Abstract
Introduction
Adalimumab, an anti-tumor necrosis factor antibody, is currently available in a 40 mg/0.8 mL formulation. The objective of this analysis was to evaluate injection site-related pain, safety, and tolerability of a 40 mg/0.4 mL formulation of adalimumab that had fewer excipients, a smaller volume, and a delivery presentation with a smaller gauge needle, versus the current 40 mg/0.8 mL formulation in patients with rheumatoid arthritis (RA).
Methods
Two identically designed, phase 2, randomized, single-blind, two-period crossover studies were conducted in Belgium and the Czech Republic (Study 1) and Australia, Canada, and Germany (Study 2). In both studies, adults with RA [biologic-naive or current users of 40 mg/0.8 mL adalimumab with an average injection site-related pain rating ≥3 cm on a visual analog scale (VAS; 0–10 cm)] were randomized to receive 40 mg/0.8 mL or 40 mg/0.4 mL adalimumab at visit 1. After 1–2 weeks (depending on patient medication schedule), patients received the other formulation at visit 2. A pain VAS [McGill Pain Questionnaire (MPQ-SF)] and the Draize scale were evaluated immediately after injection and 15 min postinjection. The primary endpoint was immediate pain after injection.
Results
64 and 61 patients were randomized in Studies 1 and 2, respectively. Both studies found a clinically relevant and statistically significant lower immediate pain after injection for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation. The mean difference on the VAS for the pooled data (−2.48 cm) was also clinically relevant. Most other endpoints in both studies favored the 40 mg/0.4 mL formulation, and its tolerability and safety profile were consistent with 40 mg/0.8 mL adalimumab.
Conclusion
A 40 mg/0.4 mL adalimumab formulation was well tolerated and associated with less injection site-related pain than the 40 mg/0.8 mL adalimumab formulation.
Trial registration
ClinicalTrials.gov identifier, NCT01561313 and NCT01502423.
Funding
AbbVie.
Electronic supplementary material
The online version of this article (doi:10.1007/s40744-016-0041-3) contains supplementary material, which is available to authorized users.
Keywords: Injections, Pain, Quality of life, Rheumatoid arthritis, Tumor necrosis factor inhibitors
Introduction
Adalimumab (Humira®; AbbVie, North Chicago, IL, USA) is a fully human, highly specific, high-affinity anti-tumor necrosis factor α monoclonal antibody approved for the treatment of patients with rheumatoid arthritis (RA) as well as other immune-mediated inflammatory diseases in over 90 countries worldwide [1–3].
Adalimumab is administered via subcutaneous injection. In placebo-controlled clinical trials in adult patients with RA, the most common adverse event (AE) with adalimumab was injection site reactions, including erythema and/or itching, hemorrhage, related pain, and swelling [4–8]. The overall incidence of such reactions was 20.3% in patients who received adalimumab versus 13.8% who received placebo; these reactions are the most commonly reported AE across all indications [4].
Pain related to subcutaneous injection can be influenced by various factors, including the inactive ingredients of the formulation (e.g., citrate buffer) [9–11], injection volume [12, 13], and needle size and sharpness [14]. A 40 mg/0.4 mL formulation of adalimumab was developed to address pain associated with injection. The active ingredient in both formulations remains adalimumab derived from the same master cell bank using identical isolation processes [15].
The 40 mg/0.4 mL formulation differs from the current formulation of adalimumab in that it has fewer excipients (Supplementary Table 1); particularly, there is no citrate buffer. Further, the citrate-free formulation has a higher concentration of adalimumab that allows a smaller injection volume (40 mg adalimumab delivered in 0.4 mL instead of 0.8 mL), and the 40 mg/0.4 mL formulation is delivered via a syringe that has a smaller (29 vs 27 gauge) needle than the 40 mg/0.8 mL formulation.
This report details the results of two phase 2, randomized crossover studies in patients with RA that assessed injection site-related pain, safety, and tolerability of the 40 mg/0.4 mL adalimumab formulation versus the 40 mg/0.8 mL formulation.
Methods
Study Designs
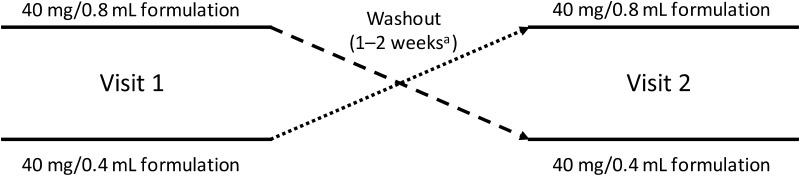
Two phase 2, randomized, single-blind, two-period crossover studies of identical design were conducted (Fig. 1). Study 1 was conducted at six sites in Belgium and the Czech Republic between March and November 2012 (ClinicalTrials.gov identifier, NCT01561313), and Study 2 was conducted at seven sites in Australia, Canada, and Germany between January and November 2012 (ClinicalTrials.gov identifier, NCT01502423).
Fig. 1.
Study design. aDependent on patient’s prescribed on-label dosing schedule
Independent ethics committees/institutional review boards reviewed and approved all study-related documents, including study protocols and any amendments, per Good Clinical Practice requirements. Studies were conducted with ethical principles in accordance with the Declaration of Helsinki. All patients provided informed consent before participating in any study-related procedures.
Formulations
Components of the 40 mg/0.8 mL and 40 mg/0.4 mL adalimumab formulations are listed in Supplementary Table 1. The 40 mg/0.8 mL formulation is delivered via a prefilled syringe with a 27-gauge needle. The 40 mg/0.4 mL presentation uses a prefilled syringe with a 29-gauge needle, a latex-free needle shield, and a plunger stopper that is coated to minimize leaching (Physiolis™; Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Patients
Injection site pain is a commonly reported AE across all populations for which adalimumab is indicated; patients with RA were selected as a representative population to investigate pain on injection for the two formulations. Eligible patients were men and nonpregnant, nonlactating women ≥18 years of age with a diagnosis of RA, as defined by the 1987 revised American College of Rheumatology (ACR) classification criteria [16] or the ACR/European League Against Rheumatism 2010 criteria [17], and who required adalimumab 40 mg every other week (eow) or every week (ew), per the local adalimumab label. Patients were either biologic-naive, or current users of 40 mg/0.8 mL adalimumab. The latter group had rated their average injection site-related pain as ≥3 cm on a pain visual analog scale (VAS) of 0–10 cm in the past month and had received ≥6 consecutive doses of adalimumab before screening. All patients had a negative tuberculosis (TB) screening assessment as determined by a tuberculin skin test (QuantiFERON-TB Gold® test; Cellestis, Inc., Valencia, CA, USA) or equivalent test, and a chest radiograph. If there was evidence of a latent TB infection, patients had to have completed ≥2 weeks of anti-TB therapy before baseline assessment. All patients must have been able and willing to provide written informed consent and comply with the requirements of the protocol. Patients were ineligible if they had an infection requiring intravenous anti-infectives within 30 days of, or oral anti-infectives within 14 days of, the first study visit; chronic recurrent infections; hepatitis B infection; an active systemic viral infection or any active infection that would make the patient an unsuitable candidate for the study based on the investigator’s assessment. Patients were excluded if they had a history of invasive infection; human immunodeficiency virus; demyelinating disease; heart disease (including moderate to severe heart failure or recent cerebrovascular accident); dysplasia or malignancy (except for successfully treated nonmetastatic cutaneous squamous cell or basal cell carcinoma or localized carcinoma in situ of the cervix); or clinically significant hematologic, renal, or liver disease. Patients were also excluded if they had received any live vaccine within 3 months of the study or if they had prior exposure to natalizumab or efalizumab. Patients who were receiving chronic medications prior to the study, including nonbiologic disease-modifying antirheumatic drugs or nonsteroidal anti-inflammatory drugs, continued on medications at their same prestudy doses.
Treatment
After a screening visit (within 30 days of baseline), patients were centrally randomized using an interactive voice response system/interactive web response system in a 1:1 ratio to one of two sequences of adalimumab [either the 40 mg/0.8 mL or 40 mg/0.4 mL formulation at visit 1, followed by the other formulation at visit 2 in a blinded manner (Fig. 1)]. The timing of the second visit was based on the regularly scheduled doses of adalimumab required by the patient’s prescribed on-label dosing schedule (eow or ew).
Assessments
Patients reported injection site pain on the four-item short-form McGill Pain Questionnaire (MPQ-SF) [18], which included (1) a VAS on which the current pain level was marked on a scale from 0 cm (no pain) to 10 cm (worst possible pain); (2) evaluation of overall pain intensity on a six-point scale [no pain (0), mild (1), discomforting (2), distressing (3), horrible (4), excruciating (5)]; (3) sensory and affective dimensions of the pain experience, rated on a four-point scale from 0 (none) to 3 (severe) for each of 15 pain descriptors (sensory: throbbing, shooting, stabbing, sharp, cramping, gnawing, hot/burning, aching, heavy, tender, and splitting; affective: tiring/exhausting, sickening, fearful, and punishing/cruel); and (4) assessment of needle pain (yes, no), including cause of pain (needle entry or solution that was injected). On each visit, items 1–3 were administered twice: immediately (within 2 min) and 15 min after the injection. Item 4 was administered to the patient after completion of item 3, immediately after the injection.
Qualified site staff used the Draize scale to score injection site hemorrhage/petechiae, erythema, edema, and pruritus (scale details are found in Supplementary Material). Assessments were performed 10 and 30 min after the injections on both visits. The study site personnel who administered injections were different from those who administered the MPQ-SF, or completed the Draize scale.
