Abstract
Objective
Liver disease markers have been associated with mortality in HIV-infected individuals, in the modern era of effective antiretroviral therapy. Our objective was to determine which markers are most predictive of mortality in HIV-monoinfected and HIV/HCV-coinfected persons.
Research Design and Methods
We measured serum albumin, total protein, calculated globulin, aspartate transaminase (AST), and alanine transaminase (ALT) in 193 HIV/HCV-coinfected and 720 HIV-monoinfected persons in the study of Fat Redistribution and Metabolic Change in HIV Infection. We evaluated associations of each marker with five-year, all-cause mortality, adjusting for cardiovascular, HIV-related factors, inflammation, renal disease, muscle, and adiposity.
Results
After 5 years of follow-up, overall mortality was 21% in HIV/HCV-coinfected and 12% in HIV-monoinfected participants. After multivariable adjustment, lower albumin and higher AST were independently associated with increased mortality. Lower albumin was associated with 49% increased odds of mortality overall (per 0.5g/dL decrease, 95%CI:1.2–1.9); the association was stronger in HIV/HCV-coinfected (OR=2.1, 95%CI:1.4–3.2) versus HIV-monoinfected (OR=1.3, 95%CI:1.0–1.7; HCV-by-albumin interaction: p=0.038). Higher AST was associated with 41% increased odds of mortality (per AST doubling; 95%CI:1.1–1.8); associations were much stronger among HIV/HCV-coinfected (OR=2.5, 95%CI:1.5–4.1) than HIV-monoinfected (OR=1.1, 95%CI:0.8–1.5; HCV-by-AST interaction: p=0.0042).
Conclusions
Lower serum albumin and higher AST appear to be important mortality risk factors in HIV/HCV-coinfection, but much less so in HIV-monoinfected individuals. The association of low albumin with mortality may reflect its role as a negative acute phase response protein. AST levels do not appear to be useful in predicting mortality in HIV-monoinfection, and should be considered primarily in the context of HCV-coinfection.
Keywords: HIV infection, HCV infection, mortality, albumin, globulin, total protein, liver enzymes
INTRODUCTION
Liver disease is a major cause of mortality, accounting for 14–18% of deaths among HIV-infected persons, and is the most common non-AIDS related cause of death[1]. Coinfection with HCV occurs in approximately 25% of HIV-infected persons, but contributes to the majority (50–75%) of liver deaths in the HIV-infected population[2]. Both HIV monoinfection and HIV/HCV coinfection are associated with perturbations in liver injury markers -- aspartate (AST) and alanine (ALT) aminotransferase -- and liver synthetic function, as denoted by serum albumin[3, 4].
The Veterans Aging Cohort Study (VACS) mortality index uses the FIB-4 score, which includes AST and ALT to predict mortality, but it is not known if the VACS index performs the same in HIV/HCV-coinfected persons as in HIV-monoinfected[5]. In HIV-infected individuals, early studies found that higher gamma globulin predicted mortality in persons with pneumocystis pneumonia[6] and it is well recognized that polyclonal hypergammaglobulinemia often accompanies HIV infection[7]. More recently, low serum albumin has been independently associated with increased mortality risk in large studies of HIV-infected veterans[8] and HIV-infected women[9]; however, it is unknown the degree to which HCV-associated liver disease is associated with lower albumin. Additionally, previous studies finding associations of albumin with mortality did not fully control for inflammation, muscle mass, and kidney function[8–12]. Albumin is a negative acute phase response protein and can also be lowered due to kidney disease.
Thus, it is unknown which liver-related markers are the strongest predictors of mortality in the setting of HIV infection in the presence or absence of HCV coinfection. Our objective was to compare the associations of serum albumin, total protein, calculated globulin (total protein minus albumin), AST, and ALT with all-cause mortality in 913 HIV-infected persons in the study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). To date, no large, nationally representative study has examined these markers in combination in the setting of infection with HIV and HCV. We hypothesized that this combination of liver markers would have stronger associations with mortality than any individual marker and would improve prediction of mortality, but that these associations would be of significantly greater magnitude in HIV/HCV-coinfected persons.
METHODS
Study population
FRAM was a large, nationally representative, multicenter study of HIV-infection, originally designed to evaluate lipodystrophy and metabolic abnormalities in HIV-infected persons. Methods, design, and sample characteristics of the FRAM cohort have been described previously in detail[13]. Between June 2000 and September 2002, 1183 HIV-infected men and women from 16 geographically diverse sites were enrolled in FRAM, with a follow-up exam conducted approximately 5 years later (FRAM-2), at which time we determined mortality status. The vital status, retention, and observation time for FRAM-2 have been described previously[14]. For the present study, we included all 913 HIV-infected participants with known vital status. The institutional review boards at all sites approved the protocols for both FRAM examinations, and informed consent was obtained from all study participants.
Predictors
Liver disease markers measured in this study included serum albumin, total protein, calculated globulin (total protein minus albumin), AST, and ALT. All measures were obtained at baseline and analyzed in a single centralized laboratory (Covance, Indianapolis, IN). We assessed the linearity assumption for each biomarker by examining spline plots generated using unadjusted generalized additive models[15] and by adding quadratic terms to the model. We evaluated markers as continuous and categorical variables, using tertiles, clinically relevant cutpoints, and cutpoints determined by inspecting spline plots. AST and ALT were log-transformed to normalize their distributions. Although albumin and AST appeared to have curvilinear associations with mortality in HIV/HCV-coinfected participants, the tests for nonlinearity did not reach statistical significance for spline fits (p=0.18 for albumin, p=0.099 for AST) or in fully adjusted analysis using quadratic terms (p=0.97 for albumin, p=0.41 for AST).
