Abstract
Subaxial cervical spine trauma is common and an often missed diagnosis. Accurate and efficient diagnosis and management is necessary to avoid devastating complications such as spinal cord injury. Several classification schemes have been devised to help categorize fractures of the subaxial spine and define treatment algorithms. The Subaxial Cervical Spine Injury Classification System (SLIC) is widely used and evaluates not only fracture morphology but also considers ligamentous injury and neurological status in surgical decision making. However, interobserver reliability is poor, which proves to be the defining pitfall of this tool. More modern classification systems have been developed, which aim to improve the interobserver reliability; however, further large-scale studies are needed for more definitive evaluation. Overall, treatment of subaxial cervical spine injuries should include a protocol with initial trauma evaluation, leading to expedient operative intervention if indicated. Surgical techniques include both anterior and posterior approaches to the cervical spine depending on fracture classification.
Keywords: Subaxial cervical spine trauma, Fracture, Ligamentous injury, Classification system
Introduction
Despite the cervical spine being injured in only 3 % of blunt trauma victims, the results can be catastrophic due to spinal cord injury [1]. The subaxial cervical spine is a common site of cervical injury with more than 50 % of injuries being located between C5 and C7 [2]. The substantial motion of the cervical spine is what predisposes it to injury or instability events. Devastating sequelae of subaxial cervical spine trauma include tetraplegia, functional loss, and permanent disability; therefore, a consistent and proven algorithm for diagnosis and management of these injuries is paramount.
Initial evaluation
Due to the potentially catastrophic nature of cervical spine instability as a result of trauma, it is necessary to perform a meticulous and standardized clinical and radiographic evaluation. A patient with suspected cervical spine trauma should be evaluated and managed through the initial standardized approach as outlined by the Advanced Trauma Life Support algorithm and beginning with assessments of the airway, breathing, and circulation. Following this evaluation, if cervical spine injury is suspected, the neck should be protected as posterior cervical palpation proceeds to identify any tenderness or step-offs. In addition, inspecting a patient’s cervical posture for angular or rotational malalignments can hint to dislocations or subluxation. The upper torso, head, and neck can be evaluated for signs of trauma as this can hint to the type of force applied to the cervical spine during the trauma event. A patient should be logrolled while maintaining cervical spine stability to evaluate the entire spinal axis.
Details of the accident can lend helpful insight into the energy pattern to produce specific subaxial cervical trauma. Furthermore, it is important to identify other comorbid conditions that have a greater propensity for injury to the cervical spine such as previous cervical spine procedures, ankylosing spondylitis (AS), diffuse idiopathic skeletal hyperostosis (DISH), or other connective tissue disorders leading to ligamentous hyperlaxity.
Diagnostic imaging
Following appropriate resuscitation, patients with suspected subaxial cervical trauma should undergo screening imaging. Modalities for imaging the cervical spine are often controversial as imaging capabilities across institutions differ based upon available resources. Traditionally, standard radiographic views including anteroposterior, lateral, and open mouth views are used to evaluate the cervical spine from the craniocervical junction to T1. Including clear visualization of the cervicothoracic junction is paramount as fracture of C7 or a fracture dislocation of C7/T1 accounts for nearly 17 % of cervical spine injuries [3]. Plain films are not only important to evaluate injury at specific cervical levels but they also demonstrate the overall alignment of the cervical spine including areas of kyphosis/lordosis, changes in disk height, or changes to the interspinous distances. Recently, however, studies have shown plain radiograph to have only 30–60 % sensitivity in evaluating for ligamentous injuries and fractures [4–6]. Traditionally, flexion-extension views were used to evaluate ligamentous stability; however, the utility of these studies have recently been questioned. Sim et al. retrospectively evaluated flexion-extension films read by a trained radiologist. They found that 95 % of these studies were deemed inadequate as a result of either inability to visualize T1 or insufficient angulation (less than 30°) from neutral likely secondary from patient guarding [7]. Similar results were demonstrated by McCracken et al., who concluded that adequate flexion-extension films are difficult to obtain and therefore are minimally helpful in the acute setting for clearance of cervical spine trauma even in the awake and alert patient [8].
