Abstract
Paragangliomas are rare neuroendocrine tumors arising from the ganglia of the sympathetic or parasympathetic nervous system. Less than 160 cases of intrapericardial or intracardiac paragangliomas have been reported in the English language peer-reviewed medical literature. Here, we report a case of intrapericardial paraganglioma, which illustrates some typical multimodality imaging features of this rare entity.
Keywords: Intrapericardial paraganglioma, Primary cardiac tumors
Case report
We report a case of a 25-year-old woman who was initially assessed with echocardiography for investigation of palpitations and mild exertional dyspnea. Transthoracic echocardiogram demonstrated a 6-cm mediastinal mass of heterogeneous echotexture in the right atrioventricular groove, compressing and deforming the right ventricle and right atrium (RA; Fig. 1).
Fig. 1.
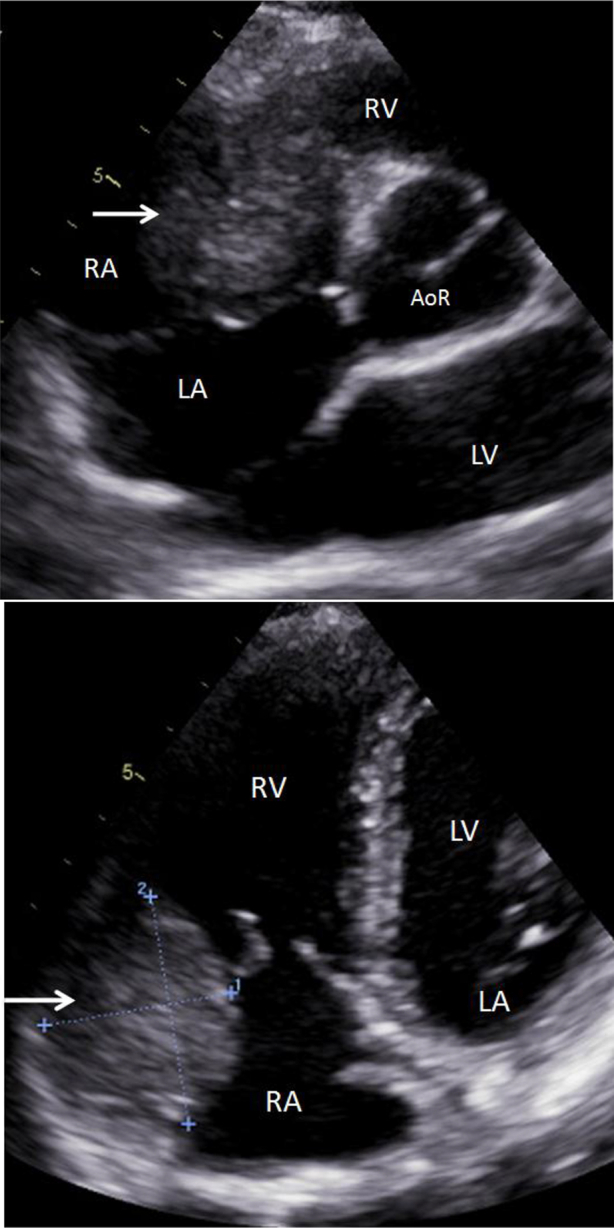
Transthoracic cardiac ultrasound demonstrating a heterogeneous mass (arrow) along the wall of the right heart chambers, at the level of the atrioventricular groove, abutting the aortic root, indenting and deforming the RA and RV. LA, left atrium; LV, left ventricle; RV, right ventricle; AoR, aortic root.
The patient then underwent a non–EKG-gated computer tomography scan of the chest with intravenous contrast. It revealed a well-defined mass in the right atrioventricular groove, indenting the RA and right ventricular outflow tract, and abutting the aortic root and distal superior vena cava (Fig. 2). The mass was hyperenhancing with a thin hypoenhancing rim and a central area of stellate hypoenhancement, reminiscent of a central scar of hepatic fibronodular hyperplasia. The lesion contained neither detectable fat nor calcifications. It appeared intrapericardial and possibly intra-atrial.
Fig. 2.
