Abstract
Peptides have gained increased interest as therapeutics during recent years. More than 60 peptide drugs have reached the market for the benefit of patients and several hundreds of novel therapeutic peptides are in preclinical and clinical development. The key contributor to this success is the potent and specific, yet safe, mode of action of peptides. Among the wide range of biologically-active peptides, naturally-occurring marine-derived cyclopolypeptides exhibit a broad range of unusual and potent pharmacological activities. Because of their size and complexity, proline-rich cyclic peptides (PRCPs) occupy a crucial chemical space in drug discovery that may provide useful scaffolds for modulating more challenging biological targets, such as protein-protein interactions and allosteric binding sites. Diverse pharmacological activities of natural cyclic peptides from marine sponges, tunicates and cyanobacteria have encouraged efforts to develop cyclic peptides with well-known synthetic methods, including solid-phase and solution-phase techniques of peptide synthesis. The present review highlights the natural resources, unique structural features and the most relevant biological properties of proline-rich peptides of marine-origin, focusing on the potential therapeutic role that the PRCPs may play as a promising source of new peptide-based novel drugs.
Keywords: proline-rich cyclic peptide, marine sponge, marine tunicate, peptide synthesis, stereochemistry, lipophilicity parameter, pharmacological activity
1. Introduction
An interesting class of marine cyclic peptides is represented by the proline-rich compounds usually containing more than six or seven amino acid residues. The role of proline in these molecules has been linked to the control of the conformation of the molecule in solution because of the restricted φ of proline. The proline-rich cyclic peptides (PRCPs) are formed by linking one end of the peptide and the other with an amide bond or other chemically-stable bonds. Some of them are used in the clinic, e.g., gramicidin S and tyrocidine with bactericidal activity, while others are in clinical trials, e.g., dehydrodidemnin B, and most of them originate from natural resources. Although the literature is enriched with reports concerned with marine-derived linear proline-rich bioactive peptides [1,2,3,4,5], e.g., dolastatin 15, kurahyne B, jahanyne, cemadotin, koshikamide A1, etc., PRCPs from marine resources are becoming popular and attracting the attention of scientists nowadays, due to their unique structural features and a wide range of the biological properties, like cytotoxicity [6], antibacterial activity [7], antifungal activity [8], immunosuppressive activity [9], anti-inflammatory activity [10], anti-HIV activity [11], repellent (antifouling) activity [12], antitubercular activity [13] and antiviral activity [14], associated with them. PRCPs include a large and heterogeneous group of small to large-sized oligopeptides characterized by the presence of proline units often constituting peculiar sequences, which confers them a typical structure that determines the various biological functions endowed by these molecules. As several features make PRCPs attractive lead compounds for drug development, as well as nice tools for biochemical research, scientists are focusing and giving diverse efforts to develop biologically-active proline-rich cyclic peptide compounds.
1.1. Natural Resources
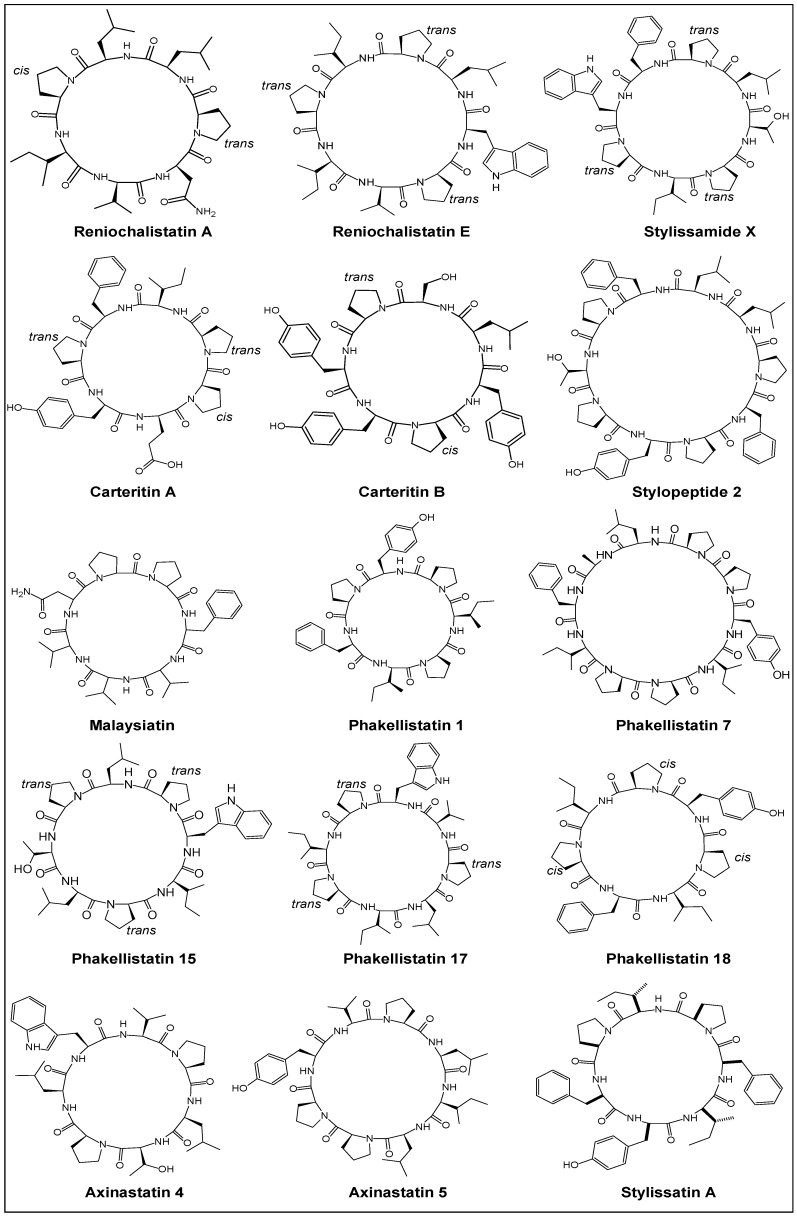
Various natural sources of PRCPs include marine sponges, ascidians, different genera of cyanobacteria and higher plants. One of the potent resources is sessile aquatic animals, i.e., sponges like Kenyan sponge Callyspongia abnormis [15], Dominican sponge Eurypon laughlini [16], Indonesian sponge Callyspongia aerizusa [17], sponge Ircinia sp. [18], Jamaican sponge Stylissa caribica [19], Yongxing Island sponge Reniochalina stalagmitis [20], Vanuatu sponge Axinella carteri [21], Korean sponge Clathria gombawuiensis [22], Fijian sponge Stylotella aurantium [23], Papua New Guinea sponge Stylissa massa [24], South China sponge Phakella fusca [25], Lithistid sponge Scleritoderma nodosum [26], Borneo sponge Pseudaxinyssa sp. [27], Philippines sponge Myriastra clavosa [28], Papua New Guinea sponge Stylotella sp. [29], Comoros sponge Axinella cf. carteri [30], Okinawan sponge Hymeniacidon sp. [31], Indo-Pacific sponges Phakellia costata and Stylotella aurantium [32], Indonesian sponge Stylissa sp. [33], Red sea sponge Stylissa carteri [34], Western Pacific Ocean sponge Hymeniacidon sp. [35], Puerto Rican sponge Prosuberites laughlini [36], Micronesian sponge Cribrochalina olemda [37], Indonesian sponge Sidonops microspinosa [38], Palau sponge Axinella sp. [39], etc. The structures of various proline-rich cyclopolypeptides from marine sponges are compiled in Figure 1.
Figure 1.
Proline-rich cyclic peptides (PRCPs) from marine sponges.
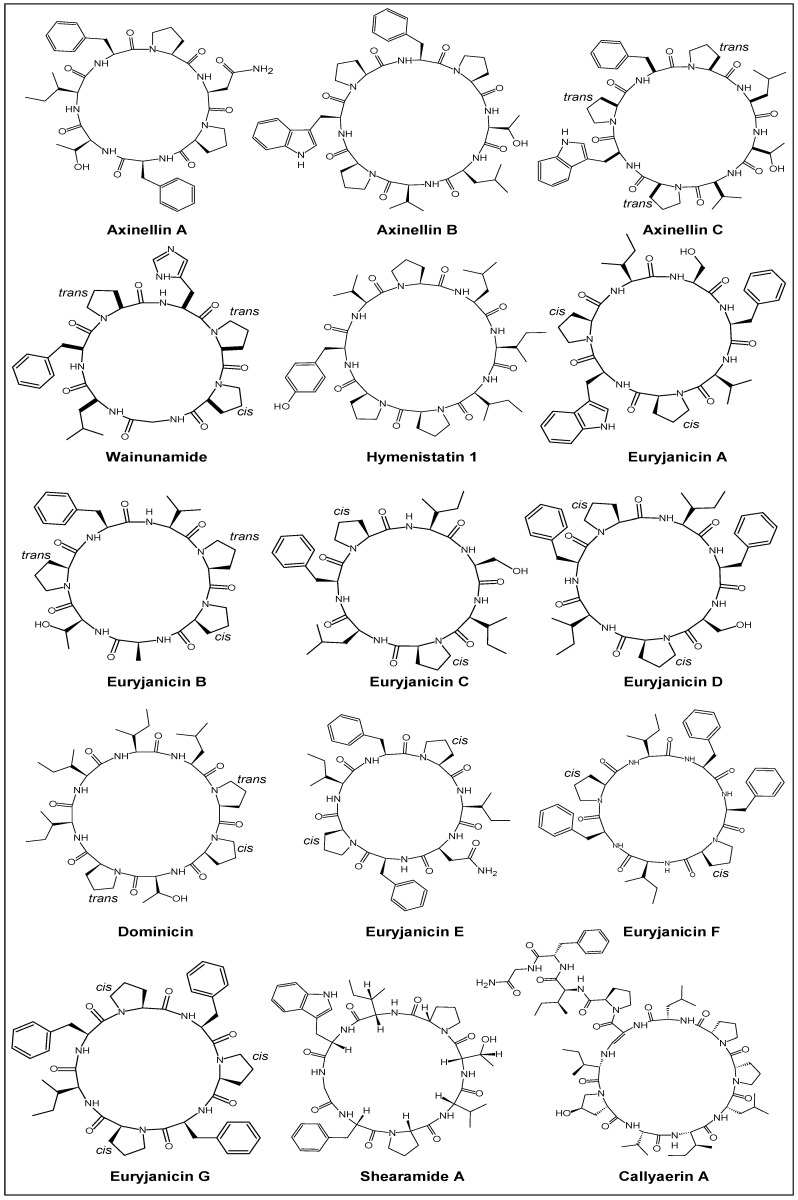
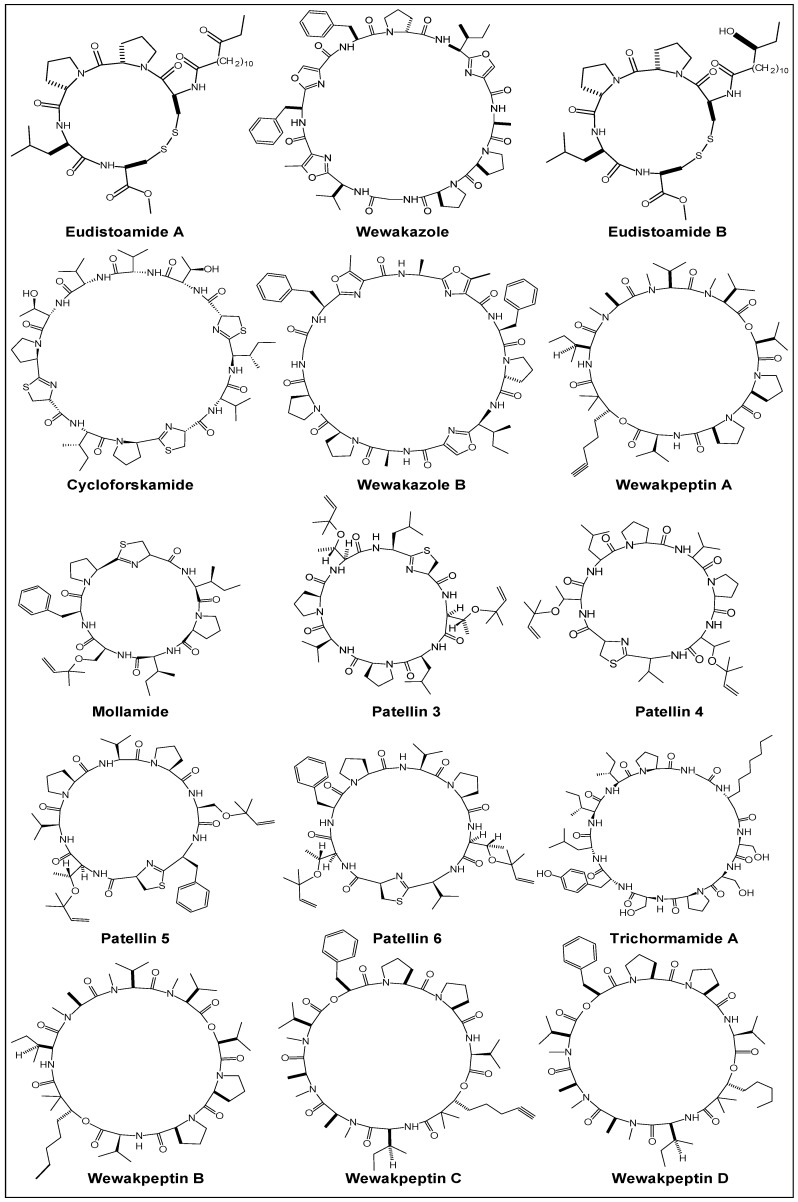
Other sources of proline-rich cyclooligopeptides are marine tunicates, like compound ascidian Didemnum molle [40], Ishigaki Island sea slug Pleurobranchus forskalii [41], Fijian ascidian Eudistoma sp. [42], Caribbean tunicate Trididemnum solidum [43], unidentified Brazilian ascidian (family Didemnidae) [44], Mediterranean ascidian Aplidium albicans [45], cyanobacteria like Papua New Guinea cyanobacterium Lyngbya semiplena [46], Red Sea cyanobacterium Moorea producens [47], Florida Everglades cyanobacterium Lyngbya sp. [48], Northern Wisconsin cyanobacterium Trichormus sp. UIC 10339 [49], toxic cyanobacterium Nostoc sp. 152 [50], Kenyan cyanobacterium Lyngbya majuscule [51], mollusks like Papua New Guinea mollusk (sea hare) Dolabella auricularia [52] and alga like Indonesian red alga (Rhodophyta) Ceratodictyon spongiosum containing the symbiotic sponge Sigmadocia symbiotica [10]. Structures of diverse proline-rich cyclopeptides from marine tunicates and cyanobacteria are tabulated in Figure 2. Besides this, proline-containing cyclooligopeptides are also obtained from roots, stems, barks, seeds, fruit peels of higher plants, as well as from bacteria and fungi [53,54,55,56,57,58,59,60,61,62,63,64,65,66].
Figure 2.
PRCPs from marine ascidians (tunicates) and cyanobacteria.
Purification procedures of PRCPs isolated from sea animals, like ascidians, sponges and mollusk, usually include initial extraction with methanol (MeOH), partitions of these extracts with organic solvents of increasing polarities to render diverse organic fractions and chromatographic steps on silica and Sephadex LH-20 columns, as well as the use of reversed phase C18 HPLC for the final purification [67].
1.2. Stability and Comparison with Linear Peptides
Linear peptides that contain less than 10 amino acid residues are especially flexible in solution. Once the length of linear peptides extends to between 10 and 20 amino acid residues, random linear peptide sequences can begin to obtain secondary structures, including α-helices, turns and β-strands. These secondary structures impose constraints that reduce the free energy of linear peptides and limit their conformations to those that may be more biologically active. The constraints imposed by cyclization force cyclic peptides to adopt a limited number of molecular conformations in solution. Generally, if cyclization limits conformations to those required for optimum receptor binding, these cyclic peptides would be more useful compared with their linear counterparts that can adopt more conformations, which are not useful for receptor binding. Cyclization has been shown to increase the propensity for β-turn formation in peptides, which is of vital utility since β-turns are often found in native proteins. Although peptide cyclization generally induces structural constraints, the site of cyclization within the sequence can affect the binding affinity of cyclic peptides.
In the case of proline, which is a proteinogenic amino acid with a secondary amine that does not follow along with the typical Ramachandran plot, the ψ and φ angles about the peptide bond have fewer allowable degrees of rotation due to the ring formation connected to the beta carbon. As a result, it is often found in “turns” of peptides/proteins, as its free entropy (ΔS) is not as comparatively large as other amino acids, and thus, in a folded form vs. unfolded form, the change in entropy is less. Furthermore, proline is rarely found in α and β structures, as it would reduce the stability of such structures, because its side chain α-N can only form one hydrogen bond.
Further, the hydroxylation of proline by prolyl hydroxylase and other additions of electron-withdrawing substituents, such as fluorine, increases the conformational stability of collagen significantly. Hence, the hydroxylation of proline is a critical biochemical process for maintaining the connective tissue of higher organisms. Polypeptide chains containing proline lack the flexibility of other peptides, because the proline ring has only one available angle for backbone rotation. Rotation occurs around the angles φ, ψ and ω [68,69].
The cyclization of linear peptide sequences can create constrained geometries that can alter the specificity of cyclic peptides to different isoforms or subtypes of targeted receptors. Peptides can be cyclized in order to reduce the overall numbers of interchanging conformers in the hope of limiting them to those selective for the desired receptors while avoiding degradation by not forming conformers susceptible to interacting with proteolytic enzymes [70].
In general, cyclization often increases the stability of peptides [71,72], which can prolong their biological activity. This prolonged activity may even be the result of additional resistance to enzymatic degradation by exoproteases that preferentially cleave near the N- or C-termini of peptide sequences. In particular, cyclization can create peptides with the ability to penetrate tumors in order to enhance the potency of anticancer drugs [73]. Cyclic peptides can potentially obtain desirable constrained geometries that are responsible for increasing their binding affinity, specificity or stability compared with their linear counterparts. Cyclic peptides are of considerable interest as potential protein ligands and might be more cell permeable than their linear counterparts due to their reduced conformational flexibility. However, it is important to note that cyclization does not necessarily lead to improvements in all of these properties, e.g., linear peptides can contain sequences that can support rigid structures without the need for cyclization [74].
2. Chemistry
2.1. Structural Features
The distinctive cyclic structure of proline’s side chain gives proline an exceptional conformational rigidity compared to other amino acids, which affects the rate of peptide bond formation between proline and other amino acids. The exceptional conformational rigidity of proline affects the secondary structure of proteins near a proline residue and may account for proline’s higher prevalence in the proteins of thermophilic organisms. Proline acts as a structural disruptor in the middle of regular secondary structure elements, such as alpha helices and beta sheets; however, proline is commonly found as the first residue of an alpha helix and also in the edge strands of beta sheets. Multiple prolines and hydroxyprolines in a row can create a polyproline helix, the predominant secondary structure in collagen [75].
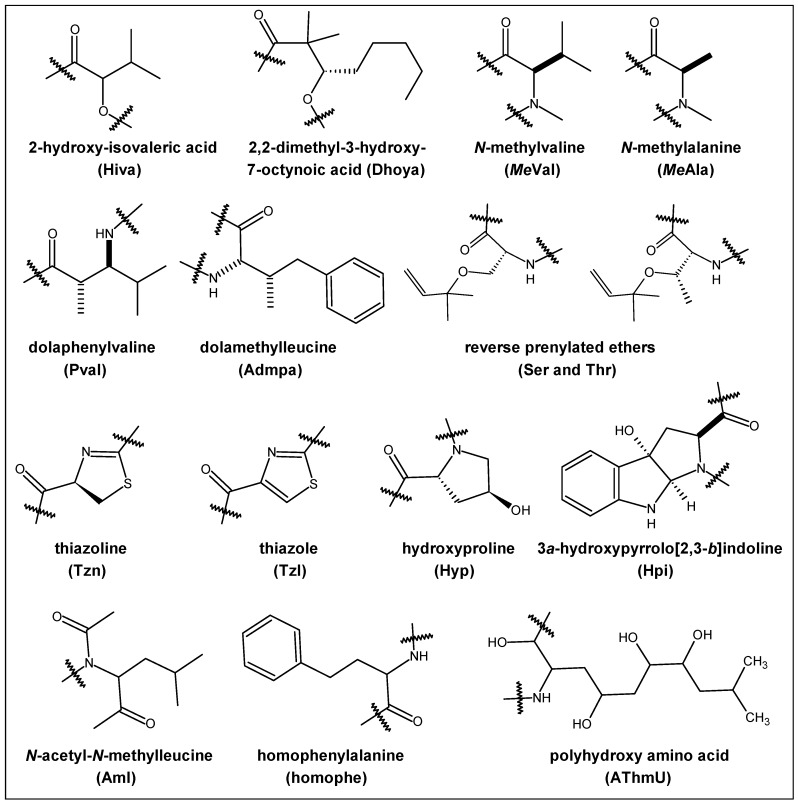
The number of proline units in a cyclic peptide structure varies from one to five (Table 1). In addition to normal hydrophobic amino acids, marine organism-derived cyclopolypeptides rich in proline units contain modified and unusual amino acid moieties and other rings, like hydroxyproline (Hyp), (Z)-2,3-diaminoacrylic acid (DAA), thiazoline (Tzn), thiazole (Tzl), oxazole, methyloxazoline, reverse prenylated ethers, i.e., serine and threonine carrying a dimethylallyl ether group, para-hydroxystyrylamide (pHSA), pyroglutamic acid (pyroGlu), 3a-hydroxypyrrolo[2,3-b]indoline (Hpi), the 12-hydroxy-tetradecanoyl moiety, 2-(1-amino-2-p-hydroxyphenylethane)-4-(4-carboxy-2,4-dimethyl-2Z,4E-propadiene)-thiazole (ACT), O-methyl-N-sulfo-d-serine, keto-allo-isoleucine, methyloxazoline, β-methoxyaspartic acid, β-aminodecanoic acid, 2,2-dimethyl-3-hydroxy-7-octynoic acid (Dhoya), β-amino acid 3-amino-2-methylbutanoic acid (Maba) and 2-Hydroxy-isovaleric acid (Hiva), O-prenyltyrosine (Ptyr) (2S,3R,5R)-3-amino-2,5-dihydroxy-8-phenyloctanoic acid (Ahoa), dolaphenvaline (Pval) and dolamethylleucine (Admpa), N-acetyl-N-methylleucine (Aml), E- and Z-dehydrobutyrines (Dhb), a homophenylalanine (homophe), (2S,3R)-β-hydroxy-p-bromophenylalanine and N,O-dimethyl tyrosine, hydroxyisovaleric acid (Hiv) (Figure 3).
Table 1.
Proline-rich cyclopolypeptides from marine resources.
| Year | Cyclic Peptide | Molecular Formula | No. of Proline Units | Composition |
|---|---|---|---|---|
| 1981 | Didemnin B [43] | C57H89N7O15 | two | cyclodepsipeptide |
| 1988 | Aplidine [45] | C57H87N7O15 | cyclodepsipeptide | |
| 1991 | Axinastatin 1 [6] | C38H56N8O8 | cycloheptapeptide | |
| 1992 | Malaysiatin [27] | C38H56N8O8 | cycloheptapeptide | |
| 1992 | Polydiscamide A [7] | C76H109BrN19O20SNa | cyclodepsipeptide | |
| 1993 | Axinastatin 4 [76] | C42H62N8O8 | cycloheptapeptide | |
| 1993 | Cyclooligopeptide [77] | C24H32N4O5 | cyclotetrapeptide | |
| 1993 | Hymenamide B [31] | C43H56N8O10 | cycloheptapeptide | |
| 1993 | Hymenamide C [8] | C43H54N8O9 | cycloheptapeptide | |
| 1993 | Hymenamide D [8] | C38H55N7O10 | cycloheptapeptide | |
| 1993 | Hymenamide E [8] | C45H55N7O10 | cycloheptapeptide | |
| 1994 | Mollamide [40] | C42H61N7O7S | cycloheptapeptide | |
| 1994 | Schizotrin A [78] | C72H107N13O21 | cycloundecapeptide | |
| 1994 | Axinastatin 2 [39] | C39H58N8O8 | cycloheptapeptide | |
| 1994 | Axinastatin 3 [39] | C40H61N8O8 | cycloheptapeptide | |
| 1995 | Stylopeptide 1 [79] | C40H61N7O8 | cycloheptapeptide | |
| 1996 | Patellin 3 [80] | C48H78N8O9S | cyclooctapeptide | |
| 1996 | Patellin 4 [80] | C47H76N8O9S | cyclooctapeptide | |
| 1996 | Patellin 5 [80] | C49H72N8O9S | cyclooctapeptide | |
| 1996 | Patellin 6 [80] | C50H74N8O9S | cyclooctapeptide | |
| 1996 | Hymenamide F [81] | C35H60N10O7S | cycloheptapeptide | |
| 1996 | Agardhipeptin B [82] | C57H69N11O8 | cyclooctapeptide | |
| 1996 | Kapakahine A [37] | C58H72N10O9 | cyclooctapeptide | |
| 1996 | Kapakahine C [37] | C58H72N10O10 | cyclooctapeptide | |
| 1996 | Kapakahine D [37] | C58H72N10O10 | cyclooctapeptide | |
| 1998 | Axinellin A [21] | C42H56N8O9 | cycloheptapeptide | |
| 1998 | Shearamide A [83] | C47H63N9O9 | cyclooctapeptide | |
| 1999 | Prenylagaramide B [84] | C49H68N8O10 | cycloheptapeptide | |
| 1999 | Nostophycin [50] | C46H64N8O10 | cycloheptapeptide | |
| 2000 | trans,trans-ceratospongamide [10] | C41H49N7O6S | cycloheptapeptide | |
| 2000 | Tamandarine A [44] | C54H87N7O14 | cyclodepsipeptide | |
| 2000 | Tamandarine B [44] | C53H82N7O14 | cyclodepsipeptide | |
| 2001 | Microspinosamide [38] | C75H109BrN18O22S | cyclodepsipeptide | |
| 2003 | Myriastramide C [28] | C42H53N9O7S | cyclooctapeptide | |
| 2004 | Scleritodermin A [26] | C42H54N7O10SNa | cyclodepsipeptide | |
| 2004 | Cyclonellin [85] | C45H62N12O12 | cyclooctapeptide | |
| 2005 | Wewakpeptin A [46] | C52H85N7O11 | cyclodepsipeptide | |
| 2005 | Wewakpeptin B [46] | C52H89N7O11 | cyclodepsipeptide | |
| 2005 | Wewakpeptin C [46] | C54H81N7O11 | cyclodepsipeptide | |
| 2005 | Wewakpeptin D [46] | C54H85N7O11 | cyclodepsipeptide | |
| 2007 | Pahayokolide A [48] | C72H105N13O20 | cycloundecapeptide | |
| 2007 | Pahayokolide B [48] | C63H90N12O18 | cycloundecapeptide | |
| 2008 | Polydiscamide B [18] | C75H110BrN18O21S | cyclodepsipeptide | |
| 2008 | Polydiscamide C [18] | C74H107BrN18O21S | cyclodepsipeptide | |
| 2008 | Polydiscamide D [18] | C73H105BrN18O21S | cyclodepsipeptide | |
| 2009 | Euryjanicin A [36] | C44H58N8O8 | cycloheptapeptide | |
| 2009 | Euryjanicin C [14] | C40H61N7O8 | cycloheptapeptide | |
| 2009 | Euryjanicin D [14] | C44H59N7O8 | cycloheptapeptide | |
| 2009 | Eudistomide A [42] | C37H61N5O8S2 | cyclolipopeptide | |
| 2009 | Eudistomide B [42] | C37H63N5O8S2 | cyclolipopeptide | |
| 2010 | Anacyclamide A10 [86] | C49H72N12O14 | cyclodecapeptide | |
| 2011 | Duanbanhuain A [87] | C43H58N8O11 | cyclooctapeptide | |
| 2011 | Duanbanhuain B [87] | C45H57N9O10 | cyclooctapeptide | |
| 2012 | Mollamide F [12] | C33H46N6O5S | cyclohexapeptide | |
| 2013 | Stylissatin A [24] | C49H63N7O8 | cycloheptapeptide | |
| 2013 | Euryjanicin E [88] | C44H60N8O8 | cycloheptapeptide | |
| 2013 | Euryjanicin F [88] | C49H63N7O7 | cycloheptapeptide | |
| 2013 | Gombamide A [22] | C38H45N7O8S2 | cyclothiohexapeptide | |
| 2013 | Cycloforskamide [41] | C54H86N12O11S3 | cyclododecapeptide | |
| 2014 | Trichormamide A [49] | C58H93N11O15 | cycloundecapeptide | |
| 2014 | Reniochalistatin A [20] | C37H62N8O8 | cycloheptapeptide | |
| 2016 | Carteritin B [34] | C46H57N7O11 | cycloheptapeptide | |
| 1990 | Hymenistatin 1 [35] | C47H72N8O9 | three | cyclooctapeptide |
| 1993 | Phakellistatin 1 [32] | C45H61N7O8 | cycloheptapeptide | |
| 1993 | Hymenamide A [31] | C46H61N11O7 | cycloheptapeptide | |
| 1993 | Phakellistatin 2 [89] | C45H61N7O8 | cycloheptapeptide | |
| 1994 | Axinastatin 5 [30] | C47H72N8O9 | cyclooctapeptide | |
| 1994 | Hymenamide G [90] | C47H72N8O9 | cyclooctapeptide | |
| 1994 | Hymenamide H [90] | C47H69N9O9 | cyclooctapeptide | |
| 1995 | Phakellistatin 11 [91] | C53H67N9O9 | cyclooctapeptide | |
| 1996 | Waiakeamide [12] | C37H49N7O8S3 | cyclohexapeptide | |
| 1998 | Axinellin B [21] | C50H67N9O9 | cyclooctapeptide | |
| 2000 | Haligramide A [92] | C37H49N7O6S3 | cyclohexapeptide | |
| 2000 | Haligramide B [92] | C37H49N7O7S3 | cyclohexapeptide | |
| 2001 | Haliclonamide A [93] | C45H60N8O9 | cyclooctapeptide | |
| 2001 | Haliclonamide B [93] | C40H52N8O9 | cyclooctapeptide | |
| 2001 | Wainunuamide [23] | C38H51N9O7 | cycloheptapeptide | |
| 2002 | Axinellin C [94] | C50H67N9O9 | cyclooctapeptide | |
| 2002 | Dolastatin 16 [52] | C47H70N6O10 | cyclodepsipeptide | |
| 2002 | Haliclonamide C [95] | C45H60N8O10 | cyclooctapeptide | |
| 2002 | Haliclonamide D [95] | C40H54N8O10 | cyclooctapeptide | |
| 2002 | Haliclonamide E [95] | C45H62N8O10 | cyclooctapeptide | |
| 2003 | Myriastramide A [28] | C45H58N8O9 | cyclooctapeptide | |
| 2003 | Myriastramide B [28] | C45H57ClN8O9 | cyclooctapeptide | |
| 2003 | Wewakazole [96] | C59H72N12O12 | cyclododecapeptide | |
| 2005 | Dominicin [16] | C43H72N8O9 | cyclooctapeptide | |
| 2006 | Stylisin 1 [19] | C45H61N7O8 | cycloheptapeptide | |
| 2009 | Euryjanicin B [14] | C36H51N7O8 | cycloheptapeptide | |
| 2010 | Phakellistatin 15 [25] | C48H71N9O9 | cyclooctapeptide | |
| 2010 | Phakellistatin 17 [25] | C49H73N9O8 | cyclooctapeptide | |
| 2010 | Phakellistatin 18 [25] | C45H61N7O8 | cycloheptapeptide | |
| 2010 | Callyaerin B [13] | C65H108N12O14 | cyclooctapeptide b | |
| 2010 | Callyaerin C [13] | C70H105N13O16 | cycloheptapeptide c | |
| 2012 | Stylissamide X [33] | C51H69N9O9 | cyclooctapeptide | |
| 2013 | Euryjanicin G [88] | C48H59N7O7 | cyclooctapeptide | |
| 2014 | Reniochalistatins E [20] | C49H73N9O8 | cyclooctapeptide | |
| 2016 | Carteritin A [34] | C44H57N7O10 | cycloheptapeptide | |
| 2016 | Stylissatin B [97] | C38H51N9O7 | cycloheptapeptide | |
| 2016 | Stylissatin C [97] | C39H55N7O9 | cycloheptapeptide | |
| 2016 | Stylissatin D [97] | C40H57N7O9 | cycloheptapeptide | |
| 2016 | Wewakazole B [47] | C58H70N12O12 | cyclododecapeptide | |
| 1968 | Antamanide [98] | C64H78N10O10 | four | cyclodecapeptide |
| 2004 | Callynormine A [15] | C61H93N11O13 | cycloheptapeptide b | |
| 2006 | Stylisin 2 [19] | C44H57N7O8 | cycloheptapeptide | |
| 2008 | Stylopeptide 2 [29] | C63H84N10O12 | cyclodecapeptide | |
| 2010 | Callyaerin A [13] | C69H108N14O14 | cyclooctapeptide c | |
| 2010 | Callyaerin E [13] | C66H94N12O13 | cycloheptapeptide c | |
| 2010 | Callyaerin H [13] | C54H81N11O10 | cycloheptapeptide a | |
| 2008 | Callyaerin G [99] | C69H91N13O12 | five | cycloheptapeptide c |
With a dipeptide, b tripeptide and c tetrapeptide side chains.
Figure 3.
Modified amino acid moieties/heterocyclic rings present in marine-derived PRCPs.
Callynormine A represents a new class of heterodetic cyclic peptides possessing an α-amido-β-aminoacrylamide cyclization functionality. Hyp forms part of the composition of cyclic endiamino peptides like callynormine A [15] and callyaerin A–D. The unusual non-proteinogenic (Z)-DAA moiety is characteristic of the callyaerin series of peptides callyaerins A–M, which links the cyclic peptide part of the callyaerins with a linear peptide side chain [13]. Indo-Pacific ascidian Didemnum molle is found to be rich in thiazole-, oxazole- and thiazoline-containing peptides, like mollamide, which share the peculiar reverse prenylated ethers of serine and threonine amino acids [40].
Furthermore, unusual amino acid residues like pHSA and pyroGlu were found to be part of the structure of cyclothiopeptide gombamide A, which possess moderate inhibitory activity against Na+/K+-ATPase [22]. Further, thiazoline-based proline containing doubly-prenylated cyclopeptides like trunkamide A contain reverse prenylated ethers of serine and threonine together in their composition. Heterocyclic amino acids like histidine and tryptophan also form part of the structures of proline-rich cyclic peptides, such as wainunuamide, phakellistatin 15, 17 and stylissatin B [23,25,97]. Moreover, cytotoxic phakellistatin 3 and isophakellistatin 3 represent a new class of proline-rich cycloheptapeptides containing an unusual amino acid unit “Hpi” that apparently derived from a photooxidation product of tryptophan [100].
Moreover, five-residue cystine-linked cyclic peptides like eudistomides A, B are flanked by a C-terminal methyl ester and a 12-oxo- or 12-hydroxy-tetradecanoyl moiety [42]. The structure of proline containing cytotoxic peptide scleritodermin A incorporates a novel conjugated thiazole moiety 2-(1-amino-2-p-hydroxyphenylethane)-4-(4-carboxy-2,4-dimethyl-2Z,4E-propadiene)-thiazole (ACT) and unusual amino acids O-methyl-N-sulfo-d-serine, keto-allo-isoleucine [26]. The proline unit may be part of a cyclic peptide and/or may be part of a side chain, e.g., scleritodermin A, didemnin B, C and plitidepsin [26,43,45], or may be part of a linear peptide, e.g., dolastatin 15 and koshikamide A1 [1,5]. The methyloxazoline ring is the part of the composition of cyclohexapeptides ceratospongamides [10]. In addition, trichormamide A contains β-amino acid residue viz. β-aminodecanoic acid, in addition to two d-amino acid residues (d-Tyr and d-Leu) [49]. The wewakpeptins, proline-rich cyclic depsipeptides contain unusual moieties, like “Dhoya”, “Maba” and “Hiva” [46], and prenylagaramides B and C contain a rare “Ptyr” unit. Moreover, nostophycin bears a novel β-amino acid moiety “Ahoa” in its structure [50]. Macrocyclic depsipeptides, homodolastatin 16 and dolastatin 16 contain the new and unusual amino acid units “Pval” and “Admpa” [51,52]. Besides this, structural features for pahayokolides A and B include a pendant N-acetyl-N-methylleucine, both E- and Z-dehydrobutyrines, a homophenylalanine and an unusual polyhydroxy amino acid [48]. Oxazole and methyloxazole rings were found to be part of the structures of cyclopolypeptides myriastramides A–C and haliclonamide A [28,93], whereas N,O-dimethyl tyrosine and “Hiv” moieties were found in the structures of cytotoxic depsipeptides, tamandarins A and B [44]. The presence of two dimethylallyl threonines (or one threonine and one serine) side chains and one thiazoline ring in the backbone of the patellins is the most important feature of these compounds termed as “cyanobactins”, which have sparked attention due to their interesting bioactivities and for their potential to be prospective candidates in the development of drugs [101,102].
2.2. Stereochemical Aspects
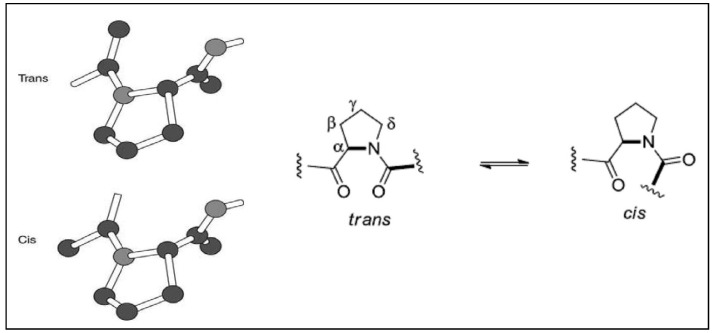
Structurally, proline is the only unusual amino acid with a secondary amino group based on a pyrrolidine, which forms a ring structure with rigid conformation and a secondary amine compared to the other twenty natural amino acids. This significantly reduces the structural flexibility of the polypeptide chain, and the nitrogen in the pyrrolidine ring cannot participate in hydrogen bonding with other residues [103]. Many biologically-important cyclic peptide sequences and natural products contain multiple proline residues. As seen previously for peptide bonds, the proline amide bond can also exist in trans or cis conformations (Figure 4). Peptide bonds to proline, and to other N-substituted amino acids, are able to populate both the cis and trans isomers. Most peptide bonds overwhelmingly adopt the trans isomer (typically 99.9% under unstrained conditions), because the amide hydrogen (trans isomer) offers less steric repulsion to the preceding Cα atom than does the following Cα atom (cis isomer). By contrast, the cis and trans isomers of the X-Pro peptide bond (where X represents any amino acid) both experience steric clashes with the neighboring substitution and are nearly equal energetically. Hence, the fraction of X-Pro peptide bonds in the cis isomer under unstrained conditions ranges from 10% to 40%; the fraction depends slightly on the preceding amino acid, with aromatic residues favoring the cis isomer slightly. Proline cis-trans isomerization plays a key role in the rate-determining steps of protein folding [104]. Furthermore, proline cis-trans isomerization controls autoinhibition of a signaling protein [105].
Figure 4.
The two possible conformations for the proline peptide bond.
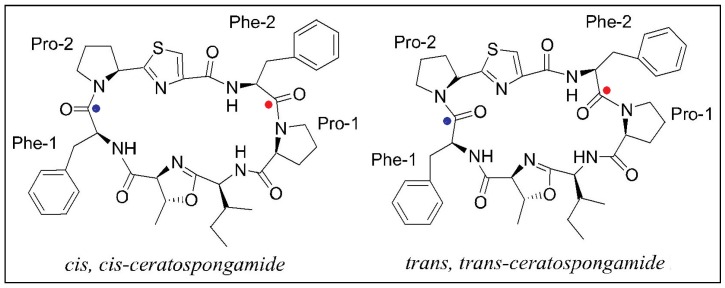
Although the trans amide bond is more common, the occurrence of cis geometry is more frequent for the proline peptide bond than for other amino acids. The frequency of the cis proline peptide bond is higher in cyclic peptides than in linear peptides. As per a statistical study performed on the Cambridge Structural Database, 57.4% of proline residues present in cyclic peptides were in the cis conformation as compared to only 5.6% in acyclic peptides [106]. The reason for this high proportion of cis proline in cyclopeptides is due to the conformational restrictions during the cyclisation step. The geometry of the proline amide can be determined on the basis of the difference in 13C chemical shifts between Cβ and Cγ signals (Δδβγ = δβ − δγ). A small 13C chemical shift difference indicates that the proline peptide bond is trans, while a large 13C chemical shift difference indicates a cis proline residue. The change in conformation of a cyclopolypeptide from “trans” to “cis” can result in loss of activity [10], e.g., the trans, trans-isomer of cyclic heptapeptide ceratospongamide showed potent inhibition of sPLA2 expression in a cell-based model for anti-inflammation, whereas the cis, cis-isomer was inactive (Figure 5). The distribution of the peptide bond angle omega for peptidyl-prolyl bonds in proteins shows significant peaks at 180° (trans peptide bond) and 0° (cis peptide bond). Investigations on “peptidyl-prolyl bonds and secondary structure” showed that trans petidyl-prolyl bonds are distributed in all types of secondary structure, whereas cis peptidyl is found primarily in bends and turns, suggesting a specific structural role for this type of bonding.
Figure 5.
Different conformers of cyclopolypeptide ceratospongamide.
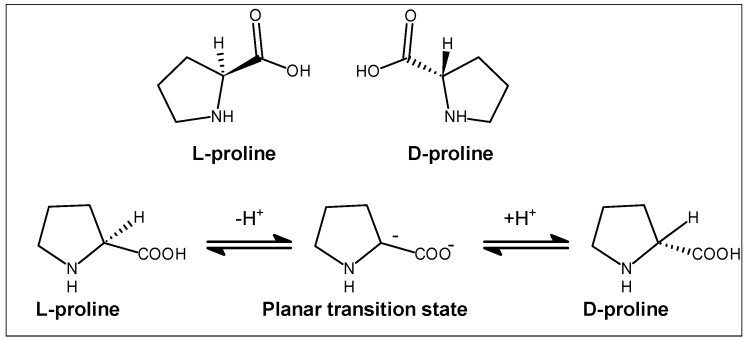
Most amino acids occur in two possible optical isomers, called d and l (Figure 6). The l-amino acids represent the vast majority of amino acids found in proteins. l-proline is a natural non-essential amino acid, and d-proline is an unnatural amino acid, with one basic and one acidic center each. In proline, only the l-stereoisomer is involved in the synthesis of mammalian peptides/proteins.
Figure 6.
General structures of l- and d-proline and their isomerization via proline racemase.
The racemization of l-proline to d-proline proceeds through a planar transition state, where the tetrahedral α-carbon becomes trigonal as a proton leaves the l-proline. The transition-state analog for this step is pyrrolidin-2-ide-2-carboxylate (2−). The absolute configuration of proline residue can be determined by Marfey’s method using reagent 1-fluoro-2,4-dinitrophenyl-5-l-alanineamide (FDAA) [107]. The absolute configuration of amino proline was determined by comparing the retention time with the standard FDAA-derivatized amino acids, e.g., the structure of cyclooctapeptide reniochalistatin E contains three l-proline units with trans conformation [20] whereas the structure of cycloheptapeptide euryjanicin E contains three l-proline units with cis conformation [88]. Further, a novel cyclic tetrapeptide isolated from a Pseudomonas sp. (strain IM-1) associated with the marine sponge Ircinia muscarum was found to contain two proline units, one with l-configuration and the other with d-configuration [77].
2.3. Steric and Lipophilicity Parameters
In order to describe the intermolecular forces of drug receptor interaction, as well as the transport and distribution of drugs in a quantitative manner, various steric and lipophilicity parameters, like molar refractivity (MR20), molar volume (MV20), parachor (Pr), index of refraction (n20), surface tension (γ20), density (d20), polarizability (α), etc., need to be calculated for natural cyclic peptides. Diverse parameters were calculated for proline-rich cyclopolypeptides of marine origin using ACD/ChemSketch software (Version 2.0, Toronto, ON, Canada) (Table S1, Supplementary Materials).
2.4. Synthetic Methodologies
Many proline-rich cyclic peptides were synthesized successfully by various research groups employing different techniques of peptide synthesis. The literature is enriched with reports explaining the synthesis of euryjanicin A [108], delavayin C [109], cherimolacyclopeptide G [110], psammosilenin A [111], hymenamide E [112], stylisin 1 [113], stylisin 2 [114], hymenistatin and yunnanin F [115], pseudostellarin B [116], segetalin E [117], rolloamide B [118] and pseudostellarin G [119] using the solution-phase method utilizing different carbodiimides as coupling agents, TEA/NMM as the base and the synthesis of euryjanicin B [120], mollamide [121], met-cherimolacyclopeptide B [122], axinellin A [123], phakellistatin 7 [124], phakellistatin 12 [125], petriellin A [126], hymenamide C [127], gombamide A [128] and scleritodermin A [129] by the solid-phase method of peptide synthesis. Solid-phase peptide synthesis (SPPS) results in high yields of pure products and works more quickly than classical synthesis, i.e., liquid-phase peptide synthesis (LPPS). Through the replacement of a complicated isolation procedure for each intermediate product with a simple washing procedure, much time is saved using SPPS. In addition, SPPS has proven possible to increase the yield in each individual step to 99.5% or better, which cannot be attained using conventional synthetic approaches. However, solution phase synthesis continues to be especially valuable for large-scale manufacturing and for specialized laboratory applications [130,131]. Moreover, in some cases, a mixed solid-phase/solution synthesis strategy is employed to accomplish total synthesis of the cyclopolypeptide [132], e.g., during the total synthesis of the naturally-occurring proline-rich cyclic octapeptide stylissamide X, the linear octapeptide was assembled first by standard Fmoc solid-phase peptide synthesis (SPPS), and cyclization was carried out subsequently by the solution method. Total synthesis can also be achieved via a convergent native chemical ligation-oxidation strategy [133], e.g., polydiscamides B–D, or utilizing diethyl phosphorocyanidate/BOP-Cl chemistry [134], e.g., axinastatins 2 and 3.
3. Biological Status
l-proline itself is an osmoprotectant and is used in many pharmaceutical and biotechnological applications, whereas the proline analogue cis-4-hydroxy-l-proline has been clinically evaluated as an anticancer drug. Although proline-rich cyclopolypeptides of marine origin are associated with a number of bioactivities, including anti-cancer, anti-tuberculosis, anti-inflammatory, anti-viral, immunosuppressive and anti-fungal activities, still the majority of them were found to exhibit cell growth inhibitory activity [135,136]. Various pharmacological activities associated marine-derived proline-rich cyclopeptides along with susceptible cell line/organism with minimum inhibitory concentration are compiled in Table 2.
Table 2.
Marine-derived proline-rich cyclopeptides with diverse bioactivities.
| PRCPs | Resource | Pharmacological Activity | |
|---|---|---|---|
| Susceptibility | MIC Value | ||
| Axinastatin 1 [6] | marine sponge | Cytotoxicity against PS leukemia cell line | 0.21 μg/mL |
| Polydiscamide A [7] | marine sponge | Antiproliferative activity against human lung cancer A549 cell line; antibacterial activity against Bacillus subtilis | 0.7 μg/mL; 3.1 μg/mL |
| Hymenamide E [8] | marine sponge | Antifungal activity against pathogenic Cryptococcus neoformans | 133 μg/mL |
| trans,trans-Ceratospongamide [10] | marine red alga | Inhibition of sPLA2 expression in a cell-based model for anti-inflammation | 0.0013 μg/mL |
| Mollamide F [12] | marine tunicate | Anti-HIV activity in cytoprotective cell-based assay and HIV integrase inhibition assay | 0.0016 and 0.0031 μg/mL |
| Callyaerin A [13] | marine sponge | Anti-TB activity against M. tuberculosis, inhibitory activity toward C. albicans | 7.37 μg/mL |
| Callyaerin B [13] | marine sponge | Anti-TB activity against Mycobacterium tuberculosis | 7.8 μg/mL |
| Callyaerin E, H [13] | marine sponge | Cytotoxicity against L5178Y cell line | 7.91 and 9.59 μg/mL |
| Euryjanicin C [14] | marine sponge | Inhibitory activity against human hepatitis B virus | 49 μg/mL |
| Polydiscamides B–D [18] | marine sponge | Agonist activity against human sensory neuron-specific G protein couple receptor (SNSR) that is involved in the modulation of pain | - |
| Axinellin A, B [21] | marine sponge | Antitumor activity against human bronchopulmonary non-small-cell lung-carcinoma lines (NSCLC-N6) | 3.0 and 7.3 μg/mL |
| Wainunuamide [23] | marine sponge | Cytotoxic activity against A2780 ovarian tumor and K562 leukemia cancer cells | 19.15 and 18.36 μg/mL |
| Stylissatin A [24] | marine sponge | Inhibition of NO production in LPS-stimulated RAW264.7 cells | 0.0011 μg/mL |
| Scleritodermin A [26] | marine sponge | Inhibition of tubulin polymerization and human tumor cell lines | - |
| Axinastatin 5 [30] | marine sponge | Cytotoxic activity against human and murine cancer cells | 0.3–3.3 μg/mL |
| Phakellistatin 1 [32] | marine sponges | Cell growth inhibitory activity against P-388 murine leukemia | 7.5 μg/mL |
| Stylissamide X [33] | marine sponge | Inhibitory activity against migration of HeLa cells | 0.001–0.1 μg/mL |
| Carteritin A [34] | marine sponge | Cytotoxicity against HeLa, HCT116 and RAW264 cells | 0.0012–0.0026 μg/mL |
| Hymenistatin 1 [35] | marine sponge | Cytotoxicity against P-388 leukemia cells | 3.5 μg/mL |
| Kapakahine A, C [37] | marine sponge | Cytotoxicity against P-388 murine leukemia cells | 5.4 and 5.0 μg/mL |
| Microspinosamide [38] | marine sponge | Anti-HIV activity in CEM-SS cells | 0.2 μg/mL |
| Axinastatin 2 [39] | marine sponge | Cytotoxicity against murine leukemia P-388 cell line | 0.02 μg/mL |
| Axinastatin 3 [39] | marine sponge | Cytotoxicity against PS leukemia cell line | 0.4 μg/mL |
| Mollamide [40] | sea squirt | Cytotoxicity against P-388 (murine leukemia) and A549 (human lung carcinoma), HT29 (human colon carcinoma) cells | 1.0–2.5 μg/mL |
| Cycloforskamide [41] | sea slug | Cytotoxicity against murine leukemia P-388 cells | 8.51 μg/mL |
| Didemnin B [43] | marine tunicate | Cytotoxic activity against human L1210 lymphocytic leukemia cell lines; pancreatic carcinoma (BX-PC3) cell lines; prostatic cancer (DU-145) cell lines; head and neck carcinoma (UMSCC10b) cell lines | 0.0025 μg/mL; 0.002 μg/mL; 0.0015 μg/mL; 0.0018 μg/mL |
| Tamandarin A [44] | marine ascidian | Cytotoxic activity against human pancreatic carcinoma (BX-PC3) cell lines; prostatic cancer (DU-145) cell lines; head and neck carcinoma (UMSCC10b) cell lines | 0.0018 μg/mL; 0.0014 μg/mL; 0.0009 μg/mL |
| Wewakpeptin A [46] | marine cyanobacterium | Cytotoxicity against NCI-H460 human lung tumor and the neuro-2a mouse neuroblastoma cell lines | 0.001 μg/mL |
| Wewakazole B [47] | marine cyanobacterium | Cytotoxicity against human MCF7 breast/H460 lung cancer cells | 8.87–15.29 μg/mL |
| Pahayokolide A [48] | marine cyanobacteria | Antibacterial activity against Bacillus megaterium, Bacillus subtilis | 5 μg/mL |
| Trichormamide A [49] | marine cyanobacteria | Antiproliferative activities against the human melanoma cell line (MDA-MB-435) and the human colon cancer cell line (HT-29) | 8.45 and 8.53 μg/mL |
| Axinastatin 4 [76] | marine sponge | Cytotoxic activity against P-388 lymphocytic leukemia cell line | 0.057 μg/mL |
| Phakellistatin 2 [89] | marine sponge | Cell growth inhibitory activity against P-388 cell line | 0.34 μg/mL |
| Phakellistatin 7–9 [137] | marine sponge | Cell growth inhibitory activity against P-388 murine leukemia | 3.0, 2.9 and 4.1 μg/mL |
| Axinellin C [94] | marine sponge | Cytotoxic activity against A2780 ovarian tumor and K562 leukemia cancer cells | 13.17 and 4.46 μg/mL |
| Callyaerin G [99] | marine sponge | Cytotoxic towards the mouse lymphoma cell line (L5178Y) and HeLa cells | 0.53 and 5.4 μg/mL |
| Stylissatin B [97] | marine sponge | Inhibitory effects against human tumor cell lines including HCT-116, HepG2, BGC-823, NCI-H1650, A2780 and MCF7 | 0.0013 μg/mL |
| Phakellistatin 10, 11 [91] | marine sponge | Cell growth inhibitory activity against murine P-388 lymphocytic leukemia | 2.1, 0.20 μg/mL |
| Stylopeptide 1 [79] | marine sponge | Cell growth inhibitory activity against murine P-388 lymphocytic leukemia | 0.01 μg/mL |
| Phakellistatin 12 [138] | marine sponge | Cell growth inhibitory activity against murine P-388 lymphocytic leukemia | 2.8 μg/mL |
3.1. Mechanism of Action
In drug development, a good antimicrobial candidate should exhibit highly specific biological activity followed by a good pharmacokinetic profile and low immunogenicity. Studies have demonstrated that the members of the proline-rich peptide group and their derivatives act with a completely divergent mechanism than the lytic amphiphilic antimicrobial peptides. Retaining highly potent antimicrobial activities, proline-rich antimicrobial peptides subsequently act in a divergent way, including stereospecific interaction with the membrane translocation system followed by intracellular targeting, compared with the more general membrane disruption mode of action of traditional antimicrobial peptides. It has been further suggested that proline-rich antimicrobial peptides stereo-specifically bind to intracellular targets, such as the bacterial heat shock DnaK protein, and this binding can be correlated with the observed antimicrobial activity. Moreover, proline-rich peptides are characterized by good water solubility, high potency against bacteria killing and low cytotoxic effects at high concentrations, making them attractive lead candidates for the development of novel antimicrobial therapeutic agents [103].
Further, proline-rich antimicrobial peptides are actively transported inside the bacterial cell where they bind and inactivate specific targets like the bacterial ribosome and, thereby, inhibit protein synthesis. This implies that they can be used as molecular hooks to identify the intracellular or membrane proteins that are involved in their mechanism of action and that may be subsequently used as targets for the design of novel antibiotics with mechanisms different from those now in use. Didemnin B is a heterodetic non-polar cyclic peptide associated with antiviral, antitumor, immunomodulating properties, potently inhibits protein and DNA synthesis by binding to the eukaryotic translation elongation factor EF-1α in a GTP-dependent manner, and the formation of the didemnin B-GTP-EF-1α complex may be responsible for the observed inhibition of protein synthesis [139]. Inhibition of protein synthesis by didemnin B occurs by stabilization of aminoacyl-tRNA to the ribosomal A-site, preventing the translocation of phenylalanyl-tRNA from the A- to the P-site, but not preventing peptide bond formation. Tamandarin A may act by the same mechanism as didemnin B. Aplidine’s (dehydrodidemnin B) mechanism of action involves several pathways, including cell cycle arrest and inhibition of protein synthesis. Aplidine induces early oxidative stress and results in a rapid and persistent activation of JNK and p38 MAPK phosphorylation with activation of both kinases occurring very rapid, long before the execution of apoptosis [140]. Didemnin B induces the death of a variety of transformed cells with apoptotic morphology, DNA fragmentation within the cytosol and the generation of DNA ladders. Scleritodermin A acts by tubulin polymerization inhibition [26].
The immunosuppressive activity of cyclolinopeptide A results from the formation of the complex with cyclophilin and inhibition of the phosphatase activity of calcineurin, a phosphatase that plays an important role in T lymphocyte signaling [141]. Cemadotin (LU103793) is a water-soluble synthetic analogue of linear peptide dolastatin 15, which is believed to act on microtubules involving binding to tubulin and strong suppression of microtubule dynamics.
3.2. Peptide Market and PRCPs in Clinical Trials
Currently, there are more than 60 U.S. Food and Drug Administration (FDA)-approved peptide medicines on the market, and this is expected to grow significantly, with approximately 140 peptide drugs currently in clinical trials and more than 500 therapeutic peptides in preclinical development. In terms of value, the global peptide drug market has been predicted to increase from US$14.1 billion in 2011 to an estimated US$25.4 billion in 2018, with an underlying increase in novel innovative peptide drugs from US$8.6 billion in 2011 (60%) to US$17.0 billion (66%) in 2018 [74]. Currently, most peptide drugs are administered by the parental route, and approximately 75% are given as injectables. However, alternative administration forms are gaining increasing traction, including oral, intranasal and transdermal delivery routes, according to the respective technology developments. The use of alternative administration forms could also enable greater usage of peptide therapeutics in other disease areas, such as inflammation, where topical administration of peptides could be the basis for highly efficacious novel treatments.
The cyclic depsipeptide didemnin B was the first marine-derived cyclopolypeptide to undergo clinical trials targeted at oncological patients. However, high toxicity, poor solubility and short life span led to the discontinuation of clinical trials of didemnin B and rendered it unsuitable for further drug development [142]. The linear depsipeptide kahalalide F is known for its antifungal and antitumor activities, and its phase II clinical trials are underway. Another cyclic depsipeptide plitidepsin (dehydrodidemnin B or aplidine) is in clinical development. In 2003, plitidepsin was granted orphan drug status by the European Medicines Agency for treating acute lymphoblastic leukemia. In 2007, it was undergoing multicenter phase II clinical trials, and in 2016, early results in a small phase I trial for multiple myeloma were announced. The two most promising peptides of antimitotic dolastatins group, dolastatin 10 and 15, were selected for development and are currently undergoing phase II clinical trials. Cemadotin, the synthetic analogue of dolastatin 15, is also in phase II clinical trials as a promising cancer chemotherapeutic agent [143,144].
4. Conclusions and Future Prospects
There is increased evidence of the emergence of resistance to conventional drugs illustrating the importance of research on natural peptide-based drug development. PRCPs have several structural features making them good drug leads, and there are several naturally-occurring cyclic peptides in clinical use and in clinical trials. In addition, biologically-active proline-rich cyclic peptides have been developed with synthetic approaches, and they are useful as therapeutics and biochemical tools. With the introduction of new high throughput screening methods, there will be more availability of marine-based PRCPs with interesting biological properties. PRCPs can work on their targets very selectively, as the interaction with the targets is very specific compared to small molecules. In addition to the merits of peptides, especially “proline-rich cyclic structures” as drug molecules, cyclopolypeptides could make even better peptide drugs for future use. Moreover, the future development of peptide drugs will continue to build upon the strengths of naturally-occurring proline-rich peptides, with the application of traditional rational design to improve their weaknesses, such as their chemical and physical properties. Further, emerging peptide technologies will help broaden the applicability of PRCPs as therapeutics. While still in the early stages of development, PRCPs drug leads have started gaining the attention of the pharmaceutical industry; however, their true potential is still very much unknown.
Acknowledgments
The authors wish to thank chief librarians of Central Drug Research Institute (CDRI), Lucknow, Uttar Pradesh, India, National Medical Library (NML), New Delhi, India, Faculty of Medical Sciences, The University of the West Indies, Trinidad and Tobago, West Indies and Wuhan University of Technology, Wuhan, China, for providing literature support.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/14/11/194/s1. Table S1: Various steric and lipophilic parameters for proline-rich cyclopolypeptides from diverse marine resources.
Author Contributions
All authors were involved in all aspects of the work done for this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bai R., Friedman S.J., Pettit G.R., Hamel E. Dolastatin 15, a potent antimitotic depsipeptide derived from Dolabella auricularia: Interaction with tubulin and effects on cellular microtubules. Biochem. Pharmacol. 1992;43:2637–2645. doi: 10.1016/0006-2952(92)90153-A. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto S., Iwasaki A., Ohno O., Suenaga K. Isolation and structure of kurahyne B and total synthesis of the kurahynes. J. Nat. Prod. 2015;78:2719–2725. doi: 10.1021/acs.jnatprod.5b00662. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A., Ohno O., Sumimoto S., Ogawa H., Nguyen K.A., Suenaga K. Jahanyne, an apoptosis-inducing lipopeptide from the marine cyanobacterium Lyngbya sp. Org. Lett. 2015;17:652–655. doi: 10.1021/ol5036722. [DOI] [PubMed] [Google Scholar]
- 4.Jordan M.A., Walker D., de Arruda M., Barlozzari T., Panda D. Suppression of microtubule dynamics by binding of cemadotin to tubulin: Possible mechanism for its antitumor action. Biochemistry. 1998;37:17571–17578. doi: 10.1021/bi9817414. [DOI] [PubMed] [Google Scholar]
- 5.Fusetani N., Warabi K., Nogata Y., Nakao Y., Matsunaga S., van Soest R.R.M. Koshikamide A1, a new cytotoxic linear peptide isolated from a marine sponge Theonella sp. Tetrahedron Lett. 1999;40:4687–4690. doi: 10.1016/S0040-4039(99)00844-8. [DOI] [Google Scholar]
- 6.Pettit G.R., Herald C.L., Boyd M.R., Leet J.E., Dufresne C., Doubek D.L., Schmidt J.M., Cerny R.L., Hooper J.N.A., Rutzler K.C. Antineoplastic agents. 219. Isolation and structure of the cell growth inhibitory constituents from the western Pacific marine sponge Axinella sp. J. Med. Chem. 1991;34:3339–3340. doi: 10.1021/jm00115a027. [DOI] [PubMed] [Google Scholar]
- 7.Gulavita N.K., Gunasekela S.P., Pomponi S.A., Robinson E.V. Polydiscamide A: A new bioactive depsipeptide from the marine sponge Discodermia sp. J. Org. Chem. 1992;57:1767–1772. doi: 10.1021/jo00032a031. [DOI] [Google Scholar]
- 8.Tsuda M., Shigemori H., Mikami Y., Kobayashi J. Hymenamides C–E, new cyclic heptapeptides with two proline residues from the Okinawan marine sponge Hymeniacidon sp. Tetrahedron. 1993;49:6785–6796. doi: 10.1016/S0040-4020(01)80422-1. [DOI] [Google Scholar]
- 9.Cebrat M., Wieczorek Z., Siemion I.Z. Immunosuppressive activity of hymenistatin 1. Peptides. 1996;17:191–196. doi: 10.1016/0196-9781(95)02123-X. [DOI] [PubMed] [Google Scholar]
- 10.Tan L.T., Williamson R.T., Gerwick W.H., Watts K.S., McGough K., Jacobs R. cis,cis- and trans,trans-Ceratospongamide, new bioactive cyclic heptapeptides from the indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J. Org. Chem. 2000;65:419–425. doi: 10.1021/jo991165x. [DOI] [PubMed] [Google Scholar]
- 11.Lu Z., Harper M.K., Pond C.D., Barrows L.R., Ireland C.M., van Wagoner R.M. Thiazoline peptides and a tris-phenethyl urea from Didemnum molle with anti-HIV activity. J. Nat. Prod. 2012;75:1436–1440. doi: 10.1021/np300270p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sera Y., Adachi K., Fujii K., Shizuri Y. A new antifouling hexapeptide from a palauan sponge, Haliclona sp. J. Nat. Prod. 2003;66:719–721. doi: 10.1021/np020271i. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim S.R., Min C.C., Teuscher F., Ebel R., Kakoschke C., Lin W., Wray V., Edrada-Ebel R., Proksch P. Callyaerins A–F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 2010;18:4947–4956. doi: 10.1016/j.bmc.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Vera B., Vicente J., Rodriguez A.D. Isolation and structural elucidation of euryjanicins B–D, proline-containing cycloheptapeptides from the Caribbean marine sponge Prosuberites laughlini. J. Nat. Prod. 2009;72:1555–1562. doi: 10.1021/np9004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berer N., Rudi A., Goldberg I., Benayahu Y., Kashman Y. Callynormine A, a new marine cyclic peptide of a novel class. Org. Lett. 2004;6:2543–2545. doi: 10.1021/ol0491787. [DOI] [PubMed] [Google Scholar]
- 16.Williams D.E., Patrick B.O., Behrisch H.W., van soest R., Roberge M., Andersen R.J. Dominicin, a cyclic octapeptide, and laughine, a bromopyrrole alkaloid, isolated from the Caribbean marine sponge Eurypon laughlini. J. Nat. Prod. 2005;68:327–330. doi: 10.1021/np049711r. [DOI] [PubMed] [Google Scholar]
- 17.Daletos G., Kalscheuer R., Koliwer-Brandl H., Hartmann R., de Voogd N.J., Wray V., Lin W., Proksch P. Callyaerins from the marine sponge Callyspongia aerizusa: Cyclic peptides with antitubercular activity. J. Nat. Prod. 2015;78:1910–1925. doi: 10.1021/acs.jnatprod.5b00266. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y., Carroll A.R., Pass D.M., Archbold J.K., Avery V.M., Quinn R.J. Polydiscamides B–D from a marine sponge Ircinia sp. as potent human sensory neuron-specific G protein coupled receptor agonists. J. Nat. Prod. 2008;71:8–11. doi: 10.1021/np070094r. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed R., Peng J., Kelly M., Hamann M.T. Cyclic heptapeptides from the jamaican sponge Stylissa caribica. J. Nat. Prod. 2006;69:1739–1744. doi: 10.1021/np060006n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan K.X., Jiao W.H., Yang F., Li J., Wang S.P., Li Y.S., Han B.N., Lin H.W. Reniochalistatins A–E, cyclic peptides from the marine sponge Reniochalina stalagmitis. J. Nat. Prod. 2014;77:2678–2684. doi: 10.1021/np5006778. [DOI] [PubMed] [Google Scholar]
- 21.Randazzo A., Piaz F.D., Orrù S., Debitus C., Roussakis C., Pucci P., Gomez-Paloma L. Axinellins A and B: New proline-containing antiproliferative cyclopeptides from the Vanuatu sponge Axinella carteri. Eur. J. Org. Chem. 1998;11:2659–2665. doi: 10.1002/(SICI)1099-0690(199811)1998:11<2659::AID-EJOC2659>3.0.CO;2-H. [DOI] [Google Scholar]
- 22.Woo J.K., Jeon J.E., Kim C.K., Sim C.J., Oh D.C., Oh K.B., Shin J. Gombamide A, a cyclic thiopeptide from the sponge Clathria gombawuiensis. J. Nat. Prod. 2013;76:1380–1383. doi: 10.1021/np4003367. [DOI] [PubMed] [Google Scholar]
- 23.Tabudravu J., Morris L.A., Kettenes-van den Bosch J.J., Jaspars M. Wainunuamide, a histidine-containing proline-rich cyclic heptapeptide isolated from the Fijian marine sponge Stylