Abstract
Hip dysplasia is a treatable developmental disorder that presents early in life but if neglected can lead to chronic disability due to pain, decreased function, and early osteoarthritis. The main causes of hip dysplasia in the young adult are residual childhood developmental dysplasia of the hip (DDH) and adolescent-onset acetabular dysplasia. These two distinct disease processes affect the growing hip during different times of development but result in a similar deformity and pathomechanism of hip degeneration. Routine screening for DDH and counseling regarding risks for acetabular dysplasia in families with a history of early hip osteoarthritis may allow early identification and intervention in these hips with anatomical risk factors for joint degeneration.
Keywords: Hip dysplasia, Acetabular dysplasia, DDH, Residual hip dysplasia, Periacetabular osteotomy, PAO
Introduction
Hip dysplasia is a treatable disease that presents early in life but if neglected can lead to chronic disability. Hip dysplasia can be present in infancy, which we term developmental dysplasia of the hip (DDH), or only arise later in adolescence or young adulthood, which we refer to as acetabular dysplasia. Although screening infants for DDH has been widely advocated, less attention has been devoted towards the risk factors, early detection of, and treatment considerations in adolescent-onset acetabular dysplasia. The poor prognosis of untreated hip dysplasia warrants careful attention towards early identification and intervention in these hips with anatomical risk factors for eventual mechanical misbehavior.
Epidemiology and natural history
Hip dysplasia is a dynamic, mechanical disorder that causes structural damage to the hip. Abnormal hip anatomy results in abnormal hip mechanical behavior, which in turn, causes more anatomical abnormalities. Hip dysplasia is an unstable ball-in-socket hip joint that is characterized by a shallow acetabulum that does not cover the femoral head sufficiently. This anatomical aberration produces instability of the hip and increased mechanical stress at the acetabular rim, leading to hypertrophy and tearing of the fibrocartilaginous acetabular labrum and more rapid degeneration of the hyaline articular cartilage. If left untreated, hip dysplasia will cause pain, decreased function, and eventually result in hip osteoarthritis.
The incidence of hip dysplasia is reported to range from 1.7 to 20 % in the general population, with most studies finding the incidence between 3 and 5 % [1–5]. Females have a two to four times increased relative risk of hip dysplasia [4, 6, 7], but males with hip dysplasia tend to have a higher incidence of concomitant hip deformities such as acetabular retroversion and a CAM deformity at the femoral head-neck junction that may need additional consideration during hip dysplasia surgery to prevent iatrogenic creation of femoroacetabular impingement [8]. A family history of hip dysplasia also increases the relative risk of having hip dysplasia by 1.4 to 1.7 % [4, 6, 7]. More than 50 % of patients with hip dysplasia have a positive family history of hip disease [9•].
Hip dysplasia is the leading cause of early onset hip osteoarthritis before the age of 60 years [1, 10–13]. In a recent study of patients under 50 years old undergoing total hip replacement for osteoarthritis, 48.4 % were found to have underlying hip dysplasia as the etiology of hip osteoarthritis [14•]. In general, the more severe the dysplasia, the higher the risk is of developing osteoarthritis. Globally dysplastic hips with combined deficiencies in both anterior and lateral coverage have a higher risk of incident osteoarthritis, with an odds ratio of 5.45 compared to 2.26 if only lateral undercoverage is present [15]. The occurrence of a tear of the acetabular labrum, the fibrocartilage ring at the acetabular periphery that provides compensatory hip stability and which is subjected to higher mechanical stresses with acetabular rim loading in hip dysplasia [16], also increases the risk of osteoarthritis [17].
Anatomic abnormalities, unfavorable mechanics, and structural damage
Hip dysplasia exists as a spectrum of mechanical instability that determines hip functionality. Overall, the more unstable the hip, the earlier the onset is of symptoms (Fig. 1a). The most severely dysplastic and unstable hip is the developmentally dislocated hip that is often caught in infancy. Without proper contact between the femoral head and the acetabulum, the dislocated hip will develop abnormally into an aspherical femoral head and a shallow, dish-shaped acetabulum. The affected leg will be functionally shorter, hip range of motion will be limited, a marked limp during gait will evolve, and ipsilateral knee and lower back pain from compensatory gait mechanics will develop over time [18].
Fig. 1.