Other Measurements
Standardized questionnaires that were validated in a general population were used to determine demographic characteristics; medical history; HIV risk factors; and tobacco use[13, 16]. Research associates interviewed participants and reviewed medical charts regarding antiretroviral (ARV) drug use. AIDS diagnosis was made by CD4 lymphocyte count<200 or history of opportunistic infection or malignancy. Whole body MRI was performed to quantify regional and total skeletal muscle and adipose tissue volumes, as described in detail previously[13, 17–19].
Hepatitis C (HCV) RNA testing was performed on frozen sera using Bayer Versant 3.0 branched DNA assay (Leverkusen, Germany) in the entire cohort. C-reactive protein (CRP), fibrinogen, and cystatin C were measured using a using a particle-enhanced immunonephelometric assay (BNII nephelometer, Dade Behring Inc, Deerfield, Illinois). Estimated glomerular filtration rate (eGFR) based on cystatin C was calculated using the Chronic Kidney Disease Epidemiology Collaboration Equation[20]. CD4 lymphocyte count and percent, HIV RNA level, and other blood specimens were analyzed in a single centralized laboratory (Covance, Indianapolis, IN). Biomarkers, plasma viremia, and all other blood specimens were measured at baseline.
Outcome
The primary outcome of this study was all-cause mortality. At the second exam, 794 HIV-infected participants were known to be alive and 128 were known to be dead. Vital status could not be determined for 261 participants where contact could not be re-established. Those who were lost to follow-up had intermediate values compared with those with known vital status in levels of serum albumin (median 4.2 vs. 4.1 vs. 3.9 g/dL for alive, lost to follow-up, and deceased respectively) and AST (median 28 vs. 30 vs. 35 U/L). Linkage to the National Death Index was not possible because of patient confidentiality constraints.
Covariates
All covariates were collected at the first examination, and vital status was determined at the second examination, five years later. Demographic-adjusted models controlled for age, gender, and race/ethnicity. Multiariable adjusted models controlled for age, gender, race/ethnicity, non-HDL (high density lipoprotein) cholesterol, smoking, arm and leg muscle volume, visceral adipose tissue, CD4 count, ARV exposure, C-reactive protein, fibrinogen, and estimated glomerular filtration rate (eGFR) by cystatin C, as in previous analyses[21]. We calculated eGFR using cystatin C rather than creatinine, because creatinine-based estimates are less sensitive than cystatin C for kidney disease diagnosis and prediction of all-cause mortality[22]. Multiple imputation using the Markov chain Monte Carlo method was used to impute missing covariates[23].
Statistical Methods
As in previous analyses [21,24,25], we analyzed cumulative 5-year mortality using multivariable logistic regression analysis, including an offset term for follow-up time. Because the exact dates of death were unknown, those who died provided left-censored observations, meaning that death was only known to have occurred sometime before the contact attempt at approximately 5 years of follow-up. We therefore used logistic regression with an offset term for follow-up time, rather than Cox proportional hazards regression as our primary analysis, because this form of regression is appropriate for left-censored events. We also tested exponential regression survival models, but found that model fit was improved using logistic regression. Follow-up time was defined as elapsed time from baseline to follow-up exam or last contact. To account for those with missing vital status, we adjusted estimates using an inverse probability weighting approach by modeling the participant’s probability of having known vital status[26]. The inverse of this probability was then used as a weight applied to persons with known vital status in the logistic regression analysis of death.
To estimate whether liver disease markers independently predicted mortality, multivariable models were adjusted for demographics, traditional cardiovascular disease (CVD) risk factors, HIV-related factors, inflammation and renal markers, and MRI-measured adiposity and muscle mass. Markers were tested both individually and jointly. To ensure that models were not overfit, we built parsimonious models using a backward stepwise procedure.
Finally, to quantify the impact of liver disease markers on mortality in HIV-infected persons at a population level, we estimated the attributable risk associated with having elevated AST (defined using the clinically established cutpoint of 40 U/L[27]) or low albumin (defined as <4 g/dL, based on our previous finding that mortality risk increases below this point in HIV-infected veterans[8]). The attributable risk accounts for not only the strength of the association of a risk factor with mortality, but also for the condition’s prevalence in the population of interest. We calculated the population attributable risk percent using incidence rates of death predicted from fully adjusted multivariable models as: 100*[(population mortality rate - mortality rate in unexposed)/population mortality rate][28]. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Baseline characteristics
Demographic and baseline clinical characteristics of the 913 HIV-infected participants with known vital status and available serum albumin are shown stratified by approximate tertile of albumin in Table 1. HIV-infected participants with low albumin were more often women, African American, smokers, antihypertensive medication users, and had higher BMI, total protein, AST, CRP, and fibrinogen. Total cholesterol was lower and there was less hypolipidemic medication use, lower eGFR, and more albuminuria among those with low albumin. Among HIV-related factors, baseline CD4 counts were lower, detectable viremia, and history of AIDS and HCV infection were more prevalent, while ARV use was less prevalent in those with lower serum albumin.
Table 1.