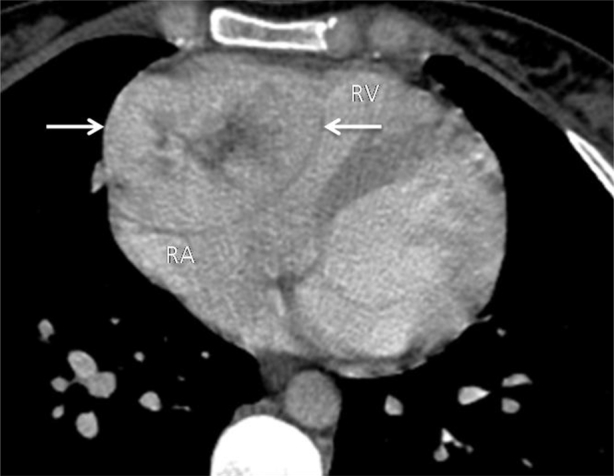
Contrast-enhanced computed tomography of the chest demonstrating a well-defined hyperenhancing mass (arrows) with a thin hypoenhancing rim, and a central area of stellate hypoenhancement, in the right atrioventricular groove, indenting the RA and right ventricle outflow tract.
The subsequent cardiac magnetic resonance imaging again demonstrated a well-defined right intrapericardial mass, showing avid enhancement with intravenous gadolinium contrast, and central stellate “scar” (Fig. 3). The appearance of the mass on cine images, and its broad base along the wall of the right cardiac chambers favored a mass centered on the epicardium.
Fig. 3.
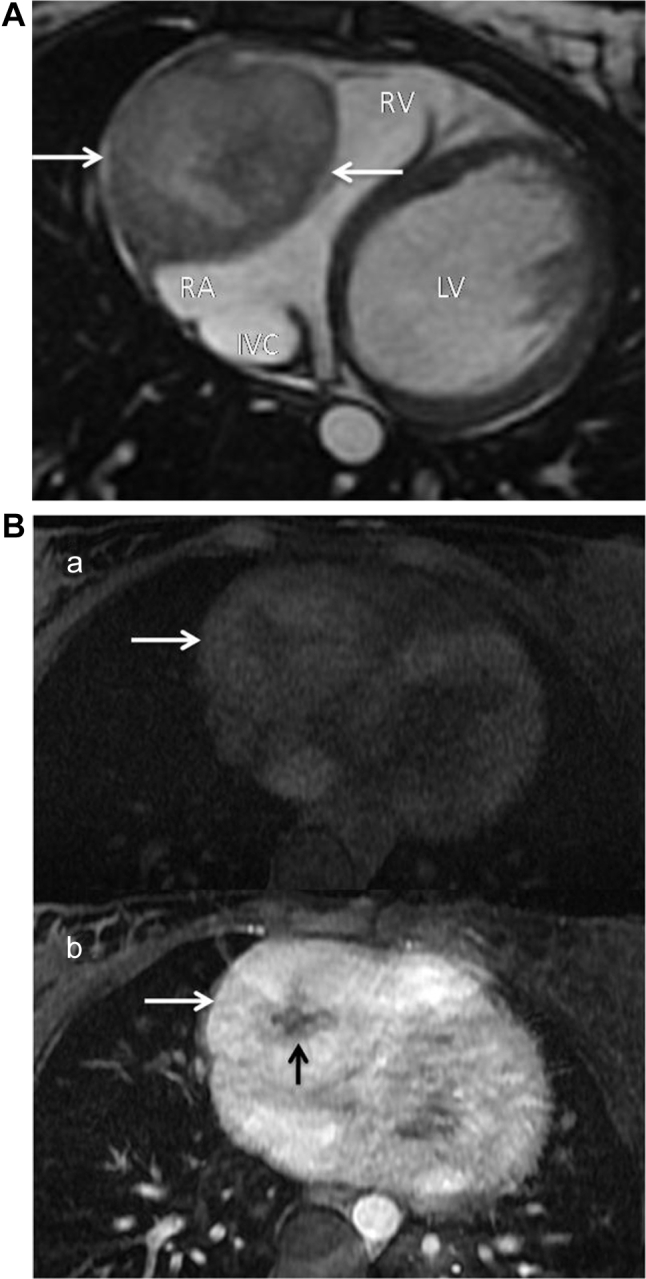
(A) Cardiac MRI, axial steady-state free precession (SSFP) image demonstrates a well-defined heterogeneous right intrapericardial mass (arrows), which is hyperintense relative to the myocardium. (B) Cardiac MRI T1 FatSat axial images pre- (a) and post-intravenous (b) gadolinium contrast administration, demonstrating avid enhancement (white arrows) of the mass, and nonenhancing central stellate “scar” (black arrow). LV, left ventricle; RV, right ventricle; IVC, inferior vena cava; MRI, magnetic resonance imaging.