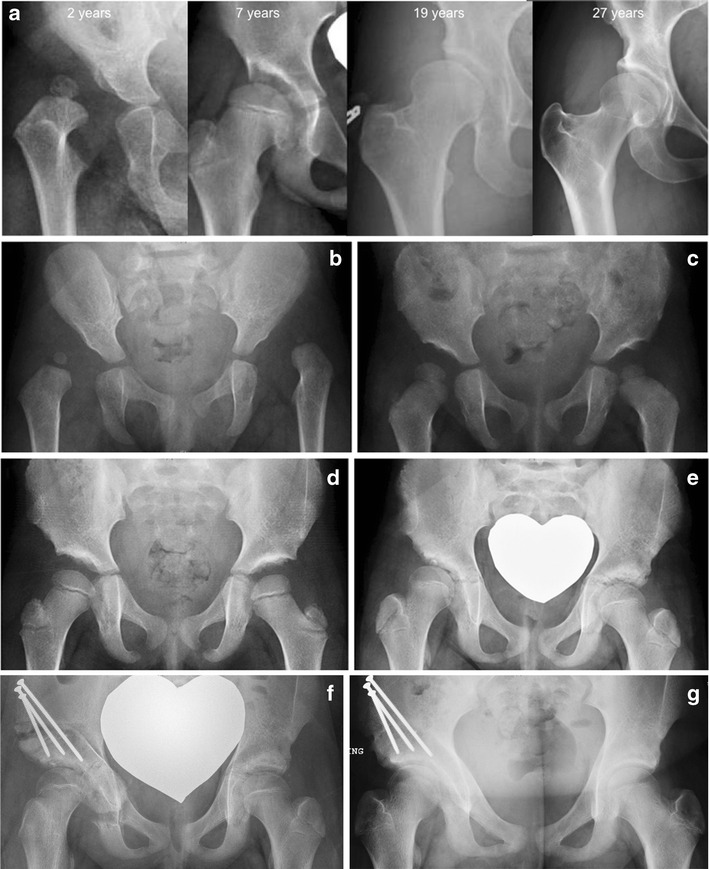
a Four different patients who presented at the ages listed above, demonstrating decreasing severity of dysplasia in the images from left to right. b A 1-year-old female child with bilateral developmentally dislocated hips that subsequently underwent bilateral open reductions and spica casting. c The same child at 3 years of age showing hips that are well-seated within the bilateral acetabula. d The same child at 5 years of age showing progression of hip growth and improvement in acetabular remodeling. e The same child returned at 10 years of age complaining of right hip pain. Although the hips appeared symmetric radiographically at the last follow-up at 5 years of age, the right hip has failed to develop adequately and the patient now had symptomatic residual acetabular dysplasia. f The right hip was treated with a periacetabular osteotomy (PAO) in order to improve femoral head coverage by the acetabulum. g The patient at 11 years of age, 1 year after PAO, with no right hip pain and return to all activities
In contrast, a less dysplastic, well-located but undercovered hip might take years to decades for the abnormal mechanics to produce enough intra-articular structural damage and extra-articular muscular compensatory motion to result in symptoms of pain and instability. This is the history with many cases of adolescent-onset acetabular dysplasia, where patients function well for many years before symptom onset.
Anatomical abnormalities involve both the acetabulum and the femur. The dysplastic acetabulum is globally, volumetrically deficient, an average of 18–19 % smaller than normal [19]. Compared to normal hips, up to 50 % of dysplastic hips requiring treatment develop a relatively hypertrophic acetabular labrum in an attempt to compensate for deficient bony acetabular coverage and to keep the femoral head within the joint [20–22]. The proximal femur is also commonly dysplastic, with an ellipsoid femoral head, a stouter femoral neck, and coxa valga [23].
CT and MRI studies have demonstrated that dysplastic hips have less surface contact area between the acetabulum and the femoral head as compared to normal hips. Using MRI, Steppacher and colleagues found the articular cartilage surface area in the dysplastic acetabulum to be 16 % smaller than normal [24•]. The dysplastic hip’s smaller acetabular cartilage surface area results in a 26 % smaller cartilage contact area between the acetabulum and the femoral head, which causes a 23 % increase in contact pressure within the joint [25]. Subluxation of the femoral head, if present, further decreases contact area between the acetabulum and the femoral head, compounding the already elevated joint contact pressure.
Over time, the increased cartilage contact stresses cause early cartilage degeneration. Compared to normal hips, dysplastic hips have higher peak and cumulative contact stresses. Mavcic and colleagues followed 58 asymptomatic hips with acetabular dysplasia for a minimum of 20 years and compared them to 48 hips without disease [26•]. They found that cumulative contact stress was the best predictor of developing osteoarthritis and that the prevalence of osteoarthritis in patients with dysplasia at age 58 was the same as in patients with normal hips at age 68. Linear extrapolation data demonstrated that the normal hips would only achieve dysplastic-level cumulative contact stresses at the age of 90 years. In other words, dysplasia effectively causes “premature aging” of the hip.
Since the rim of the dysplastic acetabulum is abnormally located directly superior to the femoral head, within the main weight-bearing zone of the hip joint, structural damage develops at the acetabular rim where abnormal loading causes concentrated contact stress, resulting in degenerative labral tears, full-thickness labral tears, intra-substance labral ganglions and paralabral cysts, and acetabular rim bone cysts and fractures [16, 27, 28]. Contact stress increases with worsening severity of acetabular dysplasia [29]. Severity of dysplasia is commonly measured by the lateral center edge angle (LCEA), which is formed by two lines from the center of the femoral head to the lateral edge of the roof of acetabulum and directly vertical, respectively. LCEA reflects the severity of dysplasia by measuring the degree of lateral femoral head coverage by the acetabulum. A similar measurement, which requires “false profile” radiographic views of the hip, is called the anterior center edge angle (ACEA), which reflects anterior coverage insufficiency in dysplasia. LCEAs less than 25° have been associated with a 4.3-fold increased risk of incident radiographic osteoarthritis [30] and increased risk of progression of such osteoarthritis over time [31]. Thomas and colleagues found that with each degree decrement in LCEA less than 28°, there was a 13 % increased risk of osteoarthritis and an 18 % increased risk of future total hip replacement [32].
Clinical presentation and diagnosis of hip dysplasia
In childhood DDH, the diagnosis is commonly made in the first few months after birth. Diagnosis of DDH is made through physical examination and ultrasound screening [33]. A positive Ortolani test, in which a dislocated femoral head is brought back into the acetabulum by abduction of the hip, indicates an unstable hip. Other findings include limited or asymmetric hip abduction, a positive Barlow test, and the appearance of uneven leg lengths with a positive Galeazzi test [34, 35]. Diagnosis is confirmed with ultrasound imaging. Despite the functional difficulties associated with DDH, children compensate well for structural deformities early in life and are often without pain.
The most common presenting symptoms of acetabular dysplasia in the skeletally mature individual are insidious onset of activity-related groin pain and/or lateral hip pain [36]. Matheney and colleagues recently found that severe dysplasia (LCEA <5°) and high activity level (UCLA score 8–10) are independent predictors of younger patient age at presentation for surgical treatment [37•]. Diagnosis is made through physical exam and radiographic findings. Up to 48 % will have a limp during gait and 97 % have a positive anterior impingement test [36]. Anteroposterior pelvic radiographs show a LCEA less than 25°, an ACEA less than 20°, and a more obliquely oriented sourcil, or weight bearing roof of the bony acetabulum, with an elevated Tönnis Angle greater than 10° [38, 39].
Childhood DDH versus adolescent-onset acetabular dysplasia
In the past, acetabular dysplasia diagnosed in adolescence or young adulthood was considered as a delayed diagnosis of childhood DDH. Although there are certainly cases of delayed diagnosis of DDH, the acetabulum continues to develop through early adolescence, so acetabular dysplasia may not be structurally present until the last stages before skeletal maturity. Despite being similarly destructive in mechanical behavior, it now appears that adolescent-onset acetabular dysplasia may be a distinct disease process from childhood DDH.
The development of DDH is thought to be due to a combination of genetic factors and intrauterine positioning. The strongest risk factor for DDH is breech presentation, which if present, confers a relative risk of 3.75 to 6.3 times normal [4, 6, 7]. Other risk factors include female gender, being the first born child, and a positive family history of DDH [4, 6, 7].
The development of adolescent-onset acetabular dysplasia has been theorized to involve delayed ossification of the triradiate cartilage and insufficient development of the lateral secondary ossification centers at the acetabular rim that usually ossify during adolescence, between 12 and 18 years of age [40, 41]. Acetabular dysplasia is also more common in females (88 %), but the female predominance is less pronounced than in DDH (98 %) [9•].
Both children with DDH and adults with acetabular dysplasia are very likely to have a positive family history of hip disease. Lee and colleagues found that of adults with hip dysplasia requiring treatment, 52 % with a history of childhood DDH and 55 % without a childhood history of DDH had a positive family history of hip disease [9•]. However, the type of hip disease present in family members differed between these two patient groups. Patients with DDH were more likely to have a first-order family member with DDH (59 %) than with early hip osteoarthritis requiring a hip replacement at an age less than 65 years (23 %). In contrast, patients with acetabular dysplasia without a childhood history of DDH were more likely to have a first-order family member with an early hip replacement (50 %) than a first-order family member with DDH (16 %).
Several other characteristics differ between DDH and adolescent-onset acetabular dysplasia. DDH affects the left hip more commonly [4, 9•]. Acetabular dysplasia appears bilaterally 61–84 % of the time, affecting both hips more often than does DDH [9•, 42]. Breech presentation appears to be a more significant risk factor for DDH than acetabular dysplasia. A history of breech presentation was found in 25 % of patients with DDH but only in 9 % of patients with acetabular dysplasia [9•].
