Abstract
Liposomes are a promising class of nanomedicine with the potential to provide site-specific chemotherapy, thus improving the quality of cancer patient care. First-generation liposomes have emerged as one of the first nanomedicines used clinically for localized delivery of chemotherapy. Second-generation liposomes, i.e. stimuli-responsive liposomes, have the potential to not only provide site-specific chemotherapy, but also triggered drug release and thus greater spatial and temporal control of therapy. Temperature-sensitive liposomes are an especially attractive option, as tumors can be heated in a controlled and predictable manner with external energy sources. Traditional thermosensitive liposomes are composed of lipids that undergo a gel-to-liquid phase transition at several degrees above physiological temperature. More recently, temperature-sensitization of liposomes has been demonstrated with the use of lysolipids and synthetic temperature-sensitive polymers. The design, drug release behavior, and clinical potential of various temperature-sensitive liposomes, as well as the various heating modalities used to trigger release, are discussed in this review.
Keywords: Drug delivery, Thermosensitive liposomes, Thermosensitive polymers, Chemotherapy
1. Introduction
Chemotherapy is the treatment of cancer with antineoplastic drugs that typically act by impairing cell mitosis, effectively targeting rapidly dividing cells that are the hallmarks of cancer [1]. Most chemotherapy is administered intravenously (i.v.), ultimately being diluted, degraded, or cleared as it travels through the circulatory system [2]. Consequently, large doses of these highly cytotoxic drugs must often be administered in order to achieve therapeutic drug levels at the tumor site. Healthy cells that divide rapidly under normal circumstances are damaged, leading to an array of adverse side effects including depression of the immune system (myelosuppression), inflammation and ulceration of the mucous membranes lining the digestive tract (mucositis), hair loss (alopecia), and organ-specific toxicities (e.g. cardiotoxicity, nephrotoxicity, etc.) [2]. These toxic effects restrict the dosages administered to patients, and cancers that survive these lower dosages oftentimes develop a resistance to chemotherapy, making it much more difficult to eradicate the tumors and save the patient [3].
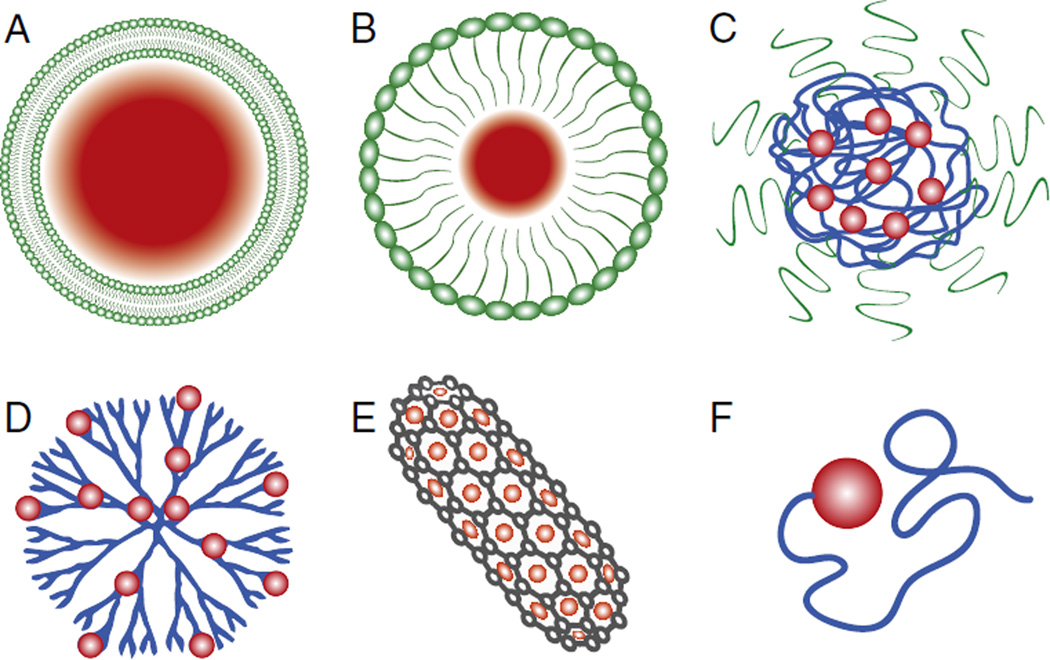
The emergence of nanomedicine – a subfield of nanotechnology where diagnostic and therapeutic structures and tools are engineered on the nanoscale – may provide the solution to the systemic toxicity issues currently limiting cancer chemotherapy. The ability to engineer nanoscale particles and tailor their composition, size, shape, modulus, surface charge, and surface functionality, has led to a rapidly expanding and diverse population of nanoscale drug delivery vehicles (Fig. 1). liposomes [4], micelles [5], polymer–drug conjugates [6], polymerosomes [7], dendrimers [8], aptamers [9], carbon nanotubes [10], lipoplexes [11], and polyplexes [12] have all been developed for the diagnosis and treatment of a broad range of indications, including various cancers. The basic rationale is that these nanosized structures have functional and structural properties that are not available from either discrete molecules or bulk materials.
Fig. 1.
Examples of current nanomedicines: (A) liposomes, (B) micelles, (C) polymeric nanoparticles, (D) dendrimers, (E) carbon nanotubes, and (F) polymer-drug conjugates.
Chemotherapeutics can be encapsulated into these nanoscale drug vehicles, and following systemic administration, can preferentially accumulate in the tumor. This phenomenon – whereby macromolecular drugs and nanomedicines of a certain size will tend to accumulate in tumor tissue more than in healthy tissue – is known as the enhanced permeability and retention (EPR) effect [13]. The EPR effect arises from the fact that tumor cells must stimulate the rapid production of blood vessels in order to keep pace with ever-increasing oxygen and nutrient demands. The hastily grown neovasculature differs greatly from normal blood vessels, with an architecture characterized by poorly aligned endothelial cells, wide fenestrations, impaired functional cell receptors, wide lumens, and absent or abnormal smooth-muscle layers, perivascular cells, and basement membranes [14]. As a result of these anatomical deficiencies, tumor blood vessels are irregularly shaped, dilated, and leaky, allowing for the extravasation of macromolecular drugs and nanomedicines into the tumor tissue. Furthermore, the tumor’s impaired lymphatic clearance of macromolecules and lipids from interstitial tissue prolongs the retention of these macromolecular species [15–21]. Other factors that promote the accumulation of drug at the tumor are the extensive angiogenesis and increased vascular densities in tumors brought on by the high nutrient and oxygen demand, as well as the increased production of vascular mediators that facilitate extravasation [15,16,22–24]. Delivery vehicles large enough (>5–6 nm [25]) to escape renal clearance but small enough (<150 nm, in the case of neutral or slightly negatively charged lipid-based carriers [26,27]) to exploit this EPR effect can be used to increase drug bioavailability and reduce off-target toxicities associated with systemic administration of free drug [28]. This fact has been the driving force behind the development of many nanoscale drug vehicles. Among these, the liposome holds the distinction of being one of the first and most widely used vehicles for chemotherapy [29].
2. Evolution of liposomes for cancer therapy
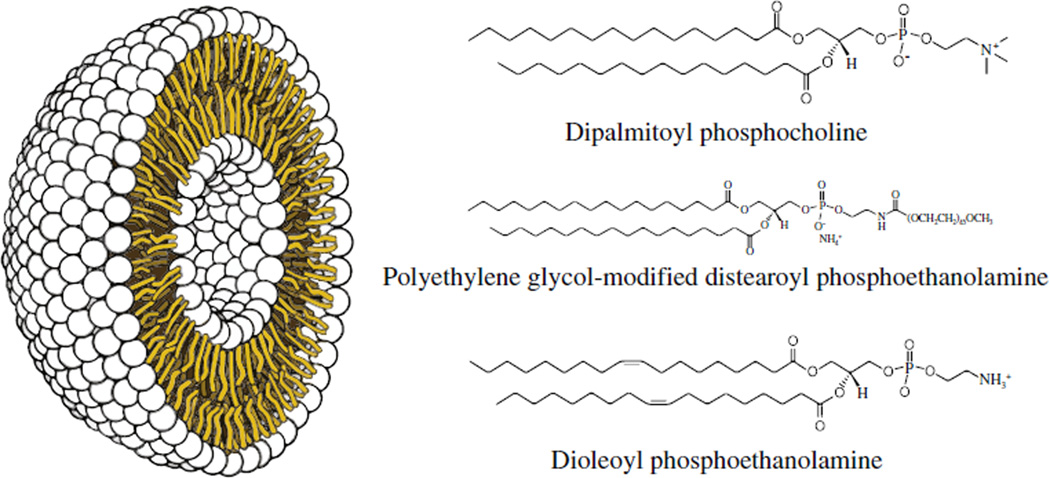
Discovered in 1961 by Alec Bangham [30], liposomes are spherical vesicles comprised of lipid bilayer shells surrounding aqueous interior cores (Fig. 2), and are formed spontaneously when amphiphilic lipids are dispersed in water. The resulting supramolecular assemblies, while not covalently bound, can stably entrap both hydrophobic and hydrophilic drugs. Moreover, liposomes are biocompatible, cause little or no antigenic, allergic, and toxic reactions, easily undergo biodegradation, protect the host from undesirable effects of the encapsulated drug, and protect the entrapped drug from premature inactivation by the physiological medium.
Fig. 2.
Schematic representation of a liposome. Single lipid units such as the phospholipid examples shown to the right form a stable bilayer around an aqueous inner core.
Early work would reveal that while liposomes fit the size criteria required to exploit the EPR effect, accumulation at the tumor was largely prevented by their rapid and efficient clearance from circulation via the phagocytic cells of the mononuclear phagocyte system (MPS) [31,32]. Many studies showed that despite liposomes having lipid compositions closely resembling those of cell membranes, between 50 and 80% of administered liposomes are recognized and adsorbed by the cells of the MPS within the first 15–30 min following intravenous administration [33]. A major breakthrough occurred with the development of long-circulating liposomes coated with polyethylene glycol (PEG) in 1990 [34]. PEG is a hydrophilic polyether that was known at the time to reduce immunogenicity and prolong circulation when attached to enzymes and growth factors [35]. The addition of a PEG–lipid conjugate to liposomal formulations was shown to significantly prolong circulation time [34,36–38]. PEG is inexpensive and can be synthesized relatively easily and in large quantities of high purity, which are distinct advantages over glycolipid surface modifiers (e.g. GM1) that had previously been used for increasing liposome circulation time. This finding formed a pivotal element in the development and translation of a number of liposomal formulations of pharmaceutical agents.
It is important to note that effective lipid-based nanomedicines for cancer must fulfill four key requirements: 1) encapsulate a therapeutically relevant drug payload, 2) effectively avoid the MPS, 3) target the tumor interstitium, and 4) once at the tumor site, release their payload at levels high enough to mediate an effective therapeutic response. Over the past four decades, significant progress has been made in the field of liposomal research, culminating in the arrival of liposomal formulations in the clinic that fulfill the first three requirements. To date, seventeen lipid-based nanomedicines have been clinically approved, with numerous more undergoing clinical evaluations (Tables 1 and 2, revised and updated from [39]). Three liposomal formulations (Doxil, DaunoXome, and Myocet) have been approved for the treatment of cancer, all of them liposomal anthracyclines (Table 1). Upon accumulation within the tumor interstitium, these lipid-based carriers rely on either the passive diffusion of drug over long periods or the slow, non-specific degradation of the lipid shell. This can be advantageous for cell cycle-specific drugs, such as vincristine, which can be effective over long exposures at relatively low concentrations [40,41].
Table 1.
List of clinically-approved lipid-based nanomedicines.
| Drug | Trade name | Company | Indication | Lipid platform | Administration |
|---|---|---|---|---|---|
| Amphotericin B | Abelcet | Enzon Pharma | Fungal infections | Lipid complex | i.v. |
| Amphotericin B | Ambisome | Gilead Sciences | Fungal and protozoal infections | Liposome | i.v. |
| Amphotericin B | Ampholip | Bharat Serums and Vaccines (India) | Fungal infections | Lipid complex | i.v. |
| Amphotericin B | Amphotec | Kadmon Pharma | Fungal infections | Lipid complex | i.v. |
| Amphotericin B | Fungisome | Lifecare Innovations (India) | Fungal infections | Liposome | i.v. |
| Bupivacaine | EXPAREL | Pacira Pharma | Postsurgical analgesia | Multivesicular liposome | i.v. |
| Cytarabine | DepoCyt | Pacira Pharma | Intrathecal treatment of lymphomatous meningitis | Multivesicular liposome | i.t. |
| Daunorubicin | DaunoXome | Gilead Sciences | HIV-related Kaposi's sarcoma | Liposome | i.v. |
| Doxorubicin | Doxil/Caelyx | Johnson & Johnson | Ovarian cancer | Liposome | i.m. |
| Multiple myeloma | |||||
| HIV-related Kaposi’s sarcoma | |||||
| Doxorubicin | Lipodox | Sun Pharma (India) | Ovarian cancer | Liposome | i.v. |
| Multiple myeloma | |||||
| HIV-related Kaposi’s sarcoma | |||||
| Doxorubicin | Myocet | Enzon Pharma | Metastatic breast cancer | Liposome | i.v. |
| Estradiol | Extrasorb | Novavax | Menopausal therapy | Micelle | Topical |
| IRIV vaccine | Epaxal | Crucell (Netherlands) | Hepatitis A | Virosome | i.m. |
| IRIV vaccine | Inflexal V | Crucell (Netherlands) | Influenza | Virosome | i.m. |
| Morphine | DepoDur | Pacira Pharma | Postsurgical analgesia | Multivesicular liposome | Epidural |
| Verteporfin | Visudyne | Valeant Pharma | Neovascular/exudative age-related macular degeneration | Liposome | i.v. |
| Pathologic myopia | |||||
| Ocular histoplasmosis | |||||
| Vincristine | Marqibo | Talon Therapeutics | Ph- acute lymphoblastic leukemia | Liposome | i.v. |
Table 2.