Safety was evaluated on the basis of AEs, physical examinations, vital signs, and standard laboratory tests. Spontaneous reports of injection site pain or reactions were considered AEs; any injection site pain or reactions reported/observed after the 30-min Draize scale evaluation was completed were also considered AEs.
Statistical Methods
Based on a prior investigation, it was reasonable to assume a mean pain level of 3.00 and 2.00 cm on the 10-cm VAS for 40 mg/0.8 mL and 40 mg/0.4 mL formulations, respectively, with a standard deviation of 2.60 for the difference. From this assumption, it was estimated that a two-sided level α = 0.05 test for superiority with 83% power would require a total of 60 patients.
Injection site pain was analyzed in the crossover intent-to-treat population, defined as all patients who were randomized and completed both periods and received study drug in each period of the study. The primary endpoint was the patient’s immediate pain after injection on the VAS. The 95% confidence intervals (CI) and P values for the difference between 40 mg/0.8 mL and 40 mg/0.4 mL formulations were calculated using a two-period crossover analysis of variance (ANOVA) model with period, sequence, and treatment as fixed effects, and subject as a random effect. The same statistical model was also used for the pooled analysis of injection site pain and for continuous endpoints in the individual studies. The two identical studies were pooled to obtain a more reliable estimate of the treatment effect. The percent difference between the two formulations in immediate pain after injection was calculated for each patient and analyzed using descriptive statistics. Based on a minimal clinically important difference (MCID) of 1.3 cm in VAS immediately following injection [19], the proportion of patients who achieved ≥1.3 cm less pain on the VAS while receiving the 40 mg/0.4 mL formulation than while receiving the 40 mg/0.8 mL formulation was calculated; a two-sided exact binomial test was performed to assess whether this proportion differed from 50%. The exploratory analyses assessing percentage differences and proportion of patients who achieved MCID were performed post hoc.
For analysis of VAS results by category, injection site pain was classified as mild (≤3 cm), moderate (>3 to <7 cm) or severe (≥7 cm) [20]. For dichotomous endpoints, Fisher’s exact version of the Mainland–Gart test was used for analysis. Demographic and baseline characteristics were summarized and compared between the two sequence groups. Homogeneity for continuous variables was assessed using a one-way ANOVA model using treatment arm as the independent factor. Homogeneity of discrete variables was evaluated using Fisher’s exact test. Unless otherwise stated, all statistical tests were conducted at an α = 0.05 level. The analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
The safety population consisted of all patients who received ≥1 dose of study drug. AEs were summarized. Treatment-emergent AEs were defined as those that began on or after the first dose of study drug and up to 70 days after the last dose of study drug.
Results
Patients
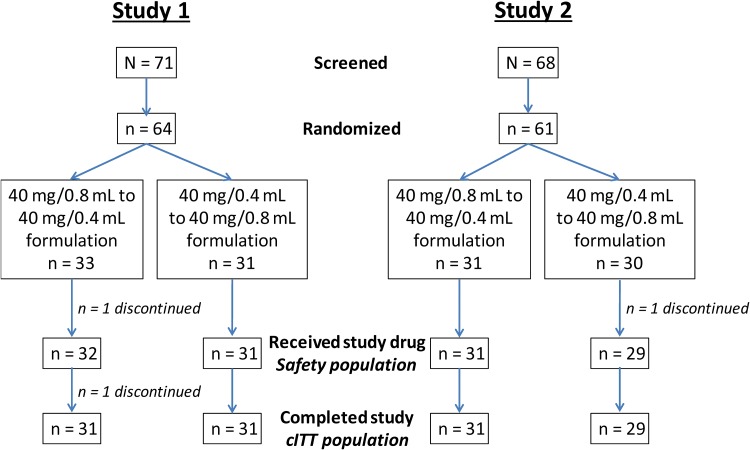
In Study 1, 71 patients were screened and 64 were randomized, 33 to the sequence of 40 mg/0.8 mL to 40 mg/0.4 mL formulation and 31 to the sequence of 40 mg/0.4 mL to 40 mg/0.8 mL formulation. In Study 2, 68 patients were screened and 61 were randomized, 31 and 30 to the respective sequences of 40 mg/0.8 mL to 40 mg/0.4 mL and 40 mg/0.4 mL to 40 mg/0.8 mL formulation (Fig. 2). One randomized patient in each study discontinued before receiving the first dose of study drug; one patient in Study 1 discontinued (due to pharyngitis) after receipt of the first dose of study drug (Fig. 2).
Fig. 2.
Patient disposition. cITT crossover intent-to-treat
Demographic and clinical characteristics at baseline within each study were similar between the sequence groups, with the exception of age and duration of RA in Study 1 (Table 1).
Table 1.