Residual hip dysplasia from childhood DDH
Not all acetabular dysplasia in the young adult is adolescent-onset. Hip dysplasia in the young adult can also be a result of unresolved childhood DDH (Fig. 1b–g). Modaressi and colleagues reported a 2.7 % (4/150) incidence of residual hip dysplasia at adolescence after successful treatment of childhood DDH [43]. Acetabular remodeling, which requires pressure of the well-seated femoral head against the acetabulum, may also be inhibited by the presence of femoral head osteonecrosis, a frequent complication of childhood treatment of DDH.
Young adults with a history of DDH and residual hip dysplasia at skeletal maturity tend to have an earlier presentation of symptoms as compared to adolescent-onset acetabular dysplasia. Okano and colleagues found that 36 % of 245 skeletally mature patients with symptomatic acetabular dysplasia had a history of DDH that had been treated in childhood. These patients had hips with more severe deformity and presented at a lower mean age (36 years) than those without prior treatment for DDH (43 years) [44].
Residual hip dysplasia, especially with subluxation, is correlated with increased incidence of arthritis. Terjesen and colleagues reported on long-term outcomes in 60 hips following treatment of late-detected DDH at mean age of 19 months. At a mean age of 45 years, 4/5 (80 %) of hips with subluxation had osteoarthritis, whereas only 4/18 (22 %) of hips with LCEA 10–19° had osteoarthritis and 2/37 (5 %) of hips with LCEA > 20° had osteoarthritis [45]. Albinana and colleagues reported long-term follow-up of 72 developmentally dislocated hips that were treated with closed reduction. At mean 40-year follow-up, there was a 29 % probability of having had a total hip replacement if LCEA 15–20° and a 49 % probability if LCEA < 15° and the hip was subluxated [46].
Successful correction of childhood DDH and careful monitoring for residual dysplasia through skeletal maturity is essential in preventing osteoarthritis later in life. Periodic radiographic monitoring has been recommended for both breech infants and children treated for DDH even after achievement of normal radiographic criteria. Imrie and colleagues found that 29 % of breech infants with normalized ultrasounds at 6 weeks of age will have signs of DDH requiring treatment on radiographs at 6 months of age [47]. For infants with the diagnosis of DDH, it has been reported that 33 % of patients have radiographic signs of persistent dysplasia at 1 year of age regardless of treatment [48]. For patients with delayed appearance of the femoral ossific nucleus, Tönnis and colleagues recommended screening for adolescent-onset acetabular dysplasia starting at 8 years of age even if prior radiographs had normalized [40].
Treatment of hip dysplasia in the young adult
Despite epidemiological differences, the end result of hip dysplasia whether from residual childhood DDH or adolescent-onset acetabular dysplasia is hip instability and increased hip contact stresses that lead to early joint destruction. Therefore, the treatment of acetabular dysplasia in the skeletally mature patient is aimed towards reconstructing normal mechanical forces within the dysplastic hip.
The periacetabular osteotomy (PAO) was first described by Professor Reinhold Ganz nearly 30 years ago [49] and has become the treatment of choice for symptomatic acetabular dysplasia in the skeletally mature patient. The PAO allows complete reorientation of the acetabulum to provide more lateral and anterior femoral head coverage by the acetabulum, reduce hip subluxation and medialize the hip joint center, redistribute the high contact stresses from the acetabular rim to the entire articular surface, and to transform the dysplastic hip’s shear stresses across the articular cartilage into compressive stresses that are more favorable for cartilage longevity [29, 50].
The short-term outcomes after PAO show that up to 71 % of patients are able to achieve the same or higher level of physical activity following surgery [51]. Novais and colleagues demonstrated that at 2-year follow-up, UCLA activity scores were on average higher and WOMAC pain scores were lower when compared to preoperative scores [52]. Younger age, preoperative higher physical activity level, and lower postoperative pain levels were independent predictors of postoperative activity level in this short-term follow-up. Competitive athletes, however, may find it more difficult to return to the same level of competitive sporting that they were capable of preoperatively. Heyworth and colleagues found that although the majority (80 %) of athletes undergoing PAO were able to return to play at 9 months postoperatively and 73 % of those patients returned to the playing at the same level, though only 58 % of competitive athletes were able to return to playing at their same competitive level [53].