List of lipid-based nanomedicines currently undergoing clinical evaluation.
| Drug | Trade name | Company | Indication | Lipid platform | Administration | Phase |
|---|---|---|---|---|---|---|
| Amikacin | Arikace | Insmed | Lung infections | Liposome | Aerosol | II/III |
| Annamycin | L-Annamycin | Callisto Pharma | Acute lymphocytic leukemia | Liposome | i.v. | I/IIa |
| Acute myeloid leukemia | I/IIa | |||||
| Belotecan | S-CKD602 | Alza | Advanced malignancies | Liposome | i.v. | I/II |
| BLP25 lipopeptide | Stimuvax | Merck KGaA | Hormone receptor-positive metastatic breast cancer | Liposome | i.v. | III |
| Cisplatin | Lipoplatin | Regulon | Non-small cell lung cancer | Liposome | i.v. | III |
| Pancreatic cancer | II/III | |||||
| Cisplatin | SLIT Cisplatin | Insmed | Osteosarcoma metastatic to lung | Liposome | Aerosol | Ib/IIa |
| Dengue DNA vaccine | Vaxfectin | Vical | Dengue infections | Liposome | i.v. | I |
| DNA encoding HLA-B7, β2 microglobulin |
Allovectin | Vical | Metastatic melanoma | Lipid complex | i.v. | III |
| Doxorubicin | Doxil/Caelyx | Johnson & Johnson | Squamous cell cancer of head and neck | Liposome | i.m. | II |
| Doxorubicin | Sarcodoxome | GP Pharm (Spain) | Soft tissue sarcoma | Liposome | i.v. | II |
| Doxorubicin | ThermoDOX | Celsion | Primary/metastatic liver cancer | Liposome | i.v. | III |
| Recurrent chest wall breast cancer | I | |||||
| Fentanyl | AeroLEF | Gilead Sciences | Postoperative analgesic | Liposome | Aerosol | II |
| Grb2 oligonucleotide | BP-100-1.01 | Bio-Path | Hematological cancers | Liposome | i.v. | I |
| Lurtotecan | OSI-211 or NX211 | OSI Pharma | Ovarian cancer | Liposome | i.v. | II |
| Squamous cell cancer of head and neck | II | |||||
| Oxaliplatin | Lipoxal | Regulon | Colorectal cancer | Liposome | i.v. | II |
| Paclitaxel | EndoTAG-1 | Medigene AG (Germany) | Pancreatic cancer | Liposome | i.v. | II/III |
| Triple negative breast cancer | II | |||||
| Paclitaxel | LEP-ETU | INSYS Therapeutics | Metastatic breast cancer | Liposome | i.v. | II |
| Prostaglandin E1 | Liprostin | Endovasc | Peripheral vascular disease | Liposome | i.v. | II/IIIa |
| RNAi | ALN-TTR02 | Alnylam | Transthyretin-mediated amyloidosis | Lipid nanoparticle | i.v. | II |
| RNAi | ALN-VSP | Alnylam | Liver cancer | Lipid nanoparticle | i.v. | I/II |
| Topotecan | Brakiva | Talon Therapeutics | Small-cell lung cancer, ovarian cancer | Liposome | i.v. | I |
| Vincristine | OncoTCS | Inex Pharma | Non-Hodgkin's lymphoma | Liposome | i.v. | II/III |
| Enzon Pharma | ||||||
| Vinorelbine | Alocrest | Talon Therapeutics | Non-small cell lung cancer, breast cancer | Liposome | i.v. | II |
However, passive release may not allow cytotoxic local concentrations of non-cell cycle specific drugs such as doxorubicin (DOX) and cisplatin [42] to be reached. Furthermore, vascular permeability between different tumor types and even within tumors can be highly variable [43–45], resulting in unpredictable liposome extravasation into tumor tissue. Due to the combination of sub-optimal drug release kinetics and unpredictable vascular permeability, only modest gains in the therapeutic index of chemotherapy have been realized with the use of liposomes for localized drug delivery [46–49]. Thus, in order to optimize the time, location, and amount of drug release, considerable effort has been dedicated towards the development of liposomal drug delivery systems that are capable of releasing drug in response to a specific stimulus at the target site (i.e., triggered release). With this goal of triggered release serving as motivation, stimuli-responsive liposomal formulations that are pH-, temperature-, ultrasound-, and photo-sensitive have been developed with varying degrees of success [50–54]. Thermosensitive liposomes (TSL) hold the distinction among these classes of triggerable liposomes of being at present the closest formulation to the clinic, with one formulation currently undergoing phase III clinical trials [53].
3. Thermosensitive liposomes
Mild hyperthermia (HT) has long been administered as an adjunctive therapy with radiation and chemotherapy [55]. A synergistic interaction between heat and chemotherapy has been validated in preclinical studies [56,57], and the combination of mild hyperthermia and chemotherapy has been shown to improve patient response when compared to chemotherapy alone [58–60]. When combined with thermosensitive liposomes, HT can increase therapeutic effectiveness by: (i) increasing tumor vascular permeability and thus accumulation of liposomes in the tumor [61], and (ii) promoting drug release from the temperature-sensitive formulations into the tumor vasculature and interstitium. These mechanisms are summarized in Fig. 3. Recent findings suggest that the mechanism of drug delivery from combination TSL and HT is dominated by intravascular release (Fig. 3C) [62].
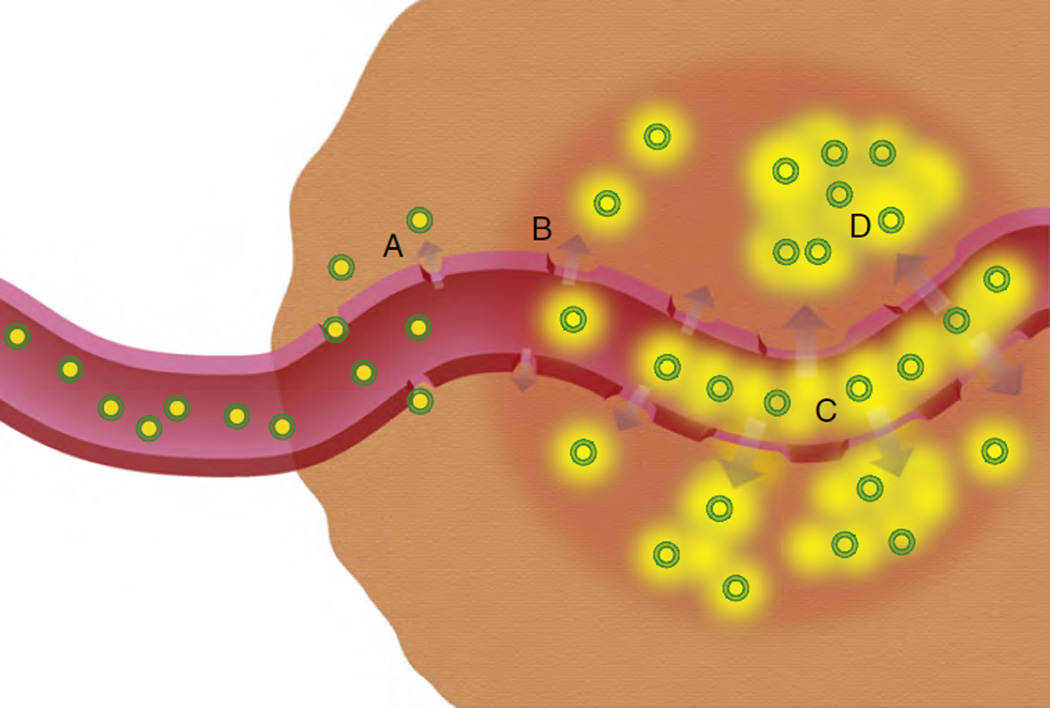
Fig. 3.
Possible mechanisms involved in combination hyperthermia and thermosensitive liposome therapy: (A) drug-loaded liposomes (green indicating the lipid shell and yellow indicating the drug) preferentially extravasate from pores in leaky tumor blood vessel walls (i.e. EPR effect), (B) applied mild hyperthermia (hyperthermia region indicated by red circle) increases tumor vessel pore size, increasing tumor liposome extravasation, (C) hyperthermia triggers drug release from temperature sensitive liposomes in the tumor vasculature as well as in (D) the tumor interstitium. Recent findings suggest the mechanism of drug delivery from combination TSL and HT is dominated by intravascular release (C) [62].
Additionally, HT can increase therapeutic effectiveness of TSL by increasing local blood flow at the exposed area [63], increasing the permeability of target cells to the released drugs [64], and being directly cytotoxic to cancer cells. For example, temperature-dependent interactions include pathways for the regulation of apoptosis, the cell cycle, DNA repair, and macromolecular synthesis [55,65]. While cancerous cells are not inherently more susceptible than healthy cells to thermal effects, within the tumor microenvironment they are stressed by low oxygen levels, higher than normal acid concentrations, and insufficient nutrients, and thus may be less able to tolerate the added stress of heat [66]. Because tumors have a disorganized and compact vascular structure, heat dissipation is often more difficult in tumor tissue, providing a possible avenue of selectively targeting cancer cells with heat.
Thus there exists great potential for the combination of TSL therapy with mild hyperthermia exposure. Here the current TSL landscape is organized into three types of formulations: (i) traditional thermosensitive liposomes (TTSL) comprised of lipids that undergo temperature-dependent phase transitions, (ii) lysolipid-containing thermosensitive liposomes (LTSL), and (iii) polymer-modified thermosensitive liposomes (PTSL). The mechanisms of thermally-triggered release, design, drug release behavior, and clinical potential of these formulations are discussed, as well as the different heating modalities used.
3.1. Traditional thermosensitive liposomes (TTSL)
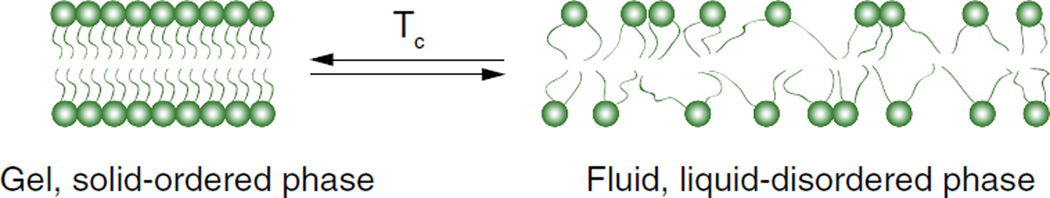
In 1978 Yatvin et al. introduced liposomes that released neomycin and inhibited bacteria protein synthesis in vitro at specific temperatures [64]. These were the first of a class of TSL now known as traditional thermosensitive liposomes (TTSL). TTSL were further developed over the next few decades, and are comprised of lipid membranes that undergo phase transitions in response to heating. Lipid membranes/liposomes have a characteristic phase transition temperature (Tc) at which they undergo a transition (‘melt’) from a gel to a liquid phase (Fig. 4). In the gel phase, lipid molecules are ordered and condensed with fully extended hydrocarbon chains, and are constrained to the two-dimensional plane of the membrane. Upon heating, the mobility of the lipid head groups gradually increases. As the temperature is further increased and approaches Tc, the orientation of the C-C single bonds in the hydrocarbon chains begins to switch from a trans to a gauche configuration. Leaky interface regions begin to develop at boundaries between still solid lipid domains and melting, liquid lipid domains. Incompatibilities in molecular packing and hydrophobic matching characterize the lipids at these interfaces [67], and the membrane initially becomes highly permeable at these locations. The increase in permeability is observed as an anomalous peak in ion permeability through the membrane at Tc [68]. At temperatures greater than the Tc, the bilayer exists fully in the liquid phase. Individual lipid molecules are still confined to the two-dimensional plane of the membrane as in the solid phase, but are able to move freely and rapidly (millions of exchanges per second) within the plane [69]. As a result, the membrane becomes fully fluidized and is permeable throughout. With TTSL, encapsulated drugs are able to leak out of the vesicle during the phase transition.
Fig. 4.
Temperature-dependent phase transition of a lipid bilayer. An increase in the lipid bilayer fluidity due to an increase in temperature above Tc is associated with an increase in drug release.
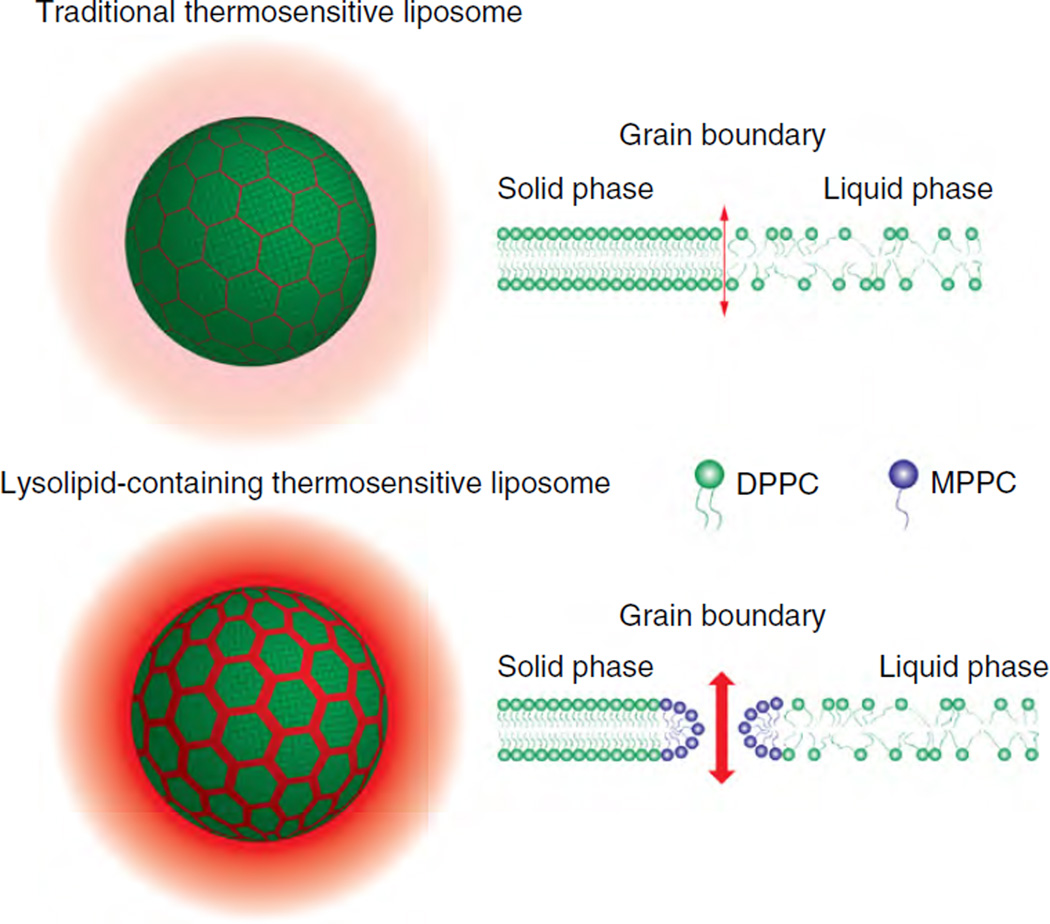
An important feature of liposomes is that the membrane does not exist as a perfectly arranged homogeneous sheet, but as a faceted, granular structure [70]. This is an artifact that comes from the liposome production process, which involves the formation of dry lipid sheets that are rehydrated at temperatures greater than Tc. This forms large multilamellar vesicles (LMV), which are then downsized to the small unilamellar vesicles (SUV) that constitute the final form of liposome formulations. During the cooling process as temperatures approach Tc, the lipid membrane does not solidify uniformly; rather, nucleates of solid domains are formed within the liquid membrane. These individual domains of solid phase lipids, or ‘grains’, orient themselves in lattice like structures of a particular orientation. Eventually as the transition nears completion and the last liquid lipid is transformed, grain boundaries are created between the solidifying domains, and the grains meet at different orientations [53]. Imperfect crystalline arrangement between grains occurs, resulting in planar defects at the boundaries. Upon heating, melting is initiated and the first solid/liquid domains are formed at these boundaries. Solid/liquid domains form during heating in planar bilayer sheets (i.e. no grain-derived defects) as well, but the presence of grain boundaries in liposomes accelerates the formation of these domains and thus enhances the permeabilization of the membrane during the phase transition.