Patients’ baseline demographic and clinical characteristics (cITT population)
| Characteristic | Study 1 | Study 2 | ||||
|---|---|---|---|---|---|---|
| 40 mg/0.8 mL to 40 mg/0.4 mL formulation (n = 31) | 40 mg/0.4 mL to 40 mg/0.8 mL formulation (n = 31) | Total (N = 62) | 40 mg/0.8 mL to 40 mg/0.4 mL formulation (n = 31) | 40 mg/0.4 mL to 40 mg/0.8 mL formulation (n = 29) | Total (N = 60) | |
| Sex, n (%) | ||||||
| Female | 21 (67.7) | 21 (67.7) | 42 (67.7) | 24 (77.4) | 23 (79.3) | 47 (78.3) |
| Male | 10 (32.3) | 10 (32.3) | 20 (32.3) | 7 (22.6) | 6 (20.7) | 13 (21.7) |
| Age, y, mean (SD) | 51.1 (12.6)a | 58.6 (13.5)a | 54.8 (13.5) | 58.3 (11.6) | 54.4 (14.1) | 56.4 (12.9) |
| Duration of RAb, y, mean (SD) | 9.3 (7.6)c | 16.8 (9.8)c | 13.0 (9.5) | 11.7 (8.0) | 12.1 (11.3) | 11.9 (9.6) |
| Biologic-naive, n (%) | 10 (32.3) | 9 (29.0) | 19 (30.6) | 10 (32.3) | 7 (24.1) | 17 (28.3) |
| Currently receiving adalimumab for ≥6 consecutive doses, n (%) | 21 (67.7) | 22 (71.0) | 43 (69.4) | 21 (67.7) | 22 (75.9) | 43 (71.7) |
| Injection site pain immediately after injection in the last month, cm, mean (SD)d,e | 4.9 (1.6) | 4.8 (1.4) | 4.8 (1.5) | 5.4 (1.9) | 5.8 (2.0) | 5.6 (1.9) |
| Adalimumab dosing frequencyd, n (%) | ||||||
| Weekly | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.3) |
| Every other week | 21 (100) | 22 (100) | 43 (100) | 19 (90.5) | 21 (95.5) | 40 (93.0) |
| Other | 0 | 0 | 0 | 1 (4.8) | 1 (4.5) | 2 (4.7) |
ANOVA analysis of variance, cITT crossover intent-to-treat, RA rheumatoid arthritis, SD standard deviation
a P < 0.05 for difference between sequence groups using one-way ANOVA
bCalculated as (date of first study drug−date of diagnosis of RA)/365.25
c P ≤ 0.001 for difference between sequence groups using one-way ANOVA
dOnly for patients currently receiving adalimumab
eAssessed on a 10-cm visual analog scale at screening (0 cm = no pain; 10 cm = worst possible pain)
Pain and Injection Site Assessment
Primary Endpoint: Immediate Pain After Injection
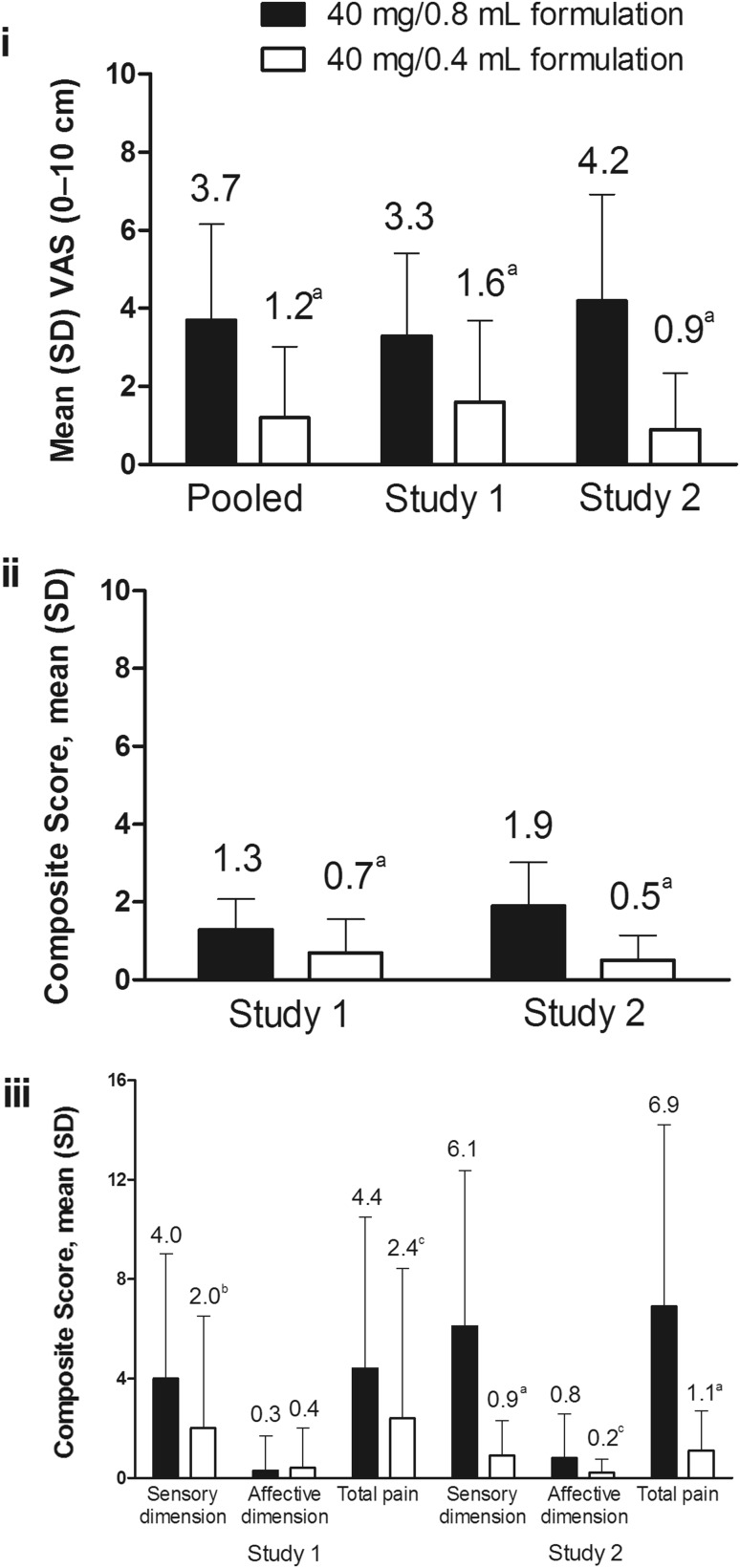
The primary endpoint was achieved in both studies. Patient-reported injection-related pain immediately after injection, as measured by VAS, was significantly lower for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation in both studies. In Study 1, the mean difference was −1.74 (95% CI −2.33 to −1.16; P < 0.001), with a 79% median reduction and a 47% mean reduction in pain; in Study 2, the mean difference was −3.25 (95% CI −4.00 to −2.49; P < 0.001) with an 89% median reduction and a 61% mean reduction in pain. The mean difference in immediate pain