Abstract
Stimulus eccentricity affects visual processing in multiple ways. Performance on a visual task is often better when target stimuli are presented near or at the fovea compared to the retinal periphery. For instance, reaction times and error rates are often reported to increase with increasing eccentricity. Such findings have been interpreted as purely visual, reflecting neurophysiological differences in central and peripheral vision, as well as attentional, reflecting a central bias in the allocation of attentional resources. Other findings indicate that in some cases, information from the periphery is preferentially processed. Specifically, it has been suggested that visual processing speed increases with increasing stimulus eccentricity, and that this positive correlation is reduced, but not eliminated, when the amount of cortex activated by a stimulus is kept constant by magnifying peripheral stimuli (Carrasco et al., 2003). In this study, we investigated effects of eccentricity on visual attentional capacity with and without magnification, using computational modeling based on Bundesen's (1990) theory of visual attention. Our results suggest a general decrease in attentional capacity with increasing stimulus eccentricity, irrespective of magnification. We discuss these results in relation to the physiology of the visual system, the use of different paradigms for investigating visual perception across the visual field, and the use of different stimulus materials (e.g. Gabor patches vs. letters).
Keywords: Spatial attention, Eccentricity, Cortical magnification, Visual processing speed, Peripheral vision
Highlights
-
•
Visual capacity for letter stimuli decreases toward the visual periphery.
-
•
The decrease in visual capacity is irrespective of cortical magnification.
-
•
Peripheral performance might depend on the specific stimuli and task demands.
1. Introduction
When attention is unguided (i.e., when stimulus location is uncued), many studies indicate that the same stimulus is processed both faster and with higher accuracy when presented at the fovea compared with the visual periphery. For instance, increasing the eccentricity of a visual target has been reported to increase reaction times and error rates (Carrasco et al., 1995, Wolfe et al., 1998), deteriorate performance in object recognition tasks (Juttner and Rentschler, 2000), attenuate the ability to quickly process emotional facial expressions (Bayle et al., 2011), and even to make it more difficult to discriminate attractive and unattractive faces (Guo et al., 2011). However, other findings indicate that some aspects of visual processing are enhanced in the peripheral visual field. In a seminal study, using a forced-choice orientation discrimination task, Carrasco and colleagues found that processing speed increases with increasing eccentricity (Carrasco et al., 2003).
While the existence of eccentricity effects is well-established, their nature is debated. Some argue that the effects are purely visual, suggesting that they can be explained by the structural layout of the human visual system (Anstis, 1998, Carrasco and Frieder, 1997). Others argue that attentional mechanisms are involved as well, holding that an anatomical explanation alone cannot adequately account for the effects observed (Wolfe et al., 1998).
Eccentricity effects have often been linked to the cortical magnification factor (Daniel and Whitteridge, 1961, Rovamo and Virsu, 1979, Virsu and Rovamo, 1979); a concept that accounts for the relationship between visual acuity and distance from the fovea. It expresses the surface area of cortex in V1 that corresponds to one degree of visual angle at different eccentricities (However, see Harvey and Dumoulin (2011), for a discussion of magnification in other areas). Since a larger cortical area is devoted to processing visual information at the fovea, rather than more eccentric locations, the fovea is said to have the largest magnification factor. By scaling stimuli according to the cortical magnification factor (M-scaling), it has been demonstrated that performance on various detection and discrimination tasks in the periphery becomes similar to the performance near or at the fovea (Carrasco and Frieder, 1997, Motter, 2009, Rovamo and Raninen, 1990). Such findings support the notion of invariance in visual processing, i.e. that stimuli are processed the same way across all locations of the visual field, predicting equal performance at all eccentricities when stimuli are scaled to achieve similar cortical representations (Yu et al., 2014). However, though scaling has accounted for eccentricity-dependent performance differences in some visual tasks, it has failed to do so in a number of other tasks (Bao et al., 2013, Valsecchi et al., 2013, Wolfe et al., 1998). Based on such findings, Wolfe (1998) argued for a model in which eccentricity effects are linked to an attentional bias for stimuli presented centrally. Such an attentional bias would enhance perception in everyday life, as we are inclined to foveate behaviorally relevant objects even though we might not be consciously aware of our limited perceptual abilities peripherally. Indeed, subjects tend to overestimate their peripheral performance (Solovey et al., 2015).
Some aspects of performance across the visual field may be given by the anatomy of the visual system or inherent central biases. However, an abundance of studies has attested to the potential for change by both environmental factors and practice. For instance, deaf individuals have been shown to allocate more attentional resources to the visual periphery, compared to hearing individuals, possibly reflecting a compensatory reorganization of spatial attention in the deaf (Dye et al., 2009b, Proksch and Bavelier, 2002). The notion of such alterations taking place is intuitively appealing, seeing that deaf individuals cannot depend on auditory cues to guide attention toward peripheral events. In the case of action video-gamers, the habitual or recent exposure to a demanding visual task have also been reported to improve visual processing speed and selective attention, especially in the far periphery (Hubert-Wallander et al., 2011). While it is suggested that a trade-off takes place between attentional resources available in central and peripheral vision in the deaf, no such trade-off has been reported for action ivideo gamers, suggesting that practice on a demanding visual task can lead to a general enhancement of visual ability across the visual field (Dye et al., 2009a, Green and Bavelier, 2007). Such evidence for the potential for change calls for a more precise characterization of eccentricity effects in order to clarify possible training prospects in the case of both healthy and clinical populations (e.g., patients with visual deficits). In addition, as previous experiments have often used simple stimuli (e.g., Gabor patches), knowledge of effects of eccentricity on the processing of more complex stimuli (e.g., letters) is needed.
In this study, we investigated the effects of stimulus eccentricity on discrete components of visual attention using the Theory of Visual Attention (TVA; Bundesen, 1990). In TVA, attention is said to comprise several distinct parameters that can be independently estimated from the same set of behavioral data. This is advantageous when seeking to understand potential differential effects of stimulus eccentricity on different components of visual attention. In one experiment, we investigated the effects of eccentricity on visual short-term memory (VSTM) capacity (), the visual perceptual threshold (), and visual processing speed (). In a subsequent experiment, we tested the effect of M-scaling on estimates of these parameters. Additionally, we manipulated expectancy of spatial location by introducing a blocked trial design in one half of the experiment, where each block contained only trials with the same stimulus eccentricity, and an intermixed design in the other. If eccentricity effects arise from magnification alone, we expect potential effects of eccentricity in Experiment 1 to be abolished by the M-scaling in Experiment 2. If attentional mechanisms are involved, we expect eccentricity effects to be diminished in the blocked part of Experiment 2, where participants know where to direct attention, compared to the intermixed part.