(18)F-fluorodeoxyglucose positron emission tomography was also performed and showed a 6-cm intensely hypermetabolic lesion in the right heart with standardized uptake value of up to 31 and central necrosis (Fig. 4).
Fig. 4.
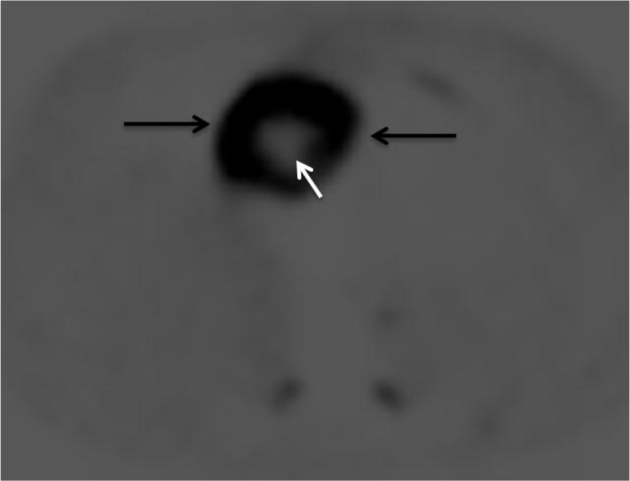
Axial image of (18)F-fluorodeoxyglucose positron emission tomography shows an intensely hypermetabolic lesion in the right heart (black arrows) with central photopenia suggestive of necrosis (white arrow).
Coronary angiography was performed next and showed the mass's blood supply from a prominent branch of the proximal right coronary artery (Fig. 5). The mass enhanced rapidly and washed out quickly via a confluence of small veins, which drained into the coronary sinus.
Fig. 5.
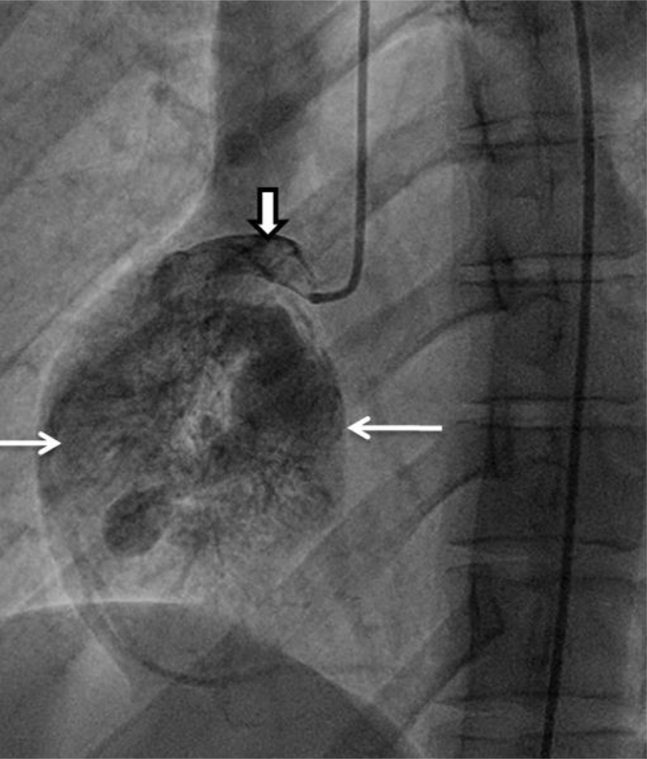
Angiography of the right coronary artery (outlined arrow) shows rapid heterogeneous contrast opacification of the mass (simple arrows).
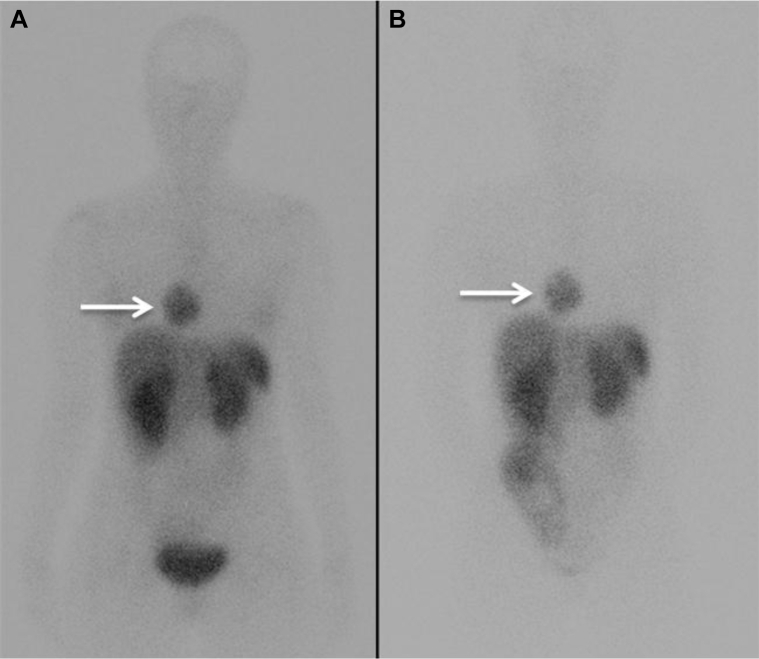
An indium-111 octreotide single-photon emission computed tomography at 4 and 24 hours postadministration of the radiopharmaceutical was performed next. The lesion was positive for octreotide uptake (Fig. 6), thus confirming the suspected diagnosis of a cardiac paraganglioma.
Fig. 6.
Indium-111 octreotide single-photon emission computed tomography at 4 hours (A) and 24 hours (B) postadministration of the radiopharmaceutical shows that the right cardiac lesion (arrows) is positive for octreotide uptake.
An iodine-131 metaiodobenzylguanidine (I-131 MIBG) scintigraphy was also performed and showed mild heterogeneous radiotracer accumulation in the RA at the site of the mass (Fig. 7).
Fig. 7.
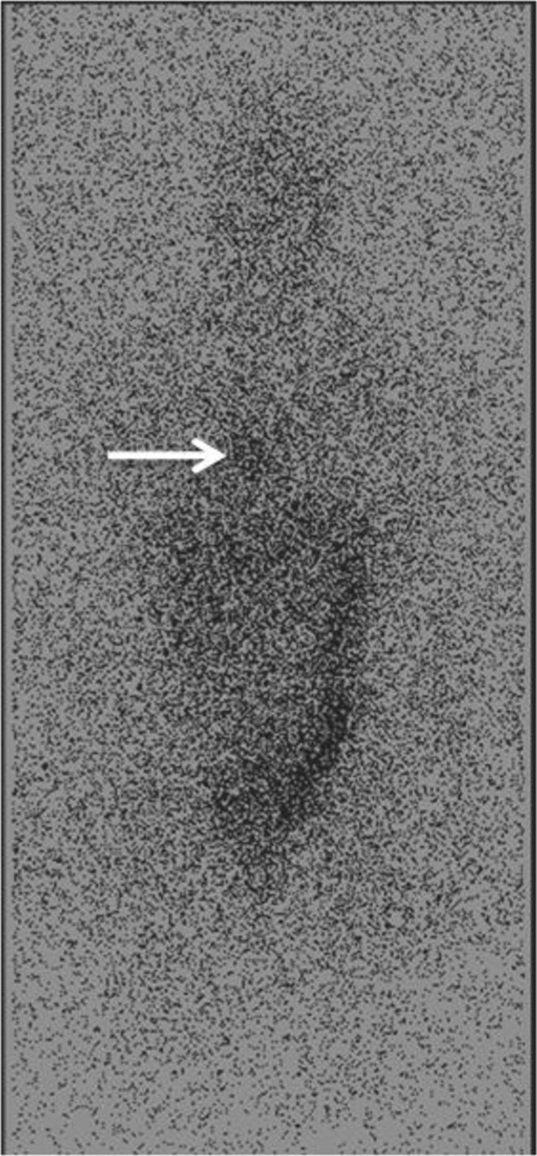
I-131 MIBG scintigraphy shows mild heterogeneous radiotracer accumulation in the right heart (arrow).
The patient eventually underwent surgical resection of the mass, with final pathology confirming the diagnosis of intrapericardial paraganglioma (IPP).
Discussion
Paragangliomas are rare neuroendocrine tumors closely related to pheochromocytomas, tumors of chromaffin-positive cells of the adrenal glands [1]. Extra-adrenal paragangliomas may arise anywhere in the body, with the majority found in the abdomen [2]. Approximately 2% of paragangliomas occur in the thorax. IPPs are very rare. In 2014 Wang et al. [3] published a review article, where they identified 158 reported cases of cardiac paragangliomas in the English language PubMed database. They found male-to-female ratio of 0.86 and mean age at diagnosis of 39.7 years.
