Abstract
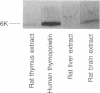
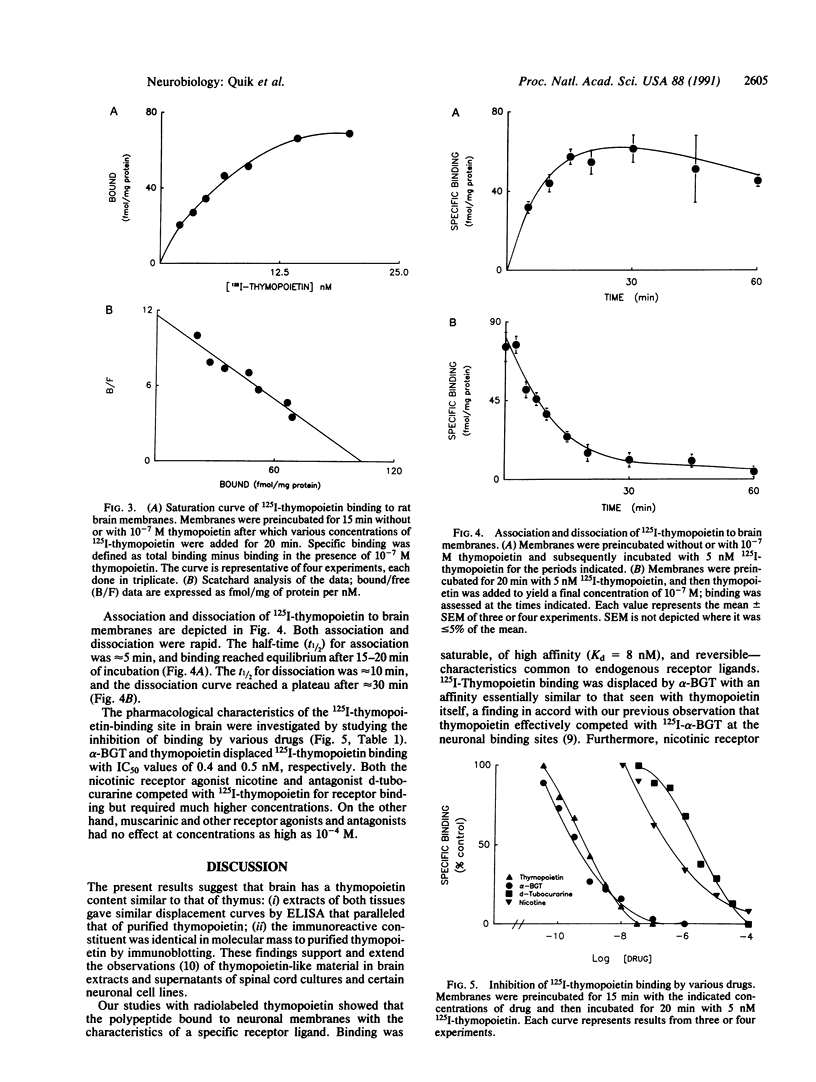
Thymopoietin, a polypeptide hormone of the thymus that has pleiotropic actions on the immune, endocrine, and nervous systems, potently interacts with the neuromuscular nicotinic acetylcholine receptor. Thymopoietin binds to the nicotinic alpha-bungarotoxin (alpha-BGT) receptor in muscle and, like alpha-BGT, inhibits cholinergic transmission at this site. Evidence is given that radiolabeled thymopoietin similarly binds to a nicotinic alpha-BGT-binding site within the brain and does so with the characteristics of a specific receptor ligand. Thus specific binding to neuronal membranes was saturable, of high affinity (Kd = 8 nM), linear with increased tissue concentration, and readily reversible; half-time was approximately 5 min for association and 10 min for dissociation. Binding of 125I-labeled thymopoietin was displaced not only by unlabeled thymopoietin but also by alpha-BGT and the nicotinic receptor ligands d-tubocurarine and nicotine; various other receptor ligands (muscarinic, adrenergic, and dopaminergic) did not affect binding of 125I-labeled thymopoietin. Thymopoietin was shown by ELISA to be present in brain extracts, displacement curves of thymus and brain extracts being parallel to the standard thymopoietin curve, and Western (immuno) blot identified in brain and thymus extracts a thymopoietin-immunoreactive polypeptide of the same molecular mass as purified thymopoietin polypeptide. We conclude that thymopoietin and thymopoietin-binding sites are present within the brain and that the receptor for thymopoietin is the previously identified nicotinic alpha-BGT-binding site of neuronal tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audhya T., Scheid M. P., Goldstein G. Contrasting biological activities of thymopoietin and splenin, two closely related polypeptide products of thymus and spleen. Proc Natl Acad Sci U S A. 1984 May;81(9):2847–2849. doi: 10.1073/pnas.81.9.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya T., Schlesinger D. H., Goldstein G. Complete amino acid sequences of bovine thymopoietins I, II, and III: closely homologous polypeptides. Biochemistry. 1981 Oct 13;20(21):6195–6200. doi: 10.1021/bi00524a044. [DOI] [PubMed] [Google Scholar]
- Audhya T., Schlesinger D. H., Goldstein G. Isolation and complete amino acid sequence of human thymopoietin and splenin. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3545–3549. doi: 10.1073/pnas.84.11.3545. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Audhya T., Talle M. A., Goldstein G. Thymopoietin radioreceptor assay utilizing lectin-purified glycoprotein from a biologically responsive T cell line. Arch Biochem Biophys. 1984 Oct;234(1):167–177. doi: 10.1016/0003-9861(84)90338-2. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Halvorsen S. W. Acetylcholine receptor. Genes encoding nicotinic receptor subtypes on neurons. Nature. 1988 Aug 4;334(6181):384–385. doi: 10.1038/334384a0. [DOI] [PubMed] [Google Scholar]
