Abstract
Background
Traumatic spinal cord injury (SCI) guidelines recommend to maintain mean arterial pressures (MAPs) above 85 mmHg for 7d following SCI to minimize spinal cord ischemia. Some doubt that patients with initially complete injuries benefit.
Objective
To assess the relationship between MAP augmentation and neurological improvement in SCI patients stratified by initial AIS score.
Methods
High-frequency MAP values of acute SCI patients admitted over a six-year period were recorded and values were correlated with degree of neurological recovery in an analysis stratified by post-resuscitation AIS score.
Results
62 patients with SCI were analyzed. 33 patients were determined to have complete injuries and of those 11 improved at least 1 AIS grade by discharge. The average MAP of initially AIS A patients who improved versus those who did not was significantly higher (96.6 +/− 0.07 mmHg vs 94.4 +/− 0.06 mmHg, respectively; p < 0.001) and the proportion of MAP values < 85mmHg was significantly lower (13.5% vs 25.6%, respectively; p<0.001). A positive correlation between MAP values and outcome was also observed in AIS B and C patients, but was not observed in patients who were initially AIS D.
Conclusion
A positive correlation was observed between MAP values and neurological recovery in AIS A, B and C patients but not AIS D patients. These data raise the possibility that patients with an initially complete SCI may derive greater benefit from MAP augmentation than patients with initial AIS D injuries.
Keywords: Spinal Cord Injury, Blood Pressure, Trauma, ASIA, AIS, Outcome, Complete
Introduction
Traumatic spinal cord injury (SCI) affects 12,000 to 20,000 new individuals per year in the United States1–2 and often leaves patients with profound neurological deficits. In addition to the personal cost of SCI, the cost to society is great; the direct and indirect costs of SCI in the United States are estimated to be $21.5 billion annually1. In one study only 11.8% of spinal cord injured patients were employed one year after the injury and only 35.2% were employed 20 years post injury vs 57.1% prior to SCI1.
In recent decades we have learned much about secondary injury processes and secondary insults which exacerbate SCI damage following the initial insult. Secondary injury involves a complex and highly inter-related series of molecular processes such as ionic dysregulation, free radical production, cytoskeletal degradation, and neuroinflammation3. Despite what we have learned about secondary injury, we are as yet without a safe and effective therapeutic which attenuates these processes3–5. Physicians have been more successful treating secondary insults which occur at the level of the organism and include hypoxia, and hypotension6–13. Indeed, supportive care aimed at preventing or reducing these insults is the mainstay of modern medical care, and is credited with the improved mortality and morbidity seen following SCI in recent decades14.
Since their inception, guidelines for the management of acute SCI have recommended not only preventing hypotension, but additionally augmenting blood pressure with vasoactive agents in the first week after injury15. Specifically, the current guidelines advise that systolic blood pressure should not be less than 90 mmHg at any time and that an average mean arterial pressure (MAP) above 85–90 mmHg be maintained for 7d following injury15. Our group recently validated this recommendation with a large dataset of high-frequency MAP measurements obtained from 100 SCI patients. We found that maintenance of MAP values above 85 mmHg correlated with improved neurological recovery3,6 and that the benefit associated with MAP augmentation decreased over time. Importantly, we also found that the proportion of values <85 mmHg correlated more strongly with outcome than mean values suggesting that efforts to prevent even brief drops below threshold are important. Our analysis did not, however, inform the relevance of these findings to patients with initially complete SCI.
Recent publications suggest a substantially higher rate of neurological improvement in initially American Spinal Injury Association Impairment Scale (AIS) A patients with contemporary management than has been seen in the past16–18. Nonetheless, patients who present with initially complete SCI (AIS A) have the poorest prognosis for neurological recovery and are vulnerable to the nihilism of treating physicians who may view aggressive care as being of little benefit in this population. To determine if the resource expenditure inherent to MAP augmentation is justified in initially AIS A patients, we performed an analysis to determine how the relationship between MAP and neurological improvement is influenced by the baseline neurological examination.
Methods
As previously described6, between the years 2005 and 2011 a computerized data acquisition system prospectively collected and stored data every minute from all patients treated in the Neurotrauma Intensive Care Unit (ICU) at San Francisco General Hospital, a high-volume level 1 trauma center. This system was HIPPA compliant and approved by the local research ethics board. Recording with this system, developed in conjunction with Aristein Bioinformatics LLC, initiated automatically and immediately following admission to the Neurotrauma ICU. We retrospectively identified patients admitted with SCI and collected their demographic and neurological data. Analyzed MAP values were measured via an arterial line. AIS scores were also collected via chart review and were obtained on initial presentation, following resuscitation, and prior to discharge. A high rate of loss to follow-up precluded an analysis of neurological recovery after discharge6.