Computed tomography (CT) scans have attained more widespread use to evaluate the cervical spine given their wide availability, rapid accessibility in the trauma bay, and robust image quality. CT scans also allow excellent visualization of the cervicothoracic and occipitocervical junctions, which are often of poor quality on plain films. Sensitivity of 99 % and specificity of 100 % have been demonstrated in some studies with use of multiplanar CT [9, 10]. Recently, the quality of CT has been deemed adequate enough to clear the cervical spine in trauma patients who are intoxicated and without gross motor deficits, therefore decreasing unnecessary immobilization and further advanced imaging [11•]. Some report the deficiencies in the ability of CT to identify purely ligamentous injuries and therefore recommend magnetic resonance imaging (MRI). However, recent studies have demonstrated that multidetector CT with sagittal and coronal reconstructions were useful without MRI as a screening tool. Although MRI frequently identifies ligamentous injuries not accounted for on CT scan, few of these injuries were found to be unstable at initial presentation or follow-up [12•]. Other studies demonstrated that CT performed similarly in classifying subaxial injury patterns when compared to CT and MRI together, and therefore, CT may be the only study that is needed in the initial triage of patients [13•].
It is evident that CT provides a powerful tool in the initial diagnostic workup, given the excellent osseous resolution; however, MRI studies are paramount for surgical planning given the excellent visualization of soft tissue compressive lesions including disk herniations and hematomas. It is also necessary to evaluate the disco-ligamentous complex (DLC), which aids in determination of subaxial stability. MRI has a high sensitivity but low specificity in evaluation for ligamentous injuries and therefore increases the potential risk for potentially unnecessary procedures. Given the propensity for false positive results which may change management, the role of MRI needs a more clearly defined role. Pourtaheri et al. attempted to define the role through a retrospective clinical and radiographic analysis to identify predisposing conditions where MRI can provided useful data. Patients older than 60, obtunded, with cervical spondylosis, polytrauma, and with neurological deficit were found to have clinical findings on MRI that changed management. The vast proportion of these management changes were as a result of MRI identifying spinal cord injury (81 %) rather than due to occult instability (19 %) [14•].
Classification
The numerous fracture and dislocation patterns of the subaxial cervical spine lend to difficulty in creating a reliable and reproducible classification system that allows concise communication, management decision making, and prognostication. Historically, classification systems relied on the use of plain radiographs; however, given the limitations in visualizing the cervical spine, these systems can be unreliable. In addition, these classification systems often failed to consider not only the neurological status of the patient but also the energy pattern used to create the specific trauma [15, 16].
Subaxial injury classification system
Of the more modern classification systems proposed by the Spinal Trauma Study Group, the Subaxial Injury Classification System (SLIC) is commonly used. This is a system based on the injury morphology, competency of the DLC, and the neurological status of the patient. Each of these categories is individually analyzed and given a score in which the sum of the score for all three categories is used for prognostication and management decision making [17]. Conservative treatment is indicated for a score of 3 or less, whereas a score of 5 or greater suggests operative intervention. A score of 4 identifies a gray area where a surgeon’s experience and other patient comorbidities may drive a decision either towards operative stabilization or conservative management.
Subaxial cervical spine morphology is comprised of compression injuries, burst patterns, distraction, or translational injuries. Compression injuries include fractures with visible height loss through the vertebral body and include burst fractures. This group also includes non-displaced or minimally displaced lateral mass and facet fractures which occur as a result of a lateral compression mechanism. More severe distraction injuries represent vertically oriented forces that result in facet subluxations as a result of disruption through the disk space or facet joint capsule. Rotational or translational injuries, which represent an even greater traumatic force to the subaxial spine, consist of unilateral or bilateral facet dislocation as a result of a horizontally directed force. Spinous process fractures and non-displaced facet fractures can be associated with any of these injury patterns but are not considered in this classification scheme. Injury morphology can be identified using traditional plain radiographs, CT, or MRI [17].
The DLC, or the consolidation of the anterior and posterior ligamentous structures, intervertebral disk, and facet joint capsule, allows for physiological cervical spine mobility while preventing instability in the setting of excessive loads. Injury to the DLC occurs with distraction or translational forces and when diagnosed as incompetent results in a SLIC score of 2. A score of 1 is given to an indeterminate assessment of the DLC and can be a source of inconsistency with surgical planning. Furthermore, as previously mentioned, the low specificity of MRI can lead to false positive findings of DLC disruption, which may result in unnecessarily elevated SLIC score and resultant surgery [17].
The neurological status of the patient, although not traditionally used in trauma systems, is of paramount importance when considering cervical spine trauma classification. The SLIC scoring system gives a higher score for incomplete injuries compared to complete and root-localized deficits. The system does not take into account symptoms of cord compression that include paresthesias, imbalance, or proprioceptive loss. In addition, there are confounding variables such as the presence of DISH, AS, or degenerative disease that may alter surgical planning and lead to unnecessary surgery in a patient with these conditions who sustained a trauma but without fracture or ligamentous injury [17].
