84 | |
| Gender (1=men) | 45 | 0–1 | |
| SES Disadvantage | 10.7 (5.3) | 0–24 | |
| BMI | 29.12 (5.9) | 15–57 | |
| Exercise MET/minutes | 1068 (1542) | 0–7359 | |
| Drinking Frequency | 1.6 (1.6) | 0–5 | |
| Current Smoker | 10 | 0–1 | |
| Chronic conditions (except diabetes) | 76 | ||
| Antidepressant Medication | 15 | 0–1 | |
| Corticosteroid Medication | 13 | 0–1 | |
Main effect analyses from Poisson regression are presented in Models 1a–d in Table 2. As expected, parental history of diabetes was associated with an almost threefold increase in diabetes risk: the RRs ranged from 2.75 to 2.79 in the different models, depending on what covariates were included (see Models 1a–d). Affective states were not significantly associated with diabetes in main effect models (p>.05 in models 1a–d).
Table 2.
A summary of Poisson regression results predicting diabetes (N=978)
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Positive Affect Only | Negative Affect Only | Depressed Affect Only | All Affect Measures | |||||
| Model 1a RR (CI) |
Model 2a RR (CI) |
Model 1b RR (CI) |
Model 2b RR (CI) |
Model 1c RR (CI) |
Model 2c RR (CI) |
Model 1d RR (CI) |
Model 2d RR (CI) |
|
|
| ||||||||
| Parental Diabetes (PD) | 2.79*** (2.05; 3.81) | 2.61*** (1.52; 4.51) | 2.75*** (2.01; 3.77) | 2.81*** (1.65; 4.87) | 2.79*** (2.04; 3.82) | 2.62*** (1.50; 4.57) | 2.77*** (2.03; 3.78) | 2.42** (1.37; 4.26) |
| Positive Affect (PA) | .99 (.79; 1.24) | 1.31 (.76; 2.26) | 1.09 (.81;1.45) | 1.51 (.71; 3.23) | ||||
| Negative Affect (NA) | 1.13 (.84; 1.53) | 1.05 (.60; 1.84) | 1.2 (.79; 1.83) | 2.01 (.77; 5.52) | ||||
| Depressed Affect (DA) | 1.11 (.66; 1.88) | .35 (.08; 1.47) | 1.05 (.60; 1.85) | .26 (.05; 1.23) | ||||
| PD × PA | .51* (.30; .84) | .46* (.23; .90) | ||||||
| PD × NA | 1.51 (.81; 2.83) | .60 (.23; 1.58) | ||||||
| PD × DA | 3.98# (.89; 17.7) | 3.90 (.77; 19.71) | ||||||
Model 1 includes PD, Affect, race, gender, age, SES, and lag between affect and diabetes assessments. Model 2 includes Model 1 plus PD × Affect, gender × Affect, age × Affect, PD × gender, age squared, PD × age, BMI, BMI × Affect, PD × BMI, exercise, exercise × Affect, PD × exercise, antidepressant medications, corticosteroid medications, drinking frequency, chronic conditions, and smoking status.
p<.001
p<.01
p<.05
p<.075
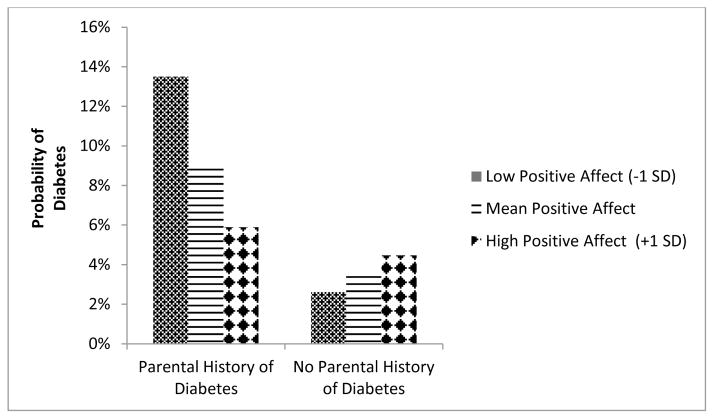
Next, we tested whether affective states moderated the relationships between parental history of diabetes and diabetes. We found a significant interaction between positive affect and parental history of diabetes on diabetes (p=.009). This pattern--a significant main effect for parental history and a significant interaction between parental history and positive affect--was unaffected by adjusting for an extensive set of sociodemographics and health covariates such as BMI, exercise, smoking, chronic conditions, drinking, and taking antidepressant or corticosteroid medications. Figure 1 illustrates the interaction: while levels of positive affect did not influence diabetes risk for participants who reported no parental history of diabetes (p=.34), higher positive affect was associated with a statistically significant lower relative risk for diabetes in participants who reported having a parental history of diabetes (RR=.66 per unit increase in positive affect; 95% CI: .47; .93). Finally, the interaction between positive affect and parental history of diabetes was minimally influenced by adding negative affect and depressed affect to the models (Model 2d; p=.024). We found no interaction between either depressed affect or negative affect and parental history of diabetes (Models 2b–d).