Baseline demographic and clinical characteristics of HIV-infected FRAM participants, stratified by serum albumin (approximate tertiles)
| Parameter | Albumin ≤ 4.0 g/dL n=335 |
4.1 – 4.3 g/dL n=259 |
Albumin ≥ 4.4 g/dL n=319 |
P-value |
|---|---|---|---|---|
| Age (y) | 43.0 (37.0–49.0) | 44.0 (37.0–50.0) | 42.0 (37.0–48.0) | 0.44 |
| Female | 144 (43%) | 84 (32%) | 46 (14%) | <.0001 |
| African-American | 191 (57%) | 95 (37%) | 86 (27%) | <.0001 |
| Caucasian | 105 (31%) | 144 (56%) | 198 (62%) | |
| Other | 39 (12%) | 20 (8%) | 35 (11%) | |
| Diabetic | 33 (10%) | 22 (8%) | 27 (8%) | 0.78 |
| Current Smoker | 180 (54%) | 96 (37%) | 105 (33%) | <.0001 |
| Past Smoker | 55 (16%) | 74 (29%) | 88 (28%) | |
| Never Smoker | 100 (30%) | 89 (34%) | 126 (39%) | |
| Antihypertensive use | 92 (27%) | 47 (18%) | 65 (20%) | 0.015 |
| ACE-I use | 37 (11%) | 20 (8%) | 29 (9%) | 0.38 |
| Hyperlipidemia Treatment | 30 (9%) | 35 (14%) | 91 (29%) | <.0001 |
| Systolic Blood Pressure (mmHg) | 114 (105–125) | 115 (105–122) | 118 (109–125) | 0.012 |
| Diastolic Blood Pressure (mmHg) | 78.0 (70.0–84.0) | 77.0 (71.0–83.0) | 79.0 (71.0–85.0) | 0.24 |
| Waist Circumference (cm) | 88.7 (79.6–96.8) | 88.3 (81.7–96.0) | 88.0 (81.5–95.8) | 0.85 |
| BMI (kg/m2) | 24.8 (22.1–29.3) | 24.7 (22.8–27.6) | 24.2 (21.9–26.8) | 0.0069 |
| Serum Albumin (g/dL) | 3.8 (3.5–3.9) | 4.2 (4.1–4.3) | 4.6 (4.4–4.7) | <.0001 |
| Total Protein (g/dL) | 8.0 (7.4–8.7) | 7.7 (7.3–8.2) | 7.9 (7.5–8.4) | 0.0013 |
| Total Protein - Albumin (g/dL) | 4.2 (3.6–4.9) | 3.5 (3.1–4.0) | 3.2 (2.9–3.7) | <.0001 |
| ALT (U/L) | 28.0 (17.0–42.0) | 25.0 (18.0–42.0) | 29.0 (21.0–47.0) | 0.037 |
| AST (U/L) | 32.0 (23.0–51.0) | 27.0 (22.0–40.0) | 29.0 (23.0–40.0) | 0.0026 |
| Total Cholesterol (mg/dL) | 178 (148–213) | 192 (165–227) | 205 (170–245) | <.0001 |
| HDL (mg/dL) | 41.0 (32.0–54.0) | 42.0 (35.0–53.0) | 40.0 (33.0–51.0) | 0.25 |
| CRP (mg/L) | 2.5 (1.1–5.2) | 1.9 (0.7–4.0) | 1.4 (0.7–3.0) | <.0001 |
| Fibrinogen (mg/dL) | 396 (318–477) | 353 (290–425) | 345 (281–405) | <.0001 |
| eGFRcys (mL/min/1.73m2) | 78 (62–97) | 87 (72–104) | 88 (74–101) | <.0001 |
| Albuminuria | 97 (29%) | 35 (14%) | 61 (19%) | <.0001 |
| Detectable HIV RNA | 217 (65%) | 128 (49%) | 101 (32%) | <.0001 |
| Current CD4 (cells/uL) | 303 (162–481) | 366 (238–566) | 423 (270–593) | <.0001 |
| HIV Duration (y) | 8.5 (5.6–11.6) | 8.0 (5.2–11.6) | 7.7 (5.3–12.0) | 0.35 |
| Hepatitis C infection | 104 (31%) | 55 (21%) | 34 (11%) | <.0001 |
| History of AIDS | 257 (77%) | 169 (65%) | 227 (71%) | 0.0088 |
| Ever use of HAART | 283 (84%) | 229 (88%) | 296 (93%) | 0.0039 |
| Current ARV | 257 (77%) | 218 (84%) | 297 (93%) | <.0001 |
Data are presented as Median (IQR) or numbers (percent).
Abbreviations: IQR, interquartile range; AST, aspartate transaminase; ALT, alanine transaminase; HDL, high-density lipoprotein; CRP, C-reactive protein; eGFRcys, estimated GFR by cystatin C; HAART, highly active antiretroviral therapy.
We also examined baseline characteristics of participants stratified by approximate tertile of AST (Supplemental Table 1). In common with the results for low albumin, HIV-infected participants with high AST were more often African American, had higher total protein, and had lower levels of albumin, total cholesterol, and eGFR. Those with higher AST also had more albuminuria, lower baseline CD4 counts, and less current ARV use, while HCV infection and history of AIDS were more prevalent compared to those who had lower levels of AST. There were differences compared to the results with low albumin in that those with higher AST had lower CRP and somewhat lower fibrinogen, and were more often men, not women. Furthermore, those with higher AST were more often older and had higher diastolic blood pressure and higher ALT. Also in contrast to those with low albumin, there was little relationship between high AST and levels of BMI, smoking, fibrinogen, and anti-hypertensive and hypolipidemic medication use.
Association of liver disease markers with five-year mortality
After 5 years of follow-up, the overall mortality rate was 14%. However, the mortality rate was 21% in HIV/HCV-coinfected participants compared to 12% in HIV-monoinfected participants (unadjusted OR=2.0 for HIV/HCV+ vs. HIV/HCV−, 95%CI: 1.4–2.9; p=0.0002). We first examined associations of individual liver disease markers with mortality. In demographic-adjusted analyses, lower levels of serum albumin were associated with greater five-year mortality risk, while higher total protein, total protein minus albumin, and AST were associated with increased mortality risk (Table 2). By contrast, the association of ALT with mortality was weak (OR=1.10 per doubling, p=0.31). The association of AST with mortality was stronger in HIV/HCV–coinfected persons than HIV-monoinfected (test for interaction: HCV x AST: p=0.023).
Table 2.