MAP values for each patient was stored in a separate Microsoft Excel file on an encrypted computer. A Matlab program was used to extract data from each of these files and to calculate average MAP values as well as the number of measurements below specified thresholds. The Matlab program allowed us to perform these analyses over specific epochs as specified by the analyst. We chose to analyze 80 different MAP thresholds (120 to 40 mmHg with 1 mmHg increments) as our group did previously6. Results were stratified by post-resuscitation AIS score and by presence or absence of subsequent neurological improvement on the final pre-discharge AIS score. Fidelity of the Matlab program was ensured by comparing to values obtained with Microsoft Excel. Microsoft Excel was used to create graphs and tables.
Statistical analysis was performed via SPSS version 23 software. For continuous data ANOVA was performed as the first statistical test for a difference between means. When significant, Tukey’s and Bonferroni’s post-hoc tests were used to compare means amongst three or more groups. Binomial logistic regression was employed for dichotomous data and Poisson regression was performed for count data. All error values and error bars presented herein reflect standard error.
Results
62 patients with high frequency arterial line blood pressure measurements were identified as having a traumatic SCI and post-resuscitation neurological examinations graded AIS A, B, C or D. 33 patients were AIS A, 17 were AIS B or C, and 12 were AIS D. We excluded 3 patients who had neurological worsening subsequent to the post-resuscitation examination due to small sample size. The AIS B and C groups were combined for analysis due to sample size limitations as only 2 patients were AIS B post-resuscitation. Although some patients achieved more than one AIS grade of improvement, sample size limitations similarly required us to truncate outcomes to those who improved one or more AIS grades vs. those who did not. Only the physiological measurements from the first three days of admission were analyzed as our group previously demonstrated a strong correlation between MAP and outcome over this period in these patients6.
The severity of the SCI, reflected by the post-resuscitation AIS score, correlated with the Injury Severity Score (ISS) in the three groups (Table 1). The AIS A patients had an average ISS of 31.6 (+/− 2.0) while the AIS B/C patients’ average ISS was 26.2 (+/− 3.8) and the AIS D patients’ average ISS was 19.2 (+/− 2.1) (p=.013). There was a significant difference in the percentage of patients who improved one or more AIS grade from admission to discharge amongst the groups (p=.0018). A total of 11 patients (33%) in the AIS A group had improvement of AIS grade from admission to discharge, with 8 of these patients improving greater than 1 AIS score. In the AIS B/C group, a total of 13 patients (76%) showed improvement from admission to discharge with the majority of patients only improving one AIS grade. In the AIS D group, 7 patients (58%) improved to AIS E by the time of discharge. The total hospital days were significantly greater for the AIS A patients with an average of 40 days (+/− 7) vs the AIS B/C patients average of 17.6 days (+/− 2.9) and the AIS D patients average of 9.9 days (+/− 1.9) (p= .036). A significantly greater proportion of patients with thoracic injuries were initially complete than incomplete (p = .036). There was no significant difference found in age, percentage of patients requiring surgery, time to surgery, requirement of two vasopressors, frequency of penetrating injury or proportion of injuries above T6 which may be associated with neurogenic shock from disruption of descending sympathetics.
Table 1. Characteristics of Studied Patients Grouped by Post-Resuscitation AIS Score.
The characteristics of analyzed patients grouped by post-resuscitation AIS score are shown. For the continuous data the averages +/− standard errors are reported. For categorical data we report the number of patients with the percentage of the total in parentheses. For continuous data ANOVA was performed for statistical analysis. For categorical data binomial logistic regression was used. For total measurements a Poisson regression was performed. Statistically significant values are denoted with red font.