Yatvin et al. [64] were the first to realize the therapeutic potential of forming liposomes from lipid membranes with Tc within the range attainable by mild HT (i.e. above physiological temperature but below 42 °C). Early versions of TTSL were based upon dipalmitoyl phosphocholine (DPPC), a phospholipid with 16-carbon saturated fatty acid chains and a Tc of 41 °C, ideal for application with mild HT. Pure DPPC liposomes showed enhanced drug release, but the rate and amount of release was relatively small [64]. Supplementation of DPPC with other lipids, predominantly distearoyl phosphocholine (DSPC) and hydrogenated soy phosphocholine (HSPC), had a positive effect on the amount and rate of drug released [71–73]. The rationale with using lipid mixtures was that the presence of multiple types of lipids would lead to an increase in packing incompatibility and thus increased permeability. Following Yatvin's initial work, a number of preclinical studies established that when DPPC-based liposomes (predominantly DPPC/DSPC liposomes) were administered in combination with hyperthermia, tumor extravasation was enhanced and an increased level of drug was available at the diseased site, corresponding with increased therapeutic efficacy. These effects were observed over a range of tumor models, including various carcinomas, sarcomas, and lymph node metastases, and over a range of drugs, including adriamycin, methotrexate, bleomycin, and cisplatin [74–80]. The advent of PEGylated liposomes in 1990 allowed for the development of the first thermosensitive, long-circulating liposomes in 1994 [81]. Long-circulating TTSL formulations demonstrated increased MPS evasion compared to non-PEGylated thermosensitive liposomes, and were more effective in delaying tumor growth. The therapeutic efficacy of PEGylated TTSL against a colon carcinoma model compared favorably with that of identical formulations modified with GM1 [72,81], with PEG having significant advantages in terms of cost and ease of preparation, as discussed previously.
While supplementing DPPC with other lipids can increase the permeability of the membrane, the inclusion of lipids with longer carbon chains than DPPC can have undesired effects on the phase transition behavior. For example, the phase transition temperature of DPPC/DSPC membranes as measured by differential scanning calorimetry (DSC) was shown to increase as a function of the molar fraction of DSPC (18-carbon chains) [82]. Furthermore, the width of the transition was shown to increase with the addition of DSPC [82,83]. Gaber et al. demonstrated that the transition temperature of DPPC and HSPC (18-carbon chains) liposomes (2:1 molar) is centered at around 46 °C with a relatively broad transition in the range of 43–48 °C [73], significantly different from the sharp peaks observed at 41 °C for pure DPPC. As a result, these formulations required temperature-triggered release in the range of 43–45 °C, a range that can put healthy tissues surrounding the tumor at risk of necrosis (discussed in further detail below), and one that is not necessarily optimized for the more clinically relevant and attainable range of mild hyperthermia (39–42 °C). Additionally, PEGylation of DPPC/HSPC liposomes (to increase circulation time) and incorporation of cholesterol (to increase serum stability) individually had negative impacts on the thermosensitivity of these liposomes [73]. Improved thermosensitivity from DPPC/HSPC liposomes containing both PEG and cholesterol was obtained, but drug release was still relatively low (~50% after 30 min of heating at 42 °C) [73].
To put the drug release behavior in a clinical context, it helps to introduce the concept of the thermal dose. As described by Sapareto et al. [84], the thermal dose takes a heat treatment at one temperature and calculates the equivalent exposure time at a reference temperature, typically 43 °C. Thermal exposures are converted to “equivalent minutes” at 43 °C as follows:
| (1) |
where t43 °C is the thermal dose of the exposure in equivalent minutes at 43 °C, Δt is the duration of the exposure (min), T is the average temperature of the exposure over Δt, and R is an empirical constant equal to 0.5 at T ≥ 43 °C and 0.25 at T < 43 °C.
Results of a number of studies [85–89] on the effects of hyperthermia have shown that the thermal dose threshold for 100% necrosis in different tissues ranges from 50 to 240 min. Meshorer et al. [85] studied the tissue effects in porcine muscle that occurred after 30 min of heating and found the onset of necrosis began at 40–43 °C, with moderate damage at 44–46 °C and severe damage at temperatures greater than 46 °C. This corresponds to 0.5–30 equivalent minutes at 43 °C for the onset of necrosis, 60–240 min for moderate damage, and more than 240 min for severe damage. The high thermal dose is sufficient to coagulate all tissue [90–92].
TTSL formulations demonstrated increased temporal control of DOX release in vivo, but required heating the tissue above 42 °C for 1 h, a thermal dose of 15 equivalent minutes at 43 °C. In vitro studies indicated that release plateaued at 40% of encapsulated drug following a thermal dose of 7.5 min, with increasing doses having no effect on release [93]. Similarly, the optimized TTSL developed by Gaber et al. [73] and discussed above required heating at 42 °C for 30 min to achieve 50% drug release (t43°C = 7.5 min). Thus, based on thermal dose metrics, these TTSL formulations required heating conditions that posed a significant threat of necrosis to healthy tissue.
The task was then to find ways to lower the thermal dose thresholds for drug release from thermosensitive liposomes to the range of mild hyperthermia (39–42 °C) while simultaneously keeping the transition sharp (i.e. a burst release). The latter property is critical in overcoming potential thresholds for efficacy, ensuring that a lethal drug dose is delivered, and in avoiding the development of multidrug resistance in cancer cells. Over the past decade and a half, two approaches emerged to prominence: (i) incorporation of lysolipids into the lipid membrane, and (ii) modification of the lipid bilayer with membrane-disruptive polymers.
3.2. Lysolipid-containing thermosensitive liposomes (LTSL)
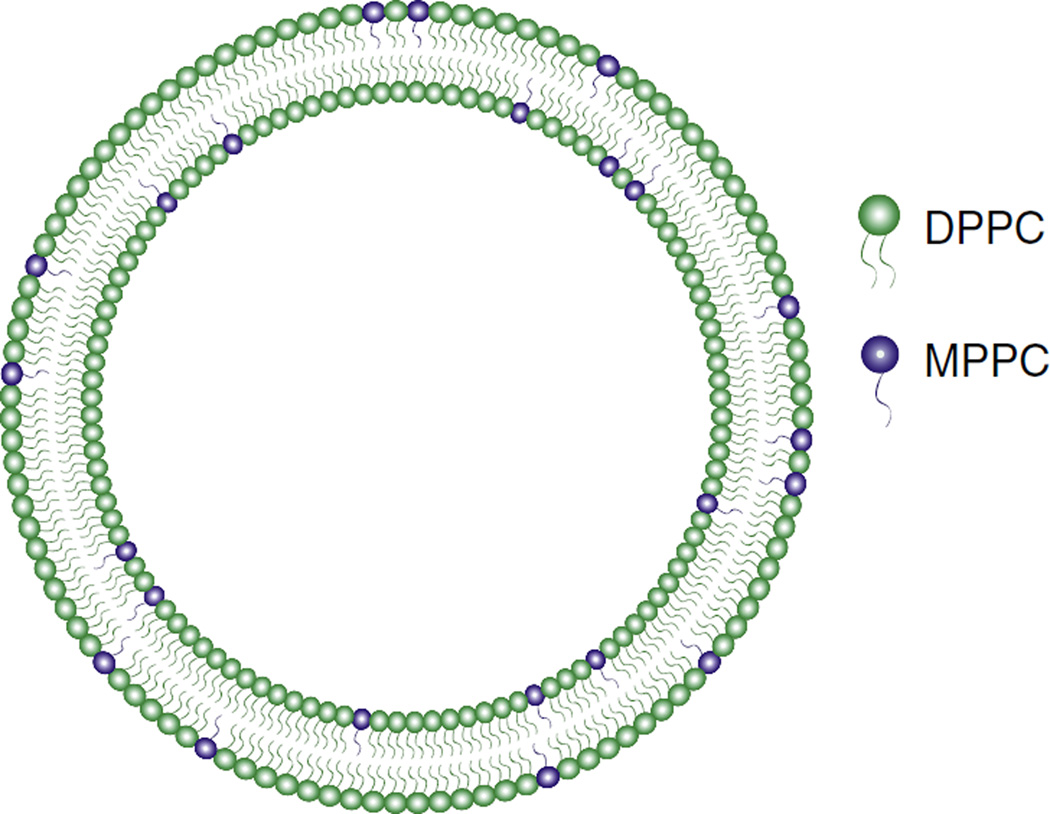
In 1999 Anyarambhatla and Needham proposed the idea of incorporating lysolipids into PEGylated DPPC membranes of traditional thermosensitive liposomes in order to lower the phase transition and promote rapid drug release (Fig. 5) [94]. Lysolipids – lipids that contain only one acyl chain – have a relatively large head group in relation to their single hydrocarbon tail, and thus a positive intrinsic curvature which favors the formation of micelles. The rationale was that as the phase transition temperature is approached and grain boundaries begin to melt, lateral lipid mobility increases which allows lysolipids to accumulate at the boundaries. There the tendency for lysolipids to form highly curved micelles would result in the formation of stabilized defects in the bilayer. It should be noted that two-tailed phosphoethanolamines, including DSPE–PEG conjugates, have a negative intrinsic curvature due to the small PE headgroups. They thus have a tendency to also form micelle structures: in this case the structures formed are inverted micelles, or hexagonal II phase, which are distinct from the micelles formed by lysolipids, which are termed hexagonal I. The hypothesis then was that lysolipids and DSPE–PEG essentially form nanopores in the bilayer during the phase transition (Fig. 6), through which entrapped drugs would rapidly be released [53].
Fig. 5.
Schematic of the lysolipid-containing thermosensitive liposomes (LTSL) developed by Needham, Dewhirst et al. [94]. Lipid bilayers are composed of DPPC and MPPC (10 mol%), a lysolipid that stabilizes grain boundary defects during the phase transition, leading to rapid drug release.
Fig. 6.
Proposed mechanisms of drug release from traditional thermosensitive liposomes (TTSL) and the lysolipid-containing thermosensitive liposomes (LTSL) developed by Needham, Dewhirst et al. [94]. Upon heating, grain boundaries form between solid and liquid domains. Melting is initiated at these boundaries, leading to drug release. In LTSL, lysolipids such as MPPC accumulate at these boundaries and form stabilized defects, leading to enhanced drug release.
Incorporation of the lysolipid monopalmitoyl phosphocholine (MPPC) at 10 mol% reduced the phase transition of traditional thermosensitive formulations from 43 °C to 39–40 °C, and provided for rapid release upon heating (nearly 50% within 20 s heating at 42 °C) [93,94]. In the context of thermal dose, incorporation of lysolipid resulted in a dramatic reduction from t43 °C = 7.5 min in TTSL to t43°C = 0.08 min (5 s) for the lysolipid-containing thermosensitive liposomes (LTSL), which is below the threshold for the onset of necrosis. Thus the LTSL formulation would be expected to have a much improved safety profile compared to traditional TSL when administered clinically. Subsequent studies in human xenograft models demonstrated that when combined with mild hyperthermia (42 °C for 1 h immediately after tail vein injection, t43 °C = 15 min), LTSL was more effective than free drug, traditional TSL, and non-thermosensitive (NTSL) formulations at reducing tumor growth [93–95]. LTSL and hyperthermia also resulted in increased tumor uptake of drug when compared to controls, and DNA-bound DOX extracted from tumors was found in significant amounts only in animals administered both LTSL and HT [95]. A recent comparative study demonstrated that DOX-loaded LTSL and HT combined therapy increased median tumor growth time compared with untreated controls, HT alone, and LTSL alone in mice inoculated with five different cancer cell lines (FaDu, HCT116, PC3, SKOV-3, and 4T07), with the slower-growing cancers (SKOV-3, PC-3) having the greatest number of complete regressions and longest tumor growth delays [96].
The lysolipid formulation developed by Needham and Dewhirst has undergone further pharmaceutical development by Celsion and is currently marketed as ThermoDOX®. Two clinical trials are currently in progress, one combining ThermoDOX with hyperthermia for patients with recurrent chest wall breast cancer (Phase I), and one combining ThermoDOX with radiofrequency ablation in patients with primary or metastatic liver cancer (Phase III) [97]. These represent the first clinical trials for the second-generation liposomal formulations (i.e. stimuli-responsive liposomes).
When investigating the mechanism of LTSL, Mills et al. were careful to show that drug release occurred through lysolipid-stabilized nanopores rather than a simple enhancement due to increased drug solubility in the bilayer. An alternative mechanism was that the lysolipids would desorb from the bilayer at Tc, leaving molecular scale defects through which drugs could escape. Mass spectrometry analysis showed that the lysolipids appeared to remain in the membranes after extensive dialysis above Tc and thus the mechanism of rapid drug release was likely due to lysolipid-stabilized pores [98].
However these experiments were donein media free of plasma proteins and cellular membrane pools, biomolecules which might have an impact on lipid desorption. Indeed, Banno et al. demonstrated that approximately 70% of lysolipids desorbed from LTSL in vivo within 1 h post-injection [99]. LTSL injected into mice and recovered in plasma samples after 1 h in circulation released 20% less drug following incubations at T > Tc, indicating that the loss of lysolipid had a negative impact on the temperature-sensitivity of the liposomes. LTSL recovered after 4 h were only able to release 50% of drug following hyperthermia [99]. Sandstrom et al. also found that in the presence of acceptor liposomes at 37 °C, lysolipids rapidly desorb from LTSL [100]. Specifically, with LTSL incubated with egg-phosphocholine (EPC) multi-lamellar vesicles (MLV) at 37 °C, approximately half the amount of lysolipids initially included in the LTSL transferred rapidly into the EPC-MLVs (50% within 10 min). Upon heating to 41.5 °C, drug release from LTSL pre-incubated with the acceptor liposomes was decreased by a factor of 1.4 when compared to LTSL that were not exposed to EPC-MLV. Furthermore, there was indirect evidence that lysolipids dissociate from LTSL upon dilution. In the presence of increasing concentrations of free lysolipid, the authors found that drug release from LTSL following mild hyperthermia increased, implying that the presence of lysolipid in the buffer solution decreased the probability of lysolipid dissociation and minimized the resulting loss of thermosensitivity.