1.1. Theory of visual attention (TVA)
TVA (Bundesen, 1990) is a computational theory of visual attention, in which attention is described as a mechanism for selecting the currently most relevant information and encoding it into VSTM. According to TVA, objects in the visual field compete for access to VSTM in a parallel processing race. Since storage capacity is limited, only objects can be encoded, assuming a slot-based model of VSTM (Luck and Vogel, 1997; but see Wilken and Ma, 2004, Bays and Husain, 2008). The probability of an object being encoded into VSTM depends on its attentional weights, reflecting the strength of the object's sensory evidence and its relevance (subjective attentional bias). In the processing race, each object in the visual field is assigned an attentional weight that determines the proportion of the total processing capacity allocated to it, and accordingly, how fast it is processed. The more processing resources an object is allotted, the higher the probability is that the object will gain access to VSTM. The total processing capacity, , is assumed to be a constant and independent of the number of objects in the visual field. Thus, the visual system is assumed to have a limited fixed processing capacity. In mathematical terms, the processing speed of an object in the visual field can be expressed as:
where is the total processing capacity, is the attentional weight assigned to object and the denominator is the sum of attentional weights across all objects in the visual field, S (see Shibuya and Bundesen (1988) for a more elaborate description of this fixed-capacity independent race model, FIRM).
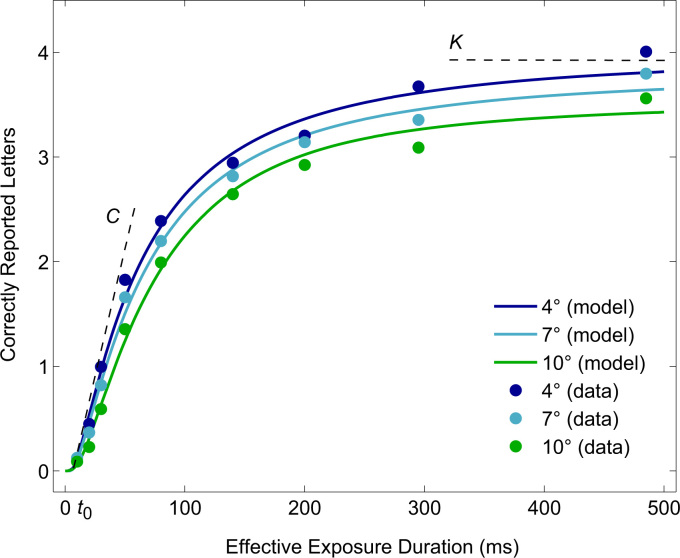
Different parameters quantifying attentional functions can be derived depending on the specific TVA-based paradigm used. We employed a whole report paradigm (see Fig. 1) with varying stimulus durations which allowed us to assess three distinct, capacity-related components of attention: , the capacity of visual short-term memory measured in number of items; , the speed of visual processing measured in items per second; and , the perceptual threshold, denoting the minimum effective exposure duration, measured in milliseconds. Stimuli, which are shown at exposure durations below the perceptual threshold, , are not perceived, however, the number of reported letters increases sharply above , with an intensity determined by the speed of visual processing, . At longer exposures, performance reaches an asymptotic level determined by the capacity of visual short-term memory, . This relationship between the three attentional parameters and whole report performance is illustrated in Fig. 2.
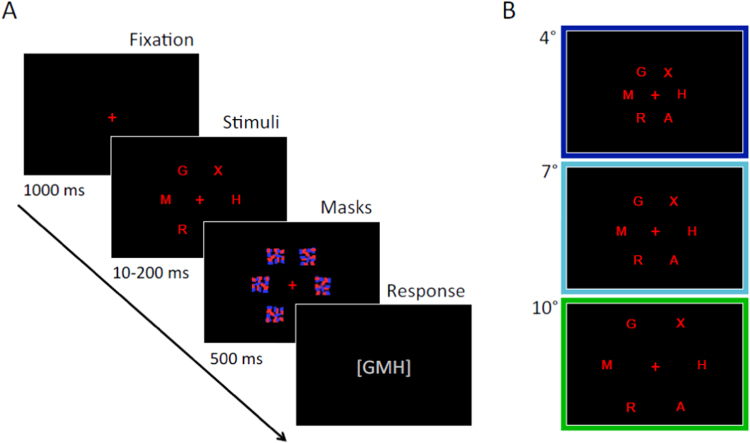
Fig. 1.
Trial outline (A) and eccentricity conditions (B) for Experiment 1. First, participants were presented with a central cross on which they were instructed to fixate throughout the trial. Then, six randomly chosen target letters were shown at an eccentricity of either 4°, 7°, or 10° of visual angle from the central fixation point. Target letters were terminated with pattern masks. Lastly, a blank screen probed participants to report the letters they had seen.
Fig. 2.
Mean number of correctly reported letters as a function of exposure duration in Experiment 1. Markers represent observed mean scores; lines represent the aggregated model fits. The two highest exposure durations represent the two unmasked conditions. For illustration purposes the subjective parameters , and are presented in relation to the aggregated 4° model fits: is the exposure duration at which the number of correctly reported letters starts to rise above 0 (i.e., the perceptual threshold), is the slope of the curve at , and is the asymptotic level of the number of correctly reported letters if the exposure duration is increased to infinity.
2. Experiment 1
2.1. 1. Materials and methods
2.1.1. Participants
16 healthy participants (, 12 women) took part in the experiment. All participants had normal or corrected-to-normal vision and received 120 DKK (16 EUR) pr. hour of testing. The experiment took approximately two hours to complete.
2.1.2. Procedure and stimuli
The experiment was a modified version of the whole report paradigm developed by Vangkilde and colleagues (Vangkilde et al., 2011). The experiment consisted of 20 blocks of 54 trials each (1080 trials in total), all of which followed the same basic outline illustrated in Fig. 1.
On each trial, six randomly chosen target letters from the set [ABDEFGHJKLMNOPRSTVXZ] were presented at one of three eccentricities (4°, 7°, or 10° of visual angle) for one of seven durations (10, 20, 30, 50, 80, 140, and 200 ms) and terminated with pattern masks. To allow sufficient time for VSTM to fill, we also included unmasked displays for the 10 and 200 ms exposure thereby prolonging the effective exposure duration of the stimulus displays (see also Luck and Vogel, 1997). The order of trials was randomized with the constraint that all possible combinations were used an equal amount of times in each block. Target locations were equidistantly spaced around a central fixation cross.
