Abstract
Of all the spontaneous fistulas that occur between the extrahepatic biliary system and the intestine, a choledochoduodenal fistula is rarely seen. When it does occur, it is most often secondary to a perforated duodenal ulcer, choledocholithiasis, or cholelithiasis. It may also be seen following complications related to iatrogenic injury or tuberculosis. Generally, choledochoduodenal fistulas are asymptomatic, but may present with vague abdominal pain, fever, and other symptoms related to cholangitis. As a result, they can be difficult to diagnose clinically before imaging is obtained. We present a case of a 74 year old, asymptomatic, female with a past medical history significant for Crohn's disease who was found to have a choledochoduodenal fistula demonstrated on MRCP, possibly secondary to her underlying inflammatory bowel disease.
Keywords: Choledochoduodenal fistula, Crohn's disease, Bilioenteric fistula
Introduction
A bilioenteric fistula is an abnormal connection between the biliary tract and the intestines. Of all the spontaneous fistulas that develop between the extrahepatic biliary ducts and intestines, cholecystoduodenal fistulas are the most common [1], [2]. Although rare, an abnormal connection between the common bile duct (CBD) and duodenum can also occur forming a choledochoduodenal fistula (CDF). A CDF most commonly arises in the setting of choledocholithiasis, cholelithiasis, or a perforated duodenal ulcer [1], [2], [3], [4], [5]. CDFs have also been reported as a complication secondary to recurrent biliary infections, adjacent neoplasm, tuberculosis, radiation, and iatrogenic injury [6], [7], [8]. To the best of our knowledge, there has been only one reported case of a CDF occurring in the setting of Crohn’s disease (CD) to date [9]. Patients with CD have an increased risk of developing gastrointestinal fistulas due to chronic inflammation of the gastrointestinal tract.
CDFs generally do not present with specific clinical symptoms. In fact, they are often found incidentally on imaging, including endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) [4], [5], [6], [10]. If symptoms are present, they are frequently related to the primary cause of the CDF.
We present a case of a 74-year-old asymptomatic woman with history of CD who presented for 1-year follow-up for surveillance magnetic resonance enterography (MRE) and MRCP and was found to have a CDF on imaging.
Case report
Our patient is a 74-year-old woman with a long history of inflammatory bowel disease (IBD) characterized by fistulizing CD. She was first diagnosed with CD by a screening colonoscopy 5 years prior. She has tried multiple drug regimens for IBD treatment; however, she has continued to experience chronic inflammation with acute exacerbations. Two years ago, her disease was further complicated by a small bowel perforation with microabscesses anterior to the terminal ileum, which required hospitalization. Surveillance MRE demonstrated persistent acute on chronic inflammation in the small bowel with an enteroenteric fistula in the right lower quadrant [Figure 1]. One year ago, the patient was started on infliximab (Remicade; Janssen Biotech, Inc, Horsham, PA) and has since remained asymptomatic. She returned 1 year later for repeat MRE, which showed resolution of the enteroenteric fistula and improved, but persistent, acute on chronic inflammation in the terminal ileum [Figure 2].
Fig. 1.

Coronal true fast imaging with steady-state free precession (TRUFI) sequence demonstrating a long segment of distal ileum with wall stratification and thickening (arrows).
Fig. 2.
T1-weighted postcontrast (coronal) sequence demonstrating hyperemia (arrow) in the same segment of distal ileum signifying acute on chronic disease.
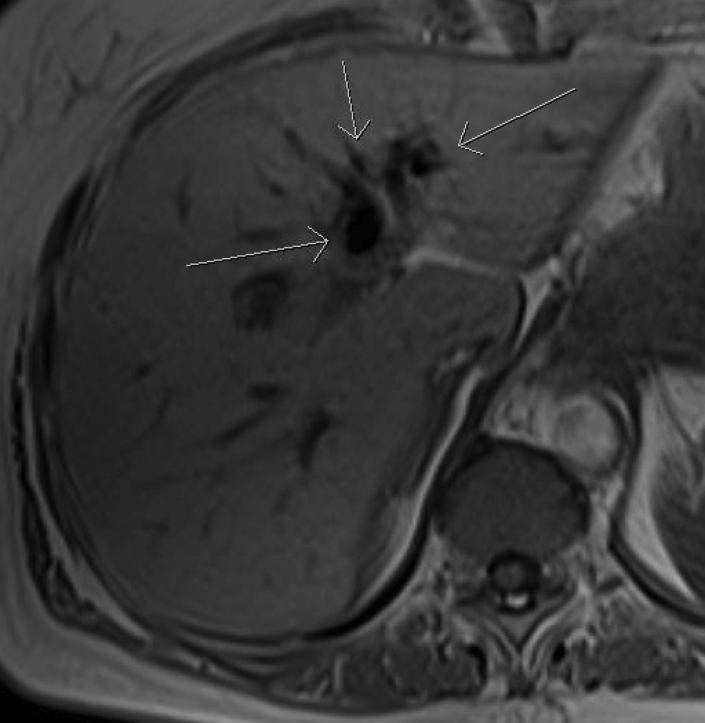
In addition, a MRCP was performed to evaluate for underlying causes of chronically elevated alkaline phosphatase. For the past 2 years, our patient’s alkaline phosphatase level ranged from 115 to 203 U/L, most recently measuring 125 U/L. Furthermore, her total bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyltransferase levels have remained within normal limits. On in-depth review of available prior imaging of our patient’s medical chart, she has never demonstrated an obstructive biliary process that could explain her isolated elevated alkaline phosphatase. On MRCP, a large fistulous connection was seen between the proximal CBD and proximal second portion of the duodenum with associated internal debris and dilation of the remainder of the CBD [Figure 3]. Pneumobilia was noted in the CBD and intrahepatic biliary radials, predominantly in the left hepatic lobe [Figure 4]. These findings, best visualized on the coronal T2-weighted sequences, are consistent with a CDF [Figure 5].
Fig. 3.

MRCP detailing the biliary tree (right hepatic duct [R], left hepatic duct [L], and common bile duct [CBD]) with fistulous connection (F) between the CBD and duodenum (D).
Fig. 4.
T1-weighted in-phase (axial) sequence showing pneumobilia (arrows).
Fig. 5.
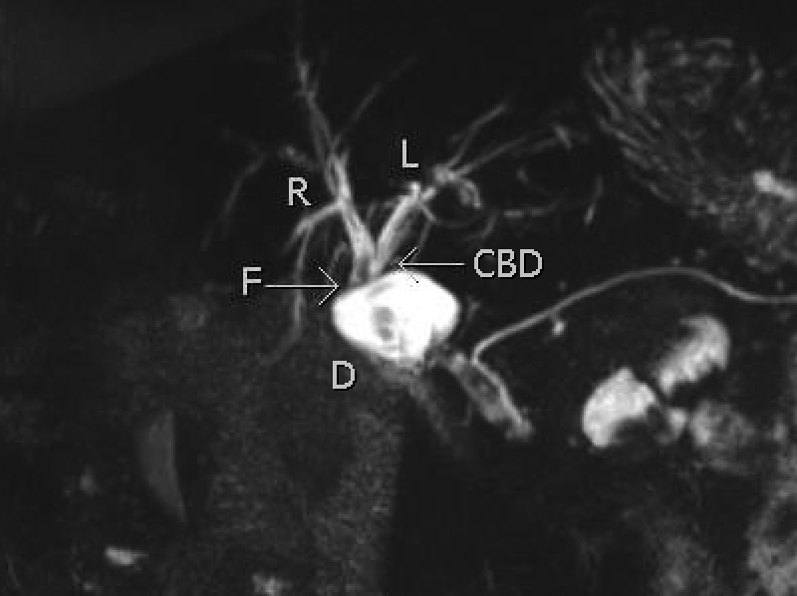
T2 sampling perfection with application optimized contrasts using different flip angle evolution (SPACE) maximal intensity projection (MIP) coronal image detailing the biliary tree with fistulous connection (F) between the common bile duct (CBD) and the duodenum (D).
On further review of our patient’s medical chart, she was diagnosed with a CDF on a remote ERCP for investigation of incidental pneumobilia. Unfortunately, no images of her ERCP are available for review. Surgical treatment for her CDF was not indicated at that time, and she was subsequently followed clinically by her gastroenterologist. Before this incidental finding, the patient had no significant history, symptoms, or imaging findings suggestive of cholelithiasis, choledocholithiasis, or duodenal ulcerative disease.
Discussion
A CDF is an abnormal connection between the CBD and duodenum. It is a rare condition, as approximately only 3.5% of all bilioenteric fistulas that occur are CDFs. CDFs likely form due to the close proximity of the first portion of the duodenum and the CBD as it courses through the hepatoduodenal ligament [1]. When CDFs occur, they do so typically in the setting of choledocholithiasis or a perforated duodenal ulcer. An abnormal, often short, fistulous tract develops secondary to inflammation from a gallstone eroding into the duodenum from within the CBD or a perforated ulcer [1], [2], [3], [4], [5].
Regardless of the etiology, CDFs are often asymptomatic [1], [2], [3], [4], [5], [6], [10]. Symptoms of ascending cholangitis are infrequently observed and previously reported cases have demonstrated evidence of a long-standing CDF without a history suggestive of cholangitis [1], [10], [11]. Our patient did not have a medical history of, imaging findings suggestive of, or typical clinical symptoms indicative of cholelithiasis, choledocholithiasis, or a duodenal ulcer. Nonetheless, these processes are difficult to definitively exclude as potential etiologies. As such, we recognize that we cannot disregard the possibility that our patient’s CDF may have been caused by remote perforated duodenal ulcer or choledocholithiasis.
Consequently, given our patient’s history, the possibility remains that her CDF could be explained by another process. She has no history of tuberculosis, radiation, or prior procedure to have caused iatrogenic injury of the CBD. She does, however, have an extensive history of CD and has had multiple complications secondary to her gastrointestinal inflammation. In CD, inflammation can occur along any part of the gastrointestinal tract, including the duodenum, which can lead to the formation of a CDF. A population-based study from the community of Olmstead, Minnesota looking at the natural history of CD found the cumulative risk of developing any type of fistula after 1, 5, 10, and 20 years after the diagnosis of CD was found to be 21%, 26%, 33%, and 50%, respectively [12]. The lifetime risk of developing a fistula in a patient with CD is between 20%-40% [13]. Our patient has a greater risk of developing a fistulous gastrointestinal tract compared with the general population given her chronic inflammatory state.
Identifying a CDF on imaging is often necessary in its diagnosis as it is frequently clinically asymptomatic [4], [5], [6], [10]. MRCP is less invasive than ERCP and has become an accepted technique in thoroughly examining the intrahepatic and extrahepatic biliary system [14]. Imaging findings are the best seen on fluid sensitive, heavily T2-weighted images obtained fast spin echo or single-shot fast spin echo. For our patient, coronal T2-weighted images show a wide, short connection between the CBD and the proximal duodenum. In addition, pneumobilia was best identified as a hypointense signal on T1-weighted in-phase images, thus further validating the presence of a CDF.
The treatment of a CDF depends on its etiology, severity, and the overall clinical condition of the patient, especially as a standard management of this condition has not been established [6]. If the CDF occurs as a complication of a perforated duodenal ulcer, the patient would need to be appropriately treated medically or surgically, depending on the severity of the ulcer. A CDF occurring as a complication of cholelithiasis or choledocholithiasis often warrants appropriate endoscopic or surgical treatment to remove the offending stones. For large CDFs causing severe clinical symptoms, surgery may be recommended to best repair the biliary tract. If a patient is asymptomatic, however, some studies suggest only regular clinical and laboratory follow-up are needed while the fistula heals [6].
Before her current presentation, our patient’s CDF was identified on a remote ERCP after an incidental finding of pneumobilia on abdominal CT (prior ERCP images were not available in patient’s medical chart). As she had no signs or symptoms to suggest cholangitis at that time, she received conservative clinical surveillance and management. She did not receive endoscopic or surgical therapy, thus, the CDF seen on the current MRCP is likely the same fistula identified in the prior ERCP. Although our patient’s IBD has appeared well controlled on current therapy over the past 2 years, she has a history of fistulizing CD, small bowel perforation, and abdominal abscess formation indicating persistent or recurrent acute on chronic inflammation throughout her gastrointestinal tract, which is the likely reason for her CDF.
Although CDFs rarely occur, it is important to understand the etiology and imaging characteristics behind these fistulas. It is important not only to advance our knowledge and understanding of this disease process, but to also be able to better manage our patients who present with this condition. We are reporting this case of a CDF to highlight its occurrence in the setting of fistulizing CD which has been infrequently documented in the past.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Jordan P., Stirrett L. Treatment of spontaneous internal biliary fistula caused by duodenal ulcer. Am J Surg. 1956;91(3):307–313. doi: 10.1016/0002-9610(56)90162-3. [DOI] [PubMed] [Google Scholar]
- 2.Page J., Dow J., Dundas D. Ulcerogenic choledochoduodenal fistula. Clin Radiol. 1989;40(1):58–60. doi: 10.1016/s0009-9260(89)80026-1. [DOI] [PubMed] [Google Scholar]
- 3.Hoppenstein J., Medoza C., Watne A. Choledochoduodenal fistula due to perforating duodenal ulcer disease. Ann Surg. 1971;173(1):145–147. doi: 10.1097/00000658-197101000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leblanc K., Barr L., Rush B. Spontaneous biliary enteric fistulas. South Med J. 1983;76(10):1249–1252. doi: 10.1097/00007611-198310000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Aziz M., Ahmed M., Siddiqui M., Shipa M., Sarker S., Arif S. Choledochoduodenal fistula secondary to duodenal ulcer disease and choledocholithiasis: report of 2 cases. J Armed Forces Med Coll, Bangladesh. 2008;4(2):38–41. [Google Scholar]
- 6.Wu M., Zhang W., Zhang Y., Mu D., Gong J. Choledochoduodenal fistula in Mainland China: a review of epidemiology, etiology, diagnosis and management. Ann Surg Treat Res. 2015;89(5):240–246. doi: 10.4174/astr.2015.89.5.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karthikeyan V., Sistla S., Ram D., Ali S., Rajkumar N., Balasubramaniam G. Spontaneous choledochoduodenal fistula with tuberculous duodenal ulceration. Ann R Coll Surg Engl. 2014;96(1):104E–105E. doi: 10.1308/003588414X13824511649292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto S., Furuse J., Maru Y., Tajiri H., Muto M., Yoshino M. Duodenal tuberculosis with a choledocho-duodenal fistula. J Gastroenterol Hepatol. 2001;16(2):235–238. doi: 10.1046/j.1440-1746.2001.02332.x. [DOI] [PubMed] [Google Scholar]
- 9.Porter J.M., Mullen D.C., Silver D. Spontaneous biliary-enteric fistulas. Surgery. 1970;68(4):597–601. [PubMed] [Google Scholar]
- 10.Isaacson S., Appleby L., Hamilton E. Choledochoduodenal fistula secondary to duodenal peptic ulcer. JAMA. 1962;179(12):969–971. [Google Scholar]
- 11.Kourias B., Chouliaras A. Spontaneous gastrointestinal biliary fistula complicating duodenal ulcer. Surg Gynaecol Obstet. 1964;119:1013–1018. [PubMed] [Google Scholar]
- 12.Schwartz D., Loftus E., Tremaine W., Pannoccione R., Harmsen W., Zinsmeister A. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122(4):875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 13.Sandborn W., Fazio V., Feagan B., Hanauer S. AGA technical review on perianal Crohn's disease. Gastroenterology. 2003;125(5):1508–1530. doi: 10.1016/j.gastro.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Griffin N., Charles-edwards G., Grant L. Magnetic resonance cholangiopancreatography: the ABC of MRCP. Insights Imaging. 2012;3(1):11–21. doi: 10.1007/s13244-011-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]