Abstract
Epidemiological models of infectious diseases are essential tools in support of risk assessment, surveillance design, and contingency planning in public and animal health. Direct pathogen transmission from host to host is an essential process of each host–pathogen system and respective epidemiological modeling concepts. It is widely accepted that numerous diseases involve indirect transmission (IT) through pathogens shed by infectious hosts to their environment. However, epidemiological models largely do not represent pathogen persistence outside the host explicitly. We hypothesize that this simplification might bias management-related model predictions for disease agents that can persist outside their host for a certain time span. We adapted an individual-based, spatially explicit epidemiological model that can mimic both transmission processes. One version explicitly simulated indirect pathogen transmission through a contaminated environment. The second version simulated direct host-to-host transmission only. We aligned the model variants by the transmission potential per infectious host (i.e., basic reproductive number R0) and the spatial transmission kernel of the infection to allow unbiased comparison of predictions. The quantitative model results are provided for the example of surveillance plans for early detection of foot-and-mouth disease in wild boar, a social host. We applied systematic sampling strategies on the serological status of randomly selected host individuals in both models. We compared between the model variants the time to detection and the area affected prior to detection, measures that strongly influence mitigation costs. Moreover, the ideal sampling strategy to detect the infection in a given time frame was compared between both models. We found the simplified, direct transmission model to underestimate necessary sample size by up to one order of magnitude but to overestimate the area put under control measures. Thus, the model predictions underestimated surveillance efforts but overestimated mitigation costs. We discuss parameterization of IT models and related knowledge gaps. We conclude that the explicit incorporation of IT mechanisms in epidemiological modeling may reward by adapting surveillance and mitigation efforts.
Keywords: indirect transmission, wildlife surveillance, wild boar, FMD, simulation model, contingency planning, environmental transmission, individual-based R0
Introduction
Host–pathogen models play an essential role in epidemiology (1). Epidemiological models are widely used to support risk assessment, surveillance design, and contingency planning (2–5). The driving force of any infectious disease is the transmission of the pathogen to susceptible hosts (6, 7), and its adequate representation in epidemiological models is therefore of crucial importance (8, 9).
The relevance of indirect transmission (IT) without a vector or reservoir, but through contaminated environment, was demonstrated for pathogenic viruses, bacteria, prions, and macroparasites. Examples include highly contagious diseases of wildlife and livestock like foot-and-mouth disease [FMD (10), reviewed in Ref. (11, 12)], classical swine fever [CSF; (13, 14)], bovine tuberculosis [bTB; (15, 16)], brucellosis (17), avian influenza [AIV; (18)], porcine reproductive and respiratory syndrome [PRRS; (19)], and chronic wasting disease [CWD; (20)]. Zoonotics and diseases of man with IT mode include infections with influenza viruses (21), cholera bacteria (22, 23), hantaviruses (24), and Salmonella bacteria (25). For several pathogens, longevity outside the host was investigated under experimental conditions [see, e.g., Ref. (26) for FMD, CSF, BVDV, and PPV; (27) review FMD; (28) review poultry diseases; (29) review CSF; (30) CSF; (31, 32) AIV; (33) Influenza A, B; (34) cholera].
The necessity to incorporate indirect environmental transmission in epidemiological models was already claimed by several authors (20, 35, 36). Despite this fact, only recent modeling studies considered this transmission mode explicitly [(18) AIV; (20) CWD; (37) cholera; (38) brucellosis]. Instead, the majority of epidemiological models follow a century-old postulate by modeling transmission proportionally to both the current number of infectious and the current number of susceptible individuals (39). Using this approach, Breban (40) elaborated the theory of incorporating IT in epidemiological models. It is not always necessary, indeed, to explicitly model all possible routes of pathogen transmission. One may argue that, for example, infectiousness of environmental contamination being short compared to the host infectious period, and then nothing is lost by summarizing everything in increased estimates of direct transmission (DT) (40). However, if empirical evidence suggests a more fundamental role of pathogen transmission through an environmental pathway, then the previous model paradigm does circumvent the explicit consideration of the biologically independent mechanisms. Such mechanisms may respond differently to interference, e.g., to measures or treatments. Summarizing transmission models, hence, do not allow inferences to be made concerning the role of pathogen stages that can persist outside of their host. Interestingly, studies assessing the impact of IT on disease dynamics or disease mitigation are rare [see, e.g., Ref. (18), for example, Ref. (41–43)].
Explicit consideration of an indirect environmental transmission mode may not only be of serious relevance to understand experimental results or the dynamics of host–pathogen systems [e.g., Ref. (40, 43, 44)]. We claim that the explicit inclusion of environmental transmission in models of wildlife diseases may be necessary for adequate predictions in the context of management activities, e.g., surveillance, mitigation, and contingency planning. Further, IT is particularly relevant in socially organized wildlife species, where direct contact is mainly restricted to the social group, and for multi-host pathogens, where direct contact between species is rare (45, 46). We addressed this hypothesis using a parameterized stochastic spatially explicit, individual-based model (SEIBM) designed for studying infectious diseases in landscape-scale populations of social (47–50) and multi-species wildlife hosts (51, 52).
We used the host–pathogen system of FMD in large wild boar (Sus scrofa) populations as a biological example. The wild boar is a social species, widely distributed in many parts of the world. It is the most abundant large mammal species in Europe (53) with increasing geographic range and population densities (53, 54) maintaining a number of infectious diseases (55, 56). FMD is one of the economically most important livestock diseases, which can be devastating in case of an incursion, like in the outbreaks in the UK in 2001 with more than 6.5 million animals culled and economic losses estimated at 5 billion £ (57, 58). FMD affects approximately 70 species of cloven-hoofed domestic and wild animals including wild boar (59). However, epidemiology of FMD in European wildlife populations is largely unknown. The FMD virus (FMDV) can survive outside the host for hours to months, depending on the environmental conditions. In pig slurry, FMDV was detectable for 14 days at 20°C and more than 100 days at 5°C in an experiment by Bøtner and Belsham (26). In a recent outbreak of FMD in wildlife and livestock in Bulgarian Thrace in 2011, wild boars were detected as being virus- and seropositive for FMD, suggesting the potential involvement of the species in FMD epidemics (59, 60).
The objective of this study was to evaluate whether infections with IT may require different surveillance and mitigation efforts than predicted by models based on DT. To this end, we extracted from the SEIBM seroprevalence time series as obtained under surveillance conditions and compared measures important for outbreak mitigation such as time to detection and the minimum sample size needed for disease surveillance.
Materials and Methods
Model Description
Overview
The FMD wildlife model was based on a spatially explicit, stochastic, individual-based demographic model for wild boars (S. scrofa) in a geographic area with suitable habitat. Superimposed is a transmission and disease course model for the FMDV. Epidemiological data on FMDV infections in wild boar are available from the field (59) and laboratory experiments (61, 62). The model is documented following the ODD protocol [Overview, Design, and Details; (63, 64)].
Purpose
The aim of the modeling study was to provide an experimental environment to test the hypothesis that neglect of pathogen persistence outside its host is an inappropriate simplification from the perspective of surveillance or contingency planning. The model was designed to compare the predictions between explicit IT and equivalently parameterized DT. For this purpose, two model variants were constructed only differing by the exclusion (DT) or inclusion (IT) of an environmental transmission model. Hence, the following model documentation is representative for all simulations performed with the submodels of direct and IT substituting each other (see Virus Transmission in Section “Details”).
State Variables and Scales
The model comprises two major components: spatial habitat units and wild boar individuals. All processes take place on a raster map of spatial habitat units. Each cell represents a functional classification of the landscape denoting habitat quality and a scalar value denoting environmental pathogen load. The cells of the model landscape represent 4 km2 (2 km × 2 km), encompassing a boar group’s core home range (65). State variables comprise boar habitat quality of the grid cell. At run time, habitat quality is interpreted as breeding capacity, i.e., the number of female boars that are allowed to have offspring [explicit density regulation; (66)]. Furthermore, an FMDV state of the habitat cell represents environmental virus load and accumulates infection pressure as shed by viremic animals.