Longer-term outcomes after PAO have used pain, development of osteoarthritis, and conversion to total hip replacement as end points for survival. Matheney and colleagues reported an overall 76 % survivorship rate in 135 hips at mean 9-year follow-up when using failure criteria of conversion to total hip replacement and a postoperative WOMAC pain score of 10 or more [54]. Using only conversion to total hip replacement as the endpoint, 96 % of hips survived at 9 years post-PAO. In 121 hips at 18 years after PAO, Wells and colleagues reported a 74 % survival rate free of total hip replacement; of the surviving hips, 53 % of hips remained asymptomatic, and 25 % were considered failed based on a WOMAC pain score of 10 or more [55•]. Following 68 hips after PAO for a mean of 20 years, Steppacher and colleagues reported a 60 % survival rate free of end-stage osteoarthritis needing total hip replacement or fusion [56]. Risk factors for failure after PAO include age greater than 25 years, minimum joint space less than 2 mm, and poor or fair joint congruency [55•].
Surgical treatment of residual dysplasia after prior childhood surgery for DDH can present more complexities than treating adolescent-onset acetabular dysplasia with no prior surgical treatment. Hips that have experienced prior surgery may have scar tissue that affects surgical technique and altered anatomy that compromises both preoperative and postoperative patient function. Following pelvic osteotomy surgery in early childhood, there is evidence in gait analysis studies that patients have hip muscular weakness, gait deviations, and increased peak axial forces within the joint itself [57, 58].
In patients who had not undergone prior pelvic surgery, the reported major complication rate was 5.9 % in 205 unilateral PAO surgeries [59]. Major complications included deep infection, nerve palsy, venous thromboembolic disease, change in acetabular position, hardware migration, and osteotomy nonunion. In comparison, Polkowski and colleagues found a 19 % overall and 11.9 % major complication rate in 67 PAOs performed in patients who had prior surgery for hip dysplasia [60]. Complications ranged from superficial wound infections and paresthesias to osteotomy nonunion and femoral and sciatic nerve palsies. Ten percent of patients had subsequent surgery for unresolved pain or deformity following PAO and 7 % converted to total hip replacement during the 2-year follow-up period. Although the average Harris Hip Score improved by 11 points, proving that PAO can improve hip function in this population, the authors cautioned that performing PAO in patients who have had previous hip surgery is a more complex endeavor that has a higher risk of perioperative complications and poor outcomes.
Conclusions
Since the hip continues growing until the early adolescent years, any disturbance to the hip’s normal course of development or growth potential early in life can lead to hip dysplasia. The main causes of hip dysplasia in the young adult are residual childhood DDH and adolescent-onset acetabular dysplasia. These two distinct disease processes affect the growing hip during different times of development but result in a similar deformity and pathomechanism of hip degeneration.
The standard treatment of hip dysplasia after skeletal maturity is hip reorienting procedures, most commonly with the periacetabular osteotomy. However, there are more complications associated with PAO in the setting of prior surgical treatment of childhood DDH. Residual DDH deformity is often more severe than adolescent-onset acetabular dysplasia, so further adjunctive intra-articular and femoral surgeries may be necessary for residual dysplasia. After PAO, patients usually experience decreased pain and improved function. Fewer hips treated with PAO go on to develop end-stage osteoarthritis at 18–20-year follow-up, which is an improvement over the natural history. However, even longer follow-up studies are necessary to show the duration of benefit of PAO surgery.
Long-term outcome studies of hip dysplasia treatment consistently show that increased likelihood of native hip “survival” is related to a younger patient age and a healthier hip at the time of intervention. Thus, we should recognize the distinct clues and risk factors for both childhood DDH and adolescent-onset acetabular dysplasia in order to identify those hips at risk for degeneration as early as possible and intervene prior to irreversible joint damage.
For DDH, current guidelines recommend clinical screening of all infants and ultrasound screening of infants with risk factors and those with clinical signs of hip instability. Knowing that residual hip dysplasia can produce significant problems at skeletal maturity, continued radiographic monitoring for residual hip dysplasia is recommended through the adolescent years.
To diagnose adolescent-onset acetabular dysplasia, it may be wise to counsel families with any family history of early onset hip osteoarthritis to closely monitor for symptoms of hip dysplasia in their first-order family members and to present for screening if any symptoms develop. If screening identifies an asymptomatic adolescent or young adult with acetabular dysplasia, it may be beneficial to counsel the patient regarding activity modifications and to return for treatment when symptoms occur but before the initiation of joint damage. Advancing knowledge of the risk factors, signs and symptoms of hip dysplasia in the young adult will promote the identification of hips at risk for early degeneration and possibly decrease the disease burden of early hip osteoarthritis.