Demographic-adjusted* associations of individual liver disease markers with all-cause mortality
| Parameter | Overall n=913) Odds of mortality (95% CI) |
HCV+/HIV+ (n=193) Odds of mortality (95% CI) |
HIV Monoinfected (n=720) Odds of mortality (95% CI) |
Test for HCV × Marker interaction P-value |
|---|---|---|---|---|
| Serum Albumin (per 0.5 g/dL decrease) | 2.2 (1.83, 2.7) p<.0001 |
2.7 (1.89, 3.9) p<.0001 |
1.98 (1.58, 2.5 p<.0001 |
0.14 |
| Total Protein (per 0.5 g/dL increase) | 1.17 (1.05, 1.31) p=0.0060 |
1.13 (0.94, 1.37) p=0.20 |
1.16 (1.01, 1.33) p=0.036 |
0.83 |
| Calculated Globulin (per 0.5 g/dL increase) | 1.39 (1.27, 1.53) p<.0001 |
1.37 (1.17, 1.61) p<.0001 |
1.37 (1.22, 1.54) p<.0001 |
0.99 |
| AST (per doubling) | 1.67 (1.38, 2.0) p<.0001 |
2.3 (1.58, 3.4) p<.0001 |
1.34 (1.01, 1.77) p=0.044 |
0.023 |
| ALT (per doubling) | 1.10 (0.92, 1.31) p=0.31 |
1.13 (0.83, 1.54) p=0.44 |
0.92 (0.72, 1.18) p=0.50 |
0.30 |
Models control for age, sex, and race/ethnicity.
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; AST, aspartate transaminase; ALT, alanine transaminase
A graphical examination showed that the mortality risk differed between HIV/HCV-coinfected and HIV-monoinfected persons, as it was pronounced and wider among those with lower albumin and higher AST (Figure 1). HIV/HCV coinfection appeared to have little association with excess mortality among those with albumin levels above 4 g/dL and among those with AST levels below 64 U/L compared to HIV-monoinfected persons. In demographic adjusted analysis (Supplemental Table 2), HIV/HCV coinfection was associated with a 2.5-fold (95%CI: 1.04–5.8) higher odds of mortality at an albumin level of 3.0 g/dL, while the odds ratio was only 1.1 (95%CI: 0.67–1.73) at an albumin level of 4.0 g/dL. Similarly, being HIV/HCV-coinfected was associated with a 2.3-fold (95%CI: 1.08–5.0) higher odds of mortality at an AST level of 120, compared with an odds ratio of 1.3 (95%CI: 0.82–2.2) at an AST level of 60.
Figure 1.
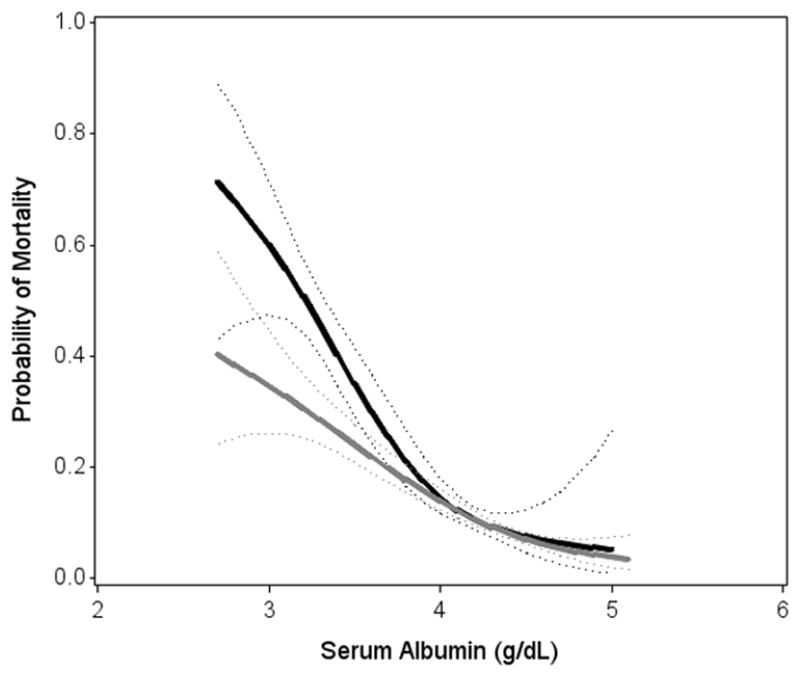
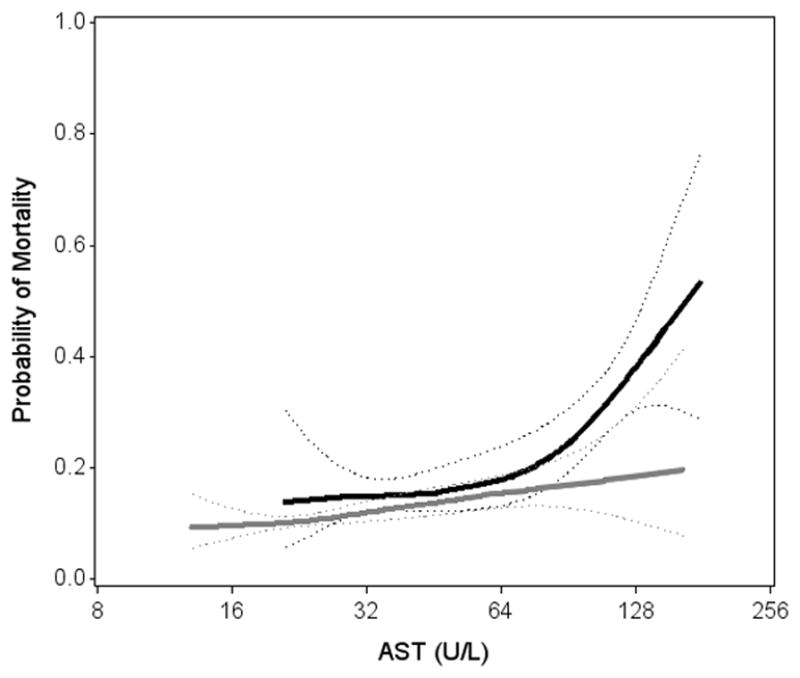
Associations of (A) Serum Albumin and (B) AST with mortality, stratified by hepatitis C status
Solid lines denotes predicted probability of mortality (with dotted 95%CI confidence bounds) calculated from unadjusted generalized additive model
Lowest and highest 2.5% are truncated.