Overall, some studies have shown that the SLIC is reproducible and reliable. A previous retrospective study by Joaquim et al. demonstrated a more than 90 % agreement between the SLIC score and treatment algorithm chosen by the surgeon [18]. In addition, good intraobserver and interobserver agreement was observed among surgeons calculating a SLIC score (0.79 and 0.98 for total SLIC score, respectively) [19–21]. However, several of these studies reporting good to excellent results are retrospective and there is a paucity of prospective data supporting these results. More recent studies have demonstrated poor intraobserver rating with regard to morphological classification with only average agreement on the integrity of the DLC. In addition, interobserver agreement concerning treatment choice based on SLIC scoring was lower than agreement based on personal surgeon preference [22]. Given these results, the reliability and reproducibility of the SLIC scoring system may improve only when morphological classification becomes less ambiguous [22].
AOSpine subaxial cervical spine injury classification
Given the disagreements in morphological classification associated with the SLIC scoring system and the inherent potential for controversies regarding management decisions, the AOSpine Knowledge Forum attempted to create a validated Subaxial Cervical Spine Injury Classification System that is more user-friendly. The classification system is based off work from the AOSpine Thoracolumbar Injury Classification System, which was been proven reliable and reproducible by several studies [23, 24•]. The goal was to develop a classification system that has high intraobserver and interobserver reliabilities similar to the AOSpine Thoracolumbar Injury Classification System. The classification describes injury patterns based on the following four criteria: injury morphology, facet injury, neurological status, and the presence of specific modifiers.
Morphology is grouped similarly to the thoracolumbar fracture classification into type A—compression fracture anteriorly with intact posterior tension band or physiologically insignificant fracture such as lamina or spinous process fractures; B—fracture with failure of the posterior or anterior tension band secondary to distraction, without evidence of malalignment, dislocation, or translation; and C—injury displacement or translation of one vertebral body relative to another in any direction. Each morphological group can be further categorized into subtypes based on specific injury mechanisms with increased bony or ligamentous disruption [24•].
Facet joint injuries are categorized to describe a spectrum of injuries to the facet complex and are classified by the highest level of injury to the facet if more than one injury pattern is present. Facet injuries can be present without an A, B, or C morphological type, and these fractures would be categorized primarily by the facet subtype. These subtypes run the spectrum from stable non-displaced fracture (F1) to more unstable fracture patterns including factures that have >1-cm displacement or inclusion of more than 40 % of the lateral mass (F2), a floating lateral mass (F3) to subluxation, or dislocated facet joints (F4) [24•].
Neurological status is graded through six subtypes ranging from neurologically intact (N0) to incomplete (N3) and complete spinal cord injuries (N4). There also exists the NX subtype to describe a neurologically indeterminate status as a result of comorbid condition preventing appropriate examination such as head trauma, intoxication or intubation, and sedation.
The classification system also includes specific modifiers such as incomplete disruption of the posterior ligamentous complex (M1); critical disk herniation (M2); vertebral artery injury(M4); and the presence of comorbid spine conditions (M3) such as osteoporosis, DISH, ossification of the posterior longitudinal ligament, and AS. The presence of these metabolic bone disease may significantly impact surgical decision making such as the need to use of longer constructs with more fixation points in poor quality bone or when a predisposition for instability exists [24•].
The reliability and reproducibility of the AOSpine Subaxial Cervical Spine Injury Classification System has been tested in numerous recent studies. The authors of the classification system observed intraobserver and interobserver reliabilities as robust for all injuries (kappa = 0.75 and 0.64, respectively) [24•]. A study by Urrutia et al. determined substantial interobserver agreement across all main types with a range of (0.57–0.64) and moderate among subtypes (0.54–0.60). The intraobserver agreement was substantial across both main types and subtypes. Overall, they concluded that this classification system is reliable and reproducible [25]. On the contrary, however, a study by Silva et al. found B type injuries and classification of facet injuries to be unreliable with low intraobserver and interobserver reliabilities [26]. Overall, this classification system may prove more reliable to reproducible than the SLIC; however, there may be difficulty with agreement across intermediate fractures and those affecting the facet. Future prospective studies may help tease out the true value of this scale as a classification tool.
Initial treatment
After stabilization of the cervical spine in rigid C collar and appropriate imaging of the C spine is obtained, patients without pain, neurological deficit, or radiographic abnormalities can be cleared of cervical spine injury and have the collar removed.
Patients presenting with dislocation should undergo timely reduction in the emergency department with the use of traction. Performing a closed reduction before obtaining MRI should only be considered in an awake patient who is neurologically intact and able to cooperate with the exam. Urgent closed reduction can also be considered in a patient who is awake and alert but presents with complete spinal cord injury, without knowing the status of the intervertebral disk given the assumption that further damage to neurological function is likely negligible. Reduction is performed with the use of Gardner-Wells tongs and the sequential addition of weight to apply traction. As increasing traction is applied, serial physical exams and radiographic evaluations should be performed. MRI should be obtained in patients who are not alert and unable to cooperate with an exam prior to reduction attempt or after a closed reduction attempt and prior to operative intervention [27].