| AIS A (n=33) | AIS B/C (n=17) | AIS D (n=12) | P value | |
|---|---|---|---|---|
| Age | 41.6 +/− 3.1 | 53.1 +/− 5.6 | 50 +/− 6.6 | .146 |
| ISS | 31.6 +/− 2 | 26.2 +/− 3.8 | 19.2 +/− 2.1 | .013 |
| Improvement AIS | 11 (33.3%) | 13 (76.5%) | 7 (58.3%) | .018 |
| 1 | 3 (27.3%) | 12 (92.3%) | 7 (100%) | .028 |
| >1 | 8 (72.7%) | 1 (7.7%) | 0 (0%) | .028 |
| Surgery | 33 (100%) | 15 (88.2%) | 10 (83.3%) | .932 |
| Timing of Surgery | 40.3 +/− 5.5h | 51.9 +/− 4.8h | 13.2 +/− 1.3h | .156 |
| Total Hospital Days | 40 +/− 7 | 17.6 +/− 2.9 | 9.9 +/− 1.9 | .036 |
| Total Measurements | 3153.6 +/− 156.4 | 3039.8 +/− 229.4 | 2894.7 +/− 362.3 | < .001 |
| Penetrating | 7 (21.2%) | 2 (11.8%) | 1 (8.3%) | .511 |
| Cervical | 18 (54.5%) | 14 (82.3%) | 8 (66.7%) | .167 |
| Thoracic | 14 (42.4%) | 1 (5.9%) | 2 (16.7%) | .036 |
| Lumbar | 1 (3%) | 2 (11.8%) | 1 (8.3%) | .514 |
| Injuries Above T6 | 22 (66.7%) | 14 (82.4%) | 9 (0.75%) | .498 |
| Required Two Vasopressors | 7 (21.2%) | 6 (35.3%) | 5 (41.7%) | .337 |
Patients who were AIS D on post-resuscitation examination had a higher proportion of MAP measures >85mmHg than the other groups (Figure 1A). In an analysis stratified by post-resuscitation AIS score (Figure 1B, C and D; Figure 2A, B, C and D) patients improving neurologically had higher MAP values than those who did not improve in both the AIS A and AIS B/C groups. The opposite relationship was seen in AIS D patients. This was true both when the proportion of MAP values below 80 different physiological thresholds (40–120 mmHg) were analyzed (Figure 1) and when the frequency of MAP values in 1 mmHg bins from 40–120 mmHg were examined (Figure 2).
Figure 1. Higher Mean Arterial Pressure Values Positively Correlate With Neurological Improvement Except in Patients Who Are AIS D on Post-Resuscitation Neurological Examination: Analysis of Thresholds.
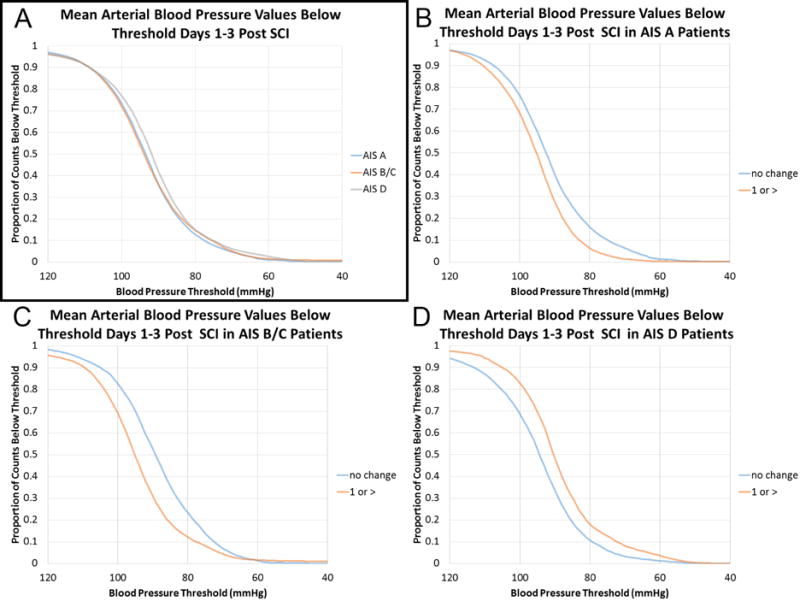
Plotted are the proportion of MAP values below 80 different MAP thresholds (40–120 mmHg in 1 mmHg increments) for 62 acute SCI patients treated at San Francisco General Hospital. A) The three groups based on post-resuscitation AIS scores show a similar distribution of MAP values though AIS D patients exhibited a higher proportion of values >85 mmHg. B) Patients who were AIS A following resuscitation are presented. Those exhibiting neurological improvement had higher MAP values than those who did not improve. C) In patients who were AIS B/C following resuscitation higher MAP values are seen in patients exhibiting neurological improvement than those who did not have an improved AIS score at the time of discharge. D) Patients who were AIS D following resuscitation demonstrate an inverse relationship between MAP values and neurological recovery.
AIS = American Spinal Injury Association Impairment Scale
MAP = mean arterial pressure
SCI = spinal cord injury
Figure 2. Higher Mean Arterial Pressure Values Positively Correlate With Neurological Improvement Except in Patients Who Are AIS D on Post-Resuscitation Neurological Examination: Frequency Plots.

Frequency plots of MAP values within 1 mmHg bins between 40 and 120 mmHg are presented. A) MAP values for the three groups based on post-resuscitation AIS score are plotted and demonstrate similar distributions. B) Patients who were AIS A on admission are plotted. Patients improving neurologically after admission exhibited higher MAP values than those who did not. C) Patients who were AIS B/C following resuscitation that had improved one or more AIS grades by the time of discharge had higher MAP values than those who did not. D) MAP values for patients who were AIS D following resuscitation are plotted. Higher MAP values were seen on those patients who did not improve neurologically by the time of discharge and compared with those who demonstrated improvement.
AIS = American Spinal Injury Association Impairment Scale
MAP = mean arterial pressure
SCI = spinal cord injury
Figure 3 (top row) displays the average MAP for AIS A, B/C, and D groups over the first three days of admission to the Neurotrauma ICU. The average MAP for initially AIS A patients who improved at least one AIS grade was significantly higher than in those who did not demonstrate improvement (96.6 +/− 0.07 mmHg vs 94.4 +/−0.06 mmHg; p < .001). The AIS B/C group showed a similar significant difference of average MAP in the improvement vs no improvement group (97.2 +/− 0.08 mmHg vs 88.4 +/− 0.1 mmHg; p < .001). In the AIS D group, patients who did not improve neurologically had a higher MAP of 96.2 +/− 0.11 mmHg compared with patients who improved one AIS grade (average MAP of 89.5 +/− 0.09 mmHg, p < .001). Figure 3 (bottom row) displays the proportion of MAP values below the 85 mmHg threshold for AIS A, B/C, and D groups over the first three days of admission to the Neurotrauma ICU. In the AIS A group there was a significantly greater proportion of MAP values recorded below the 85 mmHg threshold in the no improvement vs improvement group (25.6% +/− 1.0 vs 13.5% +/− 0.6; p < .001). Similarly, a greater proportion of MAP values below 85 mmHg was revealed in the AIS B/C group in the no improvement vs improvement groups (35.7% +/− 0.3 vs 18.6% +/− 0.6; p < .001). However, in the AIS D group those who improved had a greater proportion of MAP values below the 85 mmHg threshold than the no improvement group (30.1% +/− 0.2 vs 19.6 +/− 1.7; p < .001).
Figure 3. Average MAP and Proportion of MAP Values Below 85 mmHg for Patients Grouped By Post-Resuscitation AIS Score.

(Top) Average MAP values recorded over the first 3 days of admission to the neurological intensive care unit are presented for patients AIS A, B and C (combined), as well as D as assessed on post-resuscitation neurological examination. This epoch was examined as our group previously demonstrated a strong correlation between MAP and outcome over this time period. In patients initially AIS A as well as those initially AIS B/C, Student’s T-Test revealed significantly higher average MAP values in patients who exhibited improvement in AIS grade by the time of discharge (p < .001 in both cases). In AIS D patients significantly higher average MAP values were seen in the patients exhibiting no improvement in neurological examination (p < .001). (Bottom) The proportion of MAP values over the first 3 days that were below 85 mmHg are plotted. In patients in AIS A and AIS B/C groups, there was a significantly greater proportion of MAP values below 85 mmHg in the no improvement group vs the improvement group (p < .001 in both cases). In AIS D patients there were a significantly greater proportion of MAP values recorded over the first 3 days that were below the 85 mmHg threshold in the improvement group vs. the no improvement group (p < .001).
Error bars represent standard error in all cases.
AIS = American Spinal Injury Association Impairment Scale
MAP = mean arterial pressure
Discussion
Given that blood pressure management is one of the few beneficial interventions which physicians currently have to offer patients with acute SCI it is imperative that we increase our understanding of how MAP relates to neurological improvement. Moreover, given the substantial costs associated with the administration and monitoring of vasoactive medications in the ICU - as well as their risk for complications19 - it is important to identify patients who may benefit less or not at all from this treatment strategy. We hypothesized that patients with an initial AIS score of A as assessed on the post-resuscitation neurological exam would show a weaker correlation between MAP values and outcome than those with incomplete injuries. Our results failed to support this hypothesis. Indeed, our results suggest the opposite - that those with marked neurological deficits may benefit from MAP augmentation more than those with less severe deficits.
The Importance of Preventing Hypotension and Augmenting MAP Values
Secondary insults play an important role in exacerbating neurological injury following SCI. Indeed, hypotension is frequently seen following SCI, affecting an estimated 68% of AIS A and B patients20. Common causes of hypotension in this population include neurogenic and hemorrhagic shock21,22. Neurogenic shock results from disruption of descending sympathetics in the spinal cord (at or above the T6 neurological level) resulting in vasodilation, hypotension and bradycardia. Hemorrhagic shock is often seen with concomitant injuries which can be difficult to identify in patients with sensory deficits. It is also important to consider that neurogenic and hypovolemic shock can co-exist. The consequence of these phenomena can be hypotension and ultimately spinal cord hypoxia and ischemia5,12,23–25.
