Abstract
The importance of the acetabular labrum has been increasingly recognized, playing a critical role in both normal anatomy and abnormal pathology of the hip joint. The labrum increases acetabular surface area and volume, providing a stable and durable articulation. The fibrocartilaginous composition affords a tissue capable of a lifetime of normal function in the absence of significant osseous pathology. In the setting of femoroacetabular impingement (FAI) or dysplasia, bony biomechanics may cause labral injury, which may translate to patient symptoms. Long-term consequences of labral tears may include joint degeneration. Labral preservation surgery emphasizes retention of the form and function of the labrum, prioritizing labral repair (in the presence of reparable tissue) and reconstruction (in the absence of reparable tissue) over debridement. Patient-reported outcomes have consistently demonstrated significantly better results following labral repair versus debridement. In conjunction with correction of osseous abnormalities, labral surgery can improve short-term outcomes and potentially reduce the risk of long-term osteoarthritis.
Keywords: Labral repair, Labral reconstruction, Labral preservation, Chondrolabral junction, Hip preservation, Hip arthroscopy
Introduction
The acetabular labrum is the fibrocartilaginous seal around the osseous acetabulum. Surrounding the periphery of the acetabular rim, the labrum increases the depth, surface area, volume, congruity, and stability of the hip joint. The labrum has been shown to contribute an average of 22 % to articulating surface area and 33 % to acetabular volume [1]. By regulating fluid transport between the central and peripheral compartments, the normal labrum distributes contact pressure across the articulating surfaces, lowering the stress on the articular cartilage [2•]. This fluid seal is one of the most important functions of the labrum, as it produces a negative intra-articular pressure, significantly increasing joint stability [3].
Labral integrity comprises but one of several elements participating in joint stability (Table 1). Loss of labral function, either via tear or debridement, may induce hip microinstability, subluxation, or dislocation [4•, 5]. Breakage of the labral fluid seal is the rationale behind loss of joint stability [6]. This may occur in hips with femoroacetabular impingement (FAI) or dysplasia during flexion and rotational maneuvers, such as cutting or pivoting [7]. Although the majority of labral tears occur in the presence of osseous pathology [8–11], they may also occur without any obvious bony pathomorphology in patients performing certain sports or activities or in patients with iliopsoas impingement [12–14]. Regardless of the source of the labral tear, it is the presence of symptoms that warrants an evaluation and therapeutic intervention, as the prevalence of asymptomatic labral tear is not insignificant [15•]. Thus, in patients with pain or dysfunction attributable to a labral injury, retention of the labrum is of paramount importance in improving symptoms. This may be accomplished via labral repair, reconstruction, or limited debridement in select cases.
Table 1.
Soft tissue and bony contributions to hip stability
| Soft tissue—static | Soft tissue—dynamic | Osseous—acetabulum | Osseous—femur |
|---|---|---|---|
| Connective tissue disorder Capsule (iliofemoral, pubofemoral, ischiofemoral ligaments, zona orbicularis) Acetabular labrum Ligamentum teres Iatrogenic—unrepaired capsulotomy Iatrogenic—labral debridement Iatrogenic—ligamentum teres debridement |
Iliopsoas Rectus femoris Iliocapsularis Gluteus minimus, gluteus medius Gluteus maximus Iatrogenic—iliopsoas tenotomy |
Lateral center edge angle Anterior center edge angle Tonnis angle Sharp’s angle Femoral head extrusion index Head-center distance Acetabular version Posterior wall sign Ischial spine sign Shenton’s line Iatrogenic—excessive rim resection |
Neck-shaft angle Alpha angle Head-neck offset Head-trochanteric distance Femoral version Iatrogenic—excessive cam osteoplasty |
In order for labral repair or labral reconstruction to be successful, an adequate healing response is necessary. The labral vascular supply has been consistently demonstrated to originate from the radial branches of a periacetabular periosteal vascular ring, which emanates primarily from the superior and inferior gluteal arteries [16]. These vessels course on the periosteum, penetrate the capsule near the capsular insertion above the acetabular rim, continue in a loose connective tissue layer on the capsular side of the labrum, and terminate at the labral free edge. Thus, even in a labral tear at the chondrolabral junction, the vascular supply remains intact. There is no consistent evidence that any vascular contribution exists from the capsule, synovium, or osseous acetabular rim.
In an ovine model, the labrum is mostly avascular, with a fibrocartilage structure similar to that of humans [17]. Twelve weeks after arthroscopic repair, ovine labra grossly healed with a stable repair. However, the repair was incomplete with a superficial shallow cleft at the chondrolabral junction. This repair was primarily fibrovascular scar. In humans undergoing arthroscopic labral repair and FAI treatment, both macroscopic and histologic assessments have revealed stable repair healing (without any residual detachment) and retention of the triangular shape with a fibrocartilage composition without significant fibrovascular scar [18]. The triangular shape was retained, despite the looped suture configuration, secondary to partial incorporation of the suture into the labral body. Neovascularization was observed near the sutures. Capsulolabral adhesions were observed on the capsular side of the repair.
In addition to a vascular response, neural anatomy and the neural response to surgical intervention are an important issue in understanding a patient’s pain following surgery. The presence of nociceptive fibers in a labral repair may permit post-operative residual pain. This provides the chief justification for removal of native labral tissue and reconstruction with an aneural graft [19]. In the normal labrum, there has been a consistent demonstration of free nerve endings (nociception) and nerve end organs (proprioception), mostly from anterosuperior to posterosuperior (typical labral pathology location), with higher sensory fiber concentration anteriorly [20–22]. These fibers come primarily from the obturator nerve and the nerve to quadratus femoris. The nociceptive free nerve endings are predominantly located at the labral base, decreasing peripherally, mostly superficially on the chondral surface [21]. While removal of pain-generating nociceptive fibers is an advantage of labral reconstruction, the additional requisite removal of proprioceptive mechanoreceptors may allow patients to overly stress the graft and should be considered during rehabilitation [19, 23].
Indications/contra-Indications
The indications for labral repair include patients with symptoms that have persisted despite non-surgical treatment including, but not limited to, rest, activity modification, oral anti-inflammatory non-narcotic medications, physical therapy, and injection(s). Physical therapy should emphasize optimizing pelvic tilt to minimize dynamic FAI, via gluteus maximus activation, abductor control, transversus abdominis and rectus abdominis activation, iliopsoas stretching, adductor stretching, rectus femoris stretching, core control, and pelvic floor control. Optimizing sagittal balance with the thoracolumbosacral spine and pelvis should be stressed, as improved strength and flexibility as part of a non-surgical management program would significantly ease post-operative rehabilitation in the event of failure of non-operative treatment. In the latter, if the symptoms and/or loss of function are unacceptable to the patient, then surgical treatment is indicated. This includes treatment of not only the labral injury, but also the inciting cause (FAI and/or dysplasia). Contra-indications to labral repair, as part of a comprehensive hip preservation procedure, are relegated more to contra-indications to hip preservation rather than the labral repair itself. These include significant hip osteoarthritis (Tonnis grade 2 or 3 or in patients with less than 2 mm of joint space on a weight-bearing anteroposterior radiograph) [24•] or in combination with uncorrected acetabular dysplasia (more than borderline or mild dysplasia: break in Shenton’s line, femoral head extrusion index >25 %, lateral center edge angle less than 20 degrees, anterior center edge angle less than 20 degrees, and Tonnis angle greater than 15 degrees) [25]. Patients with asymptomatic labral tears are not indicated for labral repair. This is especially important, as the prevalence of asymptomatic labral injury may be as high as 68 %, and there is currently no role for prophylactic arthroscopic preservation, including labral repair [15•, 26•].
Author’s preferred technique for labral tear management
Pre-operative planning
The clinician needs to meticulously consider the patient’s subjective and objective evaluation, including their chief complaint, history of present illness, and physical examination. The accumulation of knowledge attained from this aspect of the assessment must be carefully corroborated with imaging (plain radiographs, magnetic resonance imaging, computed tomography)—“treat the patient, not the MRI.” In evaluation of patients with possible labral injury, these images help confirm or reject possible diagnosis(es) (Table 2). Patients with labral tears, FAI, or dysplasia typically demonstrate an intra-articular evaluation, with a complaint of deep groin pain (a “C” sign, a “between the fingers” sign), worse with flexion rather than extension, worse with sitting rather than standing, and worse with deep flexion and rotational cutting and/or pivoting maneuvers, as frequently performed during sports. A positive response to a previous intra-articular local anesthetic injection may be both diagnostic and therapeutic. Physical examination techniques include FADIR (Flexion, ADduction, Internal Rotation) anterior impingement, subspine impingement, lateral impingement, and posterior impingement maneuvers. It is important to inquire if these maneuvers exactly reproduce the patient’s subjective complaints that brought them to the clinician’s office. A loss of hip flexion and/or internal rotation may be observed. A FABER (Flexion, ABduction, External Rotation) maneuver may reveal an asymmetry in distance to the table (four or more centimeters difference).
Table 2.
Plain radiograph, magnetic resonance imaging, and computed tomography imaging evaluation parameters in patients with suspected labral injury
| Plain radiographs | Magnetic resonance imaging | Computed tomography |
|---|---|---|
| Anteroposterior (weight-bearing) Neck-shaft angle Alpha angle Head-trochanteric distance Lateral center edge angle Tonnis angle Sharp’s angle Femoral head extrusion index Head-center distance Posterior wall sign Ischial spine sign Shenton’s line False profile Alpha angle Anterior center edge angle Dunn 45 degree (90 degree, frog leg, cross-table) lateral Alpha angle Head-neck offset Head-neck offset ratio |
Labral tear Stress fracture (femoral neck, pubis, sacrum) Impingement cyst Subchondral cyst Subchondral edema Effusion Articular cartilage injury Synovial thickening Benign or malignant lesions Tendon tear (iliopsoas, abductor, proximal hamstring, adductor, rectus femoris, rectus abdominis) Osteitis pubis Intra-pelvic pathology (ovarian cyst, ascites, mass) |
Three-dimensional osseous anatomy Acetabular version Femoral version Cortical sclerosis Fracture union |
Patient positioning
The patient is positioned supine on the traction table (Advanced Supine Hip Positioning System with two Universal Hip Distractors and two Active Heel Traction Boots; Smith & Nephew, Andover, MA, USA) to optimize arthroscopic access to the hip joint. General anesthesia with complete muscle relaxation assists in reduction of force necessary to obtain sufficient distraction (less than 50 pounds) for atraumatic entry into the joint [27, 28]. A well-padded perineal post and a greater lateral vector of distraction have been associated with reduced pressure on the pudendal nerve. Although traction time has not been shown to influence the risk of a post-operative nerve event in either supine or lateral positions, the duration of time should be kept below 2 hours to reduce the risk of compressive, ischemic “tourniquet-like” injury to the nerves in the perineum [27, 29]. Hip arthroscopy has even been successfully performed without a post, using a Trendelenburg position, avoiding the perineal complications related to traction [30]. Since the most common complication of hip arthroscopy is iatrogenic chondrolabral injury (during portal placement), proper portal placement begins with perfect patient positioning [31 32•]. This risk can be minimized, or even eliminated, with certain technical pearls [33–36]. The hip arthroscopy learning curve, quantified via a reduction in risk of iatrogenic articular cartilage or labral injury, has been shown to be as few as 20 or as many as over 100 independent hip arthroscopy surgeries [37–40].
Examination under anesthesia
After induction of general anesthesia with paralysis, a thorough examination under anesthesia is performed of bilateral hips, measuring and recording hip flexion, abduction, and internal and external rotations (at 90 degrees of supine hip flexion). External rotation recoil, dial, and traction testing (with and without fluoroscopy) can reveal any capsular contribution to hip instability. Fluoroscopy can also reveal trochanteric-pelvic and ischiofemoral impingement. A vacuum sign may be observed if the labral suction seal is broken, and the negative pressure is released [4•, 13].
Portal placement and interportal capsulotomy
Traction commences with the operative limb in 5 degrees adduction, 5 degrees flexion, and internal rotation dialed to the degree of femoral anteversion. The anterolateral portal (AL) is established first, using 17-gauge spinal needle localization, entering the joint at approximately the 12:30 acetabular clock-face position (right hip reference). A 4.5-mm cannula is placed using the Seldinger technique and the 70 degree arthroscope introduced into the joint. No fluid is utilized yet at this point of the procedure. If synovial fluid or tissue obscures the view, the arthroscope may be removed, wiped, and re-inserted. Alternatively, a syringe of air may be injected through the arthroscope and this will frequently clear the field of view to visualize the anterior triangle, optimizing modified mid-anterior portal (MMAP) placement with spinal needle localization at the 3:00 position. Interportal capsulotomy is created using an arthroscopic 4.0-mm Beaver Blade (Smith & Nephew; Andover, MA, USA). The capsulotomy should be as far away as possible from acetabulum and labrum so as to maximize the amount of tissue available for capsular repair at case conclusion. The lateral component of the capsulotomy is made while viewing from the MMAP portal. At this point, the arthroscopic pump is turned on (40-mm mercury pressure) and fluid instilled in the joint. Next, the medial component of the capsulotomy is made while viewing from the AL portal. A capsular suspension technique using traction sutures significantly improves acetabular rim and anterior inferior iliac spine viewing, permitting improved acetabuloplasty technique, labral repair, and labral refixation visualization [41]. Thorough central compartment diagnostic arthroscopy ensues, with visualization and palpation of all structures.
Labral repair
The key to repairing the labrum is preparing the labrum. This begins with appropriate decortication acetabuloplasty of the rim (with an exact amount based on pre-operative imaging and intra-operative decision-making), to prepare a bleeding surface for labral healing to the bony rim. Depending on the magnitude of rim resection, the labrum does not necessarily need to be detached [42, 43]. After characterization of the location, type, and size of tear (Fig. 1), the repair may begin. Labral repair may be performed with either two portals (MMAP, AL) or with three (MMAP, AL, and DALA [distal anterolateral accessory portal]). The DALA portal is created with spinal needle localization, approximately 4 cm distal to and in line with the AL portal.
Fig. 1.
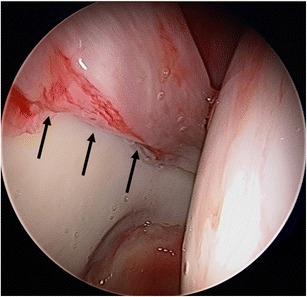
Right hip arthroscopy, anterolateral viewing portal, demonstrating a large labral tear upon placement of the arthroscope into the hip joint prior to instilling fluid into the joint (black arrows indicating the tear, a separation of the transition zone of the chondrolabral junction)
The author prefers a three portal or four portal repair. For suture anchor placement between 3:00 and 11:00, a DALA portal provides an optimal angle for anchor placement. Anterior to 3:00, the MMAP is ideal. Posterior to 11:00, a posterolateral (PL) portal is ideal. Regardless of the portal used, the anchor should be placed as close as possible to the rim so that when knot-tying, the labrum is not pulled over the rim non-anatomically. In addition, the angle must ensure that the drill bit and/or anchor does not penetrate the subchondral bone and/or articular cartilage. In addition, for anterior anchor placement, the angle must ensure that the psoas tunnel is not perforated. The author prefers to use an all-suture anchor for labral repair (Smith & Nephew; Q-Fix, 1.8 mm single-loaded all-suture anchor—drill depth 22 mm; anchor deploys to a 4.0-mm diameter quasi-sphere). Typically, three to six anchors are utilized for each labral repair, spaced approximately 5 to 7 mm apart (approximately every 45 to 60 min on the acetabular clockface).
Using the acetabular rim angle, the safety margin for anchor placement has been welldefined [44]. The acetabular rim angle is the angle subtended by the subchondral margin and the outer acetabular cortex, quantifying how much room exists to place a suture anchor completely in bone. The rim angle was greatest at 2 o’clock and smallest at 3 o’clock. Rim trimming increased the rim angle and increased drill depth decreased the rim angle. Using a similar study design, posterior suture anchor placement between 11 o’clock and 8 o’clock positions the anchor completely in bone consistently from a PL portal, but MA and DALA portal use perforated the cortex in all cases [45]. Curved drill guides and curved anchors have been utilized to circumvent iatrogenic articular penetration [46]. When articular penetration is unrecognized, bipolar articular cartilage damage may rapidly progress and total hip arthroplasty may be the only reliable solution [47]. When psoas tunnel perforation occurs, patients may complain of persistent pain (anterior impingement and resisted hip flexion) and MRI may reveal anchor penetration into the psoas tunnel [48].
After the anchor hole is drilled, the repair type suture configuration must be selected. The goals of a looped suture repair and a labral base refixation mattress repair are essentially one and the same [43, 49]. In situations of a small labrum (less than 5-mm width), a looped repair may be better than a pierced design as sufficient quality and quantity tissue may not be present to pass a penetrating instrument through the labrum and tie an arthroscopic knot with tension (Fig. 2). In medium to larger labra (greater than 5-mm width), a looped repair or a mattress labral base refixation may be equivalent, as long as restoration of the suction seal is achieved. Proponents of the labral base refixation technique espouse that the normal triangular shape is lost with the looped repair [43]. Opponents of the labral base refixation technique claim that (1) the suction seal is still restored with a looped repair; (2) that the pierced suture in base refixation may damage the substance of the labrum; and (3) that the suture itself is less than 0.5 mm in diameter, the tension of the repair may be titrated to the appropriate amount; and that the labrum may partially incorporate the suture and not “spot-weld” the triangular shape to a cylindrical shape as much as the labral base refixation technique proponents claim [49].
Fig. 2.
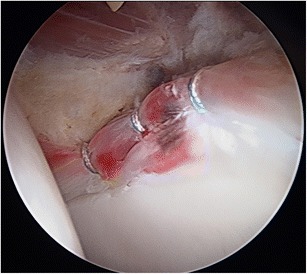
Left hip arthroscopy, anterolateral viewing portal, three anchor looped configuration labral repair, with anchors placed at approximately 3, 2, and 1 o’clock locations on the acetabular clock face. The small size of the labrum (less than 5-mm width) required a looped design to be performed, as a pierced design would be unable to gain meaningful tissue to repair
Outcomes
In comparison to labral debridement (with concomitant FAI treatment for both groups), labral repair has consistently demonstrated significantly better subjective patient-reported outcomes (Harris Hip Score, SF-12, and VAS pain) at a mean 3.5-year follow-up [50]. In addition, 92 % of subjects and 68 % of the subjects in the repair and debridement groups, respectively, reported good to excellent results. The earlier publication (17-month mean follow-up) revealed similar findings, exhibiting superiority of repair over debridement [51]. A similar comparative investigation at a separate institution randomized females to either debridement or repair with a 32-month mean follow-up [52]. Labral repair, again, demonstrated significantly better patient-reported outcomes (both Hip Outcome Score activities of daily living and sport-specific subscales).
In comparison of looped versus pierced suture techniques for labral repair, statistically and clinically equivalent Hip Outcome Scores have been demonstrated at a mean 3-year follow-up [53]. In addition, no significant difference in revision or failure rates was observed. A similar investigation at a separate institution demonstrated no statistical or clinical difference between looped and pierced techniques at 30-month mean follow-up using multiple patient-reported outcomes (modified Harris Hip Score, Hip Outcome Score, Non-Arthritic Hip Score, VAS-pain, and patient satisfaction) [54].
The largest, most recent, highest quality systematic review has analyzed 490 subjects from six unique investigations reporting labral repair outcomes [55•]. The modified Harris Hip Score, although not specifically developed or validated for non-arthritic patients with labral tears and FAI, was the most commonly reported (50 %) outcome measure. In comparison to debridement, labral repair consistently reported greater post-operative improvements in multiple patient-reported outcome scores (modified Harris Hip Score, Hip Outcome Score, Non-Arthritic Hip Score, and Merle d’Aubigne score). Study methodological quality was reported individually as fair, but GRADE recommendation as low. Future investigations should strive for high-quality, well-designed, prospective studies of subjects undergoing labral preservation with longer follow-up using both subjective patient-reported and objective clinician-measured outcome scores with appropriate psychometric properties.
Conclusions
The acetabular labrum serves a critical function in the hip. It may be damaged secondary to multiple osseous and soft tissue abnormalities, such as femoroacetabular impingement, dysplasia, extra-articular impingement, among others. Once injured, the labrum may cause significant symptoms. Due to the high prevalence of asymptomatic labral tear, the clinician must ensure that the patient’s complaints match their imaging studies (“treat the patient, not the MRI”). Once diagnosed, multiple non-surgical treatments exist that may improve pain and function. Once non-operative measures have failed and the patient finds their symptoms unacceptable, surgical treatment should consist of treating the labrum with repair (or refixation), not debridement, when sufficient tissue is present to repair. This must occur in conjunction with a complete correction of any osseous pathomorphology. Short- and mid-term outcomes have shown excellent patient-reported results after labral repair in non-arthritic subjects.
Compliance with ethical standards
Conflict of Interest
Dr. Harris reports JDH: Editorial board: Arthroscopy: The Journal of Arthroscopic and Related Surgery; Frontiers In Surgery; Research Support: Smith & Nephew; Depuy Synthes; Publication royalties: SLACK, Inc.; Committees: AOSSM Self-Assessment Committee; AAOS Osteoarthritis Pain and Function Workgroup; AANA Research Committee.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Outcomes Research in Orthopedics
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Seldes RM, et al. Anatomy, histologic features, and vascularity of the adult acetabular labrum. Clin Orthop Relat Res. 2001;382:232–40. doi: 10.1097/00003086-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 2.•.Dwyer MK, et al. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42(4):812–9. doi: 10.1177/0363546514522395. [DOI] [PubMed] [Google Scholar]
- 3.Philippon MJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722–9. doi: 10.1007/s00167-014-2874-z. [DOI] [PubMed] [Google Scholar]
- 4.•.Harris JD, et al. Microinstability of the Hip and the Splits Radiograph. Orthopedics. 2016;39:e169–75. doi: 10.3928/01477447-20151228-08. [DOI] [PubMed] [Google Scholar]
- 5.Duplantier NL, et al. Hip Dislocation or Subluxation After Hip Arthroscopy: a Systematic Review. Arthroscopy. 2016. [DOI] [PubMed]
- 6.Nepple JJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730–6. doi: 10.1007/s00167-014-2875-y. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer MK, et al. Femoroacetabular impingement negates the acetabular labral seal during pivoting maneuvers but not gait. Clin Orthop Relat Res. 2015;473(2):602–7. doi: 10.1007/s11999-014-3760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolan MM, et al. CT reveals a high incidence of osseous abnormalities in hips with labral tears. Clin Orthop Relat Res. 2011;469(3):831–8. doi: 10.1007/s11999-010-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guevara CJ, et al. Comprehensive morphologic evaluation of the hip in patients with symptomatic labral tear. Clin Orthop Relat Res. 2006;453:277–85. doi: 10.1097/01.blo.0000246536.90371.12. [DOI] [PubMed] [Google Scholar]
- 10.Peelle MW, et al. Acetabular and femoral radiographic abnormalities associated with labral tears. Clin Orthop Relat Res. 2005;441:327–33. doi: 10.1097/01.blo.0000181147.86058.74. [DOI] [PubMed] [Google Scholar]
- 11.Wenger DE, et al. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145–50. doi: 10.1097/01.blo.0000136903.01368.20. [DOI] [PubMed] [Google Scholar]
- 12.Domb, B.G., et al., Iliopsoas impingement: a newly identified cause of labral pathology in the hip. HSS J. 7(2): p. 145-50 [DOI] [PMC free article] [PubMed]
- 13.Mitchell RJ, et al. Radiographic evidence of Hip microinstability in elite ballet. Arthroscopy. 2016;32(6):1038–1044.e1. doi: 10.1016/j.arthro.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Duthon VB, et al. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy. 2013;29(3):411–9. doi: 10.1016/j.arthro.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 15.•.Frank JM, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy. 2015;31(6):1199–204. doi: 10.1016/j.arthro.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Kalhor M, et al. Vascular supply to the acetabular labrum. J Bone Joint Surg Am. 2010;92(15):2570–5. doi: 10.2106/JBJS.I.01719. [DOI] [PubMed] [Google Scholar]
- 17.Philippon MJ, Arnoczky SP, Torrie A. Arthroscopic repair of the acetabular labrum: a histologic assessment of healing in an ovine model. Arthroscopy. 2007;23(4):376–80. doi: 10.1016/j.arthro.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Audenaert EA, et al. Histologic assessment of acetabular labrum healing. Arthroscopy. 2012;28(12):1784–9. doi: 10.1016/j.arthro.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 19.White BJ, et al. Allograft use in arthroscopic labral reconstruction of the Hip with front-to-back fixation technique: minimum 2-year follow-up. Arthroscopy. 2016;32(1):26–32. doi: 10.1016/j.arthro.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Haversath M, et al. The distribution of nociceptive innervation in the painful hip: a histological investigation. Bone Joint J. 2013;95-b(6):770–6. doi: 10.1302/0301-620X.95B6.30262. [DOI] [PubMed] [Google Scholar]
- 21.Alzaharani A, et al. The innervation of the human acetabular labrum and hip joint: an anatomic study. BMC Musculoskelet Disord. 2014;15:41. doi: 10.1186/1471-2474-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhardt M, et al. Characterisation and classification of the neural anatomy in the human hip joint. Hip Int. 2012;22(1):75–81. doi: 10.5301/HIP.2012.9042. [DOI] [PubMed] [Google Scholar]
- 23.White BJ, Herzog MM. Arthroscopic labral reconstruction of the Hip using iliotibial band allograft and front-to-back fixation technique. Arthrosc Tech. 2016;5(1):e89–97. doi: 10.1016/j.eats.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.•.Philippon MJ, et al. Joint space predicts THA after hip arthroscopy in patients 50 years and older. Clin Orthop Relat Res. 2013;471(8):2492–6. doi: 10.1007/s11999-012-2779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris JD, et al. Radiographic prevalence of dysplasia, Cam, and pincer deformities in elite ballet. Am J Sports Med. 2016;44(1):20–7. doi: 10.1177/0363546515601996. [DOI] [PubMed] [Google Scholar]
- 26.•.Collins JA, Ward JP, Youm T. Is prophylactic surgery for femoroacetabular impingement indicated? A systematic review. Am J Sports Med. 2014;42(12):3009–15. doi: 10.1177/0363546513499227. [DOI] [PubMed] [Google Scholar]
- 27.Telleria JJ, et al. Risk of sciatic nerve traction injury during hip arthroscopy—is it the amount or duration? An intraoperative nerve monitoring study. J Bone Joint Surg Am. 2012;94(22):2025–32. doi: 10.2106/JBJS.K.01597. [DOI] [PubMed] [Google Scholar]
- 28.Kocaoglu H, et al. The effect of traction force and Hip abduction angle on pudendal nerve compression in Hip arthroscopy: a cadaveric model. Arthroscopy. 2015;31(10):1974–80.e6. doi: 10.1016/j.arthro.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Birmingham P. Hip arthroscopy neurapraxia: is it only about weight of traction? J Bone Joint Surg Am. 2012;94(22) doi: 10.2106/JBJS.L.01062. [DOI] [PubMed] [Google Scholar]
- 30.Mei-Dan O, McConkey MO, Young DA. Hip arthroscopy distraction without the use of a perineal post: prospective study. Orthopedics. 2013;36(1):e1–5. doi: 10.3928/01477447-20121217-10. [DOI] [PubMed] [Google Scholar]
- 31.Harris, J., et al., Complications and Re-operations During and Following Hip Arthroscopy—a Systematic Review of 92 Studies and Over 6,000 Patients. Arthroscopy, 2012.