Use of methylprednisolone
One of the more controversial topics among spine surgeons surrounds the use of methylprednisolone. Initial data from the National Acute Spinal Cord Injuries studies did not show significant difference in motor, sensory, or functional outcomes. However, post hoc analysis demonstrated that early administration of methylprednisolone within 8 h of injury provided significant recovery [28]. There has been a shift away from using methylprednisolone for acute spinal cord injuries as a result of the 2013 guidelines from the AANS/CNS, which sited level 1 evidence recommending against methylprednisolone for use in spinal cord injury [29, 30]. This recommendation was made despite recent studies demonstrating that a use of methylprednisolone concomitantly with early decompression (within 24 h) may improve recovery [31]. It is clear that no distinct guideline can be used regarding methylprednisolone use especially given the risk of adverse events as a result of high-dose administration. The surgeon must use his discretion on a case by case basis to decide when the benefits of corticosteroid administration outweigh potential risks.
Conservative treatment
There is no consensus regarding which injuries are deemed stable enough for non-operative treatment or a precise non-surgical treatment protocol. Factors to consider in decision making include the stability of the injury pattern, neurological compromise, and patient comorbidities. In general, injuries with a SLIC score of 3 or less are deemed stable and can be managed in a conservative manner. Patients with a SLIC score of 4 are indeterminately treated conservatively based on the treating surgeon’s experience and factors associated with the patient’s acute health status and chronic comorbidities. A study by Samuel et al. used SLIC to determine treatment regimens. In their study, 94 % of patients with SLIC of 3 were treated without surgery; however, only 65 % of patients with a SLIC score of 4 were treated conservatively [33]. This difference emphasizes the indeterminant nature of cases assigned a SLIC score of 4 and highlights the inherent difficulty in standardizing treatment for these patients. Overall, patients who are treated non-operatively are often prescribed a cervical orthosis, however, given these fractures are deemed stable; these collars are primarily for comfort measures. Rigid orthosis are worn for 6–12 weeks, and patients are followed regularly for clinical and radiographic evaluation.
Surgical intervention
Fractures deemed unstable or having the potential for neurological compromise in a patient who is fit for surgery should be treated with operative intervention. Prior studies have demonstrated that early decompression is associated with an improved outcome [31]. The prospective cohort study by Fehlings et al. demonstrated that nearly 20 % of patients who underwent early surgery (within 24 h) had at least a two grade improvement in the ASIA score at 6 month follow-up in comparison to only 9 % of patients showing two grade improvement in the delayed surgery group. There was no difference in adverse outcomes in either group. A follow-up study by Furlan et al. [32•] demonstrated substantial incremental cost savings per quality adjusted life years for patients with complete and incomplete spinal cord injuries [33]. This data suggest that decompression and stabilization should be performed as quickly as possible after appropriate patient resuscitation and hemodynamic stabilization.
Surgical intervention can be performed either through an anterior or posterior approach and should be based on the pathology of the injury pattern. Anterior approaches may be associated with fewer wound complications and a higher fusion rate at the risk of postoperative swallowing difficulties [34]. There are however no differences in neurological recovery or patient-reported outcome measures [34, 35]. Therefore, both posterior and anterior techniques are viable options for decompression and fusion and should be tailored according to fracture morphology and patient-specific factors.
Surgical management of specific injuries
Subaxial burst and flexion/compression injuries
Burst fractures are typically a result of axial load leading to compressive forces across the vertebrae. These fractures typically are associated with a forward flexion movement that may distract the posterior structures resulting in disco-ligamentous injury. Instability of these fractures can be suggested by pathological kyphosis, interspinous widening, and significant loss of height. Fractures reaching a SLIC score in the operative range include teardrop-type fractures and those with retropulsion of fracture fragments resulting in neurological compromise. These fractures are typically treated through an anterior approach with corpectomy, bone grafting, and anterior plating, which allows for restoration of the anterior column. Factures associated with significant disco-ligamentous complex may require combined anterior and posterior approaches [36].
Facet injuries
Facet injuries are typically the result of a flexion and distraction injury and may contain an element of rotation. These injuries can be purely ligamentous or have subantial bony involvement of the facet and lateral mass and run the spectrum from fracture and subluxation to a locked dislocation. As discussed previously, dislocations should be treated initially with attempted closed reduction under the right conditions. MRI should be used diligently especially in the operative planning to evaluate disco-ligamentous injury, specifically the disk which can be displaced in as high as 40 % of unilateral and 80 % of bilateral fact dislocations [36]. In the event of failed or unattempted closed reduction, an open reduction can be performed by both anterior and posterior approaches.