Despite the general acceptance of the blood pressure parameters recommended in the acute SCI guidelines15, the data which supports these recommendations have substantial limitations. In all cases the relationship between blood pressure and outcome was confounded by an overall aggressive care strategy, and in no case was comparison made to contemporaneous controls26–30. Although the data inherent to this work and our previous work is correlative and does not inform causation, it substantially informs the relationship between MAP and outcome. Our previous publication reports evidence supporting efforts to maintain MAP values greater than 85–90 mmHg in spinal cord injured patients as these blood pressure values correlated with greater degree of neurological improvement6.
Patients with AIS A, B and C but Not D Injuries Demonstrated a Positive Correlation Between MAP and Outcome
Although published guidelines currently recommend the same blood pressure management irrespective of initial injury severity, it is recognized that severely injured patients may be subject to nihilism and less aggressive care.31–32 In our experience, many physicians provide less aggressive care to spinal cord injured patients with complete neurological deficits because of doubts that it is of benefit. Importantly our study demonstrated that higher blood pressures correlated with improved outcome in patients with initially motor and sensory complete injuries (Figures 1, 2). While our study does not provide evidence of a causal relationship, the presence of this relationship raises this possibility. Moreover, it suggests that patients with initially complete SCIs may benefit from MAP augmentation similar to those with AIS B/C injuries. This result suggests that nihilism is best avoided in these patients.
Surprisingly, patients with post-resuscitation AIS scores of D did not show a positive correlation between MAP values and outcome. One possibility is that because these patients already had higher proportion of MAP values >85 mmHg than AIS A, B and C patients (Figure 1A), MAP augmentation in this group was not of additional benefit. The fact that AIS D patients can only improve by a single AIS score may also play a role. Another potential confound is that the AIS D patients had significantly fewer days in the hospital than the other groups potentially allowing fewer of these patients to improve neurologically by the time of their outcome assessment. Duration of hospital stay did not differ between patients who improved neurologically vs. those who did not, however (p=0.498, binomial logistic regression; R2=0.0001). Because of these issues we do not feel that these data should discourage efforts to intensively monitor and treat blood pressure in AIS D patients.
We chose to examine MAP values only within the first 3 days after SCI as our previous work demonstrated the strongest association between MAP and outcome early after SCI. The temporal relationship between MAP and neurological improvement following SCI was subject to detailed analysis in our previous publication and benefit was seen for 5–7 days with diminishing benefit over time6. An analysis of only the first 3 days post ICU admission shown here, was thus for the sake of simplicity and should not be used as evidence to conclude that a short period of MAP augmentation is appropriate.
Vasopressor Complications and Risk Inherent to Use With Complete Spinal Cord Injury
MAP augmentation with vasoactive agents is associated with a wide array of possible complications in critically injured patients including hypoperfusion of extremities and internal organs, dysrhythmias, hyperglycemia, myocardial infarction, and local effects, such as skin necrosis.33–36 As well, the prolonged ICU stay and immobility inherent in administration of vasopressors can lead to numerous complications such as thromboembolism, pressure sores and pneumonia.37 Complications associated with the administration of vasoactive agents following acute SCI were recently examined in detail in a series of SCI patients treated at our institution which overlap with those included in the present study. In this work Inoue et al found that complications of vasopressor therapy were seen in 74% of patients (most commonly tachycardia). These complications were independently associated with the overall usages of dopamine (odds ratio [OR] 8.97; p < 0.001) and phenylephrine (OR, 5.92; p = 0.004), age > 60 years old (OR, 5.16; p = 0.013), and – of great relevance to the current study – complete SCI (OR, 3.23; p = 0.028). AIS A patients are likely at greater risk of complications because many have severe injury to autonomic innervation38. This elevated risk of complications should be considered in the decision to administer vasopressor medication to AIS A patients in accordance with published guidelines, and could prompt early cessation in the context of diminishing benefit with time, especially in the elderly (Hawryluk et al, 2015).
Initially Complete Spinal Cord Injury and Nihilism
While it is true that patients with initially complete SCIs have the poorest prognosis, contemporary medical care is seeing substantial improvement in many of these patients. Our study found that 33% of patients who are AIS A at the post-resuscitation neurological examination will improve by at least 1 grade, and 24% improve by greater than one grade by the time of hospital discharge. This is similar to the conversions rates seen in the recent STASCIS and Cethrin studies16,18. While aggressive care such as early surgical decompression and MAP augmentation is unlikely to benefit all initially complete SCI patients – such as the very rare patient with a true spinal cord transection – our data demonstrate that many may benefit.