Black/solid ————: HCV/HIV coinfected
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; AST, aspartate transaminase.
Multivariable analysis of liver disease markers with five-year mortality
Table 3 presents multivariable models of mortality, adjusting for traditional CVD risk factors, HIV-related factors, inflammation, renal markers, muscle mass, and adiposity (all factors previously shown to be associated with mortality). Within the overall HIV-infected group, we found that decreased albumin and increased AST were independently associated with higher mortality risk. In this fully adjusted analysis, each 0.5 g/dL decrease in serum albumin was associated with a 49% increase in the odds of mortality (p=0.0010), while each doubling of AST was associated with a 41% increase in the odds of mortality (p=0.0076). Addition of albumin and AST to the model improved discrimination to a greater extent in HIV/HCV-coinfected (from c=0.82 to c=0.88, p=0.0088) than in HIV-monoinfected participants (from c=0.89 to c=0.89, p=0.21).
Table 3.
Multivariable-adjusted associations of serum albumin and AST with all-cause mortality
| Parameter | Overall (n=913) Odds of mortality (95% CI) |
HCV+/HIV+ (n=193) Odds of mortality (95% CI) |
HIV Monoinfected (n=720) Odds of mortality (95% CI) |
Test for HCV × factor interaction P-value |
|---|---|---|---|---|
| Serum Albumin (per 0.5 g/dL decrease) |
1.49 (1.17, 1.89) p=0.0010 |
2.1 (1.39, 3.2) p=0.0005 |
1.28 (0.98, 1.69) p=0.074 |
0.038 |
| AST (doubling) |
1.41 (1.09, 1.81) p=0.0076 |
2.5 (1.54, 4.1) p=0.0002 |
1.08 (0.77, 1.51) p=0.67 |
0.0042 |
| Current vs. Never Smoker | 3.2 (1.83, 5.5) p<.0001 |
2.5 (1.01, 6.1) p=0.046 |
3.5 (1.89, 6.6) p<.0001 |
0.46 |
| Past vs. Never Smoker | 1.68 (0.88, 3.2) p=0.12 |
1.86 (0.57, 6.1) p=0.30 |
1.64 (0.82, 3.3) p=0.16 |
0.75 |
| Arm SM Tertile 2 vs. Tertile 1 | 0.59 (0.35, 0.99) p=0.046 |
0.62 (0.25, 1.57) p=0.31 |
0.57 (0.31, 1.06) p=0.077 |
0.42 |
| Arm SM Tertile 3 vs. Tertile 1 | 0.48 (0.23, 1.00) p=0.050 |
0.53 (0.19, 1.43) p=0.21 |
0.46 (0.18, 1.15) p=0.093 |
0.70 |
| VAT Tertile 2 vs. Tertile 1 | 2.2 (1.21, 3.8) p=0.0095 |
2.3 (0.90, 5.6) p=0.082 |
2.1 (1.13, 4.0) p=0.019 |
0.69 |
| VAT Tertile 3 vs. Tertile 1 | 2.7 (1.41, 5.1) p=0.0027 |
3.5 (1.31, 9.2) p=0.013 |
2.4 (1.17, 5.0) p=0.017 |
0.36 |
| Leg SM Tertile 2 vs. Tertile 1 | 0.95 (0.55, 1.61) p=0.84 |
0.91 (0.39, 2.1) p=0.83 |
0.97 (0.53, 1.78) p=0.91 |
0.97 |
| Leg SM Tertile 3 vs. Tertile 1 | 0.44 (0.22, 0.90) p=0.025 |
0.51 (0.17, 1.52) p=0.23 |
0.41 (0.19, 0.91) p=0.029 |
0.83 |
| CD4 (per doubling) | 0.63 (0.55, 0.72) p<.0001 |
0.86 (0.67, 1.09) p=0.21 |
0.56 (0.48, 0.66) p<.0001 |
0.0049 |
| Non HDLc (per 10 mg/dL) | 0.94 (0.90, 0.98) p=0.0031 |
0.91 (0.81, 1.01) p=0.074 |
0.94 (0.90, 0.99) p=0.0099 |
0.54 |
| Amprenavir (total duration) | 2.1 (1.31, 3.5) p=0.0022 |
2.3 (0.099, 53.1) p=0.60 |
2.1 (1.30, 3.5) p=0.0026 |
0.96 |
| CRP (doubling) | 1.19 (1.04, 1.35) p=0.0095 |
1.32 (1.07, 1.61) p=0.0081 |
1.10 (0.94, 1.29) p=0.23 |
0.16 |
| Fibrinogen (doubling) | 2.1 (1.17, 3.8) p=0.013 |
1.31 (0.47, 3.6) p=0.60 |
2.5 (1.28, 4.8) p=0.0072 |
0.27 |
| eGFRcys (per 10 ml/min/1.73m2) | 0.90 (0.81, 1.00) p=0.041 |
0.89 (0.74, 1.06) p=0.19 |
0.90 (0.79, 1.02) p=0.089 |
0.92 |
Note: Multivariable-adjusted models control for age, gender, race/ethnicity, serum albumin, AST, smoking, arm SM, VAT, Leg SM, CD4 count, non-HDLc, amprenavir, CRP, Fibrinogen, and eGFRcys.