after injection for the pooled population was −2.48 (95% CI −2.97 to −2.00; P < 0.001; Fig. 3i) with an 84% median reduction and a 54% mean reduction in pain; 67% of patients experienced ≥1.3 cm less pain (MCID) following receipt of the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation (P < 0.001). No carryover effects between the two visits were noted in either study; carryover effects were not tested between pain immediately after injection and pain 15 min after injection.
Fig. 3.
Parameters assessed in the cITT population immediately after injection. Injection site pain for pooled data, Study 1, and Study 2 (i); present pain intensity in Study 1 and Study 2 (ii); and MPQ-SF total pain, sensory dimension, and affective dimension scores of pain experience scores in Study 1 and Study 2 (iii). cITT crossover intent-to-treat, MPQ-SF short-form McGill Pain Questionnaire, SD standard deviation, VAS visual analog scale. a P < 0.001. b P = 0.001. c P = 0.009
Pain Immediately After Injection by Category
Changes in patient pain perception were assessed by category based on VAS assessments of pain immediately after injection (Table 2). More patients who received the 40 mg/0.4 mL formulation reported mild pain on the VAS compared with patients who received the 40 mg/0.8 mL formulation (86.9% vs 42.6%). Most patients reported a reduction in pain to the mild category after receiving the 40 mg/0.4 mL formulation, including 15 of 17 patients who experienced severe pain and 42 of 53 patients who experienced moderate pain after receiving the 40 mg/0.8 mL formulation. Only four patients reported worse pain with the 40 mg/0.4 mL formulation than the 40 mg/0.8 mL formulation.
Table 2.
Changes in patient perception of immediate pain on the VAS by category
| VAS category, n (%) | 40 mg/0.8 mL formulation (N = 122) | 40 mg/0.4 mL formulation (N = 122) | ||
|---|---|---|---|---|
| Total | Mild (≤3 cm) | Moderate (>3 to <7 cm) | Severe (≥7 cm) | |
| Mild (≤3 cm) | 52 (42.6) | 49 (40.2) | 2 (1.6) | 1 (0.8) |
| Moderate (>3 to <7 cm) | 53 (43.4) | 42 (34.4) | 10 (8.2) | 1 (0.8) |
| Severe (≥7 cm) | 17 (13.9) | 15 (12.3) | 2 (1.6) | 0 |
| Total | 122 (100) | 106 (86.9) | 14 (11.5) | 2 (1.6) |
VAS visual analog scale
Other Endpoints
Injection-related pain 15 min after injection was significantly lower for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation in Study 2 but not in Study 1 (Table 3). For the pooled population, the mean difference in pain 15 min after injection for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation was −0.35 (95% CI −0.62 to −0.07; P = 0.014).