Participants were instructed to make an unspeeded report of the letters they were fairly certain of having seen, and refrain from pure guessing. Participants received feedback on the accuracy of their reported letters after each block, with a feedback screen stating the percentage of correctly reported letters out of the total number of letters they had reported. Before beginning the experiment, participants were instructed to aim for a report accuracy of 80–90%. In addition, participants were told that if their accuracy fell below 80%, they should aim to guess less and be more confident of the identity of reported letters. If accuracy was above 90%, they should try to be less conservative in their report. Preceding the experiment, participants completed a short practice session of 3 blocks of 15 trials each, resembling the actual experiment in every way, with the exception that only a subset of the exposure durations were used. A feedback screen was also shown after each of the practice blocks.
The fixation cross and letter stimuli were presented in red (20.82 cd/m2) on a black (0.14 cd/m2) background. The letters were written in the font Arial with a letter point size of 42. The average letter subtended 1.26 in width. Stimulus size was kept constant across the eccentricities.
The experiment was developed using the E-prime software (version 2.0.8.90) and presented on CRT monitors at 100 Hz and a screen resolution of 1024×768. Participants were seated approximately 60 cm from the monitors in a dimly lit room.
2.2. Results
2.2.1. Data analyses
All statistical analyses were carried out using IBM SPSS Statistics software (version 20.0). Both raw performance (mean scores and error rates) and TVA modeled estimates of attentional abilities were analyzed and will be reported here. We used repeated measures ANOVAs to examine if eccentricity affected raw scores and TVA parameters. In cases where the assumption of sphericity was violated, the Greenhouse-Geisser correction was applied. Repeated measures ANOVAs are presented with the effect size partial eta squared, . For post-hoc t-tests, effect sizes are given as Cohen's , calculated as the difference between the means divided by the pooled standard deviation.
2.2.2. Raw scores
The effects on the number of correctly reported letters were investigated using a repeated measures ANOVA with exposure duration and eccentricity as within-subject factors. As expected, increasing the exposure duration led to an increase in the number of correctly reported letters, . Conversely, increasing eccentricity led to a decrease in the number of correctly reported letters, . There was a significant interaction between eccentricity and exposure duration, . Plotting mean scores as a function of exposure duration separately for each eccentricity showed a pattern with higher mean scores for lower eccentricities across all exposure durations, except the lowest (10 ms; see Fig. 2).
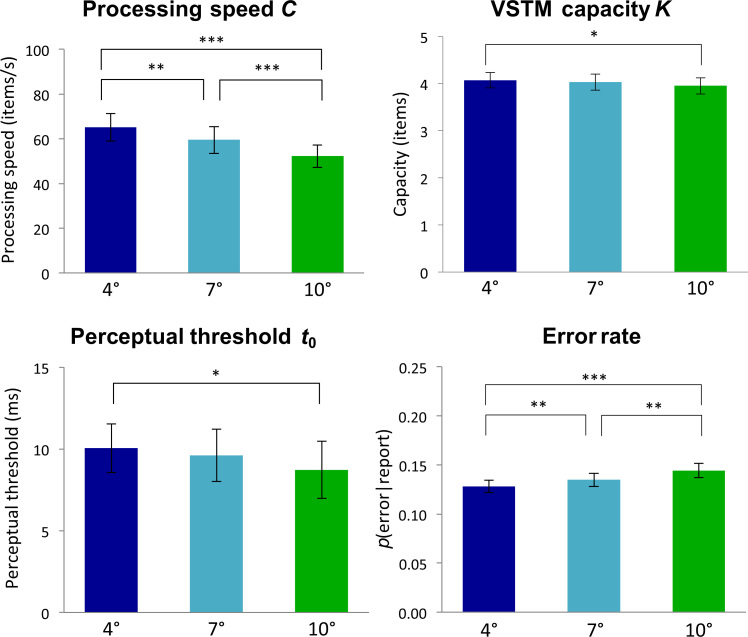
We also investigated the effects of eccentricity on error rates (percentage of reported letters that were incorrect) with a one-way repeated measures ANOVA. Error rates increased significantly with increasing eccentricity, (see Fig. 3, lower right panel). Post-hoc -tests showed that the effect was significant across all eccentricities: 4° compared to 7°, ; 7° compared to 10° ; and 4° compared to 10°,
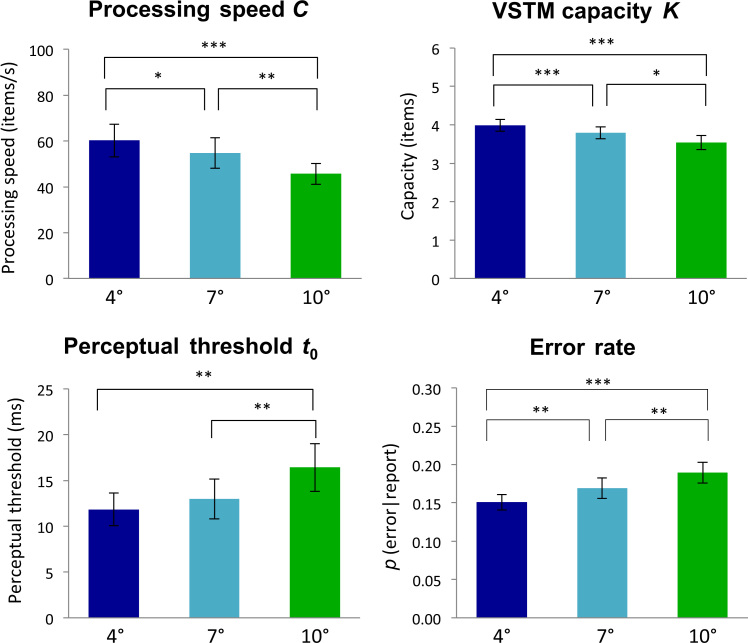
Fig. 3.
Mean TVA parameter estimates and error rates measured for each eccentricity (4°, 7°, and 10°, respectively) in Experiment 1. Upper left panel, , visual processing speed; Upper right panel, , visual short-term memory capacity; Lower left panel, , visual perceptual threshold; Lower right panel, error rate. Significant differences in post-hoc t-tests are indicated by asterisks, , , . Error bars show standard errors of the means.
2.2.3. Estimated attentional performance
TVA-parameters were estimated by a maximum-likelihood fitting procedure using the LibTVA toolbox (Dyrholm et al., 2011, Kyllingsbæk, 2006). Based on the number of correctly reported letters in each trial, the fitting procedure assessed attentional abilities in terms of three parameters1: , VSTM capacity (number of letters); , the speed of visual processing (letters/s); and , the perceptual threshold, defined as the longest ineffective exposure duration (ms). A graphical illustration of the parameters is presented in Fig. 2. In addition, the retinal decay of the unmasked displays was approximated by adding an equivalent additional exposure duration (see Loftus et al., 1985), , to the durations of the unmasked displays (see Bundesen, 1990).
