Abstract
Purpose
Addition of rolapitant to standard antiemetic therapy improved protection against chemotherapy-induced nausea and vomiting (CINV) in phase 3 trials of patients receiving highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC). Here, we assessed the impact of CINV on the daily lives of patients receiving HEC or MEC using the Functional Living Index-Emesis (FLIE).
Methods
In three double-blind phase 3 studies, patients receiving HEC or MEC were randomized 1:1 to receive oral rolapitant 180 mg or placebo prior to chemotherapy plus 5-hydroxytryptamine type 3 receptor antagonist and dexamethasone therapy. Patients completed the FLIE questionnaire on day 6 of cycle 1. Endpoints included FLIE total score, nausea and vomiting domain scores, and the proportion of patients with no impact on daily life (total score >108 [range 18–126]). We performed a prespecified analysis of the MEC/anthracycline-cyclophosphamide (AC) study and a post hoc analysis of two pooled cisplatin-based HEC studies.
Results
In the pooled HEC studies, rolapitant significantly improved the FLIE total score (114.5 vs 109.3, p < 0.001), nausea score (55.3 vs 53.5, p < 0.05), and vomiting score (59.2 vs 55.8, p < 0.001) versus control; similar results were observed in the MEC/AC study for FLIE total score (112.7 vs 108.6, p < 0.001), nausea score (54.1 vs 52.3, p < 0.05), and vomiting score (58.6 vs 56.3, p < 0.001). A higher proportion of patients reported no impact on daily life with rolapitant than with control in the MEC/AC study (73.2 vs 67.4, p = 0.027).
Conclusions
Compared with control, rolapitant improved quality of life in patients receiving HEC or MEC.
Keywords: Rolapitant, Neurokinin-1 receptor antagonist, Quality of life, Functional Living Index-Emesis, Chemotherapy-induced nausea and vomiting (CINV), Antiemetic
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a debilitating side effect of anticancer treatments associated with substantial reductions in health-related quality of life (QoL) [1–3]. Without antiemetic prophylaxis, >90 % of patients receiving highly emetogenic chemotherapy (HEC) and 30–90 % of patients receiving moderately emetogenic chemotherapy (MEC) experience emesis [4]. Prior to the adoption of current antiemetic prophylaxis strategies, patients consistently ranked nausea and vomiting among the most distressing side effects of chemotherapy, ranking these side effects worse than fatigue, depression, or the impact of chemotherapy on their family or partner [5, 6]. The risk of developing CINV is primarily related to the emetogenic potential of the chemotherapeutic regimen, although factors including chemotherapy dose, number of treatment cycles, and patient age and sex may affect the risk [7].
Uncontrolled CINV can result in malnutrition, dehydration, and electrolyte imbalance [8] and has been associated with the development of anticipatory nausea and vomiting with the potential to compromise adherence to treatment [8, 9]. Fortunately, substantial progress in our understanding of the neural mechanisms underlying CINV has led to the development of highly effective therapeutic approaches [9]. The use of 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs), particularly when combined with dexamethasone, has been found to effectively reduce CINV in patients treated with HEC or MEC, although CINV control was greatest in the acute phase (≤24 h following chemotherapy) [4]; symptoms may persist in the delayed phase (>24–120 h after chemotherapy administration) in up to 50 % of patients [10–13]. The addition of a neurokinin-1 (NK-1) RA to a 5-HT3 RA and dexamethasone has improved CINV control throughout the delayed phase, and this three-drug combination is the recommended antiemetic prophylaxis regimen for patients receiving HEC and select patients with CINV risk factors receiving MEC [4]. Despite substantial progress, complete control of CINV, particularly nausea, in the delayed phase has proven difficult [14, 15]. Furthermore, delayed-phase CINV has been found to have a greater impact on QoL than CINV experienced only in the acute phase [1, 2].