- Boulter J., Connolly J., Deneris E., Goldman D., Heinemann S., Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Boulter J., O'Shea-Greenfield A., Duvoisin R. M., Connolly J. G., Wada E., Jensen A., Gardner P. D., Ballivet M., Deneris E. S., McKinnon D. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990 Mar 15;265(8):4472–4482. [PubMed] [Google Scholar]
- Brown R. H., Jr, Schweitzer J. S., Audhya T., Goldstein G., Dichter M. A. Immunoreactive thymopoietin in the mouse central nervous system. Brain Res. 1986 Sep 3;381(2):237–243. doi: 10.1016/0006-8993(86)90072-7. [DOI] [PubMed] [Google Scholar]
- Clarke P. B., Schwartz R. D., Paul S. M., Pert C. B., Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985 May;5(5):1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin R. M., Deneris E. S., Patrick J., Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989 Oct;3(4):487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Goldstein G. Isolation of bovine thymin: a polypeptide hormone of the thymus. Nature. 1974 Jan 4;247(5435):11–14. doi: 10.1038/247011a0. [DOI] [PubMed] [Google Scholar]
- Gotti C., Ogando A. E., Clementi F. The alpha-bungarotoxin receptor purified from a human neuroblastoma cell line: biochemical and immunological characterization. Neuroscience. 1989;32(3):759–767. doi: 10.1016/0306-4522(89)90296-0. [DOI] [PubMed] [Google Scholar]
- Jacob M. H., Berg D. K. The ultrastructural localization of alpha-bungarotoxin binding sites in relation to synapses on chick ciliary ganglion neurons. J Neurosci. 1983 Feb;3(2):260–271. doi: 10.1523/JNEUROSCI.03-02-00260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G., Bentley L., McNamee M. G., Morley B. J. Purification and characterization of the alpha-bungarotoxin binding protein from rat brain. Brain Res. 1985 Nov 18;347(2):274–283. doi: 10.1016/0006-8993(85)90187-8. [DOI] [PubMed] [Google Scholar]
- Kemp G., Edge M. Cholinergic function and alpha-bungarotoxin binding in PC12 cells. Mol Pharmacol. 1987 Sep;32(3):356–363. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindstrom J., Schoepfer R., Whiting P. Molecular studies of the neuronal nicotinic acetylcholine receptor family. Mol Neurobiol. 1987 Winter;1(4):281–337. doi: 10.1007/BF02935740. [DOI] [PubMed] [Google Scholar]
- Loring R. H., Dahm L. M., Zigmond R. E. Localization of alpha-bungarotoxin binding sites in the ciliary ganglion of the embryonic chick: an autoradiographic study at the light and electron microscopic level. Neuroscience. 1985 Feb;14(2):645–660. doi: 10.1016/0306-4522(85)90316-1. [DOI] [PubMed] [Google Scholar]
- Lukas R. J., Audhya T., Goldstein G., Lucero L. Interactions of the thymic polypeptide hormone thymopoietin with neuronal nicotinic alpha-bungarotoxin binding sites and with muscle-type, but not ganglia-type, nicotinic acetylcholine receptor ligand-gated ion channels. Mol Pharmacol. 1990 Dec;38(6):887–894. [PubMed] [Google Scholar]
- Marks M. J., Burch J. B., Collins A. C. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983 Sep;226(3):817–825. [PubMed] [Google Scholar]