Table 3.
Pain parameters 15 min after injection
| Assessment, mean (SD) | 40 mg/0.8 mL formulation | 40 mg/0.4 mL formulation | Within-patient differencea (95% CI) | P valueb |
|---|---|---|---|---|
| Study 1 | N = 62 | N = 62 | ||
| Pain of injection | 1.0 (1.52) | 0.9 (1.66) | –0.09 (–0.40 to 0.23) | 0.581 |
| Present pain intensity | 0.3 (0.54) | 0.2 (0.48) | –0.05 (–0.22 to 0.13) | 0.584 |
| MPQ-SF pain experience | ||||
| Sensory dimension score | 0.8 (1.83) | 0.6 (2.14) | –0.13 (–0.73 to 0.48) | 0.671 |
| Affective dimension score | 0.1 (0.65) | 0.1 (0.58) | –0.03 (–0.24 to 0.17) | 0.754 |
| Total score | 0.9 (2.32) | 0.7 (2.64) | –0.16 (–0.93 to 0.61) | 0.678 |
| Study 2 | N = 60 | N = 60 | ||
| Pain of injection | 1.0 (1.61) | 0.4 (1.08) | –0.62 (–1.08 to –0.17) | 0.008 |
| Present pain intensity | 0.7 (0.86) | 0.2 (0.53) | –0.42 (–0.62 to –0.22) | <0.001 |
| MPQ-SF pain experience | ||||
| Sensory dimension score | 1.5 (2.84) | 0.4 (0.92) | –1.17 (–1.81 to –0.52) | <0.001 |
| Affective dimension score | 0.1 (0.25) | 0.1 (0.36) | 0 (–0.09 to 0.10) | 0.963 |
| Total score | 1.6 (2.88) | 0.5 (1.03) | –1.17 (–1.81 to –0.52) | <0.001 |
ANOVA analysis of variance, CI confidence interval, MPQ-SF short-form McGill Pain Questionnaire, SD standard deviation
aWithin-patient difference for 40 mg/0.4 mL adalimumab–40 mg/0.8 mL adalimumab from the crossover ANOVA model with period, sequence, and treatment as fixed effects, and subject as a random effect
bFor differences between treatment groups from ANOVA with period, sequence, and treatment as fixed effects, and subject as a random effect
Present pain intensity immediately after injection was significantly lower for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation in Study 1 and in Study 2 (Fig. 3ii); within-patient differences were –0.60 (95% CI –0.84 to –0.35) and –1.35 (95% CI −1.64 to −1.06), respectively (P < 0.001 for both). Present pain intensity scores 15 min after injection were significantly lower for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation in Study 2 but not in Study 1 (Table 3).
In Study 1, MPQ-SF pain experience sensory dimension and total scores immediately after injection were significantly lower for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation [within-patient differences of –2.05 (95% CI –3.25 to –0.85) and –2.03 (95% CI –3.53 to –0.54), respectively; P ≤ 0.009 for both]. Affective dimension scores were not significantly different [0.02 (95% CI –0.36 to 0.39); P = 0.932; Fig. 3iii]. Immediately after injection in Study 2, sensory dimension, affective dimension, and total scores were significantly lower for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation [within-patient differences of –5.19 (95% CI –6.69 to –3.68), –0.65 (95% CI –1.13 to –0.17), and –5.84 (95% CI –7.63 to –4.05), respectively; P ≤ 0.009 for all; Fig. 3iii]. Pain experience sensory dimension, affective dimension, and total scores 15 min after injection were not significantly lower for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation in Study 1, whereas sensory dimension and total scores were significantly lower in Study 2 (Table 3).
A subgroup of patients (n = 55 in each study) were able to discern between the pain of the needle entering the skin and the pain from the injected solution for ≥1 of the injections. In both studies, more patients attributed pain with the 40 mg/0.8 mL formulation to the solution rather than to the needle. Conversely, more patients attributed pain with the 40 mg/0.4 mL formulation to needle entry than to the solution (Supplementary Table 2).
Study staff completed the Draize scale at 10 and 30 min after injection. In both studies and with both formulations, the majority of patients had no hemorrhage/petechiae, no or very slight erythema, and no or very slight edema; pruritus was rarely observed (Supplementary Tables 3, 4).
Safety
There were few AEs in Studies 1 and 2 (Table 4). In Study 1, there were three AEs; none were serious or severe. One patient discontinued after visit 1 (after receiving the 40 mg/0.8 mL formulation of adalimumab) because of mild pharyngitis. One AE in Study 1 was considered to be at least possibly related to adalimumab [erythematous rash (mild) in a patient after receiving the 40 mg/0.8 mL formulation of adalimumab].