The Functional Living Index-Emesis (FLIE) is a validated questionnaire designed to specifically address the impact of CINV on patients’ daily lives [16, 17]. Validation of the FLIE instrument is a sensitive and reliable measure of the impact of CINV on QoL [16, 17]. Furthermore, use of the FLIE has demonstrated that beyond a reduction in the physical symptoms of nausea and vomiting, effective antiemetic prophylaxis reduces the negative impact of CINV on patients’ daily lives [8, 18].
Rolapitant (VARUBI®, TESARO, Inc.) is a highly selective, long-acting NK-1 RA indicated in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy [19]. Results of two phase 3 cisplatin-based HEC studies (HEC-1 and HEC-2) [20] and one phase 3 MEC or anthracycline and cyclophosphamide (AC)-based chemotherapy study [21] demonstrated that a single oral dose of rolapitant (180 mg) taken before chemotherapy with a 5-HT3 RA and dexamethasone resulted in superior protection against CINV in the delayed phase compared with placebo plus a 5-HT3 RA and dexamethasone. Here, we report on the impact of CINV on patients’ daily lives using the FLIE in an analysis of the rolapitant phase 3 trials.
Methods
Three global, multicenter, randomized, parallel-group, double-blind phase 3 studies (clinicaltrials.gov identifiers NCT01500213, NCT01499849, and NCT01500226) were conducted in compliance with the International Conference on Harmonisation and Good Clinical Practice (GCP) guidelines [20, 21]. Detailed methods and results regarding the incidence of CINV were previously reported [20, 21].
Patients were required to be aged ≥18 years and to have a Karnofsky performance score ≥60, a predicted life expectancy of ≥4 months, and adequate bone marrow, kidney, and liver function. Eligibility for the HEC studies required patients to be naive to cisplatin and scheduled to receive their first course of cisplatin ≥60 mg/m2. Eligibility for the MEC/AC study required patients to be naive to MEC and HEC and scheduled to receive their first course of one or more of the following agents alone or in combination: intravenous cyclophosphamide (<1500 mg/m2), doxorubicin, epirubicin, carboplatin, idarubicin, ifosfamide, irinotecan, daunorubicin, and/or intravenous cytarabine (>1 g/m2). The study protocol prespecified that ≥50 % of patients enrolled in the MEC/AC study would receive AC-based chemotherapy [21].
Prior to receiving study drug, patients were not permitted to use any of the following medications: 5-HT3 RAs, phenothiazines, benzamides, domperidone, cannabinoids, NK-1 RAs, or benzodiazepines within 48 h; palonosetron within 7 days; or systemic corticosteroids or sedative antihistamines (e.g., dimenhydrinate or diphenhydramine) within 72 h of day 1, with the exception of premedication for chemotherapy (e.g., taxanes). Patients with established nausea, vomiting, or both were permitted to take rescue medication; those who used rescue medication were considered failures for the efficacy endpoint of complete response (no emesis and no use of rescue medication).
Treatment
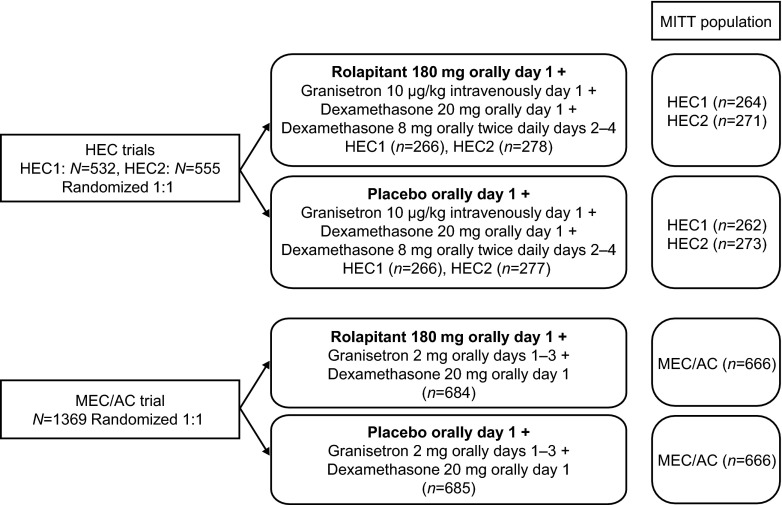
Patients were stratified by sex and randomly assigned (1:1) to either rolapitant 180 mg (rolapitant group) or placebo (control group). All patients received 5-HT3 RA and dexamethasone therapy (Fig. 1). Treatment blinding was maintained throughout the studies.