Overall surgical treatment of cervical fact fracture dislocations is variable. In the anterior approach, after discectomy is performed, distraction is applied either with the use of Caspar pins or a lamina spreader. Once distraction has “unlocked” the facet joints, a posterior directed force can be applied to reduce the dislocation [27]. In the setting of unilateral dislocation, distraction should be accompanied with rotation to allow for joint reduction. After both discectomy and reduction are obtained, interbody fusion should be performed, reserving corpectomy for larger more complex disk herniations or compressive lesions inaccessible through discectomy. In more complex fracture dislocations, a combined anterior and posterior approach is required for adequate discectomy, reduction, anterior column reconstruction with grafting, and reconstitution of the posterior tension band with stabilization and fusion [27].
Case
Cervical teardrop
The patient is a 51-year-old female with a history of COPD, who presented the emergency department after a motor vehicle accident complaining of neck pain. The patient was hemodynamically stable and noted to have no neurological deficits. She had cervical tenderness to palpation, but no step-off was appreciated. A protocoled trauma workup was performed, which included a CT scan of the cervical spine (Fig. 1). Imaging was notable for C4 burst fracture with teardrop morphology, fracture of the right C4 lamina, and associated focal kyphosis of C4/C5. An MRI demonstrated injury to the DLC including injury to the interspinous ligament from C3–C5 (Fig. 2). A SLIC score of 6 was calculated, 4 points for teardrop morphology, 2 points for DLC disruption with focal kyphosis, and 0 points for intact neurological status. The decision was made for operative intervention and included first an anterior approach (for C4 corpectomy, strut grafting, and C3–C5 anterior spinal fusion), followed by a posterior approach (for restoration of posterior tension and C3–C5 posterior spinal fusion) (Fig. 3).
Fig. 1.
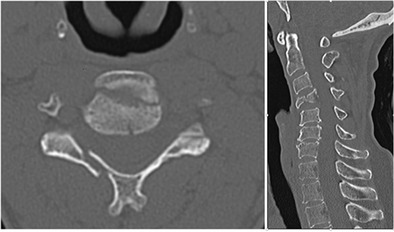
Axial and sagittal CT cuts of the cervical spine demonstrating vertebral body burst fracture (teardrop) lamina fracture and focal kyphosis at C4-C5
Fig. 2.
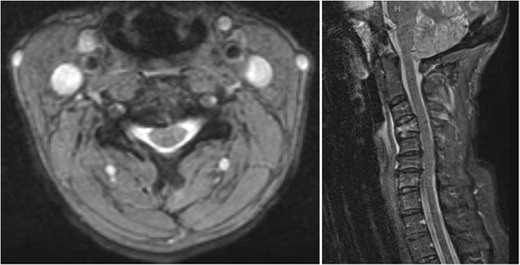
Axial and sagittal MRI cuts of the cervical spine demonstrating injury to the DLC (interspinous ligaments from C3–C5)
Fig. 3.
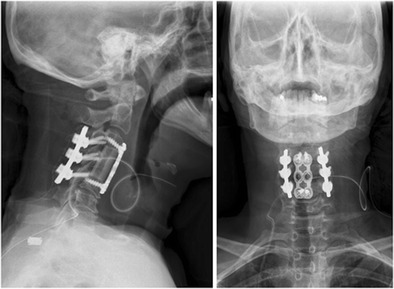
Postoperative AP and lateral plain radiographs demonstrating anterior C4 corpectomy, strut grafting, and C3–C5 posterior instrumentation
Cervical facet fracture/dislocation
The patient is a healthy 21-year-old male who presented after a 6-ft fall onto his head, complaining of neck pain and right arm numbness. After appropriate trauma triage and hemodynamic stabilization, the patient was noted to have 4/5 triceps and wrist extension weakness as well as dysesthetic pain and numbness of the forearm and hand. Plain radiographs and CT scan of the cervical spine demonstrated right C6 inferior and C7 superior facet fracture with unilateral subluxation, resulting in C6/C7 right foraminal stenosis (Figs. 4 and 5). An MRI demonstrated injury to the DLC including the disk, anterior, and posterior longitudinal ligaments (Fig. 6). A SLIC score of 7 was calculated (4 points for morphology, 2 points for DLC, and 1 point for root injury). The decision was made for operative intervention. Closed reduction in the operating room was performed under general anesthesia with the use of Gardner-Wells tongs, intraoperative monitoring, and sequential addition of weight up to 60 lb. Once reduction was confirmed radiographically, an anterior approach was utilized for discectomy, foraminal decompression, and fusion (Fig. 7).
Fig. 4.
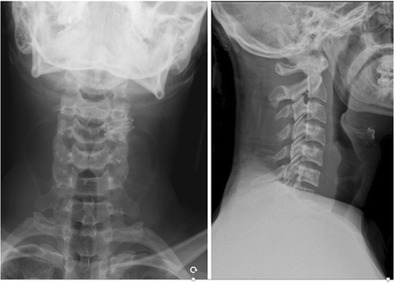
AP and lateral plain radiographs demonstrating traumatic spondylolisthesis at C6-C7. Note the difficulty of visualizing the cervicothoracic junction which can lead to difficulty in diagnosis. Suspicious findings should warrant advanced imaging
Fig. 5.
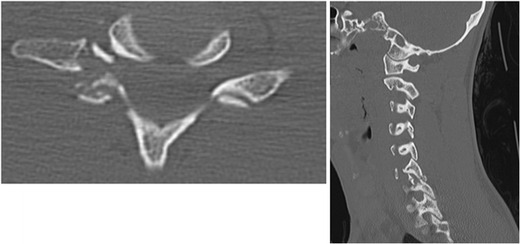
Axial and sagittal cuts of the C6/C7 facet joint demonstrating fracture and unilateral dislocation
Fig. 6.
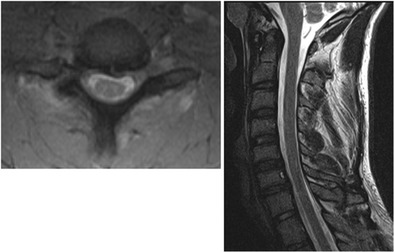
Axial and sagittal MRI cuts demonstrating DLC injury and unilateral dislocation resulting in right C7 foraminal stenosis
Fig. 7.
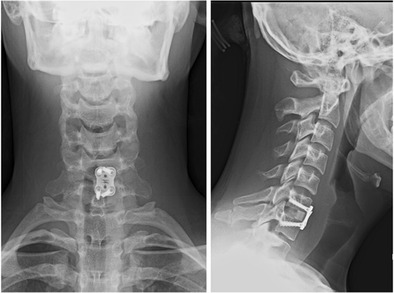
AP and lateral postoperative radiographs after anterior cervical discectomy and fusion
Conclusion
Subaxial cervical spine injuries can have devastating complications especially when subtleties in the injury pattern exist, which can lead to misdiagnosis. Fortunately, the widespread availability of high-resolution multiplanar CT has allowed for improved diagnostic accuracy and efficiency while also allowing for early access to surgical intervention, which has been shown to result in improved outcomes in the setting of spinal cord injury. Despite some advancements in the care of subaxial cervical spine injuries, some controversies still exist such as the use of methylprednisolone for associated spinal cord injuries. In addition, although the SLIC classification system has shown some promise in its reliability and reproducibility in diagnosing fractures and guiding management, it certainly has not reached complete widespread use as a result of disagreements among morphological injury classification. Although not yet validated in larger prospective studies, the AOSpine Subaxial Cervical Spine Injury Classification System does show promise in its potentially higher interobserver and intraobserver agreement. Overall, subaxial cervical spine injuries are typically complex and standardization of treatment is not always feasible. The surgeon must consider in each case not only the subaxial injury itself but also the individual patient comorbidities, surgeon experience, and available resources when guiding management.
Compliance with ethical standards
Conflict of interest
Eric Feuchtbaum declares that he has no conflict of interest.
Jacob Buchowski reports personal fees from Advance Medical, personal fees from CoreLink Inc., personal fees from DePuy Synthes, personal fees from Gerson Lehrman Group (GLG), personal fees from Globus Medical Inc., personal fees from K2M Inc., personal fees from Medtronic Inc., personal fees from Stryker Inc., personal fees from Broadwater/Vertical Health, personal fees from DePuy Synthes, personal fees from Globus Medical Inc., personal fees from Orthofix, personal fees from Stryker Inc., personal fees from Wolters Kluwer Health Inc., and personal fees from Globus Medical Inc., outside the submitted work. In addition, Dr. Buchowski has a patent CAPRI (spinal fixation device) with royalties paid to K2M Inc. and AO Foundation (parent organization to AOSpine), “other,” “teaching,” and “not for profit organization.”