Figure 1.
Positive Affect Moderates the Influence of Parental Diabetes on Diabetes Risk
Follow-up analyses explored whether the associations between affect and diabetes depended on whether participants knew their diabetes status when affect was assessed. None of the interactions between affect and self-report of diabetes in predicting diabetes were significant (ps>.1).
Discussion
This is the first study to investigate the interplay among parental history of diabetes, affect, and diabetes, and we found some support for our hypotheses. Parental history of diabetes predicted an almost three-fold increase in relative risk for diabetes, which is consistent with previous research documenting a two-to six-fold increase in diabetes risk across different ethnic groups and study designs (48). Similar to other studies (7, 8), we found that the effect of parental history of diabetes was only slightly attenuated by including potential mediators such as relevant health behaviors, highlighting the fact that while parental diabetes is a strong and independent predictor of diabetes risk, we have a limited understanding of mediating processes and subgroup differences.
Our study addressed this knowledge gap by investigating whether different affective states moderate the degree to which parental history of diabetes predicts diabetes risk. The differential susceptibility framework proposes important individual differences in susceptibility to both positive and negative experiences (36, 37). We found partial evidence for our hypotheses: people who were more vulnerable to diabetes because of parental history of diabetes were more affected than those without a parental history of diabetes by the salubrious influence of positive affect. However, we did not find evidence for disproportionate sensitivity to negative or depressed affect. This pattern of increased susceptibility to positive, but not negative, influences has been recently proposed to reflect vantage sensitivity, a concept reflecting variation in response to exclusively positive experiences as a function of individual endogenous characteristics (36). These results suggest that interventions designed to promote well-being as a vantage sensitivity factor, independent of presence of depression, could be viable targets for reducing diabetes risk among those with a parental history of diabetes.
Our findings converge with prior work showing that the effects of positive affect may confer benefits for glucoregulation (30–33) but sharpen the inquiry on individual differences in diabetes risk by demonstrating that positive affect is not beneficial for glucoregulation among all respondents, but rather is limited to those with a family history of diabetes. These results, which need to be replicated in different samples, extend previous research showing that additional risk factors such as obesity work interactively with parental history of diabetes (8, 9, 11) to influence risk for type 2 diabetes. Current etiological understanding of diabetes suggests that underlying genetic risk is compounded by other factors until clinical threshold is reached (4). Thus, a system already challenged due to genetic predisposition could be more easily influenced by a variety of factors. Our work underscores the potential role of protective influences in this multifactorial context.
The question remains, however, as to why the protective influence of positive affect was limited to people with a parental history of diabetes. Previous research suggests that individuals with a family history of diabetes perceive greater threat of diabetes and also engage in more weight control than those who do not have a family history of diabetes (49). Positive affect may be a factor that motivates a focus on health behaviors, or alternatively, it could act as a stress-buffer vis-à-vis the perceived threat of diabetes. Regarding the first possibility, we found that the protective effect of positive affect was evident even after controlling for concurrent exercise and BMI. However, it is possible that positive affect was associated with other, unmeasured aspects of healthy lifestyles, such as diet, weight, and exercise patterns throughout the lifecourse. The second possibility that positive affect serves as a stress-buffer in the face of perceived threat of diabetes is consistent with the “broaden and build” theory of positive affect (50). This idea could not be examined, given that we had no measure of perceived threat of diabetes.