Compliance with ethical standards
Conflict of interest
The author declares that she has no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Pediatric Orthopedics
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Jacobsen S, Sonne-Holm S. Hip dysplasia: a significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology (Oxford) 2005;44(2):211–218. doi: 10.1093/rheumtology/keh436. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen S, Sonne-Holm S, Søballe K, Gebuhr P, Lund B. Hip dysplasia and osteoarthrosis: a survey of 4151 subjects from the Osteoarthrosis Substudy of the Copenhagen City Heart Study. Acta Orthop. 2005;76(2):149–158. doi: 10.1080/00016470510030517. [DOI] [PubMed] [Google Scholar]
- 3.Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162–1169. doi: 10.2106/JBJS.H.01674. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz-Neira CL, Paolucci EO, Donnon T. A meta-analysis of common risk factors associated with the diagnosis of developmental dysplasia of the hip in newborns. Eur J Radiol. 2012;81(3):e344–351. doi: 10.1016/j.ejrad.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Engesæter IØ, Laborie LB, Lehmann TG, et al. Prevalence of radiographic findings associated with hip dysplasia in a population-based cohort of 2081 19-year-old Norwegians. Bone Joint J. 2013;95-B(2):279–285. doi: 10.1302/0301-620X.95B2.30744. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann HP, Hinton R, Morello P, Santoli J. Developmental dysplasia of the hip practice guideline: technical report. Committee on Quality Improvement, and Subcommittee on Developmental Dysplasia of the Hip. Pediatrics. 2000;105(4):E57. doi: 10.1542/peds.105.4.e57. [DOI] [PubMed] [Google Scholar]
- 7.Clinical practice guideline: early detection of developmental dysplasia of the hip. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. American Academy of Pediatrics. Pediatrics. 2000;105(4 Pt 1):896-905. [DOI] [PubMed]
- 8.Duncan ST, Bogunovic L, Baca G, Schoenecker PL, Clohisy JC. Are there sex-dependent differences in acetabular dysplasia characteristics? Clin Orthop Relat Res. 2015;473(4):1432–1439. doi: 10.1007/s11999-015-4155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.•.Lee CB, Mata-Fink A, Millis MB, Kim Y-J. Demographic differences in adolescent-diagnosed and adult-diagnosed acetabular dysplasia compared with infantile developmental dysplasia of the hip. J Pediatr Orthopaedics 2013. 2013;33(2):107–111. doi: 10.1097/BPO.0b013e3182745456. [DOI] [PubMed] [Google Scholar]
- 10.Stulberg SD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: In: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis (M): CV Mosby; 1975:212-228.
- 11.Cooperman DR, Wallensten R, Stulberg SD. Acetabular dysplasia in the adult. Clin Orthop Relat Res. 1983;175:79–85. [PubMed] [Google Scholar]
- 12.Aronson J. Osteoarthritis of the young adult hip: etiology and treatment. Instr Course Lect. 1986;35:119–128. [PubMed] [Google Scholar]
- 13.Murphy SB, Ganz R, Müller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77(7):985–989. doi: 10.2106/00004623-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 14.•.Clohisy JC, Dobson MA, Robison JF, et al. Radiographic structural abnormalities associated with premature, natural hip-joint failure. J Bone Joint Surg Am. 2011;93(Suppl 2):3–9. doi: 10.2106/JBJS.J.01734. [DOI] [PubMed] [Google Scholar]
- 15.Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK) Osteoarthr Cartil. 2013;21(10):1514–1521. doi: 10.1016/j.joca.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg (Br) 1991;73(3):423–429. doi: 10.1302/0301-620X.73B3.1670443. [DOI] [PubMed] [Google Scholar]
- 17.Jessel RH, Zurakowski D, Zilkens C, Burstein D, Gray ML, Kim Y-J. Radiographic and patient factors associated with pre-radiographic osteoarthritis in hip dysplasia. J Bone Joint Surg Am. 2009;91(5):1120–1129. doi: 10.2106/JBJS.G.00144. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein SL. Natural history of congenital hip dislocation (CDH) and hip dysplasia. Clin Orthop Relat Res. 1987;225:62–76. [PubMed] [Google Scholar]
- 19.van Bosse H, Wedge JH, Babyn P. How are dysplastic hips different? A three-dimensional CT study. Clin Orthop Relat Res. 2015;473(5):1712–1723. doi: 10.1007/s11999-014-4103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garabekyan T, Ashwell Z, Chadayammuri V, et al. Lateral acetabular coverage predicts the size of the hip labrum. Am J Sports Med. 2016;44(6):1582–1589. doi: 10.1177/0363546516634058. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Chandrasekaran S, Redmond JM, et al. Does labral size correlate with degree of acetabular dysplasia? Orthop J Sports Med. 2015;3(2):2325967115572573. doi: 10.1177/2325967115572573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankar WN, Beaulé PE, Clohisy JC, et al. Labral morphologic characteristics in patients with symptomatic acetabular dysplasia. Am J Sports Med. 2015;43(9):2152–2156. doi: 10.1177/0363546515591262. [DOI] [PubMed] [Google Scholar]