State variables of host individuals are the wild boar’s age in weeks [where 1 week represents the approximate FMD infectious period in wild boar; (61, 62)], resulting in age classes: piglet (<8 months ± 6 weeks), sub-adult (<2 years ± 6 weeks), and adult (67). Each host individual has a location, which denotes its home range cell on the raster grid as well as its family group. The individual host animal comprises an epidemiological status (susceptible, infected, or immune after recovery or due to transient maternal antibodies). Sub-adult wild boar may disperse during the dispersal period (i.e., early summer).
Process Overview and Scheduling
The model proceeds in weekly time steps and processes are executed in the following order (see Figure 1): virus release, infection, dispersal of subadults, reproduction, death, and aging. In the first week of each year, mortality probabilities are assigned stochastically to represent annual fluctuations in wild boar living conditions, and female wild boars are assigned to breed or not, according to the carrying capacity of their home range cell.
Figure 1.
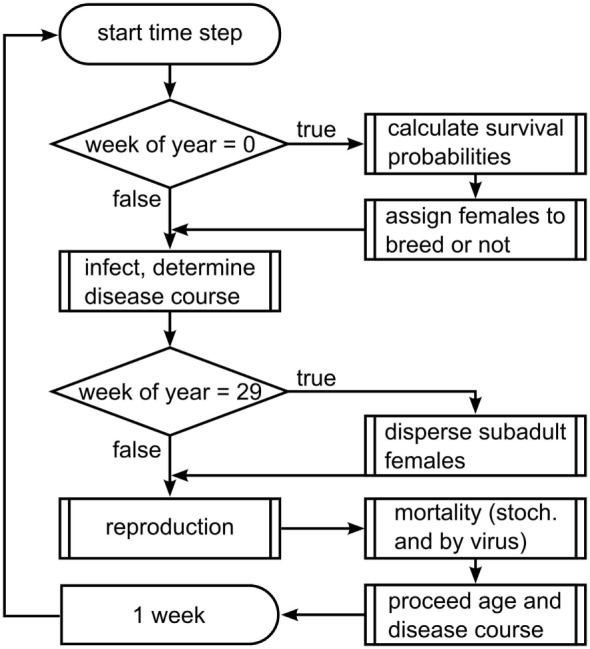
Flow chart of the scheduling of submodels.
Design Concepts
Wild boar population dynamics emerge from individual behavior, defined by age-dependent seasonal reproduction and mortality probabilities and age- and density-dependent dispersal behavior, all including stochasticity. The epidemic course in the DT model emerges from virus transmission within and between groups and wild boar dispersal. The epidemic course in the IT model emerges from virus excretion by infectious hosts, survival dynamics of infectious virus outside the host, contact to infectious doses, and wild boar dispersal.
We included stochasticity by representing demographic, behavioral, and pathogen parameters as probabilities or probability distributions. Annual fluctuations of living conditions are realized by annually varying mortality rates.
Details
Initialization
The model landscape represents 60 km × 60 km of connected wildlife habitat without barriers. The specified extent ensures that the epidemic wave does not reach the edge of the landscape before detection in any simulation. The 900 grid cells were randomly initialized with integer values of local breeding capacity in range 0, …, 3. Breeding capacity was scaled to result in an average wild boar density of 5 hosts/km2 in January, i.e., before the reproductive season (68, 69). The average population size in January was 18,000 individuals.
One boar group was released to each habitat cell, where group size is six times breeding capacity. Initial age distributions were taken from the results of a 100 years model run [see Table S1 in Supplementary Material; (48)].
Input
The applied model setup does not include any external inputs or driving variables.
Submodels
Submodels are described where essential to understand the study. The Supplementary Material contains the complete descriptions of all submodels. A list of parameters with their values and sources is given in Table S2 in Supplementary Material.
Virus Release
The virus was released to the population by infection of five wild boars, randomly selected from the nine most central habitat cells. Release takes place in the sixth year of each simulation (see Simulation Experiments) to allow population dynamics to be established. Introduction was chosen in the season of most likely establishment of the infection according to the increasing population numbers, i.e., at the start of the reproductive season of wild boar.
Disease Course
The disease course following infection is modeled for each infected individual. The infectious period of a host tinf is 1 week. After the infectious period, hosts achieve lifelong immunity. We assumed minimum case lethality (61, 62).
Virus Transmission
Direct Transmission
Direct transmission in the model is a stochastic process. Parameters determine the probability of contracting the infection from an infectious group mate and the probability of contracting the infection from an infectious animal in a neighboring group (3 × 3 neighborhood) during 1 week. For each susceptible animal, the probability of becoming infected accumulates over all infectious animals within the group and in the neighborhood:
| (1) |
where Ii is the number of infected individuals in the home group i and Ij is the number of infected individuals in wild boar groups of the eight neighboring cells j ∈ {1, …, 8}. The model iterates over all individuals and stochastically sets each susceptible individual to infected if a uniformly distributed random number r drawn from U(0, 1) is smaller than Πi of its home cell.
Indirect Transmission
We modeled indirect virus transmission via excretion of infectious material, decay of infectious material by time in the environment (i.e., outside of host individuals), and contact of hosts to infectious material in the environment. At contact, we modeled the effective infection stochastically with the event probability derived from a standard dose–response relation.
The weekly dynamics of the pathogen pool used in the model are based on parameters available from literature on a daily basis. Temporal evolution of the pathogen pool C of each cell is an exponential decay process and the term of pathogen load added to the cell:
| (2) |
with λ being the decay constant λ = ln(2)/T1/2, s being the pathogen added to the cell per time unit, and t being time in weeks. Solve
| (3) |
Within one time step, s is constant. Thus, the pathogen pool can be calculated analytically as
| (4) |
The average available dose for uptake during the weekly time step is
| (5) |
The pathogen source s for a cell is determined from the number of infectious hosts in the cell and in neighboring cells. Hosts in infectious state excrete infectious material with constant daily rate (parameter g; i.e., 7g is the weekly excretion), measured in tissue culture infective dose 50% (TCID50) per day. A host animal spends a portion of daytime (parameter pt) in contact areas, i.e., areas subsequently reached by neighboring animal groups. Accordingly, excreted infectious material is distributed to different cells: g(1 − pt) doses adding to the pool of the home cell of the host, while 1/8g pt doses are added to each of the eight neighboring cells. Therefore, the pathogen added to a cell on a weekly basis is:
| (6) |
Per host, individual contact to infectious material in the environment is determined as constant share (parameter u on a daily basis; i.e., 7u corresponds to the weekly share) of the available dose in its home range cell. The weekly contact dose CD is
| (7) |
Effective infection after contact to a particular dose of infectious material is modeled stochastically as a binomial chance process so that the individual’s weekly probability of becoming infected follows an exponential dose–response relation:
| (8) |
with PTCID50 being the probability of infection after contact to one TCID50 dose. Figure 2 shows the dose–response curve for PTCID50 = 0.003 (70, 71).
Figure 2.
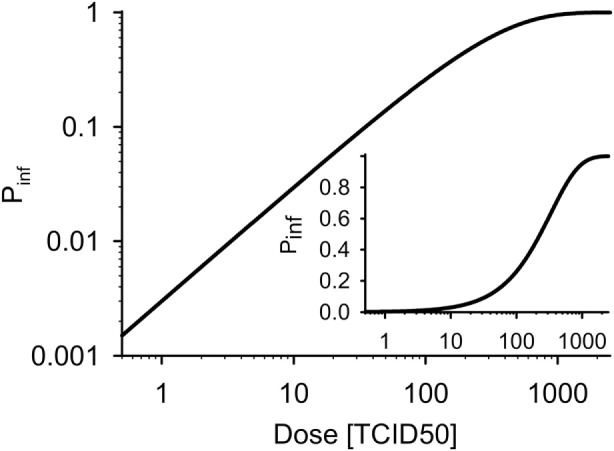
Dose–response curves for wild boar (PTCID50 = 0.003). Inset: linear ordinate.
Parameters, Simulation Experiments, and Analysis
Parameters
A complete list of all parameters with their values and sources is shown in Table S2 in Supplementary Material.