We did not find a relationship between depressed or negative affect and diabetes risk in our sample. Depression and diabetes are often co-morbid, and each condition is worsened by the other (21). Previous work has shown that people with depression have increased rates of diabetes, largely attributable to obesity-promoting health behaviors (24). However, the association is considered modest (51) and some studies have documented a non-significant relationship (52–54). Further, there is evidence for a bidirectional relationship, and studies on the differences in depressive symptoms between individuals with diagnosed and undiagnosed diabetes offer compelling evidence that diabetes can lead to higher depressive symptoms, perhaps due to the daily challenges and self-care burden of managing a chronic disease (23, 55). Importantly, the current state of the science on the association between depression and diabetes has proposed a novel paradigm shift by considering shared underlying behavioral and biological pathways that may simultaneously predispose people to both disorders (21), and future empirical examinations that model this more complex pattern might help reconcile the mixed findings in the literature.
The key predictors in our study clearly precede the assessment of diabetes: parental history of diabetes is a proxy for genetic effects present at birth and affective states were measured on average 2 years prior to the assessment of diabetes. The main limitation of our study pertains to the lack of longitudinal data on glucoregulation that could illuminate cross-time trajectories and related issues of causal directionality. Future research utilizing data from MIDUS 3 (currently in progress) will allow for modeling incidence of diabetes since MIDUS 2. Further, these future analyses will incorporate the minority subsample added at MIDUS 2 that was omitted from present analyses due to lack of baseline diabetes data in MIDUS 1. Relatedly, although we excluded participants who self-reported diabetes at MIDUS 1, it should be acknowledged that we did not have biological data to rule out undiagnosed diabetes at MIDUS 1. Finally, our analyses were modeled to capture known risk influences on type 2 diabetes, but we did not have information on whether participants with diabetes had type 1 or type 2 diabetes. Out of the 123 participants with diabetes, 67 did not self-report diabetes (or taking medications to treat diabetes) and were therefore considered “undiagnosed” which is common in type 2 diabetes, but is unusual for type 1 diabetes. The remaining 56 people who reported having diabetes at MIDUS 2 did not report having diabetes 10 years earlier at MIDUS 1. Given that type 1 diabetes is usually diagnosed in childhood and early adulthood and therefore likely to have been diagnosed before MIDUS 1 and that approximately 90–95% of people with diabetes have type 2 diabetes (56), our results may not be strongly affected by this imprecision. One notable strength of our study was that diabetes status was ascertained using current ADA criteria based on fasting glucose and HbA1c levels, reducing the concern about undiagnosed diabetes.
Type 2 diabetes is a prevalent chronic disease that is influenced by having a family history of diabetes. Family history of diabetes is currently the only genetic measure of diabetes risk that can be feasibly used on a population level, and its potential for increasing awareness and identifying individuals at high risk can be important in the prevention, early detection, and treatment of type 2 diabetes. Family history of diabetes is a powerful risk factor that is non-modifiable, underscoring the urgency to identify underlying mechanisms and effect modifiers. Our central finding that each unit increment in positive affect is associated with 34% reduced risk of diabetes among people who have a parental history of diabetes is comparable in magnitude to 31% reduced incidence of diabetes documented as the “gold standard” pharmacological treatment with metformin in the landmark Diabetes Prevention Program (57). Overall, our findings have clinical relevance and underscore the importance of assessing mental health in patients who are at risk for diabetes as well as targeting positive affect as a modifiable factor to achieve extra reduction in diabetes risk.
Acknowledgments
This work was supported by a grant from the National Institute on Aging (P01-AG020166; Carol D. Ryff, Principal Investigator) to conduct a longitudinal follow-up of the MIDUS (Midlife in the US) investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. Data collection was supported by the following grants: M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program, and 1UL1RR025011 (UW) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. The first author (VT) of this study was supported by the National Institute on Aging (K01AG041179) and by the Robert Wood Johnson Foundation. No conflict of interest.
Footnotes
The authors have no potential conflicts of interest.