Fig. 1.
Treatment schema. In the HEC and MEC/AC trials, patients received a single oral dose of rolapitant 180 mg or matching placebo capsules 1–2 h prior to the administration of chemotherapy. In the HEC trials, all patients received granisetron 10 μg/kg intravenously and dexamethasone 20 mg orally prior to the administration of chemotherapy on day 1; dexamethasone 8 mg orally twice daily was administered on days 2–4. In the MEC/AC trial, all patients received granisetron 2 mg orally plus dexamethasone 20 mg orally prior to chemotherapy administration on day 1; granisetron 2 mg orally was administered once daily on days 2 and 3. In the HEC and MEC/AC trials, patients receiving taxanes were administered dexamethasone according to the package insert. AC anthracycline and cyclophosphamide-based chemotherapy, HEC highly emetogenic chemotherapy, MEC moderately emetogenic chemotherapy, MITT modified intent-to-treat
QoL assessment
The 18-item FLIE questionnaire [16, 17] comprises two domains (nausea and vomiting); in each domain, the patient answers one question on the magnitude of the symptom (nausea or vomiting) followed by eight questions to assess the impact of the symptom on the patient’s ability to enjoy meals/liquids, prepare meals/do household tasks, perform daily functions, and engage in usual recreation/leisure activities, as well as his/her willingness to spend time with family and friends and the extent to which symptoms have caused personal hardship [16, 17]. Patient responses are recorded using a seven-point visual analogue scale (VAS), with higher scores corresponding to a higher QoL and an average item score >6 defined as no impact of CINV on daily life. Patients completed the 18-item FLIE questionnaire on day 6 (5-day recall) during cycle 1. Responses to each of nine questions on the nausea domain and nine questions on the vomiting domain were marked on a 100-mm (seven-point) VAS. FLIE responses were summed to determine the total score (range 18–126) and the nausea and vomiting domain scores (range 9–63). Higher scores indicate a greater ability to maintain daily life. The proportion of patients with no impact on daily life, defined as an average item score >6 on the seven-point VAS (FLIE total score >108) [16, 17], was also determined. If ≥5 questions were answered in a domain, the subtotal score for the domain was calculated as . If more than four of nine questions were missed in a domain, subtotal and total scores were considered missing [17].
Statistical analysis
Data were analyzed for all randomized patients in the modified intent-to-treat (MITT) population (patients who received at least one dose of study drug at a GCP-compliant site) who had a valid FLIE questionnaire obtained on day 6. Prespecified analyses were performed for the MEC/AC study, and post hoc analyses were performed for the pooled HEC studies. p values <0.05 were considered statistically significant and were not adjusted for multiplicity. Treatment comparisons were performed between the FLIE total score and nausea and vomiting domain scores using an analysis of variance model with terms for sex and study (pooled HEC analysis) in the model. For the endpoint of no impact on daily life, treatment comparisons were performed using the Cochran-Mantel-Haenszel χ 2 test stratified by sex and study (pooled HEC analysis). These analyses were not prospectively powered to demonstrate statistical significance.
Results
Patients
The MITT populations comprised 1070 patients in the pooled HEC studies and 1332 patients in the MEC/AC study. Randomized and MITT populations are detailed in Fig. 1. Patient baseline characteristics were balanced between rolapitant and control groups (Table 1). The majority of patients in the cisplatin-based HEC studies were male (63 %), and the most commonly diagnosed malignancy was lung cancer (44 %). In the MEC/AC study, most patients were female (80 %) and breast cancer was the most commonly diagnosed malignancy (63 %); patients received a range of chemotherapies, with 53 % receiving AC-based chemotherapy (now classified as HEC [4]), 30 % receiving carboplatin-based chemotherapy, and 17 % receiving other agents.