Lukas Zebala reports personal fees from K2M, personal fees from Ulrich Medical USA, personal fees from Broadwater, personal fees from K2M, non-financial support from Scoliosis Research Society, non-financial support from Depuy Synthes Spine, non-financial support from Medtronic, and non-financial support from Nuvasive, outside the submitted work.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Cervical Injuries and Treatment
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mower WR. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38:12–6. doi: 10.1067/mem.2001.116149. [DOI] [PubMed] [Google Scholar]
- 2.Aebi M. Surgical treatment of upper, middle and lower cervical injuries and non-unions by anterior procedures. Eur Spine J. 2010;19(1, suppl1):S33–9. doi: 10.1007/s00586-009-1120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg W, Mueller C, Panacek E, Tigges S, Hoffman JR, Mower WR. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38:17–21. doi: 10.1067/mem.2001.116150. [DOI] [PubMed] [Google Scholar]
- 4.Jones C, Jazayeri F. Evolving standards of practice for cervical spine imaging in trauma: a retrospective review. Australas Radiol. 2007;51:420–42. doi: 10.1111/j.1440-1673.2007.01863.x. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch PT, France J, Jones DL, et al. Helical computed tomography alone compared with plain radiographs with adjunct computed tomography to evaluate the cervical spine after high-energy trauma. J Bone Joint Surg Am. 2005;87:2388–239. doi: 10.2106/JBJS.E.00208. [DOI] [PubMed] [Google Scholar]
- 6.Schenarts PJ, Diaz J, Kaiser C, et al. Prospective comparison of admission computed tomographic scan and plain films of the upper cervical spine in trauma patients with altered mental status. J Trauma. 2001;51:663–8. doi: 10.1097/00005373-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Sim V, Bernstein MP, Frangos SG, Wilson CT, Simon RJ, McStay CM, et al. The (f)utility of flexion-extension C-spine films in the setting of trauma. Am J Surg. 2013;206(6):929–33. doi: 10.1016/j.amjsurg.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 8.McCracken B, Klineberg E, Pickard B, Wisner DH. Flexion and extension radiographic evaluation for the clearance of potential cervical spine injures in trauma patients. Eur Spine J. 2013;22(7):1467–73. doi: 10.1007/s00586-012-2598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez B, Waxman K, Jones T, Conner S, Chung R, Becerra S. Cervical spine clearance in blunt trauma: evaluation of a computed tomography-based protocol. J Trauma. 2005;59(1):179–83. doi: 10.1097/01.TA.0000171449.94650.81. [DOI] [PubMed] [Google Scholar]
- 10.Antevil JL, Sise MJ, Sack DI, Kidder B, Hopper A, Brown CV. Spiral computed tomography for the initial evaluation of spine trauma: a new standard of care? J Trauma. 2006;61(2):382–7. doi: 10.1097/01.ta.0000226154.38852.e6. [DOI] [PubMed] [Google Scholar]
- 11.• Bush L, Brookshire R, Roche B, Johnson A, Cole F, Karmy-Jones R, Long W, Martin MJ. Evaluation of cervical spine clearance by computed tomographic scan alone in intoxicated patients with blunt trauma. JAMA Surg. 2016;151(9):807–13. This prospective observational study has high potential to change medical decision making processes not only for spine surgeons but also for emergency room physicians. It provides sound evidence that CT scan can be used to clear cervical spine injuries in the intoxicated patient therefore preventing a delay in clearance and unnecessary cervical immobilization. [DOI] [PubMed]
- 12.Chew BG, Swartz C, Quigley MR, Altman DT, Daffner RH, Wilberger JE. Cervical spine clearance in the traumatically injured patient: is multidetector CT scanning sufficient alone? Clinical article. J Neurosurg Spine. 2013;19(5):576–81. doi: 10.3171/2013.8.SPINE12925. [DOI] [PubMed] [Google Scholar]
- 13.Mascarenhas D, Dreizin D, Bodanapally UK, Stein DM. Parsing the utility of CT and MRI in the Subaxial Cervical Spine Injury Classification (SLIC) System: is CT SLIC enough? AJR Am J Roentgenol. 2016;206(6):1292–7. doi: 10.2214/AJR.15.15492. [DOI] [PubMed] [Google Scholar]
- 14.Pourtaheri S, Emami A, Sinha K, Faloon M, Hwang K, Shafa E, et al. The role of magnetic resonance imaging in acute cervical spine fractures. Spine J. 2014;14(11):2546–53. doi: 10.1016/j.spinee.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 15.Allen BL, Jr, Ferguson RL, Lehmann TR, O’Brien RP. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine. 1982;7(1):1–27. doi: 10.1097/00007632-198200710-00001. [DOI] [PubMed] [Google Scholar]