Abbreviations: CI, confidence interval; SM, skeletal muscle; VAT, visceral adipose tissue; CRP, C-reactive protein
Although HCV infection was associated with mortality in unadjusted analysis (OR=2.0, p=0.0002), when included in the fully adjusted model with albumin and AST, HCV showed little association with mortality (OR=0.90, 95%CI: 0.51, 1.59, p=0.71). Even after multivariable adjustment, associations of serum albumin and AST differed by HCV status and were stronger in HIV/HCV-coinfected persons. The association of serum albumin with mortality was stronger among HIV/HCV-coinfected persons (OR=2.1 per 0.5 g/dL decrease, p=0.0005) compared to those without HCV infection (OR=1.28, p=0.074; test for interaction: p=0.038). The AST by HCV interaction was especially strong (p=0.0042). AST was associated with 2.5-fold higher odds of mortality in HIV/HCV-coinfected persons (95%CI: 1.5–4.1, p=0.0002) but only 1.08-fold odds in those without HCV infection (95%CI: 0.77–1.51, p=0.67; both per AST doubling). Calculated globulin and total protein showed weaker associations with mortality compared with albumin, and were not included in fully adjusted models.
Our multivariable models controlled for two distinct acute phase response proteins, CRP and fibrinogen, both of which showed independent associations with mortality. We found that lower albumin levels showed only modest correlations with higher acute phase response markers (e.g., the correlations with CRP were: r=−0.19, p<.0001 overall; r=−0.26, p=0.0003 for HIV/HCV-coinfected; and r=−0.23, p<.0001 for HIV-monoinfected). In contrast, higher AST levels were not associated with higher acute phase response proteins. Instead, AST was weakly associated with lower CRP overall (r=−0.11, p=0.0012), and had less association when stratified by disease category (r=−0.045, p=0.54 for HIV/HCV-coinfected; and r=−0.031, p=0.40 for HIV-monoinfected).
Population-level risk for mortality attributable to low albumin and AST elevation
We examined joint associations of albumin and AST with mortality, using clinically established cutpoints to define low serum albumin (≤4 g/dL) and elevated AST (>40 U/L) (Figure 2A). Mortality rates were highest in those who had both low albumin and elevated AST (34% for HIV/HCV-coinfected, 25% for HIV-monoinfected), and were intermediate in those with isolated low albumin (27% and 20%, respectively) or isolated elevated AST (14% and 8.4%). Mortality rates were lowest in those with normal albumin and AST (2.7% and 7.5%). Of note, the effects of both clinically increased AST and decreased albumin were associated with independent and cumulative risk in those with HIV/HCV coinfection, although tests for interaction of AST by albumin were not statistically significant (HCV+: p=0.21, HCV−: p=0.38). In contrast, having an AST >40 U/L added little increased risk to those with HIV monoinfection independent of albumin status.
Figure 2.
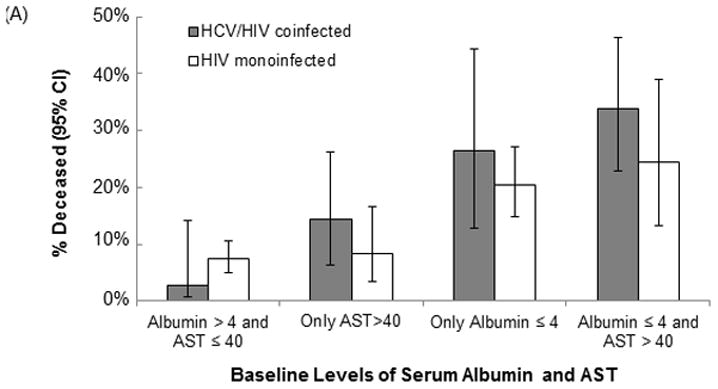
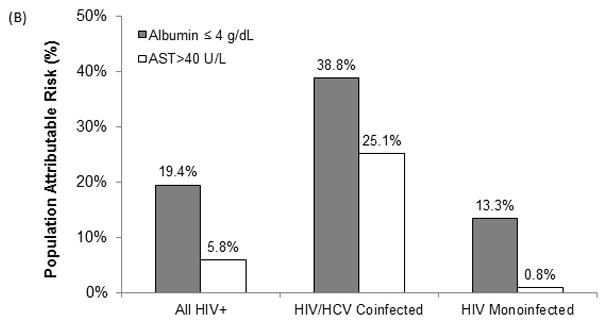
Association of low serum albumin and elevated AST with (A) mortality rates and (B) population-level attributable risk for mortality, stratified by hepatitis C status
Note: The population attributable risk (PAR) is an estimate of the percentage of the mortality rate that is attributable to having low albumin or high AST. Multivariable-adjusted models control for age, gender, race/ethnicity, serum albumin, AST, smoking, arm SM, VAT, Leg SM, CD4 count, non-HDLc, amprenavir, CRP, Fibrinogen, and eGFRcys.
Based on the fully-adjusted model presented in Table 3, we then calculated attributable risks associated with low serum albumin and with elevated AST. The population attributable risk (PAR) for having low serum albumin was 19% and for elevated AST was 5.8%, corresponding to absolute risks of 27 and 8 deaths per 1000 among all HIV-infected persons over five years, respectively. However, the PARs were substantially higher for the HIV/HCV-coinfected persons (Figure 2B).
DISCUSSION
In this nationally representative cohort of HIV-infected men and women, we found independent associations of lower serum albumin and higher AST with all-cause mortality. These associations were independent of demographics, traditional CVD factors, inflammation, limb muscle mass, visceral adiposity, and renal function. The risk was greater in HIV/HCV-coinfected than in HIV-monoinfected individuals. Decreased serum albumin alone accounted for over one-third of the population-level mortality in HIV/HCV-coinfected individuals, whereas increased AST accounted for one-fourth. In HIV-monoinfected, the attributable risk for decreased albumin was weaker and there was little risk that could be attributed to high AST.