The desorption of lysolipids from LTSL into the biological milieu and the defects left behind in the membrane may lead to premature drug leakage at physiological conditions. It has been demonstrated that LTSL release 50% of encapsulated drug within 1 h of administration in vivo [101], and from 25% up to 80% within 30 min at 37 °C in serum-containing media in vitro [102,103]. Together these results indicate that the transfer of lysolipids into biological membrane pools and their desorption upon dilution in the blood stream may: (i) compromise the thermosensitivity of LTSL in the clinic, and (ii) at worst lead to premature drug leakage at physiological conditions and a reversion to free drug behavior and its associated systemic toxicities. Consequently, application of hyperthermia after LTSL injection must be scheduled appropriately to take full advantage of the responsiveness and rapid drug release from the LTSLs prior to lysolipid desorption.
3.3. Polymer-modified thermosensitive liposomes
Another approach for sensitizing liposomes to temperature is to incorporate synthetic polymers that are membrane disruptive in response to heating. Such polymers can either add temperature-responsive functionality to nonthermosensitive formulations, or they can enhance the existing temperature-responsiveness of thermosensitive liposomes. The predominant mechanism of temperature-sensitive polymers is an energetically-driven, sharp coil-to-globule transition and phase separation at either a lower critical solution temperature (LCST) or an upper critical solution temperature (UCST). The LCST and UCST are the respective critical temperatures below and above which the polymer is completely miscible in solvent. LCST and UCST phase transitions are en-tropically and enthalpically driven processes, respectively [104].
Temperature-sensitive polymers studied for modifying liposomes predominantly present an LCST. This transition is spontaneous and endothermic, driven by the entropy gain from the release of hydrogen-bonded water molecules [105]. Below the LCST, the efficiency of hydrogen bonding between the polymer chains and water molecules is sufficient to solubilize the polymer. As the temperature is increased to the LCST, this efficiency is reduced such that H-bonding is no longer sufficient to solubilize the polymer, and phase separation occurs. From a thermodynamic perspective, the LCST corresponds to the region in the phase diagram where the enthalpy contribution of water H-bonded to the polymer chain becomes less than the entropic gain of the system as a whole [106]. The LCST is thus dependent on the hydrogen-bonding capabilities of the constituent monomer units that comprise the polymer, and in theory can be tuned to desired ranges (e.g. 38– 40 °C) by varying hydrophilic or hydrophobic monomer content.
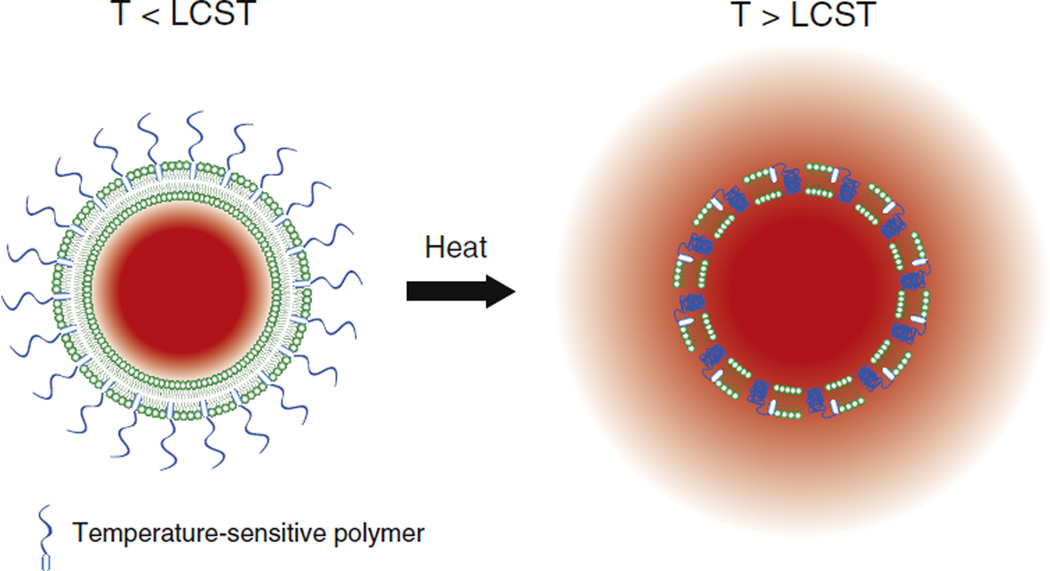
When temperature-sensitive polymers are attached to liposomes, the phase transition has been shown to be membrane disruptive [107], promoting drug release near the LCST [102,108–110] (Fig. 7). The rational design of temperature sensitive liposomes can thus be achieved by functionalization with temperature-sensitive polymers with LCST tuned to desired temperatures.
Fig. 7.
Illustration of drug release from polymer-modified thermosensitive liposomes (PTSL). At temperatures greater than the lower critical solution temperature (LCST) of the polymer, polymer chains collapse, resulting in disruption of the membrane and release of encapsulated drug.
Temperature-sensitive polymers can be broadly classified into different groups according to the chemistry of the groups: (a) poly(N-substituted acrylamides), including the most extensively studied temperature-sensitive polymer, poly(N-isopropylacrylamide) (p(NIPAAm)), (b) poly(N-vinylethers), (c) poloxamers, also known by their trade name Pluronic ®, (d) poly(N-vinylalkylamides), such as poly(N-vinyliso-butyramide) and poly(N-vinylcaprolactam), (e) poly(ethyleneglycols) (PEG) and PEG methacrylates, (f) elastin-like peptides, (g) poly(N-alkyl oxazolines), and (h) poly(N-aminoethyl methacrylates). Among these, (a–c) have been added to sensitize liposomes to temperature with some success, and these works are summarized here. Within the classes outlined above, all but the poloxamers present an LCST as the mechanism for temperature-responsive behavior. Poloxamers and their unique mechanism are discussed below.
3.3.1. poly(N-substituted acrylamides)
Poly(N-substituted acrylamides) are the most extensively studied class of temperature-sensitive polymers. Kono and coworkers were one of the first groups to recognize the potential of sensitizing liposomes to temperature by incorporating these polymers into the lipid membrane. The authors introduced the idea of fixating N-isopropylacrylamide (NIPAAm) onto liposomes by copolymerizing NIPAAm with hydrophobic monomers that would interact strongly with the hydrophobic core of the lipid membrane, and thus would not desorb in the manner of lysolipids. Their early attempts focused on the modification of pure p(NIPAAm) by free radical polymerization of NIPAAm with 1% molar octadecyl acrylate (ODA) [111]. The long alkyl chains of ODA essentially served as anchor sites for the fixation of the polymer to the lipid membrane. While unmodified p(NIPAAm) has an LCST of 32 °C, NIPAAm copolymers containing 1% ODA revealed an LCST of 27 °C. The authors functionalized nonthermosensitive egg phosphocholine (EPC) liposomes and temperature-sensitive DPPC liposomes with p(NIPAAm-co-ODA), and found enhanced release at temperatures greater than the polymer LCST but minimal release at temperatures less than the LCST in both formulations. Release was more extensive with the DPPC liposomes than with the EPC liposomes, suggesting a synergy between the inherent thermosensitivity of the DPPC membrane and the temperature-dependent membrane disruption properties of the polymer.
In a subsequent study, dioleoyl phosphoethanolamine (DOPE) was introduced into the EPC formulation, resulting in improved release [109]. A slightly different approach was presented: DOPE, a lipid with a small headgroup that tends to form inverted micelles (hexagonal II phase), does not form stable bilayers. In lipid membrane mixtures it has a destabilizing effect, one that can be negated by the presence of hydrated NIPAAm chains at temperatures less than the LCST. At temperatures greater than the LCST, the collapse of polymer chains reduces this stabilizing effect, and the liposome becomes unstable, releasing drug.
These early studies employed polymers with LCST below physiological temperature, and thus the formulations presented were not clinically practical. However they demonstrated the feasibility of anchoring temperature-sensitive polymers to lipid membranes in order to confer temperature-sensitive release properties to the liposome. More recent studies have introduced copolymers of NIPAAm and various monomers in addition to anchoring monomers, with the goal of designing polymers with LCST in the range of physiological temperature. In 1999, Hayashi et al. demonstrated that free radical copolymerization of NIPAAm with different molar concentrations of hydrophilic acrylamide (AAm) allowed for tuning of the LCST. Copolymerization with AAm monomers raised the LCST of pure poly(NIPAAm) from 32 °C to 39 °C (10% AAm), 47.2 °C (20% AAm), and 53.2 °C (30% AAm) [112]. Poly(NIPAAm-co-AAm)-modified, non-PEGylated DOPE/EPC liposomes loaded with marker drug had enhanced release above polymer LCST and minimal release below LCST. Significantly, liposomes exhibited increased Tc with increasing AAm%, demonstrating that tuning polymer LCST can in turn tune liposome Tc.
Han et al. confirmed these findings with DOX-loaded DPPC liposomes modified with p(NIPAAm-co-AAm). Copolymers of 25% AAm demonstrated LCST of 47 °C, whereas dropping the AAm% to 17% resulted in a reduction in LCST to 40 °C. The Tc of liposomes modified with these polymers corresponded closely to the polymer LCST [113]. Unmodified PEGylated DPPC liposomes released 40% of encapsulated drug at 40 °C, demonstrating the inherent thermosensitivity of the DPPC membrane. Fixation of p(NIPAAm-co-PAA) at 17% AAm resulted in 65% drug release at 40 °C, demonstrating the synergistic effects of the polymer and DPPC. In the studies by Hayashi and Han et al., drug release transitions were sharp, but no formulation demonstrated release of more than 65% of encapsulated DOX. The one exception (poly(NIPAAm-co-Aam)-modified DOPE/EPC liposomes at 10% Aam discussed in [112]) reached 80% drug release only at temperatures greater than 50 °C (Δt = 1 min, t43°C = 128 min).
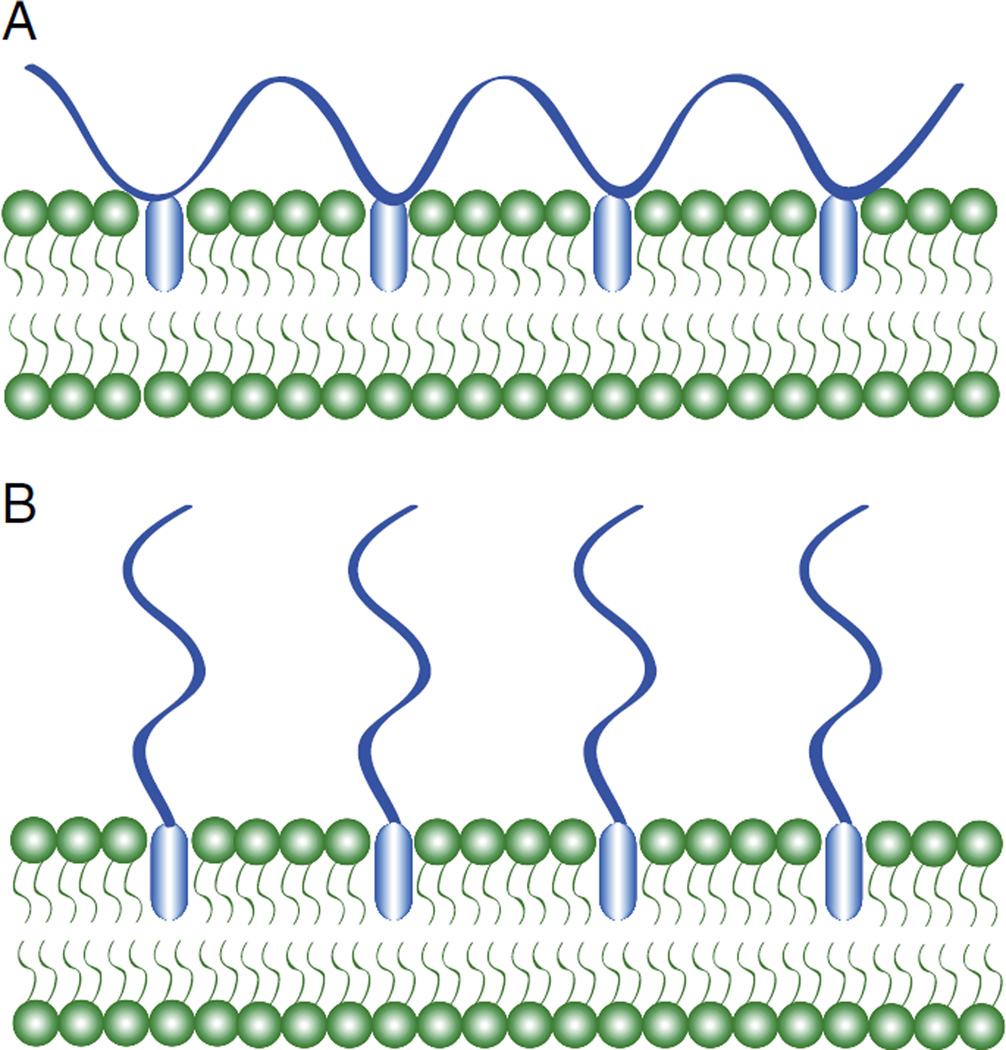
These findings brought to light that while polymer-modified liposomes could be designed to release drug at a predetermined temperature, a better understanding of the factors governing the ultimate amount of drug released was needed (e.g. polymer architecture and choice of comonomer). Kono et al. studied the effect of polymer architecture on drug release efficiency [114]. Specifically, the authors fixed thermosensitive polymers onto liposomal membranes with anchors either at arbitrary locations along the polymer backbone or at the terminal end of the chain (Fig. 8). The examples discussed thus far have involved polymers attached at multiple arbitrary locations along the backbone. Kono and coworkers observed that liposomes modified with copolymers of NIPAAm and N-acryloylpyrrolidine (Apr) anchored at the terminal end displayed a sharper enhancement of drug release in a narrow temperature region compared to liposomes modified with the same copolymer with anchors at arbitrary locations. Okano et al. observed that NIPAAm chains grafted to a solid surface at the chain end undergo hydrophilic to hydrophobic transitions more efficiently than those grafted to the same surface at multiple arbitrary locations [115,116]. This was likely due to the higher conformational freedom of the terminally-anchored chains, and provides an explanation for the enhanced release observed in liposomes modified with terminally-anchored NIPAAm chains versus those modified with chains anchored at multiple locations.
Fig. 8.
Schematic of fixation of polymer chains having anchors at (A) random positions on the polymer backbone and (B) the chain end to a liposome membrane.