- 23.Steppacher SD, Tannast M, Werlen S, Siebenrock KA. Femoral morphology differs between deficient and excessive acetabular coverage. Clin Orthop Relat Res. 2008;466(4):782–790. doi: 10.1007/s11999-008-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.•.Steppacher SD, Lerch TD, Gharanizadeh K, et al. Size and shape of the lunate surface in different types of pincer impingement: theoretical implications for surgical therapy. Osteoarthr Cartil. 2014;22(7):951–958. doi: 10.1016/j.joca.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Hipp JA, Sugano N, Millis MB, Murphy SB. Planning acetabular redirection osteotomies based on joint contact pressures. Clin Orthop Relat Res. 1999;364:134–143. doi: 10.1097/00003086-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 26.•.Mavcic B, Iglic A, Kralj-Iglic V, Brand RA, Vengust R. Cumulative hip contact stress predicts osteoarthritis in DDH. Clin Orthop Relat Res. 2008;466(4):884–891. doi: 10.1007/s11999-008-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leunig M, Podeszwa D, Beck M, Werlen S, Ganz R. Magnetic resonance arthrography of labral disorders in hips with dysplasia and impingement. Clin Orthop Relat Res. 2004;418:74–80. doi: 10.1097/00003086-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Stelzeneder D, Mamisch TC, Kress I, et al. Patterns of joint damage seen on MRI in early hip osteoarthritis due to structural hip deformities. Osteoarthr Cartil. 2012;20(7):661–669. doi: 10.1016/j.joca.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Chosa E, Totoribe K, Deng G. Effect of periacetabular osteotomy for acetabular dysplasia clarified by three-dimensional finite element analysis. J Orthop Sci. 2010;15(5):632–640. doi: 10.1007/s00776-010-1511-z. [DOI] [PubMed] [Google Scholar]
- 30.Reijman M, Hazes JMW, Pols H a P, Koes BW, Bierma-Zeinstra SMA. Acetabular dysplasia predicts incident osteoarthritis of the hip: the Rotterdam study. Arthritis Rheum. 2005;52(3):787–793. doi: 10.1002/art.20886. [DOI] [PubMed] [Google Scholar]
- 31.Wyles CC, Heidenreich MJ, Jeng J, Larson DR, Trousdale RT, Sierra RJ. The John Charnley Award: redefining the natural history of osteoarthritis in patients with hip dysplasia and impingement. Clin Orthop Relat Res. 2016 doi: 10.1007/s11999-016-4815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas GER, Palmer AJR, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthr Cartil. 2014;22(10):1504–1510. doi: 10.1016/j.joca.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Mahan ST, Katz JN, Kim Y-J. To screen or not to screen? A decision analysis of the utility of screening for developmental dysplasia of the hip. J Bone Joint Surg Am. 2009;91(7):1705–1719. doi: 10.2106/JBJS.H.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aronsson DD, Goldberg MJ, Kling TF, Roy DR. Developmental dysplasia of the hip. Pediatrics. 1994;94(2 Pt 1):201–208. [PubMed] [Google Scholar]
- 35.Roposch A, Protopapa E, Cortina-Borja M. Weighted diagnostic criteria for developmental dysplasia of the hip. J Pediatr. 2014;165(6):1236–1240.e1. doi: 10.1016/j.jpeds.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Nunley RM, Prather H, Hunt D, Schoenecker PL, Clohisy JC. Clinical presentation of symptomatic acetabular dysplasia in skeletally mature patients. J Bone Joint Surg Am. 2011;93(Suppl 2):17–21. doi: 10.2106/JBJS.J.01735. [DOI] [PubMed] [Google Scholar]
- 37.•.Matheney T, Zaltz I, Kim Y-J, et al. Activity level and severity of dysplasia predict age at Bernese periacetabular osteotomy for symptomatic hip dysplasia. J Bone Joint Surg Am. 2016;98(8):665–671. doi: 10.2106/JBJS.15.00735. [DOI] [PubMed] [Google Scholar]
- 38.Tönnis D. Congenital dysplasia and dislocation of the hip in children and adults. New York, NY: Springer; 1987. [Google Scholar]
- 39.Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90(Suppl 4):47–66. doi: 10.2106/JBJS.H.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tönnis D, Remus W. Development of hip dysplasia in puberty due to delayed ossification of femoral nucleus, growth plate and triradiate cartilage. J Pediatr Orthop B. 2004;13(5):287–292. doi: 10.1097/01202412-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245–1249. doi: 10.1097/01.TA.0000029212.19179.4A. [DOI] [PubMed] [Google Scholar]