- 16.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3(4):184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 17.Vaccaro AR, Hulbert RJ, Patel AA, Spine Trauma Study Group et al. The Subaxial Cervical Spine Injury Classification System: a novel approach to recognize the importance of morphology, neurology, and integrity of the disco-ligamentous complex. Spine. 2007;32(21):2365–74. doi: 10.1097/BRS.0b013e3181557b92. [DOI] [PubMed] [Google Scholar]
- 18.Joaquim AF, Lawrence B, Daubs M, Brodke D, Patel AA. Evaluation of the Subaxial Injury Classification System. J Craniovertebr Junction Spine. 2011;2(2):67–72. doi: 10.4103/0974-8237.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WJ, Yoon SH, Kim YJ, Kim JY, Park HC, Park CO. Interobserver and intraobserver reliability of sub-axial injury classification and severity scale between radiologist, resident and spine surgeon. J Korean Neurosurg Soc. 2012;52(3):200–3. doi: 10.3340/jkns.2012.52.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whang PG, Patel AA, Vaccaro AR. The development and evaluation of the subaxial injury classification scoring system for cervical spine trauma. Clin Orthop Relat Res. 2011;469(3):723–31. doi: 10.1007/s11999-010-1576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone AT, Bransford RJ, Lee MJ, Vlela MD, Bellabarba C, Anderson PA, et al. Reliability of classification systems for subaxial cervical injuries. Evid Based Spine Car J. 2010;1:19–26. doi: 10.1055/s-0030-1267064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Middendorp JJ, Audige L, Bartels RH, Bolger C, Deverall H, Dhoke P, et al. The Subaxial Cervical Spine Injury Classification System: an external agreement validation study. Spine J. 2013;13(9):1055–63. doi: 10.1016/j.spinee.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Kepler CK, Vaccaro AR, Koerner JD, et al. Reliability analysis of the AOSpine Thoracolumbar Spine Injury Classification System by a worldwide group of naive spinal surgeons. Eur Spine J. 2015. [DOI] [PubMed]
- 24.Vaccaro AR, Koerner JD, Radcliff KE, Oner FC, Reinhold M, Schnake KJ, et al. AOSpine Subaxial Cervical Spine Injury Classification System. Eur Spine J. 2016;25(7):2173–84. doi: 10.1007/s00586-015-3831-3. [DOI] [PubMed] [Google Scholar]
- 25.Urrutia J, Zamora T, Yurac R, Campos M, Palma J, Mobarec S, Prada C. An independent inter- and intra-observer agreement evaluation of the AOSpine Subaxial Cervical Spine Injury Classification. Spine (Phila Pa 1976). 2015. [DOI] [PubMed]
- 26.Silva OT, Sabba MF, Lira HI, Ghizoni E, Tedeschi H, Patel AA, Joaquim AF. Evaluation of the reliability and validity of the newer AOSpine Subaxial Cervical Injury Classification (C-3 to C-7). J Neurosurg Spine. 2016: 1-6. [DOI] [PubMed]
- 27.Joaquim AF, Patel AA. Subaxial cervical spine trauma: evaluation and surgical decision making. Glob Spine J. 2014;4:63–70. doi: 10.1055/s-0033-1356764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–11. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 29.Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;72(suppl 2):93–105. doi: 10.1227/NEU.0b013e31827765c6. [DOI] [PubMed] [Google Scholar]
- 30.Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(suppl 1):36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 31.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furlan JC, Craven BC, Massicotte EM, Fehlings MG. Early versus delayed surgical decompression of spinal cord after traumatic cervical spinal cord injury: a cost-utility analysis. World Neurosurg. 2016;88:166–74. doi: 10.1016/j.wneu.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 33.Samuel S, Lin JL, Smith MM, Hartin NL, Vasili C, Ruff SJ, et al. Subaxial injury classification scoring system treatment recommendations: external agreement study based on retrospective review of 185 patients. Spine (Phila Pa 1976) 2015;40(3):137–42. doi: 10.1097/BRS.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 34.Kwon BK, Fisher CF, Boyd MC, et al. A prospective randomized controlled trial of anterior compared to posterior stabilization for unilateral facet injuries of the cervical spine. J Neurosurg Spine. 2007;7(1):1–12. doi: 10.3171/SPI-07/07/001. [DOI] [PubMed] [Google Scholar]
- 35.Brodke DS, Anderson PA, Newell DW, Grady MS, Champan JR. Comparison of anterior and posterior approaches in cervical spinal cord injuries. J Spinal Disord Tech. 2003;1(3):229–35. doi: 10.1097/00024720-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Rizzolo SJ, Piazza MR, Cotler JM, et al. Intervertebral disc injury complicating cervical spine trauma. Spine (Phila Pa 1976) 1991;16:S187–9. doi: 10.1097/00007632-199106001-00002. [DOI] [PubMed] [Google Scholar]