Potential Confounding Effects
Despite the “big data” inherent to our study, the limited number of patients studied prevents us from performing meaningful multivariate analyses which could inform potential confounds relevant to our data. We have instead performed a series of univariate analyses of variables which could confound the results of our analysis (Table 1). A number of confounds which could have detracted from the benefit of MAP augmentation in AIS D patients are described above. In addition, it is important to note that all AIS A patients required surgery and that the average time to surgery was 40.3 hours in this group. This time to surgery in the AIS D group was only 13.2 hours, although this difference was not statistically significant (Table 1, p=.156). This likely reflects more severe, complex spinal injuries in AIS A patients as well as the presence of other severe injuries, which is supported by the higher ISS values noted in this group. A greater effort to perform early surgery on those felt to have greater capacity for recovery may also be at least somewhat contributory. Based largely on the findings of the STASCIS study, current recommendations are to decompress and/or stabilize traumatic SCI patients within 24 hours of injury as this practice is associated with improved neurological outcome in acute cervical SCI patients16,39.
Analysis of the proportion of patients receiving two vasopressors reveals that medical management of AIS A patients was not as aggressive as patients with less severe SCI in this study. Only 21.2 % of patients in the AIS A group received two vasopressors, while 35.3% of patients and 41.7% of patients in the AIS B/C and AIS D groups respectively received two vasopressors. This is remarkable considering the lower MAP values seen in AIS A, B and C patients compared with those AIS D on post-resuscitation neurological exam. This raises the possibility of nihilism in our institution, although alternate explanations are possible such as the presence of other injuries precluding aggressive MAP augmentation such as an aortic dissection.
Limitations
We have previously discussed many of the limitations inherent to our study6. Our stratification of patients based on baseline post-resuscitation AIS score led to a small number of patients in some groups. This mandated collapsing some of the groups in order to achieve meaningful results. Specifically, we had to collapse those with post-resuscitation AIS scores of B or C into a single group. As well, we dichotomized outcomes into patients who improved or did not improve – sample size limitations prevented a discrimination of patients with different degrees of neurological improvement. In addition, though our physiologic data were collected prospectively, the other data were collected retrospectively. Twenty-six patients were excluded because data from the neurological examination was not available. Blood pressure values obtained in the pre-hospital setting and those from the emergency department were not available for analysis; the first recorded blood pressure values were obtained in the ICU in all cases.
Conclusion
To the authors’ knowledge this is the first study to date to analyze the association between MAP and neurological improvement in patients stratified by their post-resuscitation AIS score. Our data indicates that there is a positive correlation between higher MAP values and neurological improvement in patients who are AIS A, and B/C on the post-resuscitation exam, but not those who are AIS D. Contrary to our hypothesis, these data thus suggest the possibility that AIS A patients may derive greater benefit from MAP augmentation than AIS D patients and that it is important to avoid nihilism when considering MAP augmentation in patients with initially complete SCIs. The higher rate of complications related to vasopressor administration in AIS A patients should be considered when deciding on the duration of therapy, however. Lack of apparent benefit from MAP augmentation in AIS D patients may be related to a number of possible confounds such as the higher baseline blood pressures observed in this group and should not discourage intensive MAP monitoring and treatment in these patients.
Highlights.
A retrospective study of SCI patients in a large academic level 1 trauma center
Analyzed the association of MAP and neurological improvement in patients with SCI stratified by AIS
Patients with an initially complete SCI may derive greater benefit from MAP augmentation than those with less severe injuries
Acknowledgments
Financial support and industry affiliations: None
Disclosure of funding:
This work was supported by DoD CDMRP grants SC090241 (W81XWH-10- 1-0910) and SC120259 (W81XWH-13-1-0297) to MSB and GTM. Jonathan Pan was supported by NIH T32 GM008440.
Abbreviations
- AIS
American Spinal Injury Association Impairment Scale
- ASIA
American Spinal Injury Association
- SCI
Spinal Cord Injury
- MAP
Mean Arterial Pressure
- ICU
Intensive Care Unit
- ISS
Injury Severity Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joshua Stephen Catapano, Email: Joshua.Catapano@barrowbrainandspine.com.
William Whetstone, Email: william.whetstone@emergency.ucsf.edu.
Rajiv Saigal, Email: saigalr@neurosurg.ucsf.edu.
Adam Ferguson, Email: adam.ferguson@ucsf.edu.
Jason Talbott, Email: jason.talbott@ucsf.edu.
Jacqueline Bresnahan, Email: jacqueline.bresnahan@ucsf.edu.
Sanjay Dhall, Email: dhalls@neurosurg.ucsf.edu.
Jonathan Pan, Email: panj@anesthesia.ucsf.edu.
Michael Beattie, Email: michael.beattie@ucsf.edu.
Geoffrey Manley, Email: manleyg@neurosurg.ucsf.edu.
References
- 1.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pa. Arch Phys Med Rehabil. 2014;95(5):986–995 e1. doi: 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spinal Cord Injury Facts and Figures at a Glance. The Journal of Spinal Cord Medicine. 2013;36(4):394–395. doi: 10.1179/204577211X13218754005573. 2013/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawryluk G, Garber S. Provision of nutrients after acute spinal cord injury: the implications of feast and famine. Neural Regen Res. 2015;10(7):1061. doi: 10.4103/1673-5374.160081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadotte DW, Fehlings MG. Spinal Cord Injury: A Systematic Review of Current Treatment Options. Clinical Orthopaedics and Related Research®. 2010;469(3):732–741. doi: 10.1007/s11999-010-1674-0. 2010/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casha S, Christie S. A Systematic Review of Intensive Cardiopulmonary Management after Spinal Cord Injury. J Neurotrauma. 2011;28(8):1479–1495. doi: 10.1089/neu.2009.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawryluk G, Whetstone W, Saigal R, et al. Mean Arterial Blood Pressure Correlates with Neurological Recovery after Human Spinal Cord Injury: Analysis of High Frequency Physiologic Data. J Neurotrauma. 2015;32(24):1958–1967. doi: 10.1089/neu.2014.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowland JW, Hawryluk GWJ, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. doi: 10.3171/foc.2008.25.11.e2. [DOI] [PubMed] [Google Scholar]
- 8.Borgens R, Ben Liu-Snyder P. Understanding Secondary Injury. Q Rev Biol. 2012;87(2):89–127. doi: 10.1086/665457. [DOI] [PubMed] [Google Scholar]
- 9.Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Chu D, Qiu J, Grafe M, et al. Delayed cell death signaling in traumatized central nervous system: hypoxia. Neurochem Res. 2002;27(1/2):97–106. doi: 10.1023/a:1014858707218. [DOI] [PubMed] [Google Scholar]
- 11.Stys PK. Anoxic and Ischemic Injury of Myelinated Axons in CNS White Matter: From Mechanistic Concepts to Therapeutics. J Cereb Blood Flow Metab. 1998:2–25. doi: 10.1097/00004647-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hagen E, Rekand T, Grønning M, Færestrand S. Cardiovascular complications of spinal cord injury. Tidsskr Nor Laegeforen. 2012;132(9):1115–1120. doi: 10.4045/tidsskr.11.0551. [DOI] [PubMed] [Google Scholar]
- 13.Hawryluk GWJ, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25(5):E14. doi: 10.3171/foc.2008.25.11.e14. [DOI] [PubMed] [Google Scholar]
- 14.Evans LT, Lollis SS, Ball PA. Management of Acute Spinal Cord Injury in the Neurocritical Care Unit. Neurosurg Clin N Am. 2013;24(3):339–347. doi: 10.1016/j.nec.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Walters BC, Hadley MN, Hurlbert RJ, et al. Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries. Neurosurgery. 2013;60:82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]
- 16.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus Delayed Decompression for Traumatic Cervical Spinal Cord Injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7(2):e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourassa-Moreau É, Mac-Thiong J-M, Li A. Do Patients with Complete Spinal Cord Injury Benefit from Early Surgical Decompression? Analysis of Neurological Improvement in a Prospective Cohort Study. J Neurotrauma. 2016 doi: 10.1089/neu.2015.3957. [DOI] [PubMed] [Google Scholar]
- 18.Fehlings MG, Theodore N, Harrop J, et al. A Phase I/IIa Clinical Trial of a Recombinant Rho Protein Antagonist in Acute Spinal Cord Injury. J Neurotrauma. 2011;28(5):787–796. doi: 10.1089/neu.2011.1765. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Manley GT, Patel N, Whetstone WD. Medical and Surgical Management after Spinal Cord Injury: Vasopressor Usage, Early Surgerys, and Complications. J Neurotrauma. 2014;31(3):284–291. doi: 10.1089/neu.2013.3061. [DOI] [PubMed] [Google Scholar]