Table 1.
Baseline demographics and patient characteristics of the MITT population
| Pooled HEC trials | MEC/AC trial | |||
|---|---|---|---|---|
| Characteristic | Rolapitant | Control | Rolapitant | Control |
| (n = 535) | (n = 535) | (n = 666) | (n = 666) | |
| Age, years | ||||
| Median (min, max) | 59 (21, 86) | 59 (18, 90) | 58 (22, 86) | 56 (22, 88) |
| Sex, n (%) | ||||
| Male | 337 (63) | 336 (63) | 135 (20) | 130 (20) |
| Female | 198 (37) | 199 (37) | 531 (80) | 536 (80) |
| Race, n (%) | ||||
| White | 404 (76) | 391 (73) | 508 (76) | 512 (77) |
| Black/African American | 4 (1) | 6 (1) | 24 (4) | 29 (4) |
| Asian | 95 (18) | 97 (18) | 92 (14) | 84 (13) |
| Other/multiracial/unknown | 32 (6) | 41 (8) | 42 (6) | 41 (6) |
| Geographic region, n (%) | ||||
| North America | 59 (11) | 64 (12) | 216 (32) | 229 (34) |
| Central/South America | 65 (12) | 71 (13) | 31 (5) | 32 (5) |
| Europe | 306 (57) | 299 (56) | 312 (47) | 299 (45) |
| Asia/South Africa | 105 (20) | 101 (19) | 107 (16) | 106 (16) |
| Alcohol consumption (drinks/week), n (%)a | ||||
| 0 to ≤5 | 493 (93)b | 483 (91)c | 636 (96)d | 625 (94) |
| >5 | 37 (7)b | 50 (9)c | 29 (4)d | 41 (6) |
| Primary tumor site, n (%) | ||||
| Breast | 12 (2) | 26 (5) | 417 (63) | 428 (64) |
| Colon/rectum | 2 (<1) | 0 (0) | 38 (6) | 27 (4) |
| Head and neck | 97 (18) | 100 (19) | 5 (1) | 6 (1) |
| Lung | 235 (44) | 232 (43) | 102 (15) | 118 (18) |
| Ovary | 33 (6) | 31 (6) | 33 (5) | 23 (3) |
| Stomach | 34 (6) | 34 (6) | 8 (1) | 9 (1) |
| Uterine | 11 (2) | 15 (3) | 14 (2) | 18 (3) |
| Other tumors | 111 (21) | 97 (18) | 49 (7) | 37 (6) |
AC anthracycline and cyclophosphamide-based chemotherapy, HEC highly emetogenic chemotherapy, MEC moderately emetogenic chemotherapy, MITT modified intent-to-treat
aBased on patient self-reports
b n = 530
c n = 533
d n = 665
Quality of life
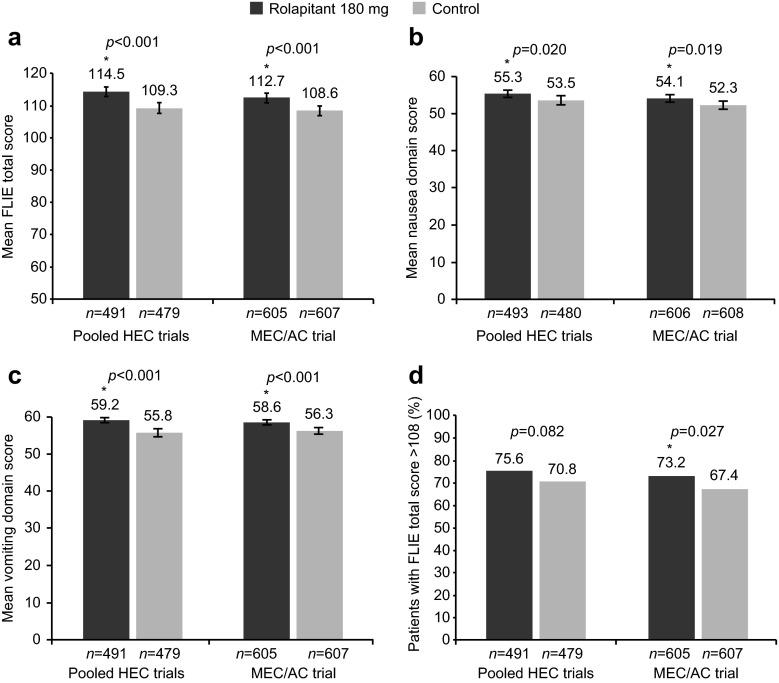
Patients in the rolapitant group reported a significantly higher FLIE total score than patients in the control group in the pooled HEC studies (mean difference = 5.2; 95 % confidence interval [CI] 2.6–7.9; p < 0.001) and in the MEC/AC study (mean difference = 4.1; 95 % CI 1.7–6.5; p < 0.001; Fig. 2a). A significant improvement in the nausea domain score was observed with rolapitant versus control in the pooled HEC studies (mean difference = 1.8; 95 % CI 0.2–3.4; p = 0.020) and the MEC/AC study (mean difference = 1.8; 95 % CI 0.3–3.3; p = 0.019; Fig. 2b), as well as in the vomiting domain score in the pooled HEC studies (mean difference = 3.4; 95 % CI 2.1–4.7; p < 0.001) and the MEC/AC study (mean difference = 2.3; 95 % CI 1.1–3.4; p < 0.001; Fig. 2c).