The role of serum albumin in liver disease is complex. Serum albumin is synthesized in the liver and plays multiple physiological roles, including maintenance of pH and normal microvascular permeability and mediation of coagulation, and has antioxidant properties[29]. While very low albumin levels are associated with end stage liver disease, albumin decreases occur at early stages due to decreased synthesis and the short albumin half-life of 18–20 days[30]. In advanced liver disease, ascitic fluid containing albumin leaks into the peritoneal cavity[31], but the resulting reduction in overall serum albumin levels is likely minimal.
The causes of low serum albumin are multifactorial. They include decreased production in the liver in the setting of liver disease as well as decreased synthesis due to poor nutrition[32]. Levels drop most rapidly during the acute phase response. Our analyses controlled for two distinct acute phase response proteins (CRP and fibrinogen) which showed independent associations with mortality and negative correlations with albumin. A portion of the association of albumin with mortality may reflect its role as a negative acute phase response protein. By contrast, AST is not part of the acute phase response and was not associated with higher levels of CRP or fibrinogen in our cohort.
Previous studies of HIV and HCV infected patients have found both AST and ALT to predict liver-related mortality, either alone or as components of indices of liver fibrosis such as FIB-4 and APRI[33, 34]. In our study, AST had stronger associations than ALT with mortality. While AST and ALT are measures of liver injury, AST is more often a marker of liver toxicity (e.g., alcohol and medications) and advanced fibrosis/cirrhosis. In the absence of HCV infection, elevated ALT is generally considered a marker of steatosis. ALT did not strongly predict mortality over the short-term five year period in our analysis. A possible explanation for this weak association is that we controlled for visceral adipose tissue (VAT) in our models. VAT is a contributor to steatosis, but also to metabolic parameters that likely make VAT a stronger predictor than ALT of mortality. It is also possible that a follow-up period longer than five years may be needed to see effects of steatosis on mortality (as in previous studies of non-alcoholic fatty liver disease[35]).
In our study, albumin values below 4 g/dL were associated with a 3.5-fold higher odds of mortality. The difference in mortality rates between HIV/HCV-coinfected and HIV-monoinfected persons appeared to diverge at 4 g/dL, with lower levels showing increasingly higher mortality rates in HIV/HCV-coinfected persons. The level of serum albumin which is considered “low” varies substantially by study[8, 10, 36, 37], but several indicate that the risk begins at the lower limit of normal. Our findings suggest that HIV/HCV-coinfected patients should be followed more closely once albumin levels drop to 4 g/dL. It should be noted however that these studies of HIV/HCV-coinfected patients were conducted prior to the availability of effective HCV antiviral therapy; it is not yet clear what effect these newer therapies may have on albumin levels. We were unable to test associations of albumin changes in our study, but Lang et al[8] reported that time-dependent models of albumin yielded especially strong associations with mortality, indicating the value of serial measurements.
The reported normal ranges for AST and ALT vary widely by lab[38], and previous studies have reported different values to be associated with increased risk of morbidity and mortality[27, 39, 40]. We found a doubling of AST to be associated with a 41% increased risk overall (2.5-fold higher in coinfection). AST values above 67 IU/L were associated with a 3-fold increased risk in HIV/HCV-coinfected patients.
The strengths of this study include: (1) use of HCVRNA to define HCV infection; (2) use of a large, geographically and ethnically diverse, population-based sample of HIV-infected persons; (3) availability of comprehensive clinical information and specialized measures of inflammation, renal function, and MRI-measured regional adiposity and muscle volume that allowed us to test the robustness of these associations.
This study had several limitations. Vital status was missing for 23% of our HIV-infected participants. However, we used inverse probability weighting to mitigate the potential bias from those with unknown death status. We did not have information regarding the cause of death, and were therefore unable to discern whether the associations of serum albumin and AST with mortality were due to liver-related or other causes. We were unable to compare our results with our control population, because there were only six deaths in the control arm of our study. An additional study limitation is that liver disease markers were collected only at baseline, which limits the sensitivity of albumin and AST as predictors of survival. We did not measure platelet count, so we could not assess APRI and FIB-4 as predictors of mortality. Mortality was assessed at five years; it is possible that the ability of these factors to predict longer term mortality in HIV-monoinfected persons might be stronger. Finally, there may have been incomplete or inadequate control for factors that may confound or explain the association between liver disease markers and mortality.
In conclusion, lower serum albumin and higher AST appear to be much more important risk factors for mortality in HIV/HCV-coinfected than in HIV-monoinfected persons. While HCV coinfection itself is a risk factor for mortality, the HCV-induced abnormities in albumin and AST are additional and strong predictors of mortality in those with HCV infection. The risk associated with low albumin may begin at higher levels than have traditionally been considered to be low and may reflect albumin’s role as a negative acute phase response protein. AST was a poor predictor of mortality in HIV monoinfection, and the effect was present almost entirely within HIV/HCV-coinfected persons. Thus, the value of these markers should be considered primarily in the context of HCV coinfection.
Supplementary Material
Acknowledgments
Supported by grants from the NIH (R01-DK57508, HL74814, and HL53359; K23 AI66943 and NIH center grants M01-RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, RR00865, and UL1 RR024131), the Albert L. and Janet A. Schultz Supporting Foundation and with resources and the use of facilities of the Veterans Affairs Medical Center, San Francisco, California. The funding agencies had no role in the collection or analysis of the data.
