Abstract
Time-resolved electronic absorption, infrared, resonance Raman, and magnetic circular dichroism spectroscopies are applied to characterization of the intermediate that is formed within 20 ps after photodissociation of CO from cytochrome a3 in reduced cytochrome oxidase. This intermediate decays with the same half-life (approximately 1 microseconds) as the post-photodissociation CU+B-CO species previously observed by time-resolved infrared. The transient UV/visible spectra, kinetics, infrared, and Raman evidence suggest that an endogenous ligand is transferred from CuB to Fea3 when CO binds to CuB, forming a cytochrome a3 species with axial ligation that differs from the reduced unliganded enzyme. The time-resolved magnetic circular dichroism results suggest that this transient is high-spin and, therefore, five-coordinate. Thus we infer that the ligand from CuB binds on the distal side of cytochrome a3 and displaces the proximal histidine imidazole. This remarkable mechanistic feature is an additional aspect of the previously proposed "ligand-shuttle" activity of the CuB/Fea3 pair. We speculate as to the identity of the ligand that is transferred between CuB and Fea3 and suggest that the ligand shuttle may play a functional role in redox-linked proton translocation by the enzyme.
Full text
PDF
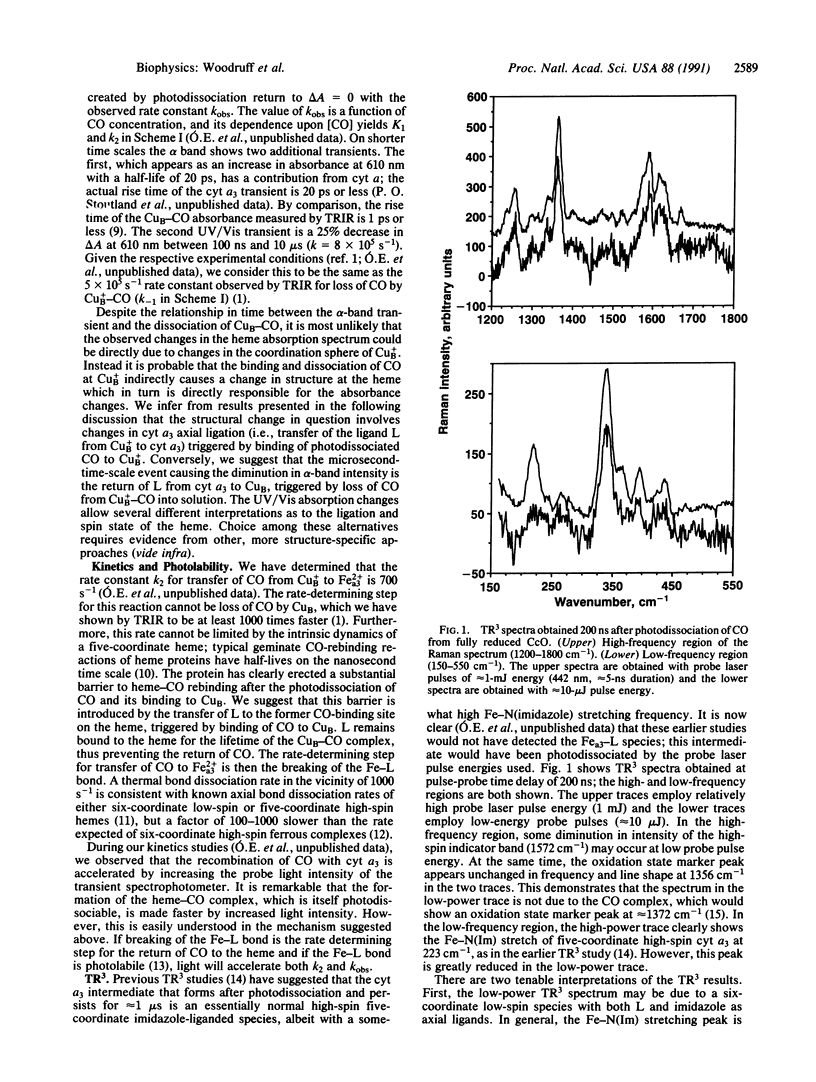
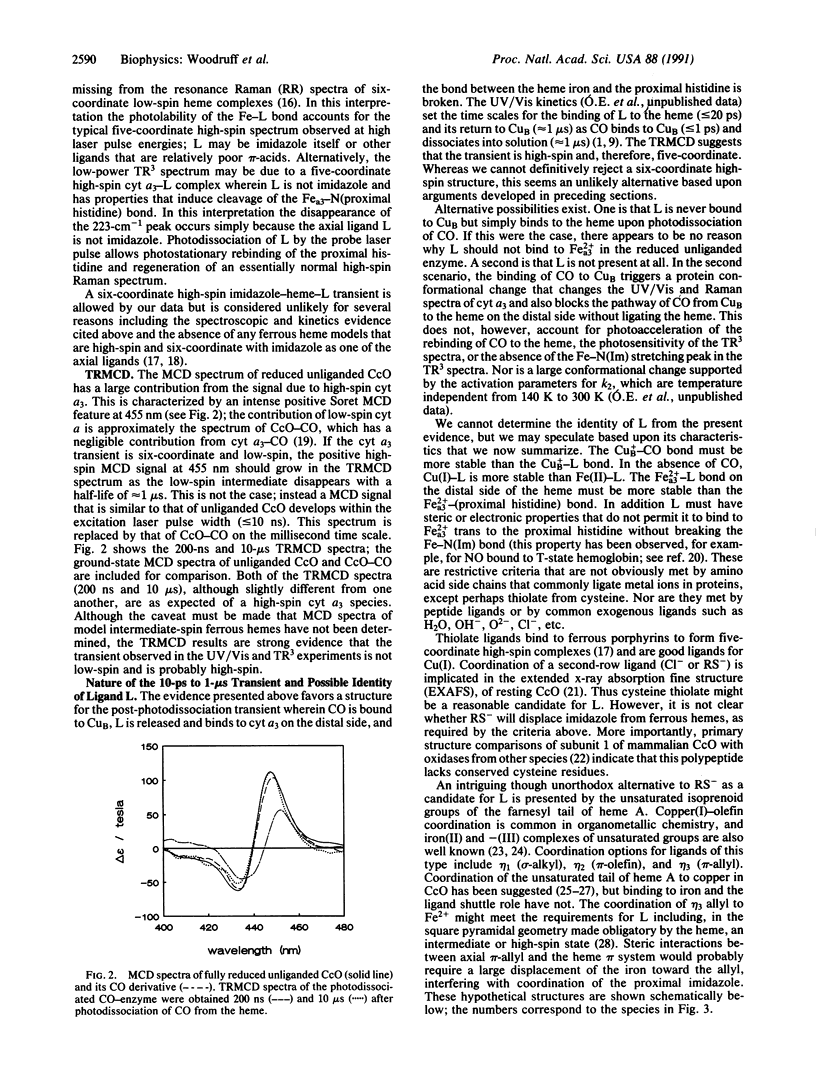
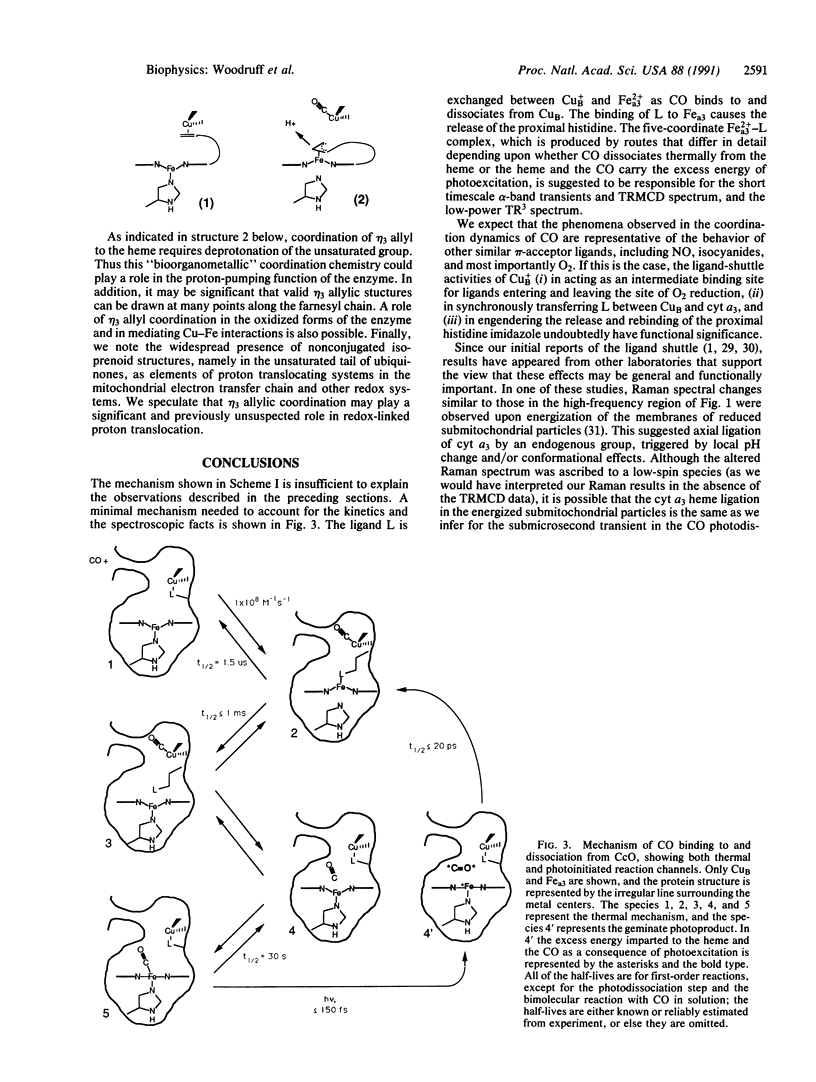

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Brzezinski P., Malmström B. G. The mechanism of electron gating in proton pumping cytochrome c oxidase: the effect of pH and temperature on internal electron transfer. Biochim Biophys Acta. 1987 Oct 29;894(1):29–38. doi: 10.1016/0005-2728(87)90209-x. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Smythe G. A., O'Keeffe D. H., Maskasky J. E., Smith M. I. Heme A of cytochrome c oxicase. Structure and properties: comparisons with hemes B, C, and S and derivatives. J Biol Chem. 1975 Oct 10;250(19):7602–7622. [PubMed] [Google Scholar]
- Chan S. I., Li P. M. Cytochrome c oxidase: understanding nature's design of a proton pump. Biochemistry. 1990 Jan 9;29(1):1–12. doi: 10.1021/bi00453a001. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Mather M. W., Springer P., Hensel S., Buse G. Isolation and partial sequence of the A-protein gene of Thermus thermophilus cytochrome c1aa3. Ann N Y Acad Sci. 1988;550:33–38. doi: 10.1111/j.1749-6632.1988.tb35319.x. [DOI] [PubMed] [Google Scholar]
- Hille R., Olson J. S., Palmer G. Spectral transitions of nitrosyl hemes during ligand binding to hemoglobin. J Biol Chem. 1979 Dec 10;254(23):12110–12120. [PubMed] [Google Scholar]