Fig. 2.
FLIE outcome assessments. a Mean FLIE total score (range 18–126), b mean nausea domain score, c mean vomiting domain score (range 9–63), and d percentage of patients with no impact on daily life. Error bars represent 95 % CIs. Presented n values are based on patients who had a valid FLIE questionnaire obtained on day 6. *Statistically significant difference versus control. AC anthracycline and cyclophosphamide-based chemotherapy, CI confidence interval, FLIE Functional Living Index-Emesis, HEC highly emetogenic chemotherapy, MEC moderately emetogenic chemotherapy
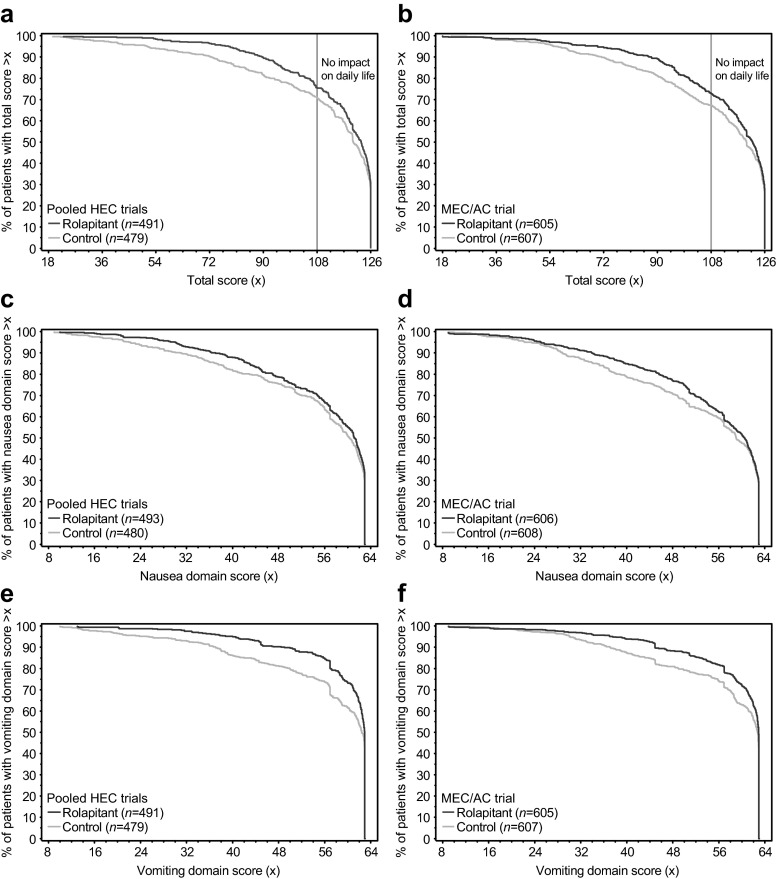
The proportion of patients with no impact on daily life, defined as FLIE total score >108 (i.e., average item score >6 on the seven-point VAS), was greater in the rolapitant group than in the control group in the pooled HEC studies and MEC/AC study. Differences reached statistical significance in the MEC/AC study (odds ratio [OR] = 1.3; 95 % CI 1.0–1.7; p = 0.027), as previously reported [21], but not in the pooled HEC studies (OR = 1.3; 95 % CI 1.0–1.7; p = 0.082; Fig. 2d). Figure 3 shows the distribution of FLIE scores for patients in the rolapitant and control groups in the pooled HEC studies and the MEC/AC study. Separation of the curves illustrates that a higher percentage of patients in the rolapitant group than in the control group achieved an improved FLIE total score, nausea domain score, and vomiting domain score in both the pooled HEC studies and the MEC/AC study.
Fig. 3.
Distribution of FLIE scores: The x-axes represent a range of FLIE scores. a Percentage of patients achieving FLIE total score >x in the pooled HEC studies and b the MEC/AC study. c Percentage of patients achieving nausea domain score >x in the pooled HEC studies and d the MEC/AC study. e Percentage of patients achieving vomiting domain score >x in the pooled HEC studies and f the MEC/AC study. Presented n values are based on patients who had a valid FLIE questionnaire obtained on day 6. AC anthracycline and cyclophosphamide-based chemotherapy, FLIE Functional Living Index-Emesis, HEC highly emetogenic chemotherapy, MEC moderately emetogenic chemotherapy