One-way repeated measures ANOVAs revealed significant effects of stimulus eccentricity on the threshold of visual perception, , , VSTM capacity, , and processing speed, .
Post-hoc -tests, comparing TVA estimates across stimulus eccentricities (see Fig. 3), showed that increasing eccentricity significantly slowed down visual processing speed, , for stimuli shown at 4° compared to 7°, , 7° compared to 10°, , and 4° compared to 10°, .
VSTM capacity, , also decreased as a function of eccentricity with significant differences between stimuli shown at 4° and 7°, , 7° and 10°, , and 4° and 10°, . Lastly, we found a significant increase in the perceptual threshold, , between stimulus eccentricities of 7° and 10°, as well as between 4° and 10°, .
2.3. Discussion
In Experiment 1 we investigated effects of eccentricity on mean scores (number of correctly reported letters out of the total number of reported letters), error rates, and estimated discrete components of attention in a whole report experiment. We found large significant effects of exposure duration and eccentricity on mean scores. With increasing eccentricity, the proportion of correctly reported letters was reduced and error rates were inflated. We also found a smaller but significant interaction effect of exposure duration and eccentricity on raw scores, which indicated that the effects of eccentricity on mean scores increased with increasing exposure duration. Taken together, these findings suggest that increasing eccentricity negatively influences performance as measured by mean scores and error rates, replicating prior findings (Carrasco et al., 1995, Carrasco and Frieder, 1997, Chen and Treisman, 2008, Hamilton et al., 2010, Steelman et al., 2013, Wolfe et al., 1998). Similarly, increasing eccentricity negatively influenced all three components of attention, estimated by use of TVA; at higher eccentricities visual processing speed slows down, VSTM capacity is reduced and the perceptual threshold is elevated.
It has been suggested that eccentricity effects observed in visual tasks can be accounted for by cortical magnification. To test whether this could be the case for the eccentricity effects found in Experiment 1, we M-scaled stimuli in Experiment 2. In addition, we introduced a blocked design in half the blocks of the experiment to test whether eccentricity effects could be partly explained by lack of spatial expectancy. It has been proposed that eccentricity effects reflect a central bias where more centrally presented stimuli are attended before peripheral stimuli, when attention is unguided (Wolfe, 1998). Thus, we employed a paradigm devoid of competition between central and peripheral stimuli, irrelevant distractors, and spatial uncertainty, to test whether the eccentricity effects observed in Experiment 1 persist under optimal conditions for visual selection.
3. Experiment 2
3.1. Materials and methods
3.1.1. Participants
24 healthy participants (, 17 women) took part in the experiment. All participants had normal or corrected-to-normal vision and received 120 DKK (16 EUR) pr. hour of testing. The experiment took approximately four hours to complete.
3.1.2. Procedure and stimuli
The general procedure was the same as in Experiment 1, and the stimuli consisted of the same set of letters. However, in Experiment 2, letters were scaled according to the cortical magnification factor (Rovamo and Virsu, 1979) in order to keep cortical representation constant across eccentricities. Following this, the average letter shown at 7° subtended 1.86° in width and the average letter shown at 10° subtended 2.48° in width. This resulted in letter point sizes of 63 and 85, respectively. In the central condition, letter size was the same as in Experiment 1 (i.e., 1.26° in width).
The experiment was divided into two parts, each consisting of 1080 trials. One part was a blocked design with one block per stimulus eccentricity, and the other part was an intermixed design, in which stimulus eccentricity varied on a trial-by-trial basis. Half of the participants began with the blocked and ended with the intermixed part, and vice versa. We chose this design to examine whether spatial uncertainty had any influence on performance, perhaps making it more difficult to distribute attention optimally when shifting between central and peripheral locations.
3.2. Results
3.2.1. Raw scores
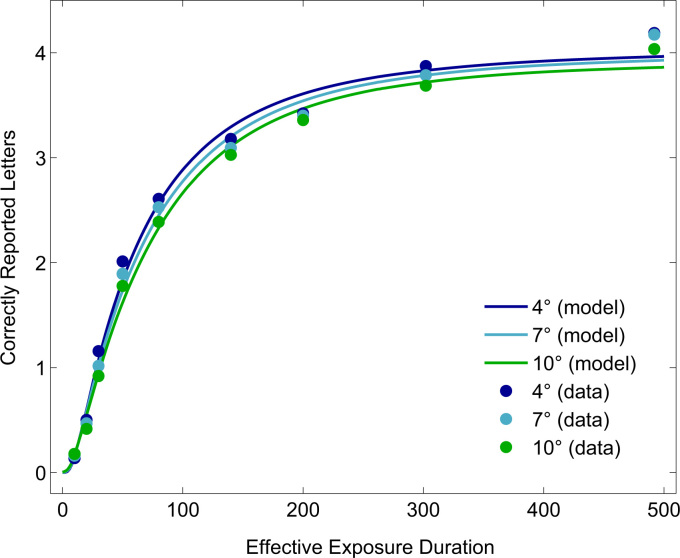
The effects on the raw scores were investigated with a repeated measures ANOVA with design (blocked vs. intermixed), eccentricity, and exposure duration as within-subject factors. The number of correctly reported letters increased with increasing exposure duration, . Conversely, increasing eccentricity led to a decrease in the number of correctly reported letters, . There was a significant interaction between eccentricity and exposure duration . Plotting mean scores as a function of exposure duration separately for each eccentricity revealed higher mean scores for lower eccentricities across exposure durations (see Fig. 4). No significant difference between performance in the blocked and intermixed design was found, . For this reason, we collapsed the data obtained in the blocked and intermixed design for each participant when analyzing the data using TVA-based modeling for Experiment 2.
Fig. 4.
Mean number of correctly reported letters as a function of exposure duration in Experiment 2. Markers represent observed mean scores; lines represent average model fits. The two highest exposure durations represent the two unmasked conditions.
As in experiment 1, we also investigated whether eccentricity had any effect on error rates. A one-way repeated measures ANOVA with eccentricity as within-subject factor revealed that error rates increased significantly with increasing eccentricity, (see Fig. 5). Post-hoc -tests showed that the effect was significant across all eccentricities: 4° compared to 7°, , 7° compared to 10° , , and 4° compared to 10°, .
Fig. 5.
Mean TVA parameter estimates and error rates measured for each eccentricity (4°, 7°, and 10°, respectively) in Experiment 2. Upper left panel, , visual processing speed; Upper right panel, , visual short-term memory capacity; Lower left panel, , visual perceptual threshold; Lower right panel, error rate. Significant differences in post-hoc -tests are indicated by asterisks, , , . Error bars show standard errors of the means.