- Jongeward K. A., Magde D., Taube D. J., Traylor T. G. Picosecond kinetics of cytochromes b5 and c. J Biol Chem. 1988 May 5;263(13):6027–6030. [PubMed] [Google Scholar]
- Li P. M., Gelles J., Chan S. I., Sullivan R. J., Scott R. A. Extended X-ray absorption fine structure of copper in CuA-depleted, p-(hydroxymercuri)benzoate-modified, and native cytochrome c oxidase. Biochemistry. 1987 Apr 21;26(8):2091–2095. doi: 10.1021/bi00382a005. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. Cytochrome oxidase: some unsolved problems and controversial issues. Arch Biochem Biophys. 1990 Aug 1;280(2):233–241. doi: 10.1016/0003-9861(90)90325-s. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray G. B., Copeland R. A., Lee C. P., Spiro T. G. Resonance Raman evidence for low-spin Fe2+ heme a3 in energized cytochrome c oxidase: implications for the inhibition of O2 reduction. Biochemistry. 1990 Apr 3;29(13):3208–3213. doi: 10.1021/bi00465a009. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Ching Y. C., Argade P. V., Rousseau D. L. Photodissociated cytochrome c oxidase: cryotrapped metastable intermediates. Biochemistry. 1988 Apr 5;27(7):2496–2502. doi: 10.1021/bi00407a036. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Caughey W. S. Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction. J Biol Chem. 1990 May 15;265(14):7945–7958. [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]