- 42.Okano K, Takaki M, Okazaki N, Shindo H. Bilateral incidence and severity of acetabular dysplasia of the hip. J Orthop Sci. 2008;13(5):401–404. doi: 10.1007/s00776-008-1252-4. [DOI] [PubMed] [Google Scholar]
- 43.Modaressi K, Erschbamer M, Exner GU. Dysplasia of the hip in adolescent patients successfully treated for developmental dysplasia of the hip. J Child Orthop. 2011;5(4):261–266. doi: 10.1007/s11832-011-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okano K, Yamaguchi K, Ninomiya Y, et al. Relationship between developmental dislocation of the hip in infant and acetabular dysplasia at skeletal maturity. Medicine (Baltimore) 2015;94(1):e268. doi: 10.1097/MD.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terjesen T. Residual hip dysplasia as a risk factor for osteoarthritis in 45 years follow-up of late-detected hip dislocation. J Child Orthop. 2011;5(6):425–431. doi: 10.1007/s11832-011-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albinana J, Dolan LA, Spratt KF, Morcuende J, Meyer MD, Weinstein SL. Acetabular dysplasia after treatment for developmental dysplasia of the hip. Implications for secondary procedures. J Bone Joint Surg (Br) 2004;86(6):876–886. doi: 10.1302/0301-620X.86B6.14441. [DOI] [PubMed] [Google Scholar]
- 47.Imrie M, Scott V, Stearns P, Bastrom T, Mubarak SJ. Is ultrasound screening for DDH in babies born breech sufficient? J Child Orthop. 2010;4(1):3–8. doi: 10.1007/s11832-009-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarkissian EJ, Sankar WN, Zhu X, Wu CH, Flynn JM. Radiographic follow-up of DDH in infants: are X-rays necessary after a normalized ultrasound? J Pediatr Orthop. 2015;35(6):551–555. doi: 10.1097/BPO.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 49.Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;232:26–36. [PubMed] [Google Scholar]
- 50.Mechlenburg I, Nyengaard JR, Rømer L, Søballe K. Changes in load-bearing area after Ganz periacetabular osteotomy evaluated by multislice CT scanning and stereology. Acta Orthop Scand. 2004;75(2):147–153. doi: 10.1080/00016470412331294395. [DOI] [PubMed] [Google Scholar]
- 51.Bogunovic L, Hunt D, Prather H, Schoenecker PL, Clohisy JC. Activity tolerance after periacetabular osteotomy. Am J Sports Med. 2014;42(8):1791–1795. doi: 10.1177/0363546514535906. [DOI] [PubMed] [Google Scholar]
- 52.Novais EN, Heyworth B, Murray K, Johnson VM, Kim Y-J, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981–988. doi: 10.1007/s11999-012-2578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heyworth BE, Novais EN, Murray K, et al. Return to play after periacetabular osteotomy for treatment of acetabular dysplasia in adolescent and young adult athletes. Am J Sports Med. 2016;44(6):1573–1581. doi: 10.1177/0363546516632743. [DOI] [PubMed] [Google Scholar]
- 54.Matheney T, Kim Y-J, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113–2123. doi: 10.2106/JBJS.G.00143. [DOI] [PubMed] [Google Scholar]
- 55.•.Wells J, Millis M, Kim Y-J, Bulat E, Miller P, Matheney T. Survivorship of the Bernese periacetabular osteotomy: what factors are associated with long-term failure? Clin Orthop Relat Res. 2016 doi: 10.1007/s11999-016-4887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steppacher SD, Tannast M, Ganz R, Siebenrock KA. Mean 20-year followup of Bernese periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1633–1644. doi: 10.1007/s11999-008-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang C-F, Wang T-M, Wang J-H, Huang S-C, Lu T-W. Adolescents after Pemberton’s osteotomy for developmental dysplasia of the hip displayed greater joint loading than healthy controls in affected and unaffected limbs during gait. J Orthop Res. 2011;29(7):1034–1041. doi: 10.1002/jor.21377. [DOI] [PubMed] [Google Scholar]
- 58.Chang C-F, Wang T-M, Wang J-H, Huang S-C, Lu T-W. Residual gait deviations in adolescents treated during infancy for unilateral developmental dysplasia of the hip using Pemberton’s osteotomy. Gait Posture. 2012;35(4):561–566. doi: 10.1016/j.gaitpost.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Zaltz I, Baca G, Kim Y-J, et al. Complications associated with the periacetabular osteotomy: a prospective multicenter study. J Bone Joint Surg Am. 2014;96(23):1967–1974. doi: 10.2106/JBJS.N.00113. [DOI] [PubMed] [Google Scholar]
- 60.Polkowski GG, Novais EN, Kim Y-J, Millis MB, Schoenecker PL, Clohisy JC. Does previous reconstructive surgery influence functional improvement and deformity correction after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(2):516–524. doi: 10.1007/s11999-011-2158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