3.2.2. Estimated attentional performance
TVA parameters were estimated as in Experiment 1. One-way repeated measures ANOVAs revealed significant main effects of stimulus eccentricity on , , as well as on , , and , .
Post-hoc -tests comparing TVA estimates across stimulus eccentricities (see Fig. 5) revealed that increasing eccentricity significantly slowed down visual processing speed, , for stimuli shown at 4° compared to 7°, , 7° compared to 10°, , and 4° compared to 10°, . VSTM capacity, , also decreased with increasing eccentricity, with a significant difference between stimuli shown at 4° and 10°, . The perceptual threshold, , also decreased with increasing eccentricity, with a significant difference between 4° and 10°, .
3.3. Discussion
In Experiment 2, we tested whether the eccentricity effects found in Experiment 1 could be accounted for by cortical magnification or spatial expectancy. We did this by M-scaling stimuli and by introducing a blocked design in half the blocks. These blocks contained only trials of the same stimulus eccentricity, and participants were informed of the eccentricity of target letters at the beginning of each block. Since no significant differences were found between performance in the blocked and intermixed part of the experiment, knowledge of where the targets would appear did not aid performance. Thus, any spatial expectancy that might have been induced in the blocked part of the experiment did not change the eccentricity effects observed. Data from the two parts of the experiment were therefore pooled before further analysis.
We found moderate to large significant effects of exposure duration and eccentricity on mean scores, showing that the number of correctly reported letters decreased with increasing eccentricity. We also found a smaller but significant interaction effect of exposure duration and eccentricity on mean scores, showing that the effects of eccentricity on mean scores increased with longer exposure durations. There was a significant effect of eccentricity on error rates, albeit much smaller than the effect found in Experiment 1. This indicates that M-scaling improved the perception of peripheral letters.
We found significant negative effects of eccentricity on processing speed and VSTM capacity, with the largest effect on processing speed. We also found a significant effect of eccentricity on the perceptual threshold, showing that the minimum effective exposure duration decreased with increasing eccentricity. This indicates that participants needed less time before they could begin to process information from the periphery, compared to the central condition. This may in fact be equivalent to findings from Carrasco et al. (2003), which will be examined in the General discussion.
Except for the opposite effect of eccentricity on the perceptual threshold, Experiment 2 yielded results comparable to the results of Experiment 1. In some cases, negative effects of eccentricity were attenuated, but in all cases the effects remained significant after M-scaling. In sum, the eccentricity effects observed were not fully accounted for by cortical magnification.
4. General results
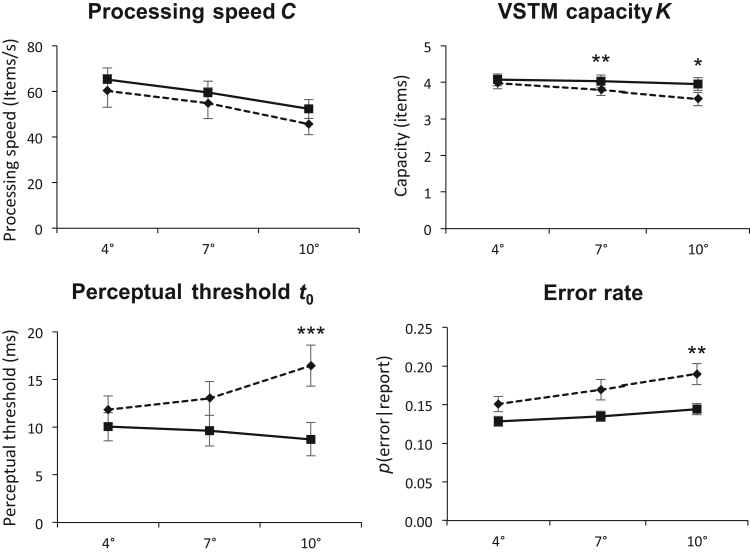
Since data was collected from two different participant groups, comparisons between the two experiments could be confounded by differences in general performance between participants who completed the same-size letter version (Experiment 1) and participants who completed the scale-size letter version (Experiment 2) of the paradigm. To control for the effect of differences in participant performance, we conducted ANCOVAs for each parameter (, , and ) with group (magnified/unmagnified) as a fixed factor, and performance on the central condition (4° eccentricity) as the covariate. Since the central condition was the same in both experiments (i.e., stimulus sizes were identical), any differences in parameter estimates at 4° eccentricity should be due to general performance differences between participants rather than stimulus magnification. Thus, by controlling for the effect of differences in participant performance at 4° eccentricity, we should get an estimate of the effect of magnification on parameter estimates at the remaining eccentricities unconfounded by general performance. As shown in Fig. 6, magnifying the letters at the outer eccentricities did not significantly affect processing speed when controlling for the effect of general performance. However, magnification did significantly affect both the estimate of VSTM capacity and the perceptual threshold, though only at the farthest eccentricity in the latter case. For VSTM capacity, magnification attenuated the negative effects of eccentricity. In the case of the perceptual threshold, magnification reversed the effects of eccentricity such that it decreased with eccentricity (rather than increase, as was the case with unmagnified stimuli).
Fig. 6.
Comparison of TVA parameter estimates and error rates from Experiment 1 (dashed lines) and Experiment 2 (solid lines) when controlling for the effect of general performance differences between participant groups. Upper left panel, , visual processing speed; Upper right panel, , visual short-term memory capacity; Lower left panel, , visual perceptual threshold; Lower right panel, error rate. All measured at 4°, 7°, and 10° eccentricity, respectively. Significant p-values for ANCOVAs are marked with asterisks,. Error bars show standard errors of the means.
5. General discussion
In this study, six target letters were presented in either central (at 4° eccentricity) or peripheral vision (at 7° or 10° eccentricity). In Experiment 1, the size of the letters was the same at all eccentricities, while in Experiment 2, letters were scaled according to cortical magnification and thus increased in size with increasing eccentricity. The study was designed to determine whether visual attentional abilities vary depending on the eccentricity of attended objects. The stimulus scaling in Experiment 2 was done to examine whether effects found in Experiment 1 could be explained in terms of the cortical magnification factor, which accounts for the relationship between visual acuity and distance from the fovea (Rovamo and Virsu, 1979, Virsu and Rovamo, 1979). The TVA model was used as the theoretical and computational framework for analyzing the results. TVA-assessment allows for the estimation of several distinct components of attention that might be differentially affected by the experimental manipulations. The components assessed were the threshold of visual perception (), visual processing speed (), and VSTM capacity ().