Discussion
Results from the FLIE questionnaire, a validated patient-reported outcome instrument [16, 17], demonstrated the efficacy of rolapitant in reducing the negative impact of CINV on patients’ daily lives. Significant improvements were observed in the FLIE total score and the nausea and vomiting domain scores for patients in the rolapitant versus control group. In these analyses, improvements relative to control were similar for patients in the HEC and MEC/AC studies. Improvements observed on the nausea domain with rolapitant were notable, as nausea, especially in the delayed phase, has proven more difficult to control than vomiting [14, 15].
Improvements in patients’ daily lives have also been reported in studies of netupitant and aprepitant, two other NK-1 RAs, using the FLIE questionnaire [10, 12, 18, 22, 23]. Recently, in a study of patients receiving AC-based chemotherapy, the proportion of patients reporting no impact on daily life overall and on the nausea and vomiting domains was significantly greater with a netupitant regimen compared with control [22]. Significant improvements over standard therapy in the proportion of patients reporting no impact on daily life have also been observed in studies of aprepitant [10, 12, 18, 23]. Whereas improvements on the nausea domain have been observed with an aprepitant regimen over control in patients receiving cisplatin [18], this has not been achieved in some aprepitant studies of patients receiving AC-based chemotherapy [12, 23].
In the rolapitant studies, the proportion of patients reporting no impact of CINV on daily life overall was significantly greater in the rolapitant group than in the control group in the MEC/AC study (p = 0.027) [21], but significance on this measure was not reached in the pooled HEC studies (p = 0.082). It is unknown whether differences in the chemotherapy received (i.e., cisplatin vs MEC/AC) and/or other CINV risk factors (i.e., proportion of male vs female patients) may have contributed to differences on this measure. It should be considered that patients had access to rescue medications throughout the 5-day postchemotherapy period in these trials; rescue medication use would mitigate symptoms of nausea and vomiting, rendering improvements in QoL more difficult to detect.