Table 4.
Adverse event summary
| AE, n (%) | Study 1 (N = 63) | Study 2 (N = 60) | ||||
|---|---|---|---|---|---|---|
| 40 mg/0.8 mL formulation | 40 mg/0.4 mL formulationa | Total | 40 mg/0.8 mL formulation | 40 mg/0.4 mL formulation | Total | |
| Any AE | 2 (3.2) | 1 (1.6) | 3 (4.8) | 8 (13.3) | 4 (6.7) | 11 (18.3) |
| Any AE at least possibly drug-related | 1 (1.6) | 0 | 1 (1.6) | 2 (3.3) | 3 (5.0) | 4 (6.7) |
| Any severe or serious AE | 0 | 0 | 0 | 0 | 0 | 0 |
| Any AE leading to discontinuation of study drug | 1 (1.6) | 0 | 1 (1.6) | 0 | 0 | 0 |
| AEs of special interest | ||||||
| Any infection | 1 (1.6) | 0 | 1 (1.6) | 6 (10.0) | 1 (1.7) | 7 (11.7) |
| Injection site reaction | 0 | 0 | 0 | 0 | 2 (3.3) | 2 (3.3) |
AE adverse event
a n = 62; one patient discontinued the study before receiving the 40 mg/0.4 mL formulation
Eleven AEs were observed in Study 2. Most were mild in severity and five were moderate [injection site reaction in one patient after receiving 40 mg/0.4 mL adalimumab; upper respiratory tract infection (URTI) in one patient after receiving the 40 mg/0.8 mL formulation of adalimumab; and lower respiratory tract infection, URTI, upper limb fracture, and asthma, all in one patient after receiving the 40 mg/0.8 mL formulation of adalimumab]. No AEs were serious, severe, or resulted in study discontinuation. Five events (in four patients) were considered by the investigator to be at least possibly related to adalimumab. After receiving the 40 mg/0.8 mL formulation of adalimumab, urinary tract infection and headache each occurred in one patient. After receiving the 40 mg/0.4 mL formulation, injection site pruritus, injection site reaction, and URTI were reported in one patient each. The injection site reaction in one patient was of moderate intensity and lasted 4 days, resolving with diphenhydramine treatment. The patient, a current adalimumab user with a history of injection site reactions, did not report any pain on the VAS at the time of the injection or 15 min post-dose. On the Draize scale, well-defined erythema was reported 10 and 30 min post-dose, and constant pruritus was reported 30 min after the injection. The second patient (biologic-naive) reported injection site reaction pruritus, which lasted for 30 min. No pain was reported on the VAS at the time of injection or 15 min after, and there was no pruritus at 10 or 30 min post-dose on the Draize scale.
Discussion
Results of these two phase 2, randomized crossover studies in patients with RA were consistently in favor of a 40 mg/0.4 mL formulation compared with the 40 mg/0.8 mL formulation of adalimumab with regard to injection site-related pain. The primary endpoint, significantly lower immediate pain after injection for the 40 mg/0.4 mL versus the 40 mg/0.8 mL formulation, was met for both studies. The mean difference on the patient-reported VAS for the pooled data (−2.48 cm) was also clinically relevant, based on assessments of the MCID in other settings. The MCID has been reported to range between 1.0 and 1.6 cm in settings of acute pain [19–21], and between 0.5 and 1.1 cm in observational studies of chronic pain in patients with RA [22].
Most other patient-reported endpoints were statistically significantly in favor of the 40 mg/0.4 mL formulation immediately after injection in Study 1 and at both time points after injection for Study 2. As observed for the primary and most other endpoints, differences between formulations were of larger magnitude in Study 2 compared with Study 1. The reasons for dissimilar pain ratings for these two identically designed studies are unclear, but they may relate to population differences where the studies were conducted (Belgium and the Czech Republic versus Australia, Canada, and Germany).
The MPQ-SF pain experience scale assesses both sensory and affective dimensions of the pain experience. It should be noted that differences that were observed between formulations were mainly related to sensory aspects of pain. Affective dimension scores were generally low for both formulations and differed significantly between formulations only in Study 2. In patients able to distinguish between the pain of needle entry versus injected solution, pain was most often attributed to the solution with the 40 mg/0.8 mL formulation and to needle entry with the 40 mg/0.4 mL formulation. The effect of volume on the ability to distinguish pain was not tested.
Results of the Draize scale, which was assessed by site staff, suggested that both formulations were similar with regard to the presence and severity of injection site hemorrhage/petechiae, erythema, edema, and pruritus. Notably, scores for each of four scale items were generally low for the 40 mg/0.4 mL and 40 mg/0.8 mL formulations of adalimumab, indicating the generally good tolerability of both formulations. There were no unexpected safety signals with the 40 mg/0.4 mL formulation.
An important strength of these studies was that the populations included patients who might be expected to experience injection site pain: 40 mg/0.8 mL users of adalimumab who had rated their average injection site pain score as ≥3 cm on a pain VAS during the month preceding the study, and patients who were biologic-naive. However, results were limited to only one injection of each formulation, administered ew or eow based on each patient’s regular adalimumab dosing schedule.
Results of both studies consistently demonstrated lower injection site-related pain with the 40 mg/0.4 mL formulation; however, it is not clear which feature(s) of the 40 mg/0.4 mL formulation (composition, volume, and/or needle size) is most responsible for pain reduction. Citrate-based buffers have been identified by others as a key ingredient in injection site pain [9–11].
Conclusion
Results of two phase 2, randomized crossover studies in patients with RA demonstrated that the 40 mg/0.4 mL formulation of adalimumab is associated with less injection site-related pain compared with the currently available formulation. Although this study was conducted in patients with RA, similar outcomes would be expected in other immune-mediated inflammatory diseases for which adalimumab is approved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
AbbVie (North Chicago, Illinois, USA) funded these studies, contributed to their design, and was involved in the collection, analysis, and interpretation of the data, and in the writing, review, and approval of the publication. AbbVie also funded the publication charges. Medical writing support was provided by Mariana Ovnic, PhD, of Complete Publication Solutions, LLC (North Wales, PA) and funded by AbbVie. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors would like to thank Monika Höbel and Hui Ding, employees of AbbVie, for statistical programming support.
Disclosures
A. Cividino has received research funding from AbbVie, Pfizer, Celgene, Takeda, Roche, and UCB, and support for the AbbVie Chair in Education in Rheumatology. P. Nash has received grants and funding for clinical trials and honoraria for advice and lectures on behalf of AbbVie. U. Arulmani is a former employee of AbbVie who may own AbbVie stock or stock options. K. Unnebrink, R. Tarzynski-Potempa, and A. N. Payne are current employees of AbbVie and may own AbbVie stock and stock options. S. Hall and J. Vanhoof have nothing to disclose.
Compliance with Ethics Guidelines
Independent ethics committees/institutional review boards reviewed and approved all study-related documents, including study protocols and any amendments, per Good Clinical Practice requirements. All patients provided informed consent before participating in any study-related procedures. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/FAE4F060049D77A7.
References
- 1.Humira (adalimumab). Summary of Product Characteristics. Maidenhead, United Kingdom: AbbVie Ltd; 2015.
- 2.Humira (adalimumab). Product Monograph. Saint-Laurent, Quebec, Canada: AbbVie Corporation; 2016.
- 3.Humira®. Adalimumab. North Chicago: AbbVie Inc; 2015.
- 4.Mease PJ. Adalimumab in the treatment of arthritis. Ther Clin Risk Manag. 2007;3:133. [DOI] [PMC free article] [PubMed]
- 5.Furst DE, Schiff MH, Fleischmann RM, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J Rheumatol. 2003;30:2563–2571. [PubMed] [Google Scholar]
- 6.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 7.van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 9.Veys N, Dhondt A, Lameire N. Pain at the injection site of subcutaneously administered erythropoietin: phosphate-buffered epoetin alpha compared to citrate-buffered epoetin alpha and epoetin beta. Clin Nephrol. 1998;49:41–44. [PubMed] [Google Scholar]
- 10.Frenken LA, van Lier HJ, Jordans JG, et al. Identification of the component part in an epoetin alfa preparation that causes pain after subcutaneous injection. Am J Kidney Dis. 1993;22:553–556. doi: 10.1016/S0272-6386(12)80928-0. [DOI] [PubMed] [Google Scholar]
- 11.Laursen T, Hansen B, Fisker S. Pain perception after subcutaneous injections of media containing different buffers. Basic Clin Pharmacol Toxicol. 2006;98:218–221. doi: 10.1111/j.1742-7843.2006.pto_271.x. [DOI] [PubMed] [Google Scholar]
- 12.Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16:971–976. doi: 10.1111/dom.12304. [DOI] [PubMed] [Google Scholar]
- 13.Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917–1923. doi: 10.1007/s00415-010-5779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaber A, Bozzato GB, Vedrine L, Prais WA, Berube J, Laurent PE. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38. doi: 10.1186/1471-2377-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tebbey PW, Varga A, Naill M, Clewell J, Venema J. Consistency of quality attributes for the glycosylated monoclonal antibody Humira(R) (adalimumab). mAbs. 2015;7:805–11. [DOI] [PMC free article] [PubMed]
- 16.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 19.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–489. doi: 10.1016/S0196-0644(96)70238-X. [DOI] [PubMed] [Google Scholar]
- 20.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher EJ, Bijur PE, Latimer C, Silver W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am J Emerg Med. 2002;20:287–290. doi: 10.1053/ajem.2002.33778. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol. 2007;34:1674–1683. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.