Experiment 1 revealed significant negative effects of eccentricity on mean scores, error rates, and all TVA-assessed components of attention. Specifically, processing speed and VSTM capacity decreased with increasing eccentricity whereas the perceptual threshold increased. In Experiment 2, we found comparable, although in some cases markedly attenuated, effects of eccentricity. Increasing the eccentricity of the target letters had less impact on the amount of correctly reported letters and the error rate in Experiment 2 than in Experiment 1. The effect of eccentricity on the VSTM capacity was also reduced in Experiment 2, whereas the effect on processing speed was similar to that found in Experiment 1 (see Fig. 6). Unlike in Experiment 1, the perceptual threshold decreased as eccentricity increased in Experiment 2. Taken together, these findings indicate that stimulus magnification improved some aspects of letter perception. However, in none of the investigated parameters were the effects of eccentricity abolished. After controlling for the effect of general performance differences between participants who partook in Experiment 1 and Experiment 2, there were no significant effects of magnification on processing speed. We found significant effects of magnification on VSTM capacity measured at 7° and 10° eccentricity. We also found significant effects of magnification on the perceptual threshold, though only at 10° eccentricity (see Fig. 6). Magnification attenuated the effects of eccentricity on VSTM capacity and, perhaps surprisingly, significantly improved the perceptual threshold.
Our results point to a general decrease in attentional abilities with increasing stimulus eccentricity, indicating that letter identification is more efficient in central vision, especially due to markedly faster processing speed (C). This is perfectly in line with previous studies reporting increased reaction times as a function of target eccentricity (Carrasco et al., 1995, Carrasco and Frieder, 1997, Chen and Treisman, 2008, Hamilton et al., 2010, Steelman et al., 2013, Wolfe et al., 1998). Notably, our TVA-based assessment provides the added advantage of unspeeded measures based on accuracy, ruling out the possibility that the effect of eccentricity on processing speed is confounded by facilitation of motor reactions to central compared to peripheral targets. Our results also show that cortical magnification did not fully account for the effect of eccentricity on any of the measures of attentional performance. Thus, our findings do not support the notion of invariance, according to which performance in central and peripheral vision is made equal by stimulus scaling (Carrasco and Frieder, 1997, Rovamo, 1983, Virsu and Rovamo, 1979, Virsu et al., 1982). Neither do our results support a purely attentional explanation of these effects (cf., Wolfe, 1998). Performance in Experiment 2 did not improve in the blocked part (blocks consisted only of trials of the same eccentricity), compared with the intermixed part (eccentricity of targets varied on a trial-by-trial basis). Thus, knowing where to direct attention did not aid performance. It is conceivable, however, that participants were unable to make use of the information given regarding the eccentricity of the targets in a specific trial; it might not be possible to cue multiple target locations using only an endogenous cue, either because it is not possible to select multiple target locations in parallel or because working memory cannot retain the spatial information accurately (see for example Close et al., 2014). Although we cannot exclude that an exogenous cue would have affected performance, the employment of an endogenous cue did not. On this basis, we suggest that eccentricity effects cannot be explained solely by either cortical magnification or mechanisms of attention. Importantly, while knowing where to direct attention did not aid performance in our experiment, evidence suggests that attentional abilities in the periphery can be enhanced with extensive practice on a demanding visual task (as seen in action video gamers; Dye et al., 2009a).
In contrast to our results, Carrasco et al. (2003) reported that information processing is more efficient toward the periphery and that M-scaling only attenuates this effect. In their study, Carrasco and colleagues used a forced-choice orientation discrimination task and asked participants to report whether a target Gabor patch was tilted 30° to the right or left from the vertical orientation. The target patch could appear alone or among 7 distractors (vertical Gabor patches) and were presented at 4° or 9° eccentricity, with or without stimulus magnification. Participants responded when a tone sounded 40–2000 ms after stimulus onset, and the time from onset to response was taken as a measure of the processing time. The results showed that a shorter amount of time was required to accumulate information about peripheral targets to achieve the same report accuracy as that of central targets. This led the authors to conclude that processing speed increases towards the periphery of the visual field. There are, however, a number of differences between our paradigm and the paradigm used by Carrasco et al. (2003). While we used the same equations for M-scaling and presented stimuli at similar eccentricities, the paradigms differ substantially in task demands and stimulus material. For instance, Carrasco et al. used simple stimuli and magnified both the size, spatial frequency and the orientation, whereas we used more complex stimuli and only magnified the size. As such, the effects of stimulus magnification found in the experiments may not be entirely comparable. Further, the use of different terminology and theoretical frameworks may also contribute to the apparent discrepancies regarding eccentricity effects on processing speed. The improved report efficiency for peripheral stimuli found by Carrasco et al. seems to pertain to what we would denote as an improvement of the perceptual threshold, t0, in the TVA framework. Indeed, the authors state that the rate of information accrual (akin to processing speed, C, in the TVA framework) is unaffected by eccentricity but that the threshold for above-chance discrimination is lower at peripheral locations, which is perfectly in line with our findings of improved perceptual thresholds in the magnified condition. However, their results are not easily reconciled with our findings of eccentricity-induced slowing of visual processing speed for both M-scaled and unscaled stimuli nor with our finding that perceptual thresholds are negatively affected by increasing eccentricity when stimuli are not scaled.
It has previously been argued that the concept of cortical magnification provides a sufficiently accurate description of acuity for the detection of simple stimuli, such as Gabor patches, but may not suffice for more complex stimuli, such as alphanumeric characters (Strasburger et al., 1991). Strasburger et al. (1991) conclude that cortical magnification does not account for performance differences at central and more peripheral locations in the visual field for the more complex functions involved in pattern recognition. Specifically, a recent review reports that peripheral vision is limited in pattern categorization by a lower representational complexity and processing speed (Strasburger et al., 2011). That eccentricity effects in our experiment were not fully eliminated by cortical magnification is in agreement with these conclusions. The question, then, is not whether cortical magnification can account for effects of eccentricity, but rather under which circumstances it does so. As Strasburger et al. (2011) describe, one characteristic shared by scalable tasks might be that they depend upon low-level processing in areas of the visual stream up to and including V1. Although the cortical magnification factor seemingly stays the same between visual areas V1 and V4 (see Motter, 2009) different visual cortical areas have been associated with different magnification factors (see Harvey and Dumoulin, 2011) and tasks depending on higher-level processing, such as alphanumerical character recognition, may require additional scaling along both spatial and non-spatial dimensions. With regard to the latter, Strasburger et al. (2011) point to contrast scaling as a key variable, which has in fact already been shown to reliably affect processing speed in TVA-based tasks (e.g., Petersen and Andersen, 2012).