The MIDUS study was approved by the Institutional Review Boards at UCLA, University of Wisconsin- Madison, Georgetown University, and Brandeis University.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: 2014. [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U. S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franks PW. Diabetes family history: a metabolic storm you should not sit out. Diabetes. 2010;59:2732–2734. doi: 10.2337/db10-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velasco Mondragon HE, Charlton RW, Peart T, et al. Diabetes risk assessment in Mexicans and Mexican Americans: effects of parental history of diabetes are modified by adiposity level. Diabetes Care. 2010;33:2260–2265. doi: 10.2337/dc10-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilding A, Eriksson AK, Agardh EE, et al. The impact of family history of diabetes and lifestyle factors on abnormal glucose regulation in middle-aged Swedish men and women. Diabetologia. 2006;49:2589–2598. doi: 10.1007/s00125-006-0402-5. [DOI] [PubMed] [Google Scholar]
- 6.Hariri S, Yoon PW, Qureshi N, et al. Family history of type 2 diabetes: a population-based screening tool for prevention? Genet Med. 2006;8:102–108. doi: 10.1097/01.gim.0000200949.52795.df. [DOI] [PubMed] [Google Scholar]
- 7.Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the US population: 6-year results from the National Health and Nutrition Examination Survey (NHANES, 1999 2004) Diabetes. 2007 doi: 10.2337/db07-0720x. [DOI] [PubMed] [Google Scholar]
- 8.InterAct C, Scott RA, Langenberg C, et al. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia. 2013;56:60–69. doi: 10.1007/s00125-012-2715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wikner C, Gigante B, Hellenius ML, de Faire U, Leander K. The risk of type 2 diabetes in men is synergistically affected by parental history of diabetes and overweight. PLoS One. 2013;8:e61763. doi: 10.1371/journal.pone.0061763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dam RM, Boer JM, Feskens EJ, Seidell JC. Parental history of diabetes modifies the association between abdominal adiposity and hyperglycemia. Diabetes Care. 2001;24:1454–1459. doi: 10.2337/diacare.24.8.1454. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Rennie DC, Dosman JA. Synergy of BMI and family history on diabetes: the Humboldt Study. Public Health Nutr. 2010;13:461–465. doi: 10.1017/S1368980009991285. [DOI] [PubMed] [Google Scholar]
- 12.Goodrich KM, Crowley SK, Lee DC, et al. Associations of cardiorespiratory fitness and parental history of diabetes with risk of type 2 diabetes. Diabetes Res Clin Pract. 2012;95:425–431. doi: 10.1016/j.diabres.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 13.Brown LC, Majumdar SR, Newman SC, Johnson JA. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care. 2005;28:1063–1067. doi: 10.2337/diacare.28.5.1063. [DOI] [PubMed] [Google Scholar]
- 14.Chen PC, Chan YT, Chen HF, Ko MC, Li CY. Population-based cohort analyses of the bidirectional relationship between type 2 diabetes and depression. Diabetes Care. 2013;36:376–382. doi: 10.2337/dc12-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan C, Silva N, Golden SH, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36:480–489. doi: 10.2337/dc12-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knol MJ, Twisk JW, Beekman AT, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 17.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Lustman PJ, Anderson RJ, Freedland KE, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz N, Gariepy G, Smith KJ, et al. Longitudinal relationships between depression and functioning in people with type 2 diabetes. Ann Behav Med. 2014;47:172–179. doi: 10.1007/s12160-013-9534-2. [DOI] [PubMed] [Google Scholar]
- 20.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt RI, de Groot M, Lucki I, et al. NIDDK international conference report on diabetes and depression: current understanding and future directions. Diabetes Care. 2014;37:2067–2077. doi: 10.2337/dc13-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 23.Nouwen A, Nefs G, Caramlau I, et al. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care. 2011;34:752–762. doi: 10.2337/dc10-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams ED, Magliano DJ, Tapp RJ, Oldenburg BF, Shaw JE. Psychosocial stress predicts abnormal glucose metabolism: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Ann Behav Med. 2013;46:62–72. doi: 10.1007/s12160-013-9473-y. [DOI] [PubMed] [Google Scholar]