- 20.Popa C, Popa F, Grigorean VT, et al. Vascular dysfunctions following spinal cord injury. J Med Life. 2010 Jul-Sep;3(3):275–285. [PMC free article] [PubMed] [Google Scholar]
- 21.Piepmeier JM, Lehmann KB, Lane JG. Cardiovascular instability following acute cervical spinal cord trauma. Cent Nerv Syst Trauma. 1985 Fall;2(3):153–160. doi: 10.1089/cns.1985.2.153. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann KG, Lane JG, Piepmeier JM, Batsford WP. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: Incidence, time course and severity. J Am Coll Cardiol. 1987;10(1):46–52. doi: 10.1016/s0735-1097(87)80158-4. [DOI] [PubMed] [Google Scholar]
- 23.Tator CH, Rowed DW, Schwartz ML. Sunnybrook cord injury scales for assessing neurological injury and neurological recovery. In: Tator CH, editor. Early Management of Acute Spinal Cord Injury. Raven Press; New York: 1982. pp. 7–24. [Google Scholar]
- 24.Yanagawa Y, Marcillo A, Garcia-Rojas R, Loor KE, Dietrich WD. Influence of Posttraumatic Hypoxia on Behavioral Recovery and Histopathological Outcome Following Moderate Spinal Cord Injury in Rats. J Neurotrauma. 2001;18(6):635–644. doi: 10.1089/089771501750291873. [DOI] [PubMed] [Google Scholar]
- 25.Hall ED, Wolf DL. A pharmacological analysis of the pathophysiological mechanisms of posttraumatic spinal cord ischemia. J Neurosurg. 1986;64(6):951–961. doi: 10.3171/jns.1986.64.6.0951. [DOI] [PubMed] [Google Scholar]
- 26.Zäch GA, Seiler W, Dollfus P. Treatment results of spinal cord injuries in the Swiss paraplegic centre of Basle. Paraplegia. 1976;14(1):58–65. doi: 10.1038/sc.1976.9. [DOI] [PubMed] [Google Scholar]
- 27.Wolf A, Levi L, Mirvis S, et al. Operative management of bilateral facet dislocation. J Neurosurg. 1991;75(6):883–890. doi: 10.3171/jns.1991.75.6.0883. [DOI] [PubMed] [Google Scholar]
- 28.Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87(2):239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- 29.Tator CH, Rowed DW, Schwartz ML, et al. Management of acute spinal cord injuries. Can J Surg. 1984 May;27(3):289–293. [PubMed] [Google Scholar]
- 30.Levi L, Wolf A, Belzberg H. Hemodynamic Parameters in Patients with Acute Cervical Cord Trauma. Neurosurgery. 1993;33(6):1007–1017. doi: 10.1227/00006123-199312000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Selecki BR. Severe injuries to the cervical cord and spine: neurosurgical management in the acute and early stage. Aust N Z J Surg. 1979 Apr;49(2):267–274. doi: 10.1111/j.1445-2197.1979.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 32.Dweik A, Van den Brande E, Kossmann T, Maas AI. History of cervical spine surgery: from nihilism to advanced reconstructive surgery. Spinal Cord. 2013 Nov;51(11):809–814. doi: 10.1038/sc.2013.107. [DOI] [PubMed] [Google Scholar]
- 33.Marik PE, Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA. 1994;272(17):1354–1357. [PubMed] [Google Scholar]
- 34.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 35.Horwitz D, Fox SM, 3D, Goldberg LI. Effects of Dopamine in man. Circ Res. 1962 Feb;10:237–243. doi: 10.1161/01.res.10.2.237. [DOI] [PubMed] [Google Scholar]
- 36.Peters JI, Utset OM. Vasopressors in shock management: Choosing and using widely. J Crit Illness. 1989;4:62. [Google Scholar]
- 37.Williams TA, Ho KM, Dobb GJ, Finn JC, Knuiman M, Webb SA, Royal Perth Hospital, I. C. U. D. L. G. Effect of length of stay in intensive care unit on hospital and long-term mortality of critically ill adult patients. Br J Anaesth. 2010;104(4):459–464. doi: 10.1093/bja/aeq025. doi:10.1093. [DOI] [PubMed] [Google Scholar]
- 38.Grimm DR, DeMeersman Re, Garofano RP, Spungen AM, Bauman WA. Effect of provocative maneuvers on heart rate variability in subjects with quadraplegia. Am J Physiol. 1995;268(6 Pt 2):H2239–H2245. doi: 10.1152/ajpheart.1995.268.6.H2239. [DOI] [PubMed] [Google Scholar]
- 39.Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of Decompressive Surgery of Spinal Cord after Traumatic Spinal Cord Injury: An Evidence-Based Examination of PreClinical and Clinical Studies. J Neurotrauma. 2011;28(8):1371–1399. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