The notion that scaling affects visual processing differentially depending on stimulus material and task demands provides an explanation for why M-scaling has been successful in equating central and peripheral performance in some tasks and has failed to do so in others. Supporting this notion, anatomical studies have shown that in some areas of the visual cortices, the central visual field is magnified even more than in V1, while the opposite bias can be observed in other areas (see for example Rosa and Elston, 1998, Rosa et al., 1997). In higher-level cortical areas, these differences are even more pronounced. For instance, areas of the ventral pathway associated with object recognition and identification processes have expanded representations for central vision, while areas of the dorsal pathway associated with spatial information processing have larger cortical areas to represent peripheral vision. Distinct functions for the central and peripheral visual field could therefore result from the specific cortical networks recruited for a particular task (see Yu et al., 2014).
Cells of the visual streams projecting to the ventral and dorsal pathways have also been suggested to process different aspects of the visual input. The majority of the projections to the dorsal stream stem from M cells in the fast-conducting magnocellular pathway (Callaway, 2005), which are especially sensitive to the temporal properties of objects and changes in luminance contrast (Kaplan, 2004, Omtzigt et al., 2002). The projections to the ventral stream, however, stem not only from M cells but also from P cells of the parvocellular pathway which is suggested to play an important role in object recognition and identification processes, including letter-identification (Omtzigt et al., 2002). The ratio of P to M cells changes with retinal eccentricity such that more M cells are available in the periphery. Thus, in addition to differences in cortical activation equated by appropriate magnification, eccentricity dependent changes in performance could also arise from differences in the relative contribution of P and M cells. This could explain why faster processing speed is found for orientation discrimination in the periphery compared to central vision (Carrasco et al., 2003). However, as Carrasco and colleagues noted, the effects of eccentricity found in their study were larger than expected from neurophysiological constraints alone.
In conclusion, we have shown that the processing of complex visual information is negatively affected by increasing eccentricity even when stimuli are scaled in size to account for cortical magnification. However, the general anatomy of the visual system, the experimental task demands, the stimulus material used, and individual factors, such as the amount of training on a visual attention task, are all important factors, contributing to the effects of stimulus eccentricity on visual perception. Variations in these factors may account for variations in the eccentricity effects observed in different studies examining visual ability across the visual field.
Conflicts of interest
The authors report no conflicts of interest.
Acknowledgments
The authors would like to thank Valdemar Hennesser Borgen Uhre for helpful discussions and advice on the manuscript. Camilla Staugaard was supported by a scholarship awarded by the Department of Psychology, University of Copenhagen, funded by a grant from the Danish Council for Independent Research under the Sapere Aude program (project no. 10-089757. "Modeling Visual Cognition - Perception, Attention, and Short-Term Memory). Signe Vangkilde was supported by a grant from the Danish Council for Independent Research under the Sapere Aude program (project no. 11-104180, “Attentive Mind”). Anders Petersen and Signe Vangkilde were both supported through a European Union FP7 Marie Curie ITN grant (606901).
Footnotes
In total, the fitted model had 14 degrees of freedom (df): K, 5 dfs (the K value reported is the expected K given a particular distribution of the probability that on a given trial K=1, 2, …, 6); , 1 df; , 2 dfs (the perceptual threshold was assumed to be drawn trial by trial from a normal distribution with a given mean and standard deviation); Six attentional weights, 5 dfs (one weight for each stimulus location under the restriction that the relative weights sum to 1); (additional effective exposure duration for unmasked letter displays), 1 df.
References
- Anstis S. Picturing peripheral acuity. Perception. 1998;27(7):817–825. doi: 10.1068/p270817. [DOI] [PubMed] [Google Scholar]
- Bao Y., Lei Q., Fang Y., Tong Y., Schill K., Poppel E., Strasburger H. Inhibition of return in the visual field the eccentricity effect is independent of cortical magnification. Exp. Psychol. 2013;60(6):425–431. doi: 10.1027/1618-3169/a000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle D.J., Schoendorff B., Henaff M.A., Krolak-Salmon P. Emotional facial expression detection in the peripheral visual field. PLoS One. 2011;6(6):e21584. doi: 10.1371/journal.pone.0021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays P.M., Husain M. Dynamic shifts of limited working memory resources in human vision. Science. 2008;321(5890):851–854. doi: 10.1126/science.1158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundesen C. A theory of visual attention. Psychol. Rev. 1990;97(4):523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Callaway E.M. Structure and function of parallel pathways in the primate early visual system. J. Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Frieder K.S. Cortical magnification neutralizes the eccentricity effect in visual search. Vis. Res. 1997;37(1):63–82. doi: 10.1016/s0042-6989(96)00102-2. [DOI] [PubMed] [Google Scholar]
- Carrasco M., Evert D.L., Chang I., Katz S.M. The eccentricity effect: target eccentricity affects performance on conjunction searches. Percept. Psychophys. 1995;57(8):1241–1261. doi: 10.3758/bf03208380. [DOI] [PubMed] [Google Scholar]
- Carrasco M., McElree B., Denisova K., Giordano A.M. Speed of visual processing increases with eccentricity. Nat. Neurosci. 2003;6(7) doi: 10.1038/nn1079. 699-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Treisman A. Distractor inhibition is more effective at a central than at a peripheral location. Percept. Psychophys. 2008;70(6):1081–1091. doi: 10.3758/pp.70.6.1081. [DOI] [PubMed] [Google Scholar]
- Close A., Sapir A., Burnett K., d’Avossa G. Attention to multiple locations is limited by spatial working memory capacity. J. Vis. 2014;14(9):1–14. doi: 10.1167/14.9.17. [DOI] [PubMed] [Google Scholar]
- Daniel P.M., Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J. Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye M.W., Green C., Bavelier D. Increasing speed of processing with action video games. Curr. Dir. Psychol. Sci. 2009;18(6):321–326. doi: 10.1111/j.1467-8721.2009.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye M.W., Hauser P.C., Bavelier D. Is visual selective attention in deaf individuals enhanced or deficient? The case of the useful field of view. PLoS One. 2009;4(5):e5640. doi: 10.1371/journal.pone.0005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrholm M., Kyllingsbæk S., Espeseth T., Bundesen C. Generalizing parametric models by introducing trial-by-trial parameter variability: the case of TVA. J. Math. Psychol. 2011;55:416–429. [Google Scholar]