Parameterization of Transmission
In the DT model, the transmission is defined by scaling the two parameters and . In the IT model, an analog to Pinf can be calculated from Eq. 8 and the dose available from one infectious host. To calculate the available dose, Eq. 5 is applied for 1 week after infection (i.e., parameter infectious period) including the excretion into the environment (i.e., s > 0) and for infinite time without further excretion. The total available dose over time is
| (9) |
where C+(t) is the pathogen pool with pathogen excretion starting with C0 = 0 (Eq. 3). C−(t) is the pathogen pool without pathogen excretion for an initial pool equal to the value after the first week [i.e., C0 = C+(1)]. Solve
| (10) |
or, without stressing mathematics, it is the product of added material s and average lifetime of the pathogen in the environment τ = 1/λ.
| (11) |
| (12) |
with newly added pathogen si = 7g(1 − pt) for within-group transmission and se = 7g(1/8)pt for between-group transmission.
By choosing and , both models produce the same basic reproductive number R0 (for validation, see Figure 3).
Figure 3.
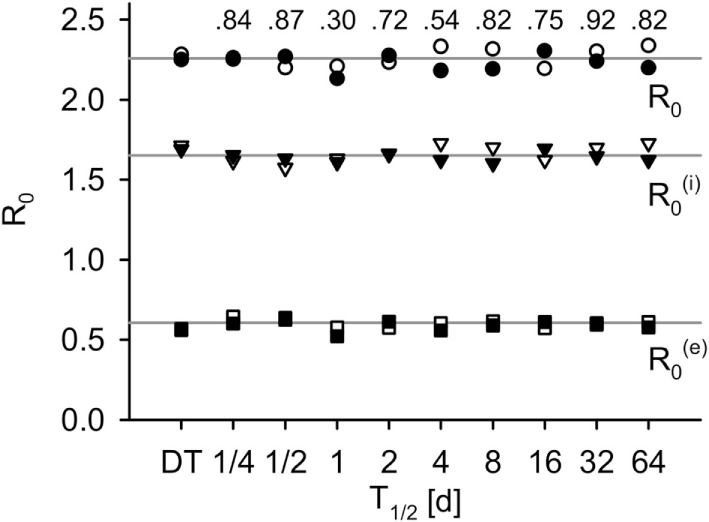
R0 measured in simulations for the DT model and for the IT model with different pathogen half-life, in total, within-group and between-group component (average of 500 simulations). Black: without population dynamics, white: with population dynamics. Lines indicate the theoretical values, and . Numbers indicate p-values of two-sided Mann–Whitney U tests of total R0 without population dynamics against DT model (H0: not different from DT).
Parallel of R0 in DT and IT Models
The DT model was parameterized to mimic the IT model in terms of the basic reproduction number R0. Accounting for transmission within and between groups, R0 was calculated for both scales of spatial transmission separately. This gives the expected number of infections from one case to its group-mates and to the animals of neighboring groups , summing up to .
In the DT model with an infectious period of 1 week, R0 is a linear function of Pinf:
| (13) |
| (14) |
Si is the number of susceptible hosts in the group of the infectious individual. Se is the number of susceptible hosts in its neighboring groups.
We can calculate R0 from the parameters of the IT model using Eqs 11 and 13 for within-group transmission and Eqs 12 and 14 for between-group transmission:
| (15) |
| (16) |
The exponent in Eqs 15 and 16 can be transformed to 7us/λ = 7usT1/2/ln(2). Thus, R0 in the IT model can be kept constant over arbitrary pathogen half-life T1/2 by compensatory scaling of the uptake u, i.e., u × T1/2 is constant (see Figure 4). With pathogen half-life approaching 0, the IT model becomes equivalent to the DT model as pathogen uptake becomes instantaneous.
Figure 4.
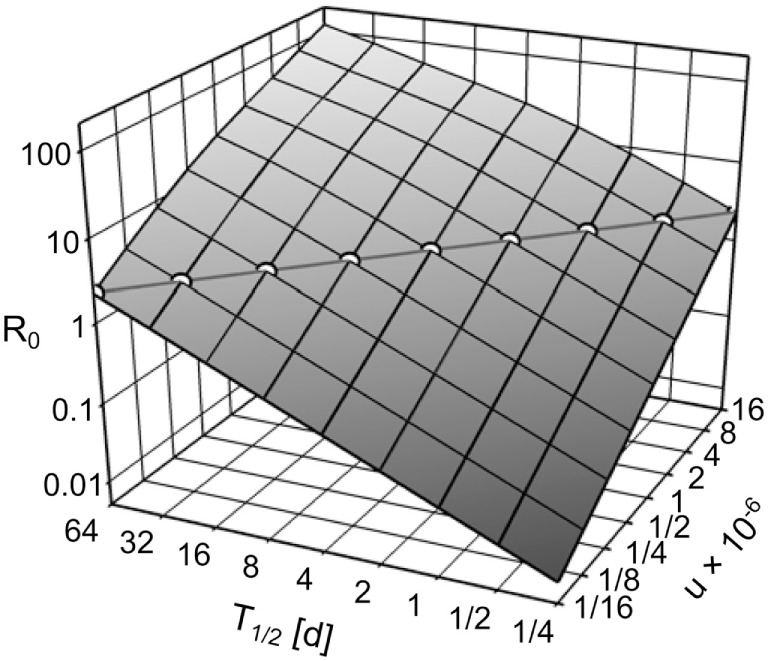
R0, depending on the environmental pathogen half-life T1/2 and on the uptake rate u. Circles along the diagonal line show the realizations of T1/2 and u used in the simulation experiments (u × T1/2 = 4 × 10−6), resulting in R0 = 2.259.
Independent Variables
The primary independent variable was the pathogen half-life T1/2.
Simulation Experiments
We performed simulations for the IT model with environmental pathogen half-life T1/2 ∈ {1/8, 1/4, 1/2, …, 32, 64} days (Figure 4). To keep R0 constant over all IT simulations, we scaled u according to u = 4 × 10−6/T1/2. All parameter combinations resulted in R0 = 2.259. For comparison, we repeated the simulations with the DT model. To achieve the same R0 as the IT model, transmission parameters were scaled to and . Each parameter set was repeated 500 times.
We performed supplementary simulations to measure an individual-based equivalent of R0 (20) in order to verify accordance of the transmission model with the theoretical calculations for the basic reproduction number. This was achieved by allowing only the first disease case per model run to be infectious and count of the number of secondary infection in the initially infected cell and in its neighboring cells. The theoretical calculations neglect population turnover, therefore in the third set of simulations, reproduction and mortality were deactivated from the week of pathogen introduction onward. The model runs for 100 times the pathogen half-life after the initial infection to make sure that the environmental reservoir completely decayed and no secondary infections were missed in the analysis.
Dependent Variables
We recorded seroprevalence time series for each run on a weekly basis as the first order dependent variable. These prevalence time series were then used to determine second-order dependent variables: (1) time to detection for fixed weekly sample sizes, (2) size of the outbreak at the time of detection, and (3) sample sizes needed to detect the disease within an a priori specified time frame. For second-order dependent variables, see Section “Analysis.”
Analysis
We mimicked systematic surveillance on the seroprevalence outcome p of the DT and the IT model deriving the following second-order dependent variables from prevalence time series.
Time to Detection
Given a weekly sample size n and seroprevalence p, the probability to not find any seropositives in a particular week t is
| (17) |
The probability of not finding any seropositives until the given week can be determined as
| (18) |
Hence, the probability to detect the disease until the given week is
| (19) |
For each model run, the first week of PD(t) ≥ 0.95 determines the time of detection. Subtracting the week of virus incursion, this gives the time to detection tD of the individual run. The geometric mean of the distribution over the runs gives the time to detection tD with 95% confidence.
Sample sizes for the underlying surveillance scheme were determined on a monthly basis according to the following equation (72):
| (20) |
with true population size N. Parameters of interest were CL = 95%, p = 5% and 1%. The required sample size was 58.3 per month (14 per week) for p = 5% and 295.6 per month (69 per week) for p = 1%.
Outbreak Size
The area affected by the disease (area of cells infected) before detection Aaff was determined as a measure of the spatial extent of the outbreak.
Required Sample Size
The probability to detect the disease before the given week is calculated according to Eq. 19. This gives the weekly sample size needed to detect the disease in a given time frame t for a given seroprevalence time series:
| (21) |
We calculated the required weekly sample sizes for each model run.