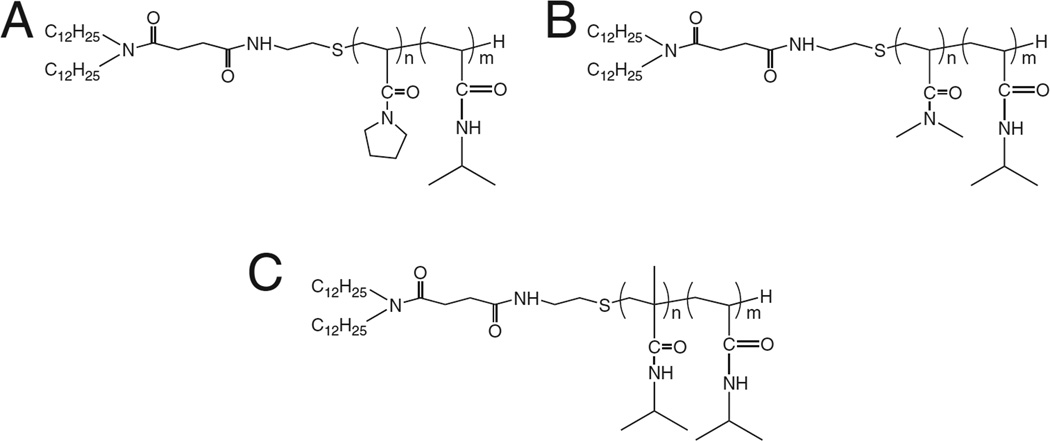
To study the effect of comonomer choice on the efficiency of release, Yoshino and coworkers synthesized three copolymers of NIPAAm, each with an LCST near 40 °C but containing structurally distinct comonomers: N-acryloylpyrrolidine (Apr), N,N-dimethylacrylamide (DMAM), and N-isopropylmethacrylamide (NIPMAM) (Fig. 9) [117]. Their hypothesis was that the distinct structural differences in the comonomers would affect polymer interaction with the liposome membrane, and thus polymer performance would differ between the polymers even if their phase transition temperatures were identical. This was indeed the case: all three polymers exhibited LCST near 40 °C, but when attached to EPC liposomes, the corresponding Tc and extent of drug release were strikingly different. The authors measured polymer LCST via two methods: cloud point measurement (i.e. measurement of amount of light that travels through an aqueous solution of polymer), and differential scanning calorimetry (DSC). Yoshino and coworkers demonstrated that the LCST determined from DSC were almost identical to the LCST determined via light scattering; however, the intensities of the DSC peaks varied between polymers. By integrating the DSC curves, the enthalpy of transition (ΔH) of each polymer was obtained, with the trend in transition enthalpies being Apr < DMAM < NIPMAM. Since the ΔH is mainly related to the destruction of water around the hydrophobic groups, the results suggested that NIPAAm copolymerized with NIPMAM formed the most hydrophobic domain above the LCST. Copolymerization with DMAM produced a polymer with an intermediate hydrophobic domain, and copolymerization with Apr produced a polymer with the least hydrophobic domain. Polymers that form more hydrophobic domains would be expected to interact more strongly with the lipid membrane, resulting in greater membrane disruption and drug release. The results confirmed this hypothesis, with drug release increasing with polymer ΔH (i.e. Apr < DMAM < NIPMAM). These findings highlight the importance of monomer choice when designing temperature-responsive liposomes with NIPAAm copolymers. The choice of monomer determines not only the resulting LCST of the polymer and the corresponding liposomal Tc, but also the extent of drug release. Release from liposomes modified with p(NIPAAm-co-NIPMAM) demonstrated rapid release at 42 °C, but also a significant amount of drug release at physiological temperature (50% at 37 °C within 15 min).
Fig. 9.
Copolymers of NIPAAm and (A) N-acryloylpyrrolidine (Apr), (B) N,N-dimethylacrylamide (DMAM), and (C) N-isopropylmethacrylamide (NIPMAM) discussed in [117]. Two dodecyl groups at the terminal end of the chains allow fixation to the hydrophobic core of the lipid membrane. Copolymers presented similar LCST (~40 °C) but varying transition enthalpies (Apr < DMAM < NIPMAM). Drug release from polymer-modified liposomes increased with increasing ΔH.
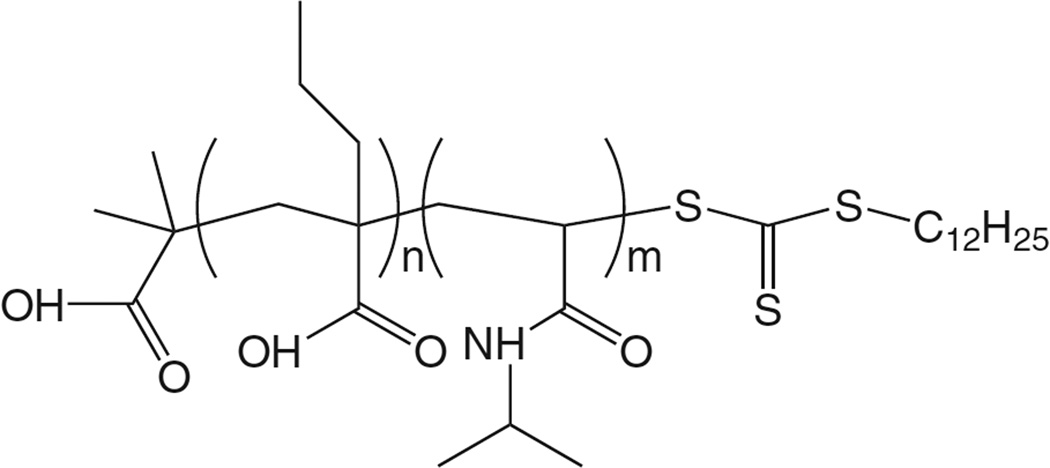
The NIPAAm-based polymers discussed thus far were synthesized via free radical polymerization, a technique that lacks control over molecular weight, yields polymers of high polydispersity index, and restricts the choice of architecture of the polymer chain [118]. Recently, we introduced liposomes modified with copolymers of NIPAAm and propyl acrylic acid (PAA) (Fig. 10) [102]. Copolymers were synthesized via reversible addition-fragmentation chain transfer (RAFT) chemistry, a type of reversible-deactivation radical polymerization (also known as living free radical polymerization) that yields polymers of low poly-dispersity and provides high control over molecular weights. Copolymerization with 5% PAA yielded polymers with an LCST of 42 °C. DPPC liposomes modified with terminally anchored polymers released 70% of drug after 5 min heating at 40 °C (t43°C = 5 s), with 100% release achieved following 5 min heating at 42 °C (t43°C = 1.25 min). Liposomes were stable in serum, with minimal release observed at 37 °C. Additionally, PAA contains an ionizable carboxyl group (pKa = 6.7) that allows the LCST to be reduced at slightly acidic pH. This is due to the fact that at pH < pKa, PAA is protonated and loses its net negative charge. The overall hydrophobicity of the polymer increases, and this allows the polymer to more readily undergo its coil-to-globule phase transition. Polymer LCST is reduced and thus drug release is increased at acidic pH. This pH-sensitivity is applicable to cancer therapy, as the tumor microenvironment is slightly acidic [119,120]. Such dual-sensitive formulations have the potential to provide greater release efficiency while remaining stable at physiological temperature and pH.
Fig. 10.
Structure of p(NIPAAm-co-PAA) synthesized via RAFT chemistry [102]. The dual-sensitive polymer (temperature, pH) was fixated onto liposomes, and drug release triggered by both heating and acidic pH. The pH-sensitivity is applicable to cancer therapy, as tumors are known to be slightly acidic.
One of the major drawbacks of the poly(N-substituted acrylamides) discussed thus far are that they are not biodegradable. As an alternative, poly(N-(2-hydroxypropyl)methacrylamide) (p(HPMA)) polymers have been widely studied as biodegradable macromolecular carriers for anticancer drugs [121]. Poly(HPMA) based copolymers have been recently synthesized with tunable LCST ranging from 13 to 65 °C, and added to DOPE/EPC and DPPC liposomes [122]. Drug release transitions were broad, from 37 to 45 °C, with 50% release occurring at 43–45 °C. Current work with liposomes modified with HPMA polymers is in its early stages, but may provide for a biodegradable polymer-modified thermosensitive liposome based on a poly(N-substituted acrylamide).
3.3.2. Poly(N-vinylethers)
Polymer-modified TSL research has traditionally been dominated by NIPAAm-based polymers. As an alternative, poly(N-vinylethers) synthesized via living cationic polymerization have been studied for the temperature-sensitization of liposomes. As with RAFT and other living polymerization techniques, living cationic polymerization yields polymers of low polydispersity and provides high control over molecular weights.
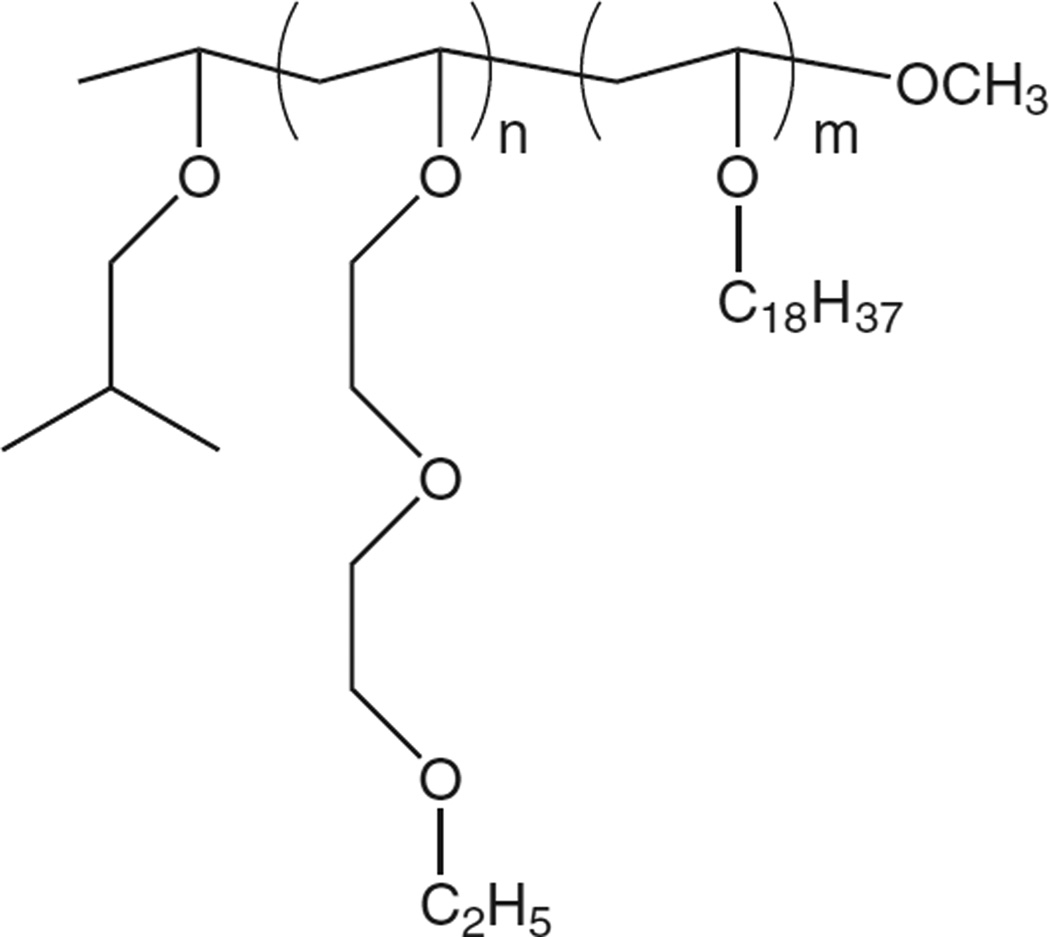
Poly(N-vinylethers) derive their thermosensitive properties from the dehydration of polymer chains, i.e. a similar mechanism to that of NIPAAm-based copolymers. As with NIPAAm copolymers, poly(N-vinylethers) present LCSTs which can be tuned by copolymerization with hydrophobic monomers that double as anchor moieties for the fixation of these polymers onto the lipid membrane [118,123,124]. Kono et al. demonstrated that block copolymers of (2-ethoxy)ethoxyethyl vinyl ether (EOEOVE) and anchor group octadecyl vinyl ether (ODVE) (Fig. 11) could be synthesized with LCST 40 °C, and that the extent of drug release was affected by varying the chain length of the poly(EOEOVE) block [118]. Increasing the chain length of poly(EOEOVE) resulted in more intensive drug release, indicating that the longer polymer chains enhance dehydration through the formation of more highly hydrophobic domains. This was supported by transition enthalpy measurements: block copolymers with the longest EOEOVE chains had the greatest measured ΔH. These findings reinforced results from earlier studies with NIPAAm which first highlighted the relationship between ΔH and extent of drug release.
Fig. 11.
Structure of p(EOEOVE-s-ODVE) synthesized via living cationic polymerization [118,123,124].
While there has a been a relative dearth of published in vivo data on thermosensitive liposomes modified with p(NIPAAm)-based copolymers, recent studies have reported in vivo success with EOEOVE-based polymers [123,124]. DOX-loaded EPC/cholesterol liposomes modified with block copolymers of poly(EOEOVE) and poly(ODVE) suppressed tumor growth in mice when combined with heating for 10 min at 45 °C (t43°C = 40 min) [123]. In another study, EPC/cholesterol liposomes coencapsulating DOX and gadolinium chelates significantly delayed tumor growth, while also providing the capability of tracking the particles via MR imaging [124].
3.3.3. Poloxamers
Poloxamers (trade name Pluronics ®) is a collective name for a large group of triblock copolymers consisting of a core hydrophobic block (polypropylene oxide, PPO) flanked by hydrophilic end blocks (polyethylene oxide, PEO). These polymers present a distinct temperature-sensitive mechanism from the LCST-presenting temperature-sensitive polymers discussed thus far. In an aqueous environment at temperatures below what is called their critical micellar temperature (CMT), poloxamers remain as individual non-associated copolymers. Above the CMT, the co-polymers become more hydrophobic and form micelles with the hydrophobic PPO groups forming the micellar core. This behavior can be used to impart thermosensitivity to liposomes. At temperatures below CMT, liposome encapsulated poloxamers do not associate with the lipid bilayer. Above the CMT, poloxamers partition into the bilayer, causing defects and disrupting the membrane, resulting in release of encapsulated drug [125].