The National Comprehensive Cancer Network evidence-based guidelines recommend NK-1 RA triple therapy to protect against CINV in patients with cancer receiving HEC and in selected patients receiving MEC. Rolapitant has recently been endorsed (category 1 recommendation) as part of an effective NK-1 RA triple regimen [4]. In phase 3 studies, complete response in the delayed phase (the primary endpoint) in the rolapitant and control groups was 71 and 60 %, respectively, in the pooled HEC studies (p < 0.001) and 71 and 62 %, respectively, in the MEC/AC study (p < 0.001) [20, 21]. Despite a relative risk reduction of 21–36 % with rolapitant relative to control in patients administered HEC or MEC, a portion of rolapitant-treated patients still experienced delayed-phase CINV [20, 21]. Since rolapitant blocks >90 % of NK-1 receptors in the brain for at least 5 days [24], it is likely that other biochemical pathways contribute to delayed-phase CINV. This underscores the challenge of providing every patient with complete CINV protection throughout the delayed phase.
Although even greater control of nausea and vomiting may be possible, the progress made in protecting against CINV and improving QoL for patients receiving anticancer treatment is noteworthy. In a study reported in 1983 by Coates et al. [5], vomiting was ranked by patients as the first and nausea as the second most troublesome chemotherapy-associated side effects. In contrast, in a 2014 study [25], Russo et al. reported that nonphysical side effects such as “affects my family or partner” or “constantly tired” were ranked as the most severe patient-perceived side effects; in addition, vomiting was no longer considered a major issue, although nausea remained among the top 10 patient-perceived side effects.
Nausea, even in the absence of vomiting, has been shown to adversely affect dietary intake and contribute to malnutrition and weight loss [26]. Patients’ nutritional status, as assessed by the Patient-Generated Subjective Global Assessment, a tool that identifies malnutrition in ambulatory oncology patients, was shown to predict the magnitude of change in QoL [27]. Future studies may consider using this tool in combination with the FLIE to explore the impact of rolapitant on nutritional status and assess the contribution that changes in nutritional status may have on QoL outcomes.
A limitation of this study is that, although the FLIE questionnaire has been validated and used widely in QoL research associated with antiemetic therapies, the questions are brief and do not address other side effects that patients may experience (e.g., fatigue). In addition, as patients did not complete the FLIE questionnaire at baseline, improvement over time within the same arm could not be evaluated, although it should be noted that the goal of this clinical trial was to assess between-group differences in mean FLIE scores (nausea, vomiting, and total) and in the proportion of patients achieving a total FLIE score >108.
In summary, the addition of rolapitant, which provided superior protection against delayed-phase CINV compared with control in phase 3 studies [20, 21], reduced the negative impact of CINV on the QoL of patients receiving HEC or MEC. These results support the benefit of adding rolapitant to a 5-HT3 RA and dexamethasone to improve the daily lives of patients with cancer who are administered HEC or MEC.
Acknowledgments
We thank the patients, clinical investigators, and site personnel who participated in these studies. Medical writing and editorial assistance, funded by TESARO, Inc., were provided by Michelle Yochum, PhD, Joanna Bloom, PhD, Dena McWain, and Joshua Safran of Infusion Communications.
Compliance with ethical standards
Conflict of interest
IS has served on an advisory board for TESARO. BR has received honoraria for speaking engagements from MSD and Roche Malaysia; he has been a consultant and has had travel/accommodations paid for by MSD and TESARO. DP is an employee of TESARO. SA has received contracting fees from TESARO during these studies and outside the submitted work. LS has served as a consultant for Eisai, Helsinn, and TESARO. All remaining authors have declared no conflicts of interest.
Funding
The phase 3 studies were designed through a collaboration of academic researchers and the study sponsor, TESARO, Inc. Study data were collected by clinical investigators, and trial conducts were monitored by TESARO, Inc. Statistical analyses were managed by TESARO, Inc., according to a predefined statistical plan. This manuscript was developed with full author participation and assistance from a medical writer in accordance with Good Publication Practice 3 (GPP3) and International Committee of Medical Journal Editors (ICMJE) guidelines. All authors had access to full data and analyses presented in this manuscript.