Statistical Analysis
For each simulated value of T1/2 in the IT model, we compared distributions of time to detection tD and weekly sample size needed nD to the outcome of the DT model using the Mann–Whitney U test (H0: distribution with IT not greater than distribution with DT). Similarly, distributions of Aaff were compared to the outcome of the DT model using the Mann–Whitney U test (H0: distribution with IT not less than distribution with DT). Significance was defined as p-value < 0.01.
Results
Basic Reproduction Number
The individual-based equivalent to R0 did not differ systematically from the theoretical calculations (compare points to lines in Figure 3). Differences between IT and DT models were not significant (Mann–Whitney U, without population dynamics: p ≥ 0.3, black fill and numbers in Figure 3; with population dynamics: p ≥ 0.35, white fill in Figure 3).
Seroprevalence
Seroprevalence increased most rapidly in the DT model (Figure 5). The first maximum was reached after less than 40 weeks. In the IT model with equal R0, the increase of seroprevalence slowed down with increasing pathogen half-life (Figure 5, numbers).
Figure 5.
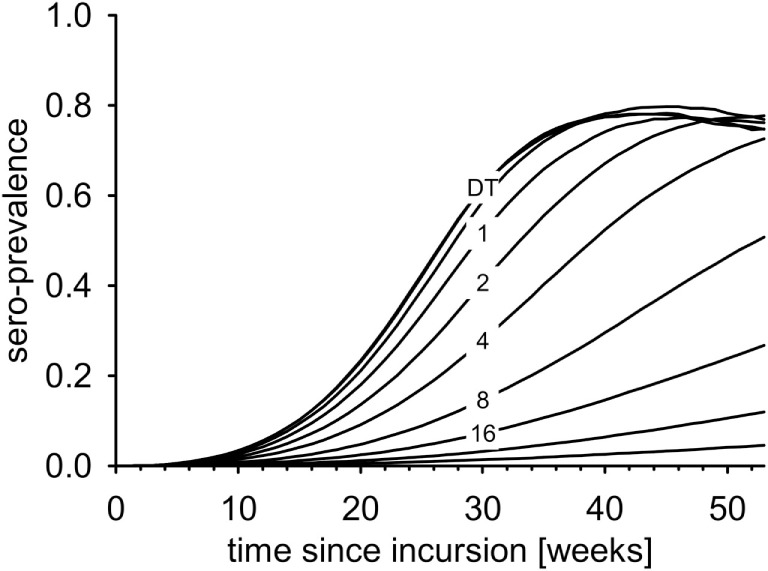
Average seroprevalence over the first year after virus incursion for the DT model and for the IT model with different pathogen half-life (numbers, in days).
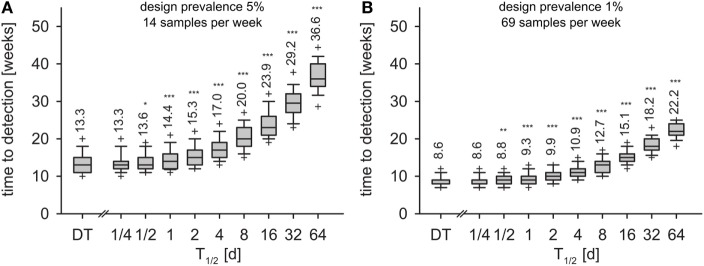
Time to Detection
In the first experiment, i.e., detection of 5% seroprevalence with 95% confidence within one month of sampling, the surveillance design required 14 samples per week. Applying this sample size to the time series of the DT model, the disease was detected 13.3 weeks after incursion with 95% confidence (geometric mean, Figure 6A, left-most box). With the IT model, time to detection depended on the half-life of pathogen T1/2 (Figure 6A). Already at T1/2 > 1 day, detection times were significantly longer than in the DT model (Mann–Whitney U test, p < 0.01). For half-life of 16 days, time to detection increased to 23.9 weeks. When half-life was 64 days (maximum simulated), time to detection more than doubled compared to the DT model and reached 36.6 weeks.
Figure 6.
Time to detection for monthly design prevalence of 5% (A) and 1% (B) for the DT model and for the IT model with different pathogen half-life. Outlier symbols (+) show 5 and 95% quantiles. Text shows geometric means. Asterisks show significance of Mann–Whitney U test against DT model (H0: not greater than DT; *p < 0.05, **p < 0.01, ***p < 0.001).
In the second experiment (detection of 1% seroprevalence with 95% confidence within 1 month of sampling, 69 samples per week), the DT model resulted in detection within 8.6 weeks (Figure 6B, left-most box). Increase of time to detection was significant for T1/2 > 1 day (Figure 6B). T1/2 = 16 days resulted in 15.1 weeks and T1/2 = 64 days in 22.2 weeks to outbreak detection.
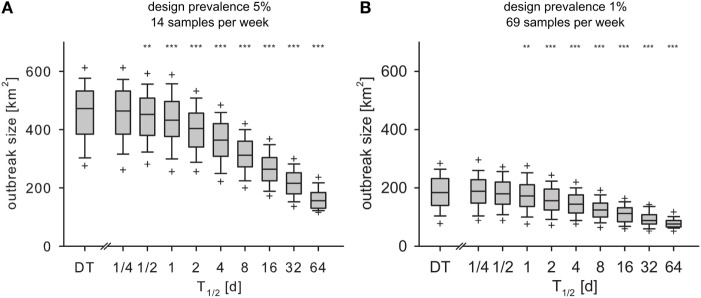
Outbreak Size
In both experiments (design prevalence of 5 and 1%), the spatial extent of the outbreaks Aaff in the IT model decreased significantly compared to the DT model for T1/2 > 1/2 and T1/2 > 1 day, respectively (Figures 7A,B).
Figure 7.
Outbreak size at the time of detection Aaff for monthly design prevalence of 5% (A) and 1% (B) for the DT model and for the IT model with different pathogen half-life. Outlier symbols (+) show 5 and 95% quantile. Asterisks show significance of Mann–Whitney U test against DT model (H0: not less than DT; *p < 0.05, **p < 0.01, ***p < 0.001).
Required Sample Size
We calculated the weekly sample size for detection within 9 weeks with 95% confidence. In the DT model, an average of 69 samples per week was necessary for detection with 95% confidence (Figure 8, left-most box). With the IT model and for pathogen half-life T1/2 > 1/2 day, the required sample size increased exponentially (Figure 8). With T1/2 = 16 days, the required sample size was 406 per week. For the maximum half-life of 64 days, 828 samples per week were required for detection within 9 weeks.
Figure 8.
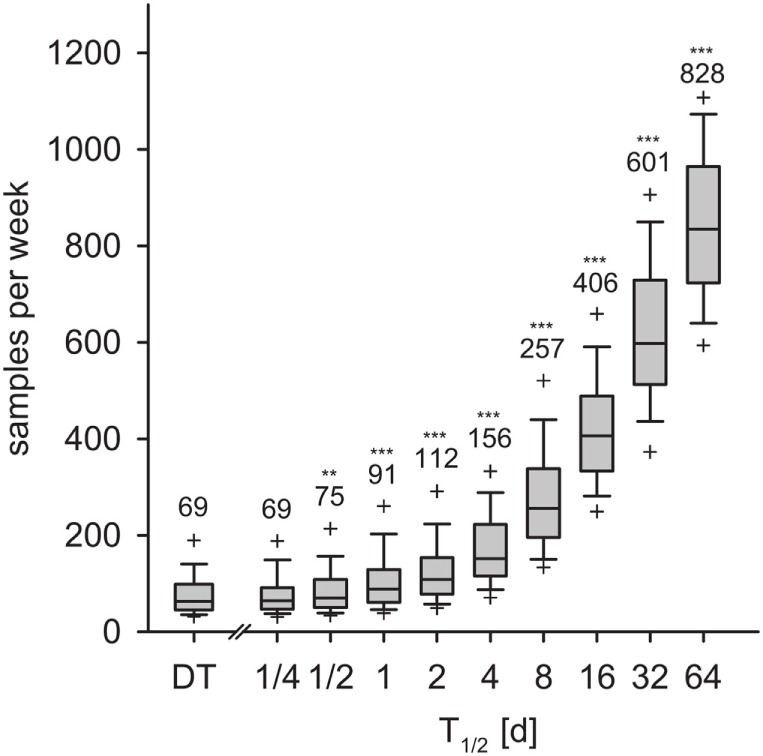
Required sample size to detect the disease within 9 weeks for the DT model and for the IT model, depending on pathogen half-life. Outlier symbols (+) show 5 and 95% quantiles. Text shows the average, i.e., the expected sample size for detection with 95% confidence in 9 weeks. Asterisks show significance of Mann–Whitney U test against DT model (H0: not greater than DT; *p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
For a wildlife host–pathogen system with a social host species, we investigated the consequences of an a priori assumption of direct host-to-host transmission in models for surveillance design. We show that the simplified, DT model underestimated necessary sampling efforts by up to one order of magnitude, but overestimated the outbreak area that would receive control or mitigation measures. Thus, simplifying transmission risk as being proportional to the abundance of infectious and susceptible individuals hindered estimation of the most appropriate surveillance and contingency parameters.