- Mitsuka M., Hatanaka H. Selective loss of acetylcholine sensitivity in a nerve cell line cultured in hormone-supplemented serum-free medium. J Neurosci. 1983 Sep;3(9):1785–1790. doi: 10.1523/JNEUROSCI.03-09-01785.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley B. J., Kemp G. E., Salvaterra P. alpha-Bungarotoxin binding sites in the CNS. Life Sci. 1979 Mar 5;24(10):859–872. doi: 10.1016/0024-3205(79)90335-7. [DOI] [PubMed] [Google Scholar]
- Oswald R. E., Freeman J. A. Alpha-bungarotoxin binding and central nervous system nicotinic acetylcholine receptors. Neuroscience. 1981;6(1):1–14. doi: 10.1016/0306-4522(81)90239-6. [DOI] [PubMed] [Google Scholar]
- Quik M., Afar R., Audhya T., Goldstein G. Thymopoietin, a thymic polypeptide, specifically interacts at neuronal nicotinic alpha-bungarotoxin receptors. J Neurochem. 1989 Oct;53(4):1320–1323. doi: 10.1111/j.1471-4159.1989.tb07431.x. [DOI] [PubMed] [Google Scholar]
- Quik M., Afar R., Geertsen S., Audhya T., Goldstein G., Trifaró J. M. Thymopoietin, a thymic polypeptide, regulates nicotinic alpha-bungarotoxin sites in chromaffin cells in culture. Mol Pharmacol. 1990 Jan;37(1):90–97. [PubMed] [Google Scholar]
- Quik M., Fournier S., Trifaró J. M. Modulation of the nicotinic alpha-bungarotoxin site in chromaffin cells in culture by a factor(s) endogenous to neuronal tissue. Brain Res. 1986 Apr 30;372(1):11–20. doi: 10.1016/0006-8993(86)91453-8. [DOI] [PubMed] [Google Scholar]
- Quik M., Geertsen S. Neuronal nicotinic alpha-bungarotoxin sites. Can J Physiol Pharmacol. 1988 Aug;66(8):971–979. doi: 10.1139/y88-160. [DOI] [PubMed] [Google Scholar]
- Quik M., Geertsen S., Trifaró J. M. Marked up-regulation of the beta-bungarotoxin site in adrenal chromaffin cells by specific nicotinic antagonists. Mol Pharmacol. 1987 Apr;31(4):385–391. [PubMed] [Google Scholar]
- Quik M., Lamarca M. V. Blockade of transmission in rat sympathetic ganglia by a toxin which co-purifies with alpha-bungarotoxin. Brain Res. 1982 Apr 29;238(2):385–399. doi: 10.1016/0006-8993(82)90112-3. [DOI] [PubMed] [Google Scholar]
- Revah F., Mulle C., Pinset C., Audhya T., Goldstein G., Changeux J. P. Calcium-dependent effect of the thymic polypeptide thymopoietin on the desensitization of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1987 May;84(10):3477–3481. doi: 10.1073/pnas.84.10.3477. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Steinbach J. H., Ifune C. How many kinds of nicotinic acetylcholine receptor are there? Trends Neurosci. 1989 Jan;12(1):3–6. doi: 10.1016/0166-2236(89)90145-8. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian K., Audhya T., Goldstein G. Binding of thymopoietin to the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(10):3171–3174. doi: 10.1073/pnas.83.10.3171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Whiting P., Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci U S A. 1987 Jan;84(2):595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]