References
- 1.Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 2.Ballatori E, Roila F, Ruggeri B, et al. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2007;15:179–185. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- 3.Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15:497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Antiemesis. Version 2.2015
- 5.Coates A, Abraham S, Kaye SB, et al. On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 6.de Boer-Dennert M, de Wit R, Schmitz PI, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer. 1997;76:1055–1061. doi: 10.1038/bjc.1997.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 8.Ballatori E, Roila F. Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. Health Qual Life Outcomes. 2003;1:46. doi: 10.1186/1477-7525-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249–262. doi: 10.1007/s40265-013-0019-1. [DOI] [PubMed] [Google Scholar]
- 10.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin. The Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 11.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 12.Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–2830. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer. 2010;18:423–431. doi: 10.1007/s00520-009-0680-9. [DOI] [PubMed] [Google Scholar]
- 14.Escobar Y, Cajaraville G, Virizuela JA, et al. Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer. 2015;23:2833–2840. doi: 10.1007/s00520-015-2809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majem M, Moreno ME, Calvo N, et al. Perception of healthcare providers versus patient reported incidence of chemotherapy-induced nausea and vomiting after the addition of NK-1 receptor antagonists. Support Care Cancer. 2011;19:1983–1990. doi: 10.1007/s00520-010-1042-3. [DOI] [PubMed] [Google Scholar]
- 16.Lindley CM, Hirsch JD, O'Neill CV, Transau MC, Gilbert CS, Osterhaus JT. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res. 1992;1:331–340. doi: 10.1007/BF00434947. [DOI] [PubMed] [Google Scholar]
- 17.Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients’ daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer. 2003;11:522–527. doi: 10.1007/s00520-003-0482-4. [DOI] [PubMed] [Google Scholar]
- 18.Martin AR, Carides AD, Pearson JD, et al. Functional relevance of antiemetic control. Experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. Eur J Cancer. 2003;39:1395–1401. doi: 10.1016/S0959-8049(03)00299-5. [DOI] [PubMed] [Google Scholar]
- 19.VARUBI® (rolapitant) tablets, for oral use [prescribing information] (2015)
- 20.Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079–1089. doi: 10.1016/S1470-2045(15)00035-2. [DOI] [PubMed] [Google Scholar]
- 21.Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol. 2015;16:1071–1078. doi: 10.1016/S1470-2045(15)00034-0. [DOI] [PubMed] [Google Scholar]
- 22.Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328–1333. doi: 10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo W, Mo FK, Suen JJ, et al. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Res Treat. 2009;113:529–535. doi: 10.1007/s10549-008-9957-9. [DOI] [PubMed] [Google Scholar]
- 24.Poma A, Christensen J, Davis J, Kansra V, Martell RE, Hedley ML. Phase 1 positron emission tomography (PET) study of the receptor occupancy of rolapitant, a novel NK-1 receptor antagonist. J Clin Oncol. 2014;32:e20690. [Google Scholar]
- 25.Russo S, Cinausero M, Gerratana L, et al. Factors affecting patient’s perception of anticancer treatments side-effects: an observational study. Expert Opin Drug Saf. 2014;13:139–150. doi: 10.1517/14740338.2013.830710. [DOI] [PubMed] [Google Scholar]
- 26.Davidson W, Teleni L, Muller J, et al. Malnutrition and chemotherapy-induced nausea and vomiting: implications for practice. Oncol Nurs Forum. 2012;39:E340–E345. doi: 10.1188/12.ONF.E340-E345. [DOI] [PubMed] [Google Scholar]
- 27.Isenring E, Bauer J, Capra S. The scored patient-generated subjective global assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur J Clin Nutr. 2003;57:305–309. doi: 10.1038/sj.ejcn.1601552. [DOI] [PubMed] [Google Scholar]