The outcomes of a DT model were compared to results from equivalently parameterized IT models with different environmental pathogen persistence. In abstract models, the DT model is a special version of IT assuming persistence time of infectious pathogen in the environment being 0 (40). Here, we are talking about explicit process models tailored to surveillance design in the field. In the field, direct and IT modes correspond with different biological mechanisms that need adequate representation in a model to allow targeted manipulations (see model documentation). The inclusion of environmental transmission is no longer a matter of model re-parameterization but corresponds to a structural change in the model. In this sense both models, the direct and the IT model become fundamentally different. Our results pinpoint the relevance of a decision on whether environmental transmission needs to be represented in a model or not already prior to making predictions. In the logic of our analysis, however, it was necessary to allow seamless transition between models in spite of two alternative transmission mechanisms involved. We have achieved the virtual equivalence of the models while keeping the transmission potential per infected host unchanged.
Environmental transmission in a disease model might be represented assuming prolonged infectiousness of infected hosts along with prolonged half-life of the pathogen in the environment. Logically then, prolonged pathogen persistence in the environment leads to increased transmission potential of the average infected host in turn changing disease dynamics [see, e.g., Ref. (40)]. Here, we were not interested in theoretical variation of the infectious potential of infected hosts across alternative pathogens. Rather, we were addressing alternative models of the same infection, e.g., a pathogen with R0 established in experiments. This approach was fundamental to the presented comparative assessment of model predictions on a particular disease, i.e., when the DT and the IT version of the model are aligned by the R0 value.
We focused the comparative assessment of the different transmission models on three measurements for two surveillance schemes: (1) time to detection of an outbreak tD, (2) spatial extent of the outbreak Aaff at the time of detection, and (3) the sample size required for outbreak detection within a prescribed time frame.
Indirect transmission slowed down the increase in seroprevalence compared to DT with equal R0. An IT route through the environment results in prolonged infectiousness beyond the infectious period of the host. This causes delayed infections compared to the DT mode, where the infectious period of the hosts limits the time span for new infections. Outbreaks governed by IT may progress much slower and hence less obvious.
Time to detection tD is a central measure to be minimized by a surveillance scheme (73). The underestimated time to detection in the DT model will impede the realized probability of detection of a given surveillance design. Therefore, a surveillance scheme based on the estimates from the DT model [e.g., Ref. (74)] would not meet its aim of detecting an outbreak within the time horizon it was designed for. The pathogen would circulate undetected in the wildlife population longer than expected, therewith increasing the risk of infection of other hosts, e.g., livestock, and the risk of far range spread by transportation or airborne aerosols [e.g., reviewed for FMD in Ref. (75)].
The spatial extent of the outbreak Aaff reflects the area under intervention measures to be implemented after outbreak detection. Aaff was overestimated by the DT model. With IT but equal R0, the disease spread slower than with DT and also has more time to spread due to later detection. Due to the continuous surveillance scheme with accumulation of chance of detection over time, the longer period of undetected pathogen circulation could not completely compensate the slower spread, thus outbreak size at detection was smaller. Control and restriction zones would be oversized if designed on estimates of undetected spread from a DT model. Thereby, the applied measures would be overly expensive and an unnecessary burden for the livestock sector (76).
The DT model underestimated the required sample size per time unit for disease detection within a given time frame. This measure quantifies the effort that is actually necessary to achieve the original aim of the surveillance program, namely, outbreak detection within a prescribed time horizon with given confidence. The extreme increase of the sample size for long pathogen persistence suggests that other methods than testing host individuals for seropositivity may be necessary for the surveillance of certain diseases (77, 78).
Remarkably, time to detection and required sample size differed from the predictions of the DT model for pathogen half-life as short as 1 day. This time span is by almost one order of magnitude shorter than the infectious period of 1 week. This fact emphasizes the relevance of IT, even in absence of extreme pathogen longevity.
The model used in this study has been previously applied for risk assessment (47), for assessment of disease control measures (79), and to contribute to the understanding of wildlife host–pathogen systems (48, 49, 51). In this study, we extend our previous work by the integration of IT and compared surveillance-related predictions of different model versions.
We restricted the model versions to either DT or IT, but did not combine both. Although DT is likely to play a role in most host–pathogen systems with IT mode, we were interested in the differences between the two modes. As the IT model with short pathogen half-life resembles the DT model, we nonetheless examined a continuous transition between the aggregation to DT and the explicit IT model.
Numerous empirical and modeling studies dealt with the quantification of indirect, particularly airborne transmission of FMD and other diseases between domestic livestock holdings [e.g., Ref. (71, 80–84), reviewed for FMD in Ref. (85)], but IT of FMDV in wildlife animals has, to our knowledge, not yet been quantified. We developed a modeling approach that breaks down IT into components that are accessible to experimental measurements, namely pathogen shedding, survival/decay in the environment, contact with infectious material, and infection according to a dose–response relationship. Although some experiments quantified pathogen excretion and secretion of FMDV [reviewed in Ref. (12)] and other pathogens [see, e.g., Ref. (86) for CSF] by domestic animals, knowledge for wildlife is rare (87). The large differences between domestic animal species regarding the shedding rates of FMDV (12, 75) call for further attention to this issue. The same applies for the susceptibility of different species, i.e., the dose–response relation (12, 75). Some quantification for domestic animals can be found in the literature [e.g., Ref. (70, 88) for FMD], but the qualitative relation between dose in the environment and probability of infection is often unclear (89, 90). Survival outside the host has been investigated for several pathogens in animal products and excrements under laboratory conditions (for references, see INTRODUCTION), but further research is necessary for environmental factors that influence pathogen survival. The contact of animals with viral contamination in the environment remains the most uncertain parameter. Here, an inverse parameter fitting approach could aid the quantification. Given assumptions for the other parameters, contact to viral contamination could be estimated from the probability of infection.
Experimental investigations of virus survival outside the host depict striking dependence on temperature and humidity [see, e.g., Ref. (26) for FMD, CSF, BVDV, and PPV; (91) for PRRS virus; (92) for influenza A]. This fact gives rise to seasonal fluctuations of the magnitude of IT. Indeed, for several viral diseases, fluctuations of their transmission were associated with climatic seasonality, partly related to virus survival outside the host [see, e.g., Ref. (92, 93) for influenza viruses; (94) for hepatitis A]. Therefore, climatic factors are expected to play a role in regional variations of the epidemiology of infectious diseases with an IT mode.
With this work, we contribute to the research on IT, which is still in an early stage but attracting increasing attention. Previous work focused on the impact of IT on key figures of host–pathogen systems such as the basic reproductive number (20), disease persistence (41), and formal conditions of relevance for modeling (40).
Our results resemble findings by Wearing et al. (1) and Almberg et al. (20), which show that a neglect of prolonged infectiousness, e.g., through environmental pathogen stages or inappropriate assumptions about the infectious period, may result in an underestimate of R0, if derived from the prevalence growth rate. Reciprocal, in our study prevalence growth rates decreased under IT despite equal reproductive potential (R0). Thus, we transferred the findings regarding the relevance of IT from a theoretical underestimation of infection dynamics, i.e., R0, to the application-oriented context of designing surveillance of any particular wildlife disease, i.e., R0 being fixed.
We conclude that a simplified aggregation of transmission processes, particularly a neglect of environmental pathogen stages, may considerably bias model predictions of the performance of disease surveillance and mitigation strategies. We state that this applies even for pathogens with an average environmental survival time that is comparatively short compared to the infectious period of the host.
Author Contributions
ML and H-HT conceived and designed the experiments; ML performed the experiments and analyzed the data; and ML, SK-S, and H-HT developed the model and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
ML was partially funded by the European Food Safety Agency (EFSA).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fvets.2016.00110/full#supplementary-material.
References
- 1.Wearing HJ, Rohani P, Keeling MJ. Appropriate models for the management of infectious diseases. PLoS Med (2005) 2(7):e174. 10.1371/journal.pmed.0020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thulke HH. Application of recent approaches in modelling for animal health. Prev Vet Med (2011) 99(1):1–3. 10.1016/j.prevetmed.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 3.Bolzoni L, Real L, De Leo G. Transmission heterogeneity and control strategies for infectious disease emergence. PLoS One (2007) 2(8):e747. 10.1371/journal.pone.0000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elderd BD, Dukic VM, Dwyer G. Uncertainty in predictions of disease spread and public health responses to bioterrorism and emerging diseases. Proc Natl Acad Sci U S A (2006) 103(42):15693–7. 10.1073/pnas.0600816103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pineda-Krch M, O’Brien JM, Thunes C, Carpenter TE. Potential impact of introduction of foot-and-mouth disease from wild pigs into commercial livestock premises in California. Am J Vet Res (2010) 71(1):82–8. 10.2460/ajvr.71.1.82 [DOI] [PubMed] [Google Scholar]
- 6.Thulke HH, Selhorst T, Müller T. Pseudorabies virus infections in wild boar: data visualisation as an aid to understanding disease dynamics. Prev Vet Med (2005) 68:35–48. 10.1016/j.prevetmed.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 7.Begon M, Bennett M, Bowers RG, French NP, Hazel SM, Turner J. A clarification of transmission terms in host-microparasite models: numbers, densities and areas. Epidemiol Infect (2002) 129(1):147–53. 10.1017/S0950268802007148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershenbaum A, Stone L, Ostfeld RS, Blaustein L. Modelling transmission of vector-borne pathogens shows complex dynamics when vector feeding sites are limited. PLoS One (2012) 7(5):e36730. 10.1371/journal.pone.0036730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCallum H, Barlow N, Hone J. How should pathogen transmission be modelled? Trends Ecol Evol (2001) 16(6):295–300. 10.1016/S0169-5347(01)02144-9 [DOI] [PubMed] [Google Scholar]
- 10.Eblé PL, de Koeijer A, Bouma A, Stegeman A, Dekker A. Quantification of within- and between-pen transmission of foot-and-mouth disease virus in pigs. Vet Res (2006) 37:647–54. 10.1051/vetres:2006026 [DOI] [PubMed] [Google Scholar]
- 11.Donaldson AI, Alexandersen S, Sørensen JH, Mikkelsen T. Relative risks of the uncontrollable (airborne) spread of FMD by different species. Vet Rec (2001) 148:602–4. 10.1136/vr.148.19.602 [DOI] [PubMed] [Google Scholar]
- 12.Alexandersen S, Zhang Z, Donaldson AI, Garland AJ. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol (2003) 129(1):1–36. 10.1016/S0021-9975(03)00041-0 [DOI] [PubMed] [Google Scholar]
- 13.Dewulf J, Laevens H, Mintiens K, De Kruif A, Koenen F. Airborne transmission of classical swine fever virus under experimental conditions. Vet Rec (2000) 147(26):735–8. 10.1136/vr.147.26.735 [DOI] [PubMed] [Google Scholar]
- 14.Ribbens S, Dewulf J, Koenen F, Maes D, De Kruif A. Evidence of indirect transmission of classical swine fever virus through contacts with people. Vet Rec (2007) 160:687–90. 10.1136/vr.160.20.687 [DOI] [PubMed] [Google Scholar]
- 15.Hutchings MR, Harris S. Quantifying the risks of TB infection to cattle posed by badger excreta. Epidemiol Infect (1999) 122(1):167–73. 10.1017/S0950268898001897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer MV, Waters WR, Whipple DL. Shared feed as a means of deer-to-deer transmission of Mycobacterium bovis. J Wildl Dis (2004) 40(1):87–91. 10.7589/0090-3558-40.1.87 [DOI] [PubMed] [Google Scholar]
- 17.Aune K, Rhyan JC, Russel R, Roffe TJ, Corso B. Environmental persistence of Brucella abortus in the Greater Yellowstone Area. J Wildl Manage (2011) 76(2):253–61. 10.1002/jwmg.274 [DOI] [Google Scholar]
- 18.Rohani P, Breban R, Stallknecht DE, Drake JM. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc Natl Acad Sci U S A (2009) 106(25):10365–9. 10.1073/pnas.0809026106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brockmeier SL, Lager KM. Experimental airborne transmission of porcine reproductive and respiratory syndrome virus and Bordetella bronchiseptica. Vet Microbiol (2002) 89(4):267–75. 10.1016/S0378-1135(02)00204-3 [DOI] [PubMed] [Google Scholar]
- 20.Almberg ES, Cross PC, Johnson CJ, Helsey DM, Richards BJ. Modeling routes of chronic wasting disease transmission: environmental prion persistence promotes deer population decline and extinction. PLoS One (2011) 6(5):e19896. 10.1371/journal.pone.0019896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buxton Bridges C, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis (2003) 37(8):1094–101. 10.1086/378292 [DOI] [PubMed] [Google Scholar]
- 22.Colwell RR, Huq A. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann N Y Acad Sci (1994) 740(1):44–54. 10.1111/j.1749-6632.1994.tb19852.x [DOI] [PubMed] [Google Scholar]
- 23.Kaper JB, Morris JG, Jr, Levine MM. Cholera. Clin Microbiol Rev (1995) 8(1):48–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallio ER, Klingström J, Gustafsson E, Manni T, Vaheri A, Henttonen H, et al. Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. J Gen Virol (2006) 87:2127–34. 10.1099/vir.0.81643-0 [DOI] [PubMed] [Google Scholar]
- 25.Österberg J, Sternberg Lewerin S, Wallgren P. Direct and indirect transmission of four Salmonella enterica serotypes in pigs. Acta Vet Scand (2010) 52:30. 10.1186/1751-0147-52-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bøtner A, Belsham GJ. Virus survival in slurry: analysis of the stability of foot-and-mouth disease, classical swine fever, bovine viral diarrhoea and swine influenza viruses. Vet Microbiol (2012) 157(1–2):41–9. 10.1016/j.vetmic.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 27.Bartley LM, Donnelly CA, Anderson RM. Review of foot-and-mouth disease virus survival in animal excretions and on fomites. Vet Rec (2002) 151:667–9. 10.1136/vr.151.22.667 [DOI] [PubMed] [Google Scholar]
- 28.Soos C, Padilla L, Iglesias A, Gottdenker N, Cruz B, don M, et al. Comparison of pathogens in broiler and backyard chikens on the Gal Pagos Islands: implications for transmission to wildlife. Auk (2008) 125(2):445–55. 10.1525/auk.2008.06235 [DOI] [Google Scholar]
- 29.Edwards S. Survival and inactivation of classical swine fever virus. Vet Microbiol (2000) 73:175–81. 10.1016/S0378-1135(00)00138-3 [DOI] [PubMed] [Google Scholar]
- 30.Weesendorp E, Stegeman A, Loeffen WLA. Survival of classical swine fever virus at various temperatures in faeces and urine derived from experimentally infected pigs. Vet Microbiol (2008) 132(3–4):249–59. 10.1016/j.vetmic.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 31.Tiwari A, Patnayak DP, Chander Y, Parsad M, Goyal SM. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis (2006) 50:284–7. 10.1637/7453-101205R.1 [DOI] [PubMed] [Google Scholar]