- 26.Heraclides A, Chandola T, Witte DR, Brunner EJ. Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. Diabetes Care. 2009;32:2230–2235. doi: 10.2337/dc09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark LA, Watson D, Leeka J. Diurnal Variation in the Positive Affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- 28.Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- 29.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 30.Richman LS, Kubzansky L, Maselko J, et al. Positive emotion and health: going beyond the negative. Health Psychol. 2005;24:422–429. doi: 10.1037/0278-6133.24.4.422. [DOI] [PubMed] [Google Scholar]
- 31.Tsenkova V, Love G, Singer B, Ryff C. Coping and positive affect predict longitudinal change in glycosylated hemoglobin. Health Psychol. 2008;27:S163–171. doi: 10.1037/0278-6133.27.2(Suppl.).S163. [DOI] [PubMed] [Google Scholar]
- 32.Nefs G, Pouwer F, Denollet J, et al. Suboptimal glycemic control in type 2 diabetes: a key role for anhedonia? J Psychiatr Res. 2012;46:549–554. doi: 10.1016/j.jpsychires.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Boehm JK, Trudel-Fitzgerald C, Kivimaki M, Kubzansky LD. The Prospective Association Between Positive Psychological Well-Being and Diabetes. Health Psychol. 2015 doi: 10.1037/hea0000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okely JA, Gale CR. Well-Being and Chronic Disease Incidence: The English Longitudinal Study of Ageing. Psychosom Med. 2015 doi: 10.1097/PSY.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moskowitz JT, Epel ES, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychol. 2008;27:S73–82. doi: 10.1037/0278-6133.27.1.S73. [DOI] [PubMed] [Google Scholar]
- 36.Pluess M, Belsky J. Vantage sensitivity: individual differences in response to positive experiences. Psychol Bull. 2013;139:901–916. doi: 10.1037/a0030196. [DOI] [PubMed] [Google Scholar]
- 37.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 38.Steptoe A, Wardle J. Positive affect and biological function in everyday life. Neurobiol Aging. 2005;26(Suppl 1):108–112. doi: 10.1016/j.neurobiolaging.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 40.Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. J Pers Soc Psychol. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- 42.Kessler R, Mickelson KD, Walters EE, Zhao S, Hamilton L. In: How healthy are we? A national study of well-being at midlife. Brim OG, Ryff C, Kessler R, editors. The University of Chicago Press; 2004. [Google Scholar]
- 43.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 44.Wolf HU, Lang W, Zander R. Alkaline haematin D-575, a new tool for the determination of haemoglobin as an alternative to the cyanhaemiglobin method. II. Standardisation of the method using pure chlorohaemin. Clin Chim Acta. 1984;136:95–104. doi: 10.1016/0009-8981(84)90251-1. [DOI] [PubMed] [Google Scholar]
- 45.Vimalananda VG, Palmer JR, Gerlovin H, et al. Depressive symptoms, antidepressant use, and the incidence of diabetes in the Black Women’s Health Study. Diabetes Care. 2014;37:2211–2217. doi: 10.2337/dc13-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123:1001–1006. doi: 10.1016/j.amjmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 47.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 48.Harrison TA, Hindorff LA, Kim H, et al. Family history of diabetes as a potential public health tool. American Journal of Preventive Medicine. 2003;24:152–159. doi: 10.1016/s0749-3797(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 49.Forsyth LH, Goetsch VL. Perceived threat of illness and health protective behaviors in offspring of adults with non-insulin-dependent diabetes mellitus. Behav Med. 1997;23:112–121. doi: 10.1080/08964289709596367. [DOI] [PubMed] [Google Scholar]
- 50.Fredrickson BL. What Good Are Positive Emotions? Rev Gen Psychol. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabak AG, Akbaraly TN, Batty GD, Kivimaki M. Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol. 2014;2:236–245. doi: 10.1016/S2213-8587(13)70139-6. [DOI] [PubMed] [Google Scholar]
- 52.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med. 2004;164:1873–1880. doi: 10.1001/archinte.164.17.1873. [DOI] [PubMed] [Google Scholar]
- 53.van den Akker M, Schuurman A, Metsemakers J, Buntinx F. Is depression related to subsequent diabetes mellitus? Acta Psychiatr Scand. 2004;110:178–183. doi: 10.1111/j.1600-0447.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 54.Everson-Rose SA, Meyer PM, Powell LH, et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27:2856–2862. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- 55.Mezuk B, Johnson-Lawrence V, Lee H, et al. Is ignorance bliss? Depression, antidepressants, and the diagnosis of prediabetes and type 2 diabetes. Health Psychol. 2013;32:254–263. doi: 10.1037/a0029014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]