- Green C.S., Bavelier D. Action-video-game experience alters the spatial resolution of vision. Psychol. Sci. 2007;18(1):88–94. doi: 10.1111/j.1467-9280.2007.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K., Liu C.H., Roebuck H. I know you are beautiful even without looking at you: discrimination of facial beauty in peripheral vision. Perception. 2011;40(2):191–195. doi: 10.1068/p6849. [DOI] [PubMed] [Google Scholar]
- Hamilton R.H., Stark M., Coslett H.B. Increased effect of target eccentricity on covert shifts of visual attention in patients with neglect. Cortex. 2010;46(1):68–76. doi: 10.1016/j.cortex.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B.M., Dumoulin S.O. The relationship between cortical magnification factor and population receptive field size in human visual cortex: constancies in cortical architecture. J. Neurosci. 2011;31(38):13604–13612. doi: 10.1523/JNEUROSCI.2572-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert-Wallander B., Green C.S., Sugarman M., Bavelier D. Changes in search rate but not in the dynamics of exogenous attention in action videogame players. Atten. Percept. Psychophys. 2011;73(8):2399–2412. doi: 10.3758/s13414-011-0194-7. [DOI] [PubMed] [Google Scholar]
- Juttner M., Rentschler I. Scale-invariant superiority of foveal vision in perceptual categorization. Eur. J. Neurosci. 2000;12(1):353–359. doi: 10.1046/j.1460-9568.2000.00907.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E. The M, P, and K pathways of the primate visual system. In: Chalupa L.M., Werner J.S., editors. Vol. 1. The MIT press; Cambridge: 2004. pp. 481–494. (The Visual Neurosciences). [Google Scholar]
- Kyllingsbæk S. Modeling visual attention. Behav. Res. Methods. 2006;38(1):123–133. doi: 10.3758/bf03192757. [DOI] [PubMed] [Google Scholar]
- Loftus G.R., Johnson C.A., Shimamura A.P. How much is an icon worth? J. Exp. Psychol.: Hum. Perform. Percept. 1985;11(1):1–13. doi: 10.1037//0096-1523.11.1.1. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Vogel E.K. The capacity of visual working memory for features and conjunctions. Nature. 1997;390(6657):279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Motter B.C. Central V4 receptive fields are scaled by the V1 cortical magnification and correspond to a constant sized sampling of the V1 surface. J. Neurosci. 2009;29(18):5749–5757. doi: 10.1523/JNEUROSCI.4496-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omtzigt D., Hendriks A.W., Kolk H.H. Evidence for magnocellular involvement in the identification of flanked letters. Neuropsychologia. 2002;40(12):1881–1890. doi: 10.1016/s0028-3932(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Petersen A., Andersen T.S. The effect of exposure duration on visual character identification in single, whole, and partial report. J. Exp. Psychol. Hum. Percept. Perform. 2012;38(2):498–514. doi: 10.1037/a0026728. [DOI] [PubMed] [Google Scholar]
- Proksch J., Bavelier D. Changes in the spatial distribution of visual attention after early deafness. J. Cogn. Neurosci. 2002;14(5):687–701. doi: 10.1162/08989290260138591. [DOI] [PubMed] [Google Scholar]
- Rosa M.G., Elston G.N. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus): middle temporal area, middle temporal crescent, and surrounding cortex. J. Comp. Neurol. 1998;393(4):505–527. [PubMed] [Google Scholar]
- Rosa M.G., Fritsches K.A., Elston G.N. The second visual area in the marmoset monkey: visuotopic organisation, magnification factors, architectonical boundaries, and modularity. J. Comp. Neurol. 1997;387(4):547–567. doi: 10.1002/(sici)1096-9861(19971103)387:4<547::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rovamo J. Cortical magnification factor and contrast sensitivity to luminance-modulated chromatic gratings. Acta Physiol. Scand. 1983;119(4):365–371. doi: 10.1111/j.1748-1716.1983.tb07351.x. [DOI] [PubMed] [Google Scholar]
- Rovamo J., Virsu V. An estimation and application of the human cortical magnification factor. Exp. Brain Res. 1979;37(3):495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- Rovamo J., Raninen A. Cortical acuity and the luminous flux collected by retinal ganglion cells at various eccentricities in human rod and cone vision. Vis. Res. 1990;30(1):11–21. doi: 10.1016/0042-6989(90)90124-4. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Bundesen C. Visual selection from multielement displays: measuring and modeling effects of exposure duration. J. Exp. Psychol.: Hum. Percept. Perform. 1988;14(4):591–600. doi: 10.1037//0096-1523.14.4.591. [DOI] [PubMed] [Google Scholar]
- Solovey G., Graney G.G., Lau H. A decisional account of subjective inflation of visual perception at the periphery. Atten. Percept. Psychophys. 2015;77(1):258–271. doi: 10.3758/s13414-014-0769-1. [DOI] [PubMed] [Google Scholar]
- Steelman K.S., McCarley J.S., Wickens C.D. Great expectations: top-down attention modulates the costs of clutter and eccentricity. J. Exp. Psychol. Appl. 2013;19(4):403–419. doi: 10.1037/a0034546. [DOI] [PubMed] [Google Scholar]
- Strasburger H., Harvey L.O., Jr., Rentschler I. Contrast thresholds for identification of numeric characters in direct and eccentric view. Percept. Psychophys. 1991;49(6):495–508. doi: 10.3758/bf03212183. [DOI] [PubMed] [Google Scholar]
- Strasburger H., Rentschler I., Juttner M. Peripheral vision and pattern recognition: a review. J. Vis. 2011;11(5):13. doi: 10.1167/11.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi M., Toscani M., Gegenfurtner K.R. Perceived numerosity is reduced in peripheral vision. J. Vis. 2013;13(13):7. doi: 10.1167/13.13.7. [DOI] [PubMed] [Google Scholar]
- Vangkilde S., Bundesen C., Coull J.T. Prompt but inefficient: nicotine differentially modulates discrete components of attention. Psychopharmacology. 2011;218(4):667–680. doi: 10.1007/s00213-011-2361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virsu V., Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Exp. Brain Res. 1979;37(3):475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- Virsu V., Rovamo J., Laurinen P., Nasanen R. Temporal contrast sensitivity and cortical magnification. Vis. Res. 1982;22(9):1211–1217. doi: 10.1016/0042-6989(82)90087-6. [DOI] [PubMed] [Google Scholar]
- Wilken P., Ma W.J. A detection theory account of change detection. J. Vis. 2004;4(12):1120–1135. doi: 10.1167/4.12.11. [DOI] [PubMed] [Google Scholar]
- Wolfe J.M., O’Neill P., Bennett S.C. Why are there eccentricity effects in visual search? Visual and attentional hypotheses. Percept. Psychophys. 1998;60(1):140–156. doi: 10.3758/bf03211924. [DOI] [PubMed] [Google Scholar]
- Yu H.H., Chaplin T.A., Rosa M.G. Representation of central and peripheral vision in the primate cerebral cortex: Insights from studies of the marmoset brain. Neurosci. Res. 2014 doi: 10.1016/j.neures.2014.09.004. [DOI] [PubMed] [Google Scholar]