- 32.Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis (2007) 50:285–9. 10.1637/7636-042806R.1 [DOI] [PubMed] [Google Scholar]
- 33.Bean B, Moore M, Sterner B, Peterson LR, Gerding DN, Balfour HH, Jr. Survival of influenza viruses on environmental surfaces. J Infect Dis (1982) 146(1):47–51. 10.1093/infdis/146.1.47 [DOI] [PubMed] [Google Scholar]
- 34.Xu HS, Roberts N, Singleton FL, Attwell RW, Grimes DJ, Colwell RR. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol (1982) 8:313–23. 10.1007/BF02010671 [DOI] [PubMed] [Google Scholar]
- 35.Caley P, Marion G, Hutchings MR. Assessment of transmission rates and routes, and the implications for management. In: Delahay RJ, Smith GC, Hutchings MR, editors. Management of Disease in Wild Mammals. Tokyo, Berlin, Heidelberg, New York: Springer; (2009). p. 31–52. [Google Scholar]
- 36.Marcé C, Ezanno P, Weber MF, Seegers H, Pfeiffer DU, Fourichon C. Invited review: modeling within-herd transmission of Mycobacterium avium subspecies paratuberculosis in dairy cattle: a review. J Dairy Sci (2010) 93(10):4455–70. 10.3168/jds.2010-3139 [DOI] [PubMed] [Google Scholar]
- 37.Joh RI, Wang H, Weiss H, Weitz JS. Dynamics of indirectly transmitted infectious diseases with immunological threshold. Bull Math Biol (2008) 71(4):845–62. 10.1007/s11538-008-9384-4 [DOI] [PubMed] [Google Scholar]
- 38.Aïnseba B, Benosman C, Magal P. A model for ovine brucellosis incorporating direct and indirect transmission. J Biol Dyn (2010) 4(1):2–11. 10.1080/17513750903171688 [DOI] [PubMed] [Google Scholar]
- 39.Eisenberg JNS, Brookhart MA, Rice G, Brown M, Colford JM, Jr. Disease transmission models for public health decision making: analysis of epidemic and endemic conditions caused by waterborne pathogens. Environ Health Perspect (2002) 110(8):783–90. 10.1289/ehp.02110783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breban R. Role of environmental persistence in pathogen transmission: a mathematical modeling approach. J Math Biol (2013) 66:535–46. 10.1007/s00285-012-0520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauvage F, Langlais M, Yoccoz NG, Pontier D. Modelling hantavirus in fluctuating populations of bank voles: the role of indirect transmission on virus persistence. J Anim Ecol (2003) 72(1):1–13. 10.1046/j.1365-2656.2003.00675.x [DOI] [Google Scholar]
- 42.Ivanek R, Lahodny GE. From the bench to modeling – R0 at the interface between empirical and theoretical approaches in epidemiology of environmentally-transmitted infectious diseases. Prev Vet Med (2015) 118(2–3):196–206. 10.1016/j.prevetmed.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 43.Fuller E, Elderd BD, Dwyer G. Pathogen persistence in the environment and insect-baculovirus interactions: disease-density thresholds, epidemic burnout, and insect outbreaks. Am Nat (2012) 179(3):E70–96. 10.1086/664488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Jong MCM, Hagenaars TJ, Eble P. Transmission of FMDV within and between species: quantification and comparisons. Proceedings of ISVEE Maastricht Wageningen: Wageningen Academic Publishers (2012). 47 p. [Google Scholar]
- 45.Woolhouse MEJ, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science (2001) 292:1109–12. 10.1126/science.1059026 [DOI] [PubMed] [Google Scholar]
- 46.Pedersen AB, Altizer S, Poss M, Cunningham AA, Nunn CL. Patterns of host specificity and transmission among parasites of wild primates. Int J Parasitol (2005) 35:647–57. 10.1016/j.ijpara.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 47.Fernández N, Kramer-Schadt S, Thulke HH. Viability and risk assessment in species restoration: planning reintroductions for the wild boar, a potential disease reservoir. Ecol Soc (2006) 11(1):6. 10.5751/ES-01560-110106 [DOI] [Google Scholar]
- 48.Kramer-Schadt S, Fernández N, Eisinger D, Grimm V, Thulke HH. Individual variations in infectiousness explain long-term disease persistence in wildlife populations. Oikos (2009) 118:199–208. 10.1111/j.1600-0706.2008.16582.x [DOI] [Google Scholar]
- 49.Lange M, Kramer-Schadt S, Blome S, Beer M, Thulke HH. Disease severity declines over time after a wild boar population has been affected by classical swine fever – legend or actual epidemiological process? Prev Vet Med (2012) 106:185–95. 10.1016/j.prevetmed.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 50.Alban L, Andersen MM, Asferg T, Boklund A, Fernández N, Goldbach SG, et al. Classical Swine Fever and Wild Boar in Denmark: A Risk Analysis. Copenhagen: Danish Institute for Food and Veterinary Research; (2005). 118 p. [Google Scholar]
- 51.Lange M. Spatial spread and maintenance of foot-and-mouth disease virus infections in wildlife populations of Thrace region applying epidemiological modelling. Scientific report submitted to EFSA. EFSA Supporting Publications. EFSA J (2012) 9(4):EN–264, 28. 10.2903/sp.efsa.2012.EN-264 [DOI] [Google Scholar]
- 52.Dhollander S, Depner K, Belsham GJ, Salman M, Willgert K, Thulke HH, et al. Evaluating the potential spread and maintenance of foot-and-mouth disease virus in wildlife; general principles and application to a specific scenario in Thrace. Transbound Emerg Dis (2016) 63(2):165–174. 10.1111/tbed.12240 [DOI] [PubMed] [Google Scholar]
- 53.Artois M, Depner KR, Guberti V, Hars J, Rossi S, Rutili D. Classical swine fever (hog cholera) in wild boar in Europe. Rev Sci Tech (2002) 21(2):287–303. 10.20506/rst.21.2.1332 [DOI] [PubMed] [Google Scholar]
- 54.Saez-Royuela DC, Telleria JL. The increased population of the wild boar (Sus scrofa) in Europe. Mamm Rev (1986) 16(2):97–101. 10.1111/j.1365-2907.1986.tb00027.x [DOI] [Google Scholar]
- 55.Martin C, Pastoret PP, Brochier B, Humblet MF, Saegerman C. A survey of the transmission of infectious diseases/infections between wild and domestic ungulates in Europe. Vet Res (2011) 42:70. 10.1186/1297-9716-42-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz-Fons F, Segalés J, Gortázar C. A review of viral diseases of the European wild boar: effects of population dynamics and reservoir rôle. Vet J (2008) 176(2):158–69. 10.1016/j.tvjl.2007.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson D, Muriel P, Russell D, Osborne P, Bromley A, Rowland M, et al. Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Rev Sci Tech (2002) 21(3):675–87. 10.20506/rst.21.3.1353 [DOI] [PubMed] [Google Scholar]
- 58.Haydon DT, Kao RR, Kitching RP. The UK foot-and-mouth disease outbreak – the aftermath. Nat Rev Microbiol (2004) 2:675–81. 10.1038/nrmicro960 [DOI] [PubMed] [Google Scholar]
- 59.EFSA AHAW Panel. Scientific opinion on foot and mouth disease in Thrace. EFSA J (2012) 10(4):2635. 10.2903/j.efsa.2012.2635 [DOI] [Google Scholar]
- 60.Alexandrov T, Stefanov D, Kamenov P, Miteva A, Khomenko S, Sumption KJ, et al. Surveillance of foot-and-mouth disease (FMD) in susceptible wildlife and domestic ungulates in Southeast of Bulgaria following a FMD case in wild boar. Vet Microbiol (2013) 166(1–2):84–90. 10.1016/j.vetmic.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 61.Breithaupt A, Depner K, Haas B, Axandrov T, Polihronova L, Georgiev G, et al. Experimental infection of wild boar and domestic pigs with a foot and mouth disease virus strain detected in the southeast of Bulgaria at the end of 2010. Vet Microbiol (2012) 159(1–2):33–9. 10.1016/j.vetmic.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 62.Mohamed F, Swafford S, Petrowski H, Bracht A, Schmit B, Fabian A, et al. Foot-and-mouth disease in feral swine: susceptibility and transmission. Transbound Emerg Dis (2011) 58(4):358–71. 10.1111/j.1865-1682.2011.01213.x [DOI] [PubMed] [Google Scholar]