Footnotes
Clinicaltrials.gov ID: NCT00331448
References
- 1.Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soriano V, Vispo E, Fernandez-Montero JV, Labarga P, Barreiro P. Update on HIV/HCV coinfection. Curr HIV/AIDS Rep. 2013;10:226–234. doi: 10.1007/s11904-013-0169-5. [DOI] [PubMed] [Google Scholar]
- 3.Tien PC, Kotler DP, Overton ET, Lewis CE, Rimland D, Bacchetti P, et al. Regional adipose tissue and elevations in serum aminotransferases in HIV-infected individuals. J Acquir Immune Defic Syndr. 2008;48:169–176. doi: 10.1097/QAI.0b013e3181685700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonello VS, Kliemann DA, Rigel Santos B, Tovo CV. HAART and liver: is it safe? J Infect Dev Ctries. 2014;8:1444–1450. doi: 10.3855/jidc.5012. [DOI] [PubMed] [Google Scholar]
- 5.Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewig S, Bauer T, Schneider C, Pickenhain A, Pizzulli L, Loos U, et al. Clinical characteristics and outcome of Pneumocystis carinii pneumonia in HIV-infected and otherwise immunosuppressed patients. Eur Respir J. 1995;8:1548–1553. [PubMed] [Google Scholar]
- 7.Coker WJ, Jeter A, Schade H, Kang Y. Plasma cell disorders in HIV-infected patients: epidemiology and molecular mechanisms. Biomark Res. 2013;1:8. doi: 10.1186/2050-7771-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang J, Scherzer R, Weekley CC, Tien PC, Grunfeld C, Shlipak MG. Serum albumin and short-term risk for mortality and cardiovascular disease among HIV-infected veterans. AIDS. 2013;27:1339–1343. doi: 10.1097/QAD.0b013e32835f1dd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman JG, Burns DN, Gange SJ, Bacchetti P, Cohen M, Anastos K, et al. Serum albumin as a predictor of survival in HIV-infected women in the Women’s Interagency HIV study. AIDS. 2000;14:863–870. doi: 10.1097/00002030-200005050-00013. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses. 2006;22:14–21. doi: 10.1089/aid.2006.22.14. [DOI] [PubMed] [Google Scholar]
- 11.Dao CN, Peters PJ, Kiarie JN, Zulu I, Muiruri P, Ong’ech J, et al. Hyponatremia, hypochloremia, and hypoalbuminemia predict an increased risk of mortality during the first year of antiretroviral therapy among HIV-infected Zambian and Kenyan women. AIDS Res Hum Retroviruses. 2011;27:1149–1155. doi: 10.1089/AID.2010.0345. [DOI] [PubMed] [Google Scholar]
- 12.Shah S, Smith CJ, Lampe F, Youle M, Johnson MA, Phillips AN, et al. Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretroviral therapy era: relationships with gender. HIV Med. 2007;8:38–45. doi: 10.1111/j.1468-1293.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 13.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockerham L, Scherzer R, Zolopa A, Rimland D, Lewis CE, Bacchetti P, et al. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53:102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hastie T, Tibshirani R. Generalized Additive Models (Chapman & Hall/CRC Monographs on Statistics & Applied Probability) New York: Chapman and Hall; 1990. [Google Scholar]
- 16.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 18.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 21.Scherzer R, Heymsfield SB, Lee D, Powderly WG, Tien PC, Bacchetti P, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25:1405–1414. doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilks WR, Richardson S, DJS . Markov chain Monte Carlo in practice. London: Chapman & Hall; 1996. [Google Scholar]
- 24.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and Mortality in HIV-Infected Adults: Analysis of the FRAM Study Cohort. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, et al. Cystatin C, Albuminuria, and 5-Year All-Cause Mortality in HIV-Infected Persons. Am J Kidney Dis. 2010 Aug 13; doi: 10.1053/j.ajkd.2010.05.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 27.Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology. 2006;43:1145–1151. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 28.Luepker RV, Evans A, McKeigue P, Reddy KS. Cardiovascular Survey Methods. 3. Geneva: World Health Organization; 2004. [Google Scholar]
- 29.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 30.Vincent JL. Relevance of albumin in modern critical care medicine. Best Pract Res Clin Anaesthesiol. 2009;23:183–191. doi: 10.1016/j.bpa.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology, Part III: Albumin and disease states. JPEN J Parenter Enteral Nutr. 1991;15:476–483. doi: 10.1177/0148607191015004476. [DOI] [PubMed] [Google Scholar]
- 32.Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc Nutr Soc. 1989;48:347–354. doi: 10.1079/pns19890050. [DOI] [PubMed] [Google Scholar]
- 33.Vinikoor MJ, Sinkala E, Mweemba A, Zanolini A, Mulenga L, Sikazwe I, et al. Elevated AST-to-platelet ratio index is associated with increased all-cause mortality among HIV-infected adults in Zambia. Liver Int. 2015 doi: 10.1111/liv.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez F, Van den Eynde E, Perez-Hoyos S, Navarro J, Curran A, Burgos J, et al. Liver stiffness and aspartate aminotransferase levels predict the risk for liver fibrosis progression in hepatitis C virus/HIV-coinfected patients. HIV Med. 2014 doi: 10.1111/hiv.12197. [DOI] [PubMed] [Google Scholar]
- 35.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373–375. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang J, Scherzer R, Tien PC, Parikh CR, Anastos K, Estrella MM, et al. Serum albumin and kidney function decline in HIV-infected women. Am J Kidney Dis. 2014;64:584–591. doi: 10.1053/j.ajkd.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monga HK, Rodriguez-Barradas MC, Breaux K, Khattak K, Troisi CL, Velez M, et al. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;33:240–247. doi: 10.1086/321819. [DOI] [PubMed] [Google Scholar]
- 38.Ceriotti F, Henny J, Queralto J, Ziyu S, Ozarda Y, Chen B, et al. Common reference intervals for aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) in serum: results from an IFCC multicenter study. Clin Chem Lab Med. 2010;48:1593–1601. doi: 10.1515/CCLM.2010.315. [DOI] [PubMed] [Google Scholar]
- 39.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schindhelm RK, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ, et al. Alanine aminotransferase and the 6-year risk of the metabolic syndrome in Caucasian men and women: the Hoorn Study. Diabet Med. 2007;24:430–435. doi: 10.1111/j.1464-5491.2007.02100.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