A minority of patients with IPP (about 8%) may be completely asymptomatic, and lesions might be found incidentally on imaging [4]. When present, symptoms in patients with IPP are usually secondary to excessive catecholamine excretion by the tumor, resulting in headaches, palpitations, diaphoresis, and systemic arterial hypertension [3].
On cross-sectional imaging, IPP usually appear as large, 3-8 cm, well-defined and often encapsulated heterogeneous masses [5]. Internal calcifications can be occasionally present [5]. IPP are hypervascular, demonstrating avid enhancement and rapid washout on contrast studies, and a hypoenhancing central area due to necrosis [6]. Most of the IPPs arise from the epicardium, and the majority are found in the interatrial and atrioventricular grooves or at the root of the great vessels, areas where parasympathetic ganglia normally exist [3].
The IPP are supplied by the coronary arteries, the majority (57.9%) fed by the right coronary artery [3].
At echocardiography, paragangliomas usually appear as echogenic masses and could be mistaken for myxomas, when arising from the interatrial septum [6]. Unlike myxomas, however, paragangliomas have a broad base [6]. Compression of adjacent vascular structures and encasement of the coronary arteries may be seen [6].
On magnetic resonance imaging, IPP usually show high-signal intensity on T2-weighted images, similar to abdominal paragangliomas, and T1 signal isointense to muscle [5].
I-123 or I-131 MIBG scintigraphy is commonly used for localization of neuroendocrine tumors. The reported sensitivity for IPP detection with MIBG screening is 75% [3]. MIBG scintigraphy must be interpreted with caution, as multiple drugs, such as calcium channel blockers, tricyclic antidepressants, labetalol, and sympathomimetics can interfere with the uptake of MIBG causing false-negative results [7].
IPP are expected to be positive on positron emission tomography with reported sensitivity of 100% [3].
Indium-111 octreotide is currently the agent of choice for nuclear medicine imaging of paragangliomas [8]. Octreotide binds to somatostatin receptors which most paragangliomas express. The largest published analysis of In-111 octreotide scanning for IPP detection showed 100% sensitivity [3].
The treatment of symptomatic cases of IPP is surgical. Because the tumors are highly vascular and tend to involve the coronary arteries, surgical resection is often difficult [4]. In cases where surgical resection is impossible, cardiac transplantation is considered a potential treatment option [2]. In all patients, life-long surveillance for recurrence should be performed.
In conclusion, IPPs are very rare tumors of the heart, making them unusual suspects during the initial evaluation of a mediastinal or cardiac mass. Multimodality imaging is essential for diagnosis, treatment, and surgical planning as well as post-treatment surveillance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.DeLellis R.A., Lloyd R.V., Heitz P.U., Eng C., editors. WHO Classification of Tumours. IARC press; Lyon, France: 2004. Pathology and Genetics of Tumours of the Endocrine Organs. [Google Scholar]
- 2.Bravo E.L., Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev. 2003;24(4):539–553. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- 3.Wang J.G., Han J., Jiang T., Li Y.J. Cardiac paragangliomas. J Card Surg. 2015;30(1):55–60. doi: 10.1111/jocs.12455. [DOI] [PubMed] [Google Scholar]
- 4.Brown M.L., Zayas G.E., Abel M.D., Young W.F., Jr., Schaff H.V. Mediastinal paragangliomas: the mayo clinic experience. Ann Thorac Surg. 2008;86(3):946–951. doi: 10.1016/j.athoracsur.2008.04.105. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton B.H., Francis I.R., Gross B.H., Korobkin M., Shapiro B., Shulkin B.L. Intrapericardial paragangliomas (pheochromocytomas): imaging features. AJR Am J Roentgenol. 1997;168(1):109–113. doi: 10.2214/ajr.168.1.8976931. [DOI] [PubMed] [Google Scholar]
- 6.Araoz P.A., Mulvagh S.L., Tazelaar H.D., Julsrud P.R., Breen J.F. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20(5):1303–1319. doi: 10.1148/radiographics.20.5.g00se121303. [DOI] [PubMed] [Google Scholar]
- 7.Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069–4079. doi: 10.1210/jc.2007-1720. [DOI] [PubMed] [Google Scholar]
- 8.Kwekkeboom D.J., van Urk H., Pauw B.K., Lamberts S.W., Kooij P.P., Hoogma R. Octreotide scintigraphy for the detection of paragangliomas. J Nucl Med. 1993;34:873–878. [PubMed] [Google Scholar]