- 63.Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V, Giske J, et al. A standard protocol for describing individual-based and agent-based models. Ecol Modell (2006) 192:115–26. 10.1016/j.ecolmodel.2006.04.023 [DOI] [Google Scholar]
- 64.Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J, Railsback SF. The ODD protocol: a review and first update. Ecol Modell (2010) 221(23):2760–8. 10.1016/j.ecolmodel.2010.08.019 [DOI] [Google Scholar]
- 65.Leaper R, Massei G, Gorman ML, Aspinall R. The feasibility of reintroducing wild boar (Sus scrofa) to Scotland. Mamm Rev (1999) 29(4):239–58. 10.1046/j.1365-2907.1999.2940239.x [DOI] [Google Scholar]
- 66.Jedrzejewska B, Jedrzejewski W, Bunevich AN, Milkowski L, Krasinski ZA. Factors shaping population densities and increase rates of ungulates in Bialowieza Primeval Forest (Poland and Belarus) in the 19th and 20th centuries. Acta Theriol (1997) 42(4):399–451. 10.4098/AT.arch.97-39 [DOI] [Google Scholar]
- 67.Keuling O, Stier N, Roth M. Annual and seasonal space use of different age classes of female wild boar Sus scrofa L. Eur J Wildl Res (2008) 54(3):403–12. 10.1007/s10344-007-0157-4 [DOI] [Google Scholar]
- 68.Sodeikat G, Pohlmeyer K. Escape movements of family groups of wild boar Sus scrofa influenced by drive hunts in Lower Saxony, Germany. Wildl Biol (2003) 9(Suppl 1):43–9. [Google Scholar]
- 69.EFSA Panel on Animal Health and Welfare. Control and eradication of Classic Swine Fever in wild boar. EFSA J (2009) 7(1):932, 199. 10.2903/j.efsa.2009.932 [DOI] [Google Scholar]
- 70.French NP, Kelly L, Jones R, Clancy D. Dose-response relationships for foot and mouth disease in cattle and sheep. Epidemiol Infect (2002) 128:325–32. 10.1017/S0950268801006446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garner MG, Hess GD, Yang X. An integrated modelling approach to assess the risk of wind-borne spread of foot-and-mouth disease virus from infected premises. Environ Model Assess (2006) 11:195–207. 10.1007/s10666-005-9023-5 [DOI] [Google Scholar]
- 72.Cannon RM. Sense and sensitivity – designing surveys based on an imperfect test. Prev Vet Med (2001) 49(3–4):141–63. 10.1016/S0167-5877(01)00184-2 [DOI] [PubMed] [Google Scholar]
- 73.Dürr S, Zu Dohna H, Di Labio E, Carpenter TE, Doherr MG. Evaluation of control and surveillance strategies for classical swine fever using a simulation model. Prev Vet Med (2013) 108(1):73–84. 10.1016/j.prevetmed.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 74.Hone J, Pech RP, Yipo P. Estimation of the dynamics and rate of transmission of classical swine fever (hog cholera) in wild pigs. Epidemiol Infect (1992) 108:377–86. 10.1017/S0950268800049840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitching RP, Hutber AM, Thrusfield MV. A review of foot-and-mouth disease with special consideration for the clinical and epidemiological factors relevant to predictive modelling of the disease. Vet J (2005) 169:197–209. 10.1016/j.tvjl.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 76.Morris RS, Sanson RL, Stern MW, Stevenson M, Wilesmith JW. Decision-support tools for foot and mouth disease control. Rev Sci Tech (2002) 21(3):557–67. 10.20506/rst.21.3.1363 [DOI] [PubMed] [Google Scholar]
- 77.Mouchantat S, Haas B, Böhle W, Globig A, Lange E, Mettenleiter TC, et al. Proof of principle: non-invasive sampling for early detection of foot-and-mouth disease virus infection in wild boar using a rope-in-a-bait sampling technique. Vet Microbiol (2014) 172(1–2):329–33. 10.1016/j.vetmic.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 78.de Carvalho Ferreira HC, Weesendorp E, Quak S, Stegeman JA, Loeffen WLA. Suitability of faeces and tissue samples as a basis for non-invasive sampling for African swine fever in wild boar. Vet Microbiol (2014) 172(3–4):449–54. 10.1016/j.vetmic.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 79.Lange M, Kramer-Schadt S, Thulke HH. Efficiency of spatio-temporal vaccination regimes in wildlife populations under different viral constraints. Vet Res (2012) 43(1):37. 10.1186/1297-9716-43-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donaldson AI, Alexandersen S. Predicting the spread of foot and mouth disease by airborne virus. Rev Sci Tech (2002) 21(3):569–75. 10.20506/rst.21.3.1362 [DOI] [PubMed] [Google Scholar]
- 81.Hagenaars TJ, Dekker A, de Jong MC, Eble PL. Estimation of foot and mouth disease transmission parameters, using outbreak data and transmission experiments. Rev Sci Tech (2011) 30(2):467–81. [PubMed] [Google Scholar]
- 82.Sørensen JH, Mackay DKJ, Jensen C, Donaldson AI. An integrated model to predict the atmospheric spread of foot-and-mouth disease virus. Epidemiol Infect (2000) 124:577–90. 10.1017/S095026889900401X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikkelsen T, Alexandersen S, Astrup P, Champion HJ, Donaldson AI, Dunkerley FN, et al. Investigation of airborne foot-and-mouth disease virus transmission during low-wind conditions in the early phase of the UK 2001 epidemic. Atmos Chem Phys (2003) 3(6):2101–10. 10.5194/acp-3-2101-2003 [DOI] [Google Scholar]
- 84.Hess GD, Garner MG, Yang XA. Sensitivity analysis of an integrated modelling approach to assess the risk of wind-borne spread of foot-and-mouth disease virus from infected premises. Environ Model Assess (2008) 13:209–20. 10.1007/s10666-007-9097-3 [DOI] [Google Scholar]
- 85.Donaldson AI, Sellers RF, Lacey J. Quantitative data on airborne foot-and-mouth disease virus: its production, carriage and deposition [and discussion]. Philos Trans R Soc B Biol Sci (1983) 302(1111):529–34. 10.1098/rstb.1983.0072 [DOI] [Google Scholar]
- 86.Weesendorp E, Stegeman A, Loeffen WLA. Dynamics of virus excretion via different routes in pigs experimentally infected with classical swine fever virus strains of high, moderate or low virulence. Vet Microbiol (2009) 133(1–2):9–22. 10.1016/j.vetmic.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 87.Thomson GR, Vosloo W, Bastos ADS. Foot and mouth disease in wildlife. Virus Res (2003) 91:145–61. 10.1016/S0168-1702(02)00263-0 [DOI] [PubMed] [Google Scholar]
- 88.Alexandersen S, Brotherhood I, Donaldson AI. Natural aerosol transmission of foot-and-mouth disease virus to pigs: minimal infectious dose for strain O1 Lausanne. Epidemiol Infect (2002) 128:301–12. 10.1017/S0950268801006501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coleman M, Marks H. Topics in dose-response modeling. J Food Prot (1998) 61(11):1550–9. [DOI] [PubMed] [Google Scholar]
- 90.Buchanan RL, Smith JL, Long W. Microbial risk assessment: dose-response relations and risk characterization. Int J Food Microbiol (2000) 58(3):159–72. 10.1016/S0168-1605(00)00270-1 [DOI] [PubMed] [Google Scholar]
- 91.Hermann J, Hoff S, Munoz-Zanzi C, Yoon KJ, Roof A, Burkhardt A, et al. Effect of temperature and relative humidity on the stability of infectious porcine reproductive and respiratory syndrome virus in aerosols. Vet Res (2007) 38:81–93. 10.1051/vetres:2006044 [DOI] [PubMed] [Google Scholar]
- 92.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog (2007) 3(10):e151. 10.1371/journal.ppat.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steel J, Palese P, Lowen AC. Transmission of a 2009 pandemic influenza virus shows a sensitivity to temperature and humidity similar to that of an H3N2 seasonal strain. J Virol (2011) 85(3):1400–2. 10.1128/JVI.02186-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mbithi JM, Springthorpe VS, Sattar SA. Effect of relative humidity and air temperature on survival of hepatitis A virus on environmental surfaces. Appl Environ Microbiol (1991) 57(5):1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.