Poloxamer CMT can be tuned by varying the molecular weight, the hydrophilic–lipophilic balance, and polymer concentration [125,126]. Encapsulation in liposomes yields Tc typically within a 5 °C range of the polymer CMT. Chandaroy et al. developed a temperature-sensitive liposome by modifying PEGylated dioleoyl phosphocholine (DOPC) liposomes with Pluronic F-127, a poloxamer with MW 12,600 Da and composition PEO98–PPO67–PEO98 (Fig. 12). The authors found that incorporation of 50% cholesterol resulted in much sharper transitions, and that liposome Tc decreased with increasing F127 concentration. The latter observation proved to be an issue, as even at low F127 concentrations (0.04% w/v) liposome Tc was generally too low for clinical applications (32–34 °C). There was one exception: liposomes encapsulating a large MW drug (bovine serum albumin conjugated to FITC) at low F127 concentration (0.04%) exhibited a narrow transition at 38– 42 °C. Liposome Tc appeared to be affected by the size of the encapsulated drug, as the same formulation substituting BSA for carboxyfluorescein exhibited a Tc near 32 °C [125].
Fig. 12.
Structure of pluronic F-127. Hydrophilic polyethylene oxide blocks flank the hydrophobic polypropylene block. The polymer forms micelles at elevated temperatures, and when encapsulated in liposomes can disrupt the lipid membrane, providing a means of temperature-triggered release [125,127].
Wells et al. demonstrated that F127-modified DOPC/cholesterol liposomes were able to deliver a small molecule fluorescent marker to cancer cells in vitro and in vivo [127]. Furthermore, cell-bound markers increased as cells or tumors were heated from 30 to 42 °C, with tumors heated to 42 °C exhibiting 2.5-fold greater fluorescence compared to controls. In vitro release curves showed a broad transition from 30 to 42 °C, correlating well with cell uptake measurements. However, roughly 50% of encapsulated marker was released at 37 °C. In general, F127 modified liposomes appear to have Tc too low for use as delivery vehicles for small molecular weight drugs.
3.4. Heating modalities for triggering release from thermosensitive liposome systems
Thermosensitive liposome research has been centered on the careful design and optimization of liposome properties (e.g. size, stability, circulation time, responsiveness to temperature). The first TSL engineered for delivery of chemotherapy is close to entering the market, marking a major milestone in the development of these triggerable carriers. These developments have raised the issue of how to most effectively and safely administer these formulations and trigger drug release. TSL as a localized therapy depends on the ability to accurately maintain controlled temperature elevations in target regions (e.g. tumor tissue), while minimizing temperature elevations in surrounding healthy tissue. Thus, the modalities used for heat-triggered release of drug from TSL are critical to the success of the drug delivery platform. Here the various modes of heating that have been used to trigger release from TSL are reviewed and evaluated.
3.4.1. Regional hyperthermia
The majority of studies with TSL in preclinical models have applied regional, superficial heating with heated water baths [74,78,79,93,95,128–131]. Animals were typically positioned in specially designed holders that allowed for the isolation and placement of the target area into a heated water bath. This method of heating is limited to superficial tumors, and suffers from poor localization of heating, i.e. it is impossible to heat the tumor without heating surrounding non-tumor tissue.
3.4.2. External electromagnetic applicators for localized hyperthermia
Superficial tumors can be heated locally by means of external antennas or applicators that emit microwaves or radiowaves. Several types of applicators, such as waveguide applicators (e.g. ring, horn, spiral, and current sheet), have been used in preclinical models with liposomes. For example, Hauck et al. studied the effects of hyperthermia applied from spiral and annular-phased array microwave applicators on the efficacy of TSL against macroscopic soft tissue canine tumors [132]. Commercially available applicators have a typical emitting diameter of 15 cm at a frequency of 150–430 MHz and a typical maximum therapeutic depth of 3 cm. On irregular surfaces such as the head and neck, the supraclavicular region, and the axilla, therapeutic depth is further limited. Thus the utility of external applicators for local hyperthermia and triggered drug release from TSL can depend strongly upon tumor location. Intratumoral temperature is controlled by both the output of the power generator and also by the positioning of the applicator.
3.4.3. Localized interstitial hyperthermia
One approach to access and heat more deeply seated tumors is to insert antennas or applicators directly within the tumor, often with the help of ultrasonographic guidance. Minimally-invasive antenna types include microwave antennas, radiofrequency electrodes, heat sources (ferromagnetic seeds, heated water), and laser fibers [55]. Several antenna types have been explored for tumor-localized heating in preclinical studies, including heated water sources run through implanted catheters to complement LTSL administration [133,134] and radiofrequency electrodes to complement Doxil administration [135,136]. Interstitial hyperthermia is typically more variable than heating provided by external microwave applicators. In order to ensure therapeutic temperatures at all points of the target volume, multiple applicators are used and are positioned in close proximity to one another, a practice that is very invasive. To minimize this, electrodes with expandable prongs have been used (Fig. 13). Interstitial hyperthermia is effective for tumors less than 5 cm in diameter, but is restricted to locations that are feasible for insertion (e.g. head and neck, prostate).
Fig. 13.
Schematic of interstitial hyperthermia showing single radiofrequency probe with expandable prongs for more uniform heating.
3.4.4. Focused ultrasound for localized hyperthermia
High-intensity focused ultrasound (FUS; frequencies greater than 20 kHz) can be used to heat deep tissue in a relatively small focal zone (mm) compared to other hyperthermia devices (e.g. microwaves). FUS also allows for heating with a high degree of temporal control, as the rate of heating depends on the magnitude and duration of the ultrasound exposure, which can be readily controlled. The application of FUS occurs at a distance remote from the transducer, thus providing a noninvasive means of heating.
Because of these attributes, FUS has emerged as a leading modality for heat-triggered drug release from TSL, and recent reports have demonstrated the potential of FUS-TSL systems for localized heat-triggered drug release [137–139]. In particular, Dromi et al. demonstrated in a preclinical model that LTSL combined with hyperthermia from FUS enhanced drug deposition at the tumor and delayed tumor growth relative to liposome treatment without FUS [137]. However, in these and other studies, temperature measurement was limited to point measurements made by invasive thermocouples or optical probes. Thus, with conventional FUS systems, there remains a need for noninvasive, online monitoring of heating throughout the whole target volume.
3.4.5. Magnetic resonance-guided focused ultrasound (MRgFUS)
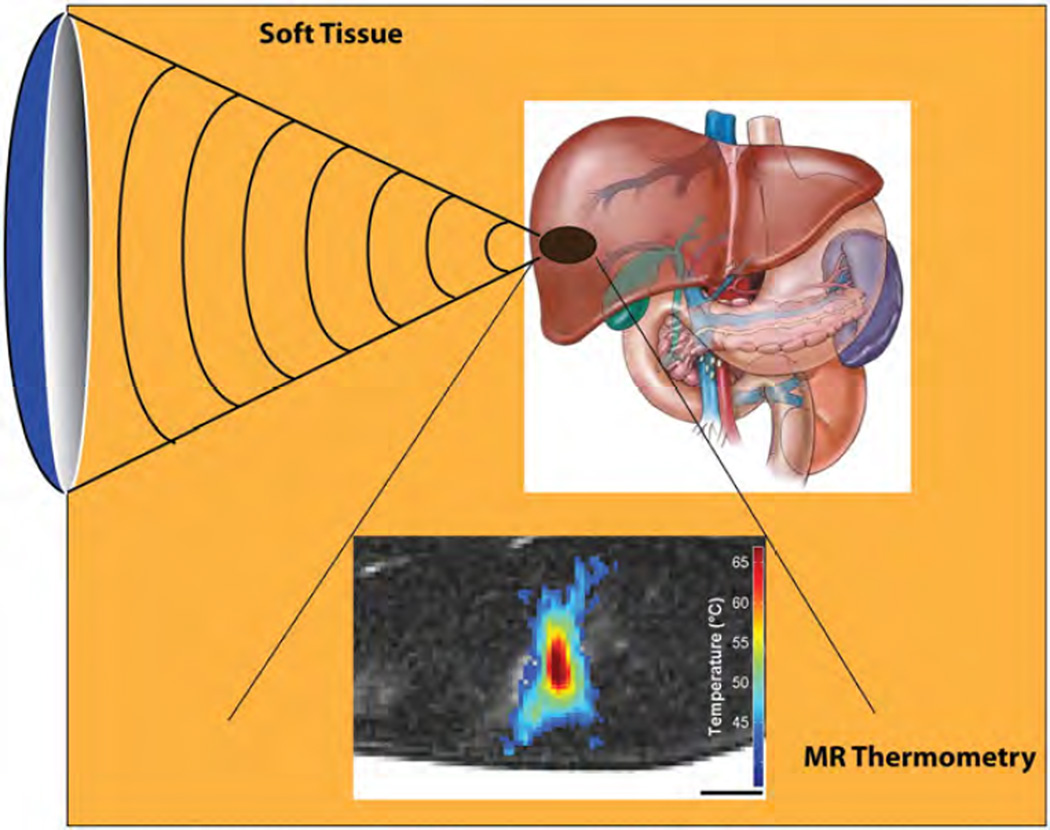
Recent advances have been made in the use of MR-compatible FUS transducers for MR-guided application of focused ultrasound (MRgFUS). The integration of MR guidance during FUS exposures allows for simultaneous: (i) MR-imaging to guide the treatment, a modality which offers superior tissue differentiation compared to ultrasound imaging, and (ii) MR thermometry to noninvasively monitor temperature changes and provide feedback in real-time (Fig. 14).
Fig. 14.
The development of MR-compatible focused ultrasound transducers has led to the emergence of MR-guided focused ultrasound as a very effective tool for image-guided thermal therapy. Focused ultrasound transmitted through the skin can heat tumors rapidly (i.e. heating in less than a second) without damaging intervening tissue. Two-dimensional maps of ultrasound-mediated heating are possible with MR thermometry, thus allowing for feedback control of the focused ultrasound system. The image-guided system can be used for sustained, controlled heating of tumors above the threshold for triggered release of chemotherapeutics from thermosensitive liposomes.
Very recently, several groups have demonstrated the effectiveness of MRgFUS as a heating modality for thermosensitive liposomes. In rat [140] and rabbit [141] experiments, tumors in treatment groups receiving both MRgFUS and drug-loaded liposome demonstrated the greatest uptake of drug when compared to controls (liposome only and/or free drug). Furthermore, in studies of traditional and lysolipid-containing formulations coencapsulating DOX with Gd-HP-DO3A, a clinically approved T1-weighted MRI contrast agent, DOX release measured via fluorescence correlated well with Gd-HP-DO3A release measured via NMR and/or MR spectroscopy [140,142]. Thus, there is the potential with these multifunctional liposomes to probe for drug release in situ by measuring the relaxation time of co-released MRI contrast agents.
Ranjan et al. demonstrated MRgFUS-enhanced distribution of DOX in tumors, with drug fluorescence observed at greater penetration depths in tumors of animals that were administered a combination of MRgFUS and liposomes as compared to tumors in animals given only liposome or free drug, in which drug remained in the tumor periphery [141]. In a non-cancer rabbit model, Staruch et al. demonstrated the ability to localize drug accumulation in areas of the bone marrow by targeting these areas with MRgFUS exposure in the presence of drug-loaded liposomes [143]. The results of these studies demonstrate the potential of MRgFUS to provide localized chemotherapy when used in combination with thermosensitive liposomal therapy, but evidence demonstrating improved clinical efficacy of these systems is currently limited.
4. Summary and conclusions
Traditional thermosensitive liposomes (TTSL) are comprised of lipids that undergo gel-to-liquid phase changes in response to temperature. These liposomes are typically composed of DPPC, a lipid with phase transition temperature (Tc) of 41 °C, and some combination of cholesterol, DSPC, HSPC, and/or PEG-conjugated lipid for prolonged blood circulation. Initial iterations of pure DPPC formulations resulted in liposomes that did not completely release drug, a problem that was addressed by the inclusion of DSPC or HSPC. However, these modified DPPC liposomes typically exhibited Tc at around 43 °C, which resulted in thermal dose requirements that can put non-cancerous tissue at risk of necrosis during potential clinical administration of TTSL.
In an effort to improve the thermal response of thermosensitive liposomes, lysolipid-containing thermosensitive liposomes (LTSL) were developed. Studies suggest that the presence of lysolipids at a low molar % in DPPC bilayers results in the formation of stabilized defects in the membrane during the phase transition, leading to enhanced release. LTSL formulations were optimized for stability and thermal response, resulting in formulations with Tc of 39–40 °C and very rapid release of drug (complete drug release within tens of seconds). The combination of LTSL with external energy sources for localized heat-induced drug release has shown tremendous promise for increasing intratumoral drug concentration, and the efficacy and potential adverse effects of the approach are currently being assessed in clinical trials.
As an alternative to lysolipids, which may desorb from liposomal shells and thus compromise the stability and/or thermosensitivity of the liposome, temperature-sensitive polymers have been utilized to improve the responsiveness of thermosensitive liposomes. These polymers typically present a lower critical solution temperature (LCST) at which they undergo coil-to-globule phase transitions. Anchored to lipid membranes via hydrophobic interactions, they dehydrate and collapse at the LCST, becoming membrane disruptive and inducing drug release. Polymer LCST can be tuned by copolymerization with a variety of monomers, the choice of which also affects the extent of drug release. Polymer-modified thermosensitive liposomes (PTSL) have thus far shown great promise, but their temperature-responsiveness has not yet been optimized: formulations typically are not able to completely release all of the encapsulated drug, or have broad release transitions such that significant concentrations of drug are released at 37 °C. Typical syntheses techniques such as standard free radical polymerization of NIPAAm have several disadvantages, such as lack of control over molecular weight, high polydispersity, and limited freedom in choosing the architecture of the polymer chain. Recently, living polymerization techniques have been used to synthesize temperature-sensitive polymers with low polydispersity and high control of molecular weight for the design of PTSL. The resulting formulations have demonstrated optimal release properties in vitro, and in vivo studies demonstrating increased efficacy are ongoing.
Lastly, current heating modalities for triggering drug release from TSL have generally suffered from limited penetration depth, invasiveness, insufficient spatial/temporal control of heating, and insufficient/invasive monitoring of heating. Recently, focused ultrasound (FUS) has shown the potential to accurately and noninvasively apply heating to tumor tissue while minimizing heating to non-cancerous tissue. Furthermore, MR-guided focused ultrasound (MRgFUS) has shown the potential for noninvasive, real-time monitoring of tumor temperature during administration.