- 32.•.Weber AE, Harris JD, Nho SJ. Complications in Hip arthroscopy: a systematic review and strategies for prevention. Sports Med Arthrosc. 2015;23(4):187–93. doi: 10.1097/JSA.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 33.Domb B, Hanypsiak B, Botser I. Labral penetration rate in a consecutive series of 300 hip arthroscopies. Am J Sports Med. 2012;40(4):864–9. doi: 10.1177/0363546512437152. [DOI] [PubMed] [Google Scholar]
- 34.Domb BG, Botser IB. Iatrogenic labral puncture of the hip is avoidable. Arthroscopy. 2012;28(3):305–7. doi: 10.1016/j.arthro.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Badylak JS, Keene JS. Do iatrogenic punctures of the labrum affect the clinical results of hip arthroscopy? Arthroscopy. 2011;27(6):761–7. doi: 10.1016/j.arthro.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Aoki SK, Beckmann JT, Wylie JD. Hip arthroscopy and the anterolateral portal: avoiding labral penetration and femoral articular injuries. Arthrosc Tech. 2012;1(2):e155–60. doi: 10.1016/j.eats.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(Suppl 2):52–6. doi: 10.2106/JBJS.J.01587. [DOI] [PubMed] [Google Scholar]
- 38.Lee YK, et al. Learning curve of basic hip arthroscopy technique: CUSUM analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1940–4. doi: 10.1007/s00167-012-2241-x. [DOI] [PubMed] [Google Scholar]
- 39.Hoppe DJ, et al. The learning curve for hip arthroscopy: a systematic review. Arthroscopy. 2014;30(3):389–97. doi: 10.1016/j.arthro.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Park MS, et al. Hip arthroscopy for femoroacetabular impingement: the changing nature and severity of associated complications over time. Arthroscopy. 2014;30(8):957–63. doi: 10.1016/j.arthro.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Federer AE, et al. Capsular suspension technique for Hip arthroscopy. Arthrosc Tech. 2015;4(4):e317–22. doi: 10.1016/j.eats.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redmond JM, et al. Arthroscopic acetabuloplasty and labral refixation without labral detachment. Am J Sports Med. 2015;43(1):105–12. doi: 10.1177/0363546514555330. [DOI] [PubMed] [Google Scholar]
- 43.Fry R, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy. 2010;26(9 Suppl):S81–9. doi: 10.1016/j.arthro.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Lertwanich P, et al. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(Suppl):111S–6S. doi: 10.1177/0363546511413746. [DOI] [PubMed] [Google Scholar]
- 45.Foster AD, et al. Safe suture anchor insertion for anterior and posterior hip labral repair. J Hip Preserv Surg. 2015;2(2):170–4. doi: 10.1093/jhps/hnv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nho SJ, et al. Computed tomographic analysis of curved and straight guides for placement of suture anchors for acetabular labral refixation. Arthroscopy. 2013;29(10):1623–7. doi: 10.1016/j.arthro.2013.07.262. [DOI] [PubMed] [Google Scholar]
- 47.Matsuda DK, et al. Anchor-induced chondral damage in the hip. J Hip Preserv Surg. 2015;2(1):56–64. doi: 10.1093/jhps/hnv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Degen RM, et al. Psoas tunnel perforation—an unreported complication of hip arthroscopy. J Hip Preserv Surg. 2015;2(3):272–9. doi: 10.1093/jhps/hnv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lertwanich P, Ejnisman L, Philippon MJ. Comments on “Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair”. Arthroscopy. 2011;27(3):303–4. doi: 10.1016/j.arthro.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Larson CM, Giveans MR, Stone RM. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement: mean 3.5-year follow-up. Am J Sports Med. 2012;40(5):1015–21. doi: 10.1177/0363546511434578. [DOI] [PubMed] [Google Scholar]
- 51.Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369–76. doi: 10.1016/j.arthro.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Krych AJ, et al. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46–53. doi: 10.1016/j.arthro.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Sawyer GA, et al. Clinical outcomes after arthroscopic Hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683–8. doi: 10.1177/0363546515581469. [DOI] [PubMed] [Google Scholar]
- 54.Jackson TJ, et al. Acetabular labral base repair versus circumferential suture repair: A matched-paired comparison of clinical outcomes. Arthroscopy. 2015;31(9):1716–21. doi: 10.1016/j.arthro.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 55.•.Ayeni OR, et al. Surgical management of labral tears during femoroacetabular impingement surgery: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):756–62. doi: 10.1007/s00167-014-2886-8. [DOI] [PubMed] [Google Scholar]