The clinical approval of Doxil in 1995 was a landmark event in the advancement of liposomal technology for the treatment of cancer. Temperature-sensitive liposomes with designs based on the gel-to-liquid phase transition of their lipid membranes are now in advanced clinical trials, and promise to offer the ability for triggered drug release and site-specific delivery. Polymer-modified liposomes that achieve temperature response via temperature-dependent interactions between polymers and the lipid membrane have been extensively studied, with multiple groups demonstrating improved performance in vitro and increased efficacy in vivo. These have the potential to provide triggered drug release at clinically attainable temperatures with increased efficiency, and without the stability issues present in existing formulations of thermosensitive liposomes. Preclinical studies with polymer-modified thermosensitive liposomes show great promise, and may lead to the development of formulations that achieve site-specific delivery and triggered release of therapeutically relevant doses of chemotherapy.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Skeel RT, Khleif SN. Handbook of Cancer Chemotherapy. 8th. Philadelphia: Lippincott Williams and Wilkins; 2011. [Google Scholar]
- 3.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discovery. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 4.Eliaz RE, Szoka CF., Jr Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61:2592–2601. [PubMed] [Google Scholar]
- 5.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp. Biol. Med. 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan R, Vicent MJ, Greco F, Nicholson RI. Polymer-drug conjugates: towards a novel approach for the treatment of endrocine-related cancer. Endocr.-Relat. Cancer. 2005;12(Suppl. 1):S189–S199. doi: 10.1677/erc.1.01045. [DOI] [PubMed] [Google Scholar]
- 7.Discher DE, Ortiz V, Srinivas G, Klein ML, Kim Y, Christian D, Cai S, Photos P, Ahmed F. Emerging applications of polymersomes in delivery: from molecular dynamics to shrinkage of tumors. Prog. Polym. Sci. 2007;32:838–857. doi: 10.1016/j.progpolymsci.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR., Jr PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7:572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 9.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, Loffler K, Fechtner M, Rohl T, Fisch G, Dames S, Arnold W, Giese K, Klippel A, Kaufmann J. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 2006;13:1360–1370. doi: 10.1038/sj.gt.3302778. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Lee S, Kim J-H, Park K, Kim K, Kwon IC. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008;33:113–137. [Google Scholar]
- 13.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 14.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 15.Maeda H, Matsumura Y. Tumoritropic and lymphotropic principles of macro-molecular drugs. Crit. Rev. Ther. Drug Carrier Syst. 1989;6:193–210. [PubMed] [Google Scholar]
- 16.Maeda H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 2001;46:169–185. doi: 10.1016/s0169-409x(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 18.Iwai K, Maeda H, Konno T. Use of oily contrast medium for selective drug targeting to tumor: enhanced therapeutic effect and X-ray image. Cancer Res. 1984;44:2115–2121. [PubMed] [Google Scholar]
- 19.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwai K, Maeda H, Konno T, Matsumura Y, Yamashita R, Yamasaki K, Hirayama S, Miyauchi Y. Tumor targeting by arterial administration of lipids: rabbit model with VX2 carcinoma in the liver. Anticancer. Res. 1987;7:321–327. [PubMed] [Google Scholar]
- 21.Maeda H, Matsumoto T, Konno T, Iwai K, Ueda M. Tailor-making of protein drugs by polymer conjugation for tumor targeting: a brief review on smancs. J. Protein Chem. 1984;3:181–193. [Google Scholar]
- 22.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 23.Folkman J, Shing Y. Angiogenesis. J. Biol. Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 24.Maeda H. Polymer conjugated macromolecular drugs for tumor-specific targeting. In: Domb AJ, editor. Polymer Site-specific Pharmacotherapy. New York: John Wiley and Sons, Inc.; 1994. pp. 95–116. [Google Scholar]
- 25.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 27.Nomura T, Koreeda N, Yamashita F, Takakura Y, Hashida M. Effect of particle size and charge on the disposition of lipid carriers after intratumoral injection into tissue-isolated tumors. Pharm. Res. 1998;15:128–132. doi: 10.1023/a:1011921324952. [DOI] [PubMed] [Google Scholar]
- 28.Maeda H, Seymour LW, Miyamoto Y. Conjugates of anticancer agents and polymers: advantages of macromolecular therapeutics in vivo. Bioconjug. Chem. 1992;3:351–362. doi: 10.1021/bc00017a001. [DOI] [PubMed] [Google Scholar]
- 29.Needham D, Ponce A. Nanoscale drug delivery vehicles for solid tumors: a new paradigm for localized drug delivery using temperature sensitive liposomes. In: Amiji MM, editor. Nanotechnology for Cancer Therapy. Boca Raton: CRC Press; 2007. pp. 677–719. [Google Scholar]
- 30.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 31.Beaumier PL, Hwang KJ. Effects of liposome size on the degradation of bovine brain sphingomyelin/cholesterol liposomes in the mouse liver. Biochim. Biophys. Acta. 1983;731:23–30. doi: 10.1016/0005-2736(83)90393-0. [DOI] [PubMed] [Google Scholar]
- 32.Gregoriadis G, Ryman BE. Fate of protein-containing liposomes injected into rats: an approach to the treatment of storage diseases. Eur. J. Biochem. 1972;24:485–491. doi: 10.1111/j.1432-1033.1972.tb19710.x. [DOI] [PubMed] [Google Scholar]
- 33.Torchilin V. Liposomes in drug delivery. In: Siepmann J, Siegel RA, Rathbone MJ, editors. Fundamentals and Applications of Controlled Release Drug Delivery. New York: Springer Science and Business Media; 2012. pp. 289–328. [Google Scholar]
- 34.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 35.Beauchamp CO, Gonias SL, Menapace DP, Pizzo SV. A new procedure for the synthesis of polyethylene glycol-protein adducts; effects on function, receptor recognition, and clearance of superoxide dismutase, lactoferrin, and alpha 2-macroglobulin. Anal. Biochem. 1983;131:25–33. doi: 10.1016/0003-2697(83)90131-8. [DOI] [PubMed] [Google Scholar]
- 36.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 37.Allen TM, Austin GA, Chonn A, Lin L, Lee KC. Uptake of liposomes by cultured mouse bone marrow macrophages: influence of liposome composition and size. Biochim. Biophys. Acta. 1991;1061:56–64. doi: 10.1016/0005-2736(91)90268-d. [DOI] [PubMed] [Google Scholar]
- 38.Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim. Biophys. Acta. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 40.Krishna R, Webb MS, St Onge G, Mayer LD. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J. Pharmacol. Exp. Ther. 2001;298:1206–1212. [PubMed] [Google Scholar]
- 41.Allen TM, Newman MS, Woodle MC, Mayhew E, Uster PS. Pharmacokinetics and anti-tumor activity of vincristine encapsulated in sterically stabilized liposomes. Int. J. Cancer. 1995;62:199–204. doi: 10.1002/ijc.2910620215. [DOI] [PubMed] [Google Scholar]
- 42.Bandak S, Goren D, Horowitz A, Tzemach D, Gabizon A. Pharmacological studies of cisplatin encapsulated in long-circulating liposomes in mouse tumor models. Anticancer Drugs. 1999;10:911–920. doi: 10.1097/00001813-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am. J. Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 44.Seynhaeve AL, Hoving S, Schipper D, Vermeulen CE, de Wiel-Ambagtsheer G, van Tiel ST, Eggermont AM, Ten Hagen TL. Tumor necrosis factor alpha mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response. Cancer Res. 2007;67:9455–9462. doi: 10.1158/0008-5472.CAN-07-1599. [DOI] [PubMed] [Google Scholar]
- 45.Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JS. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001;7:243–254. [PubMed] [Google Scholar]
- 46.Garcia AA, Kempf RA, Rogers M, Muggia FM. A phase II study of Doxil (lipo-somal doxorubicin): lack of activity in poor prognosis soft tissue sarcomas. Ann. Oncol. 1998;9:1131–1133. doi: 10.1023/a:1008439013169. [DOI] [PubMed] [Google Scholar]
- 47.Ellerhorst JA, Bedikian A, Ring S, Buzaid AC, Eton O, Legha SS. Phase II trial of doxil for patients with metastatic melanoma refractory to frontline therapy. Oncol. Rep. 1999;6:1097–1099. doi: 10.3892/or.6.5.1097. [DOI] [PubMed] [Google Scholar]
- 48.Gill PS, Wernz J, Scadden DT, Cohen P, Mukwaya GM, von Roenn JH, Jacobs M, Kempin S, Silverberg I, Gonzales G, Rarick MU, Myers AM, Shepherd F, Sawka C, Pike MC, Ross ME. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 1996;14:2353–2364. doi: 10.1200/JCO.1996.14.8.2353. [DOI] [PubMed] [Google Scholar]
- 49.Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 50.Dass CR, Walker TL, Burton MA, Decruz EE. Enhanced anticancer therapy mediated by specialized liposomes. J. Pharm. Pharmacol. 1997;49:972–975. doi: 10.1111/j.2042-7158.1997.tb06025.x. [DOI] [PubMed] [Google Scholar]
- 51.Schroeder A, Honen R, Turjeman K, Gabizon A, Kost J, Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J. Control. Release. 2009;137:63–68. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Shum P, Kim JM, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv. Drug Deliv. Rev. 2001;53:273–284. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 53.Landon CD, Park JY, Needham D, Dewhirst MW. Nanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karanth H, Murthy RS. pH-sensitive liposomes: principle and application in cancer therapy. J. Pharm. Pharmacol. 2007;59:469–483. doi: 10.1211/jpp.59.4.0001. [DOI] [PubMed] [Google Scholar]
- 55.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 56.Bull JM. An update on the anticancer effects of a combination of chemotherapy and hyperthermia. Cancer Res. 1984;44:4853s–4856s. [PubMed] [Google Scholar]
- 57.Dahl O. Interaction of hyperthermia and chemotherapy. Recent Results Cancer Res. 1988;107:157–169. doi: 10.1007/978-3-642-83260-4_23. [DOI] [PubMed] [Google Scholar]
- 58.Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, Caldarera E, Pavone-Macaluso M. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J. Clin. Oncol. 2003;21:4270–4276. doi: 10.1200/JCO.2003.01.089. [DOI] [PubMed] [Google Scholar]
- 59.Franckena M, De Wit R, Ansink AC, Notenboom A, Canters RA, Fatehi D, Van Rhoon GC, Van Der Zee J. Weekly systemic cisplatin plus locoregional hyperthermia: an effective treatment for patients with recurrent cervical carcinoma in a previously irradiated area. Int. J. Hyperth. 2007;23:443–450. doi: 10.1080/02656730701549359. [DOI] [PubMed] [Google Scholar]
- 60.Issels R, Lindner LH, Wendtner CM, Hohenberger P, Reichardt P, Daugaard S, Mansmann U, Hiddemann W, Blay JY, Verweij J. 1LBA Impact of regional hy-perthermia (RHT) on response to neo-adjuvant chemotherapy and survival of patients with high-risk soft-tissue sarcoma (HR-STS): results of the randomized EORTC-ESHO intergroup trial (NCI-00003052) EJC. 2009;(Suppl. 7):2. [Google Scholar]
- 61.Huang SK, Stauffer PR, Hong K, Guo JW, Phillips TL, Huang A, Papahadjopoulos D. Liposomes and hyperthermia in mice: increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res. 1994;54:2186–2191. [PubMed] [Google Scholar]
- 62.Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, ten Hagen TL, Needham D, Dewhirst MW. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nano-particle delivery: effect of particle size. Cancer Res. 2000;60:4440–4445. [PubMed] [Google Scholar]
- 64.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 65.Sakaguchi Y, Stephens LC, Makino M, Kaneko T, Strebel FR, Danhauser LL, Jenkins GN, Bull JM. Apoptosis in tumors and normal tissues induced by whole body hyperthermia in rats. Cancer Res. 1995;55:5459–5464. [PubMed] [Google Scholar]
- 66.Freeman C, Halperin EC, Brady LW, Wazer DE. Perez and Brady's Principles and Practice of Radiation Oncology. Philadelphia: Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 67.Kim KY. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomedicine. 2007;3:103–110. doi: 10.1016/j.nano.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Papahadjopoulos D, Jacobson K, Nir S, Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 1973;311:330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 69.Berg HC. Random Walks in Biology. Princeton: Princeton University Press; 1993. [Google Scholar]
- 70.Kim DH, Costello MJ, Duncan PB, Needham D. Mechanical properties and mi-crostructure of polycrystalline phospholipid monolayer shells: novel solid mi-croparticles. Langmuir. 2003;19:8455–8466. [Google Scholar]
- 71.Bassett JB, Anderson RU, Tacker JR. Use of temperature-sensitive liposomes in the selective delivery of methotrexate and cis-platinum analogues to murine bladder tumor. J. Urol. 1986;135:612–615. doi: 10.1016/s0022-5347(17)45761-2. [DOI] [PubMed] [Google Scholar]
- 72.Maruyama K, Unezaki S, Takahashi N, Iwatsuru M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim. Biophys. Acta. 1993;1149:209–216. doi: 10.1016/0005-2736(93)90203-c. [DOI] [PubMed] [Google Scholar]
- 73.Gaber MH, Hong K, Huang SK, Papahadjopoulos D. Thermosensitive sterically stabilized liposomes: formulation and in vitro studies on mechanism of doxorubicin release by bovine serumand human plasma. Pharm. Res. 1995;12:1407–1416. doi: 10.1023/a:1016206631006. [DOI] [PubMed] [Google Scholar]
- 74.Yatvin MB, Muhlensiepen H, Porschen W, Weinstein JN, Feinendegen LE. Selective delivery of liposome-associated cis-dichlorodiammineplatinum(II) by heat and its influence on tumor drug uptake and growth. Cancer Res. 1981;41:1602–1607. [PubMed] [Google Scholar]
- 75.Zou YY, Ueno M, Yamagishi M, Horikoshi I, Yamashita I, Tazawa K, Gu XQ. Targeting behavior of hepatic artery injected temperature sensitive liposomal adriamycin on tumor-bearing rats. Sel. Cancer Ther. 1990;6:119–127. doi: 10.1089/sct.1990.6.119. [DOI] [PubMed] [Google Scholar]
- 76.Weinstein JN, Magin RL, Yatvin MB, Zaharko DS. Liposomes and local hyper-thermia: selective delivery of methotrexate to heated tumors. Science. 1979;204:188–191. doi: 10.1126/science.432641. [DOI] [PubMed] [Google Scholar]
- 77.Tacker JR, Anderson RU. Delivery of antitumor drug to bladder cancer by use of phase transition liposomes and hyperthermia. J. Urol. 1982;127:1211–1214. doi: 10.1016/s0022-5347(17)54299-8. [DOI] [PubMed] [Google Scholar]
- 78.Maekawa S, Sugimachi K, Kitamura M. Selective treatment of metastatic lymph nodes with combination of local hyperthermia and temperature-sensitive liposomes containing bleomycin. Cancer Treat. Rep. 1987;71:1053–1059. [PubMed] [Google Scholar]
- 79.Nishimura Y, Ono K, Hiraoka M, Masunaga S, Jo S, Shibamoto Y, Sasai K, Abe M, Iga K, Ogawa Y. Treatment of murine SCC VII tumors with localized hyperthermia and temperature-sensitive liposomes containing cisplatin. Radiat. Res. 1990;122:161–167. [PubMed] [Google Scholar]
- 80.Iga K, Hamaguchi N, Igari Y, Ogawa Y, Gotoh K, Ootsu K, Toguchi H, Shimamoto T. Enhanced antitumor activity in mice after administration of thermosensitive liposome encapsulating cisplatin with hyperthermia. J. Pharmacol. Exp. Ther. 1991;257:1203–1207. [PubMed] [Google Scholar]
- 81.Unezaki S, Maruyama K, Takahashi N, Koyama M, Yuda T, Suginaka A, Iwatsuru M. Enhanced delivery and antitumor activity of doxorubicin using long-circulating thermosensitive liposomes containing amphipathic polyethylene glycol in combination with local hyperthermia. Pharm. Res. 1994;11:1180–1185. doi: 10.1023/a:1018949218380. [DOI] [PubMed] [Google Scholar]
- 82.Nibu Y, Inoue T, Motoda I. Effect of headgroup type on the miscibility of homologous phospholipids with different acyl chain lengths in hydrated bilayer. Biophys. Chem. 1995;56:273–280. doi: 10.1016/0301-4622(95)00041-u. [DOI] [PubMed] [Google Scholar]
- 83.Ganesan MG, Schwinke DL, Weiner N. Effect of Ca2+ on thermotropic properties of saturated phosphatidylcholine liposomes. Biochim. Biophys. Acta. 1982;686:245–248. doi: 10.1016/0005-2736(82)90119-5. [DOI] [PubMed] [Google Scholar]
- 84.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 85.Meshorer A, Prionas SD, Fajardo LF, Meyer JL, Hahn GM, Martinez AA. The effects of hyperthermia on normal mesenchymal tissues: application of a histologic grading system. Arch. Pathol. Lab. Med. 1983;107:328–334. [PubMed] [Google Scholar]
- 86.Jung H. A generalized concept for cell killing by heat. Radiat. Res. 1986;106:56–72. [PubMed] [Google Scholar]
- 87.Lyons BE, Obana WG, Borcich JK, Kleinman R, Singh D, Britt RH. Chronic histological effects of ultrasonic hyperthermia on normal feline brain tissue. Radiat. Res. 1986;106:234–251. [PubMed] [Google Scholar]
- 88.Borrelli MJ, Thompson LL, Cain CA, Dewey WC. Time-temperature analysis of cell killing of BHK cells heated at temperatures in the range of 43.5 degrees C to 57.0 degrees C. Int. J. Radiat. Oncol. Biol. Phys. 1990;19:389–399. doi: 10.1016/0360-3016(90)90548-x. [DOI] [PubMed] [Google Scholar]
- 89.Jansen W, Haveman J. Histopathological changes in the skin and subcutaneous tissues of mouse legs after treatment with hyperthermia. Pathol. Res. Pract. 1990;186:247–253. doi: 10.1016/S0344-0338(11)80542-X. [DOI] [PubMed] [Google Scholar]
- 90.Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J. Acoust. Soc. Am. 1994;95:1641–1649. doi: 10.1121/1.408550. [DOI] [PubMed] [Google Scholar]
- 91.Chung AH, Jolesz FA, Hynynen K. Thermal dosimetry of a focused ultrasound beam in vivo by magnetic resonance imaging. Med. Phys. 1999;26:2017–2026. doi: 10.1118/1.598707. [DOI] [PubMed] [Google Scholar]
- 92.McDannold N, Hynynen K, Wolf D, Wolf G, Jolesz F. MRI evaluation of thermal ablation of tumors with focused ultrasound. J. Magn. Reson. Imaging. 1998;8:91–100. doi: 10.1002/jmri.1880080119. [DOI] [PubMed] [Google Scholar]
- 93.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 94.Anyarambhatla GR, Needham D. Enhancement of the phase transition permeability of DPPC liposomes by incorporation of MPPC:a new temperature-sensitive liposome for use with mild hyperthermia. J. Liposome Res. 1999;9:491–506. [Google Scholar]
- 95.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 96.Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D, Viglianti BL, Dewhirst MW. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int. J. Hyperth. 2010;26:485–498. doi: 10.3109/02656731003789284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koning GA, Eggermont AM, Lindner LH, ten Hagen TL. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm. Res. 2010;27:1750–1754. doi: 10.1007/s11095-010-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mills JK, Needham D. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. Biochim. Biophys. Acta. 2005;1716:77–96. doi: 10.1016/j.bbamem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Banno B, Ickenstein LM, Chiu GN, Bally MB, Thewalt J, Brief E, Wasan EK. The functional roles of poly(ethylene glycol)-lipid and lysolipid in the drug retention and release from lysolipid-containing thermosensitive liposomes in vitro and in vivo. J. Pharm. Sci. 2010;99:2295–2308. doi: 10.1002/jps.21988. [DOI] [PubMed] [Google Scholar]
- 100.Sandstrom MC, Ickenstein LM, Mayer LD, Edwards K. Effects of lipid segregation and lysolipid dissociation on drug release from thermosensitive liposomes. J. Control. Release. 2005;107:131–142. doi: 10.1016/j.jconrel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Chiu GN, Abraham SA, Ickenstein LM, Ng R, Karlsson G, Edwards K, Wasan EK, Bally MB. Encapsulation of doxorubicin into thermosensitive liposomes via complexation with the transition metal manganese. J. Control. Release. 2005;104:271–288. doi: 10.1016/j.jconrel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 102.Ta T, Convertine AJ, Reyes CR, Stayton PS, Porter TM. Thermosensitive liposomes modified with poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules. 2010;11:1915–1920. doi: 10.1021/bm1004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Smet M, Langereis S, van den Bosch S, Grull H. Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. J. Control. Release. 2010;143:120–127. doi: 10.1016/j.jconrel.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Ward MA, Georgiou TK. Thermoresponsive polymers for biomedical applications. Polymers. 2011;3:1215–1242. [Google Scholar]
- 105.Yin X, Hoffman AS, Stayton PS. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules. 2006;7:1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- 106.Pennadam SS, Firman K, Alexander C, Gorecki DC. Protein-polymer nano-machines: towards synthetic control of biological processes. J. Nanobiotechnol. 2004;2:8. doi: 10.1186/1477-3155-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Polozova A, Winnik FM. Mechanism of the interaction of hydrophobically-modified poly-(N-isopropylacrylamides) with liposomes. Biochim. Biophys. Acta. 1997;1326:213–224. doi: 10.1016/s0005-2736(97)00025-4. [DOI] [PubMed] [Google Scholar]
- 108.Kono K. Thermosensitive polymer-modified liposomes. Adv. Drug Deliv. Rev. 2001;53:307–319. doi: 10.1016/s0169-409x(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 109.Kono K, Henmi A, Yamashita H, Hayashi H, Takagishi T. Improvement of temperature-sensitivity of poly(N-isopropylacrylamide)-modified liposomes. J. Control. Release. 1999;59:63–75. doi: 10.1016/s0168-3659(98)00180-1. [DOI] [PubMed] [Google Scholar]
- 110.Kim JC, Bae SK, Kim JD. Temperature-sensitivity of liposomal lipid bilayers mixed with poly(N-isopropylacrylamide-co-acrylic acid) J. Biochem. 1997;121:15–19. doi: 10.1093/oxfordjournals.jbchem.a021558. [DOI] [PubMed] [Google Scholar]
- 111.Kono K, Hayashi H, Takagishi T. Temperature-sensitive liposomes: liposomes bearing poly (N-isopropylacrylamide) J. Control. Release. 1994;30:69–75. [Google Scholar]
- 112.Hayashi H, Kono K, Takagishi T. Temperature sensitization of liposomes using copolymers of N-isopropylacrylamide. Bioconjug. Chem. 1999;10:412–418. doi: 10.1021/bc980111b. [DOI] [PubMed] [Google Scholar]
- 113.Han HD, Shin BC, Choi HS. Doxorubicin-encapsulated thermosensitive liposomes modified with poly(N-isopropylacrylamide-co-acrylamide): drug release behavior and stability in the presence of serum. Eur. J. Pharm. Biopharm. 2006;62:110–116. doi: 10.1016/j.ejpb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 114.Kono K, Nakai R, Morimoto K, Takagishi T. Thermosensitive polymer-modified liposomes that release contents around physiological temperature. Biochim. Biophys. Acta. 1999;1416:239–250. doi: 10.1016/s0005-2736(98)00226-0. [DOI] [PubMed] [Google Scholar]
- 115.Takei YG, Aoki T, Sanui K, Ogata N, Sakurai Y, Okano T. Dynamic contact angle measurement of temperature-responsive surface properties for poly(N-isopropylacrylamide) grafted surfaces. Macromolecules. 1994;27:6163–6166. [Google Scholar]
- 116.Yakushiji T, Sakai K, Kikuchi A, Aoyagi T, Sakurai Y, Okano T. Graft architectural effects on thermoresponsive wettability changes of poly(N-isopropylacrylamide)-modified surfaces. Langmuir. 1998;14:4657–4662. [Google Scholar]
- 117.Yoshino K, Kadowaki A, Takagishi T, Kono K. Temperature sensitization of li-posomes by use of N-isopropylacrylamide copolymers with varying transition endotherms. Bioconjug. Chem. 2004;15:1102–1109. doi: 10.1021/bc034205j. [DOI] [PubMed] [Google Scholar]
- 118.Kono K, Murakami T, Yoshida T, Haba Y, Kanaoka S, Takagishi T, Aoshima S. Temperature sensitization of liposomes by use of thermosensitive block copolymers synthesized by living cationic polymerization: effect of copolymer chain length. Bioconjug. Chem. 2005;16:1367–1374. doi: 10.1021/bc050004z. [DOI] [PubMed] [Google Scholar]
- 119.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int. J. Hyperth. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 120.Ojugo AS, McSheehy PM, McIntyre DJ, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 121.Kopecek J, Kopeckova P, Minko T, Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 122.Paasonen L, Romberg B, Storm G, Yliperttula M, Urtti A, Hennink WE. Temperature-sensitive poly(N-(2-hydroxypropyl)methacrylamide mono/dilactate)-coated liposomes for triggered contents release. Bioconjug. Chem. 2007;18:2131–2136. doi: 10.1021/bc700245p. [DOI] [PubMed] [Google Scholar]
- 123.Kono K, Ozawa T, Yoshida T, Ozaki F, Ishizaka Y, Maruyama K, Kojima C, Harada A, Aoshima S. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials. 2010;31:7096–7105. doi: 10.1016/j.biomaterials.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 124.Kono K, Nakashima S, Kokuryo D, Aoki I, Shimomoto H, Aoshima S, Maruyama K, Yuba E, Kojima C, Harada A, Ishizaka Y. Multi-functional liposomes having temperature-triggered release and magnetic resonance imaging for tumor-specific chemotherapy. Biomaterials. 2011;32:1387–1395. doi: 10.1016/j.biomaterials.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 125.Chandaroy P, Sen A, Hui SW. Temperature-controlled content release from li-posomes encapsulating Pluronic F127. J. Control. Release. 2001;76:27–37. doi: 10.1016/s0168-3659(01)00429-1. [DOI] [PubMed] [Google Scholar]
- 126.Alexandridis P, Hatton TA. Association of pluronic polyols in aqeuous solutions. In: Pillai V, Shah DO, editors. Dynamic Properties of Interfaces and Associated Structures. Champaign: AOCS Press; 1996. pp. 231–262. [Google Scholar]
- 127.Wells J, Sen A, Hui SW. Localized delivery to CT-26 tumors in mice using thermosensitive liposomes. Int. J. Pharm. 2003;261:105–114. doi: 10.1016/s0378-5173(03)00290-4. [DOI] [PubMed] [Google Scholar]
- 128.Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: extravasation and release of contents in tumor mi-crovascular networks. Int. J. Radiat. Oncol. Biol. Phys. 1996;36:1177–1187. doi: 10.1016/s0360-3016(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 129.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61:3027–3032. [PubMed] [Google Scholar]
- 130.Chen Q, Tong S, Dewhirst MW, Yuan F. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol. Cancer Ther. 2004;3:1311–1317. [PubMed] [Google Scholar]
- 131.Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliv. Rev. 2001;53:285–305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 132.Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, Pruitt AF, Klein A, Case B, Thrall DE, Needham D, Dewhirst MW. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin. Cancer Res. 2006;12:4004–4010. doi: 10.1158/1078-0432.CCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 133.Viglianti BL, Ponce AM, Michelich CR, Yu D, Abraham SA, Sanders L, Yarmolenko PS, Schroeder T, MacFall JR, Barboriak DP, Colvin OM, Bally MB, Dewhirst MW. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn. Reson. Med. 2006;56:1011–1018. doi: 10.1002/mrm.21032. [DOI] [PubMed] [Google Scholar]
- 134.Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, Bally MB, Dewhirst MW. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J. Natl. Cancer Inst. 2007;99:53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- 135.Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int. J. Hyperth. 2004;20:781–802. doi: 10.1080/02656730410001711655. [DOI] [PubMed] [Google Scholar]
- 136.Solazzo SA, Ahmed M, Schor-Bardach R, Yang W, Girnun GD, Rahmanuddin S, Levchenko T, Signoretti S, Spitz DR, Torchilin V, Goldberg SN. Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology. 2010;255:62–74. doi: 10.1148/radiol.09091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti SK, Li KC, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin. Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patel PR, Luk A, Durrani A, Dromi S, Cuesta J, Angstadt M, Dreher MR, Wood BJ, Frenkel V. In vitro and in vivo evaluations of increased effective beam width for heat deposition using a split focus high intensity ultrasound (HIFU) transducer. Int. J. Hyperth. 2008;24:537–549. doi: 10.1080/02656730802064621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O'Neill BE, Li KC. Augmentation of targeted delivery with pulsed high intensity focused ultrasound. Int. J. Hyperth. 2008;24:506–520. doi: 10.1080/02656730802093661. [DOI] [PubMed] [Google Scholar]
- 140.de Smet M, Heijman E, Langereis S, Hijnen NM, Grull H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. J. Control. Release. 2011;150:102–110. doi: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 141.Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, Wood BJ, Dreher MR. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J. Control. Release. 2012;158:487–494. doi: 10.1016/j.jconrel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Negussie AH, Yarmolenko PS, Partanen A, Ranjan A, Jacobs G, Woods D, Bryant H, Thomasson D, Dewhirst MW, Wood BJ, Dreher MR. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int. J. Hyperth. 2011;27:140–155. doi: 10.3109/02656736.2010.528140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Staruch R, Chopra R, Hynynen K. Hyperthermia in bone generated with MR imaging-controlled focused ultrasound: control strategies and drug delivery. Radiology. 2012;263:117–127. doi: 10.1148/radiol.11111189. [DOI] [PubMed] [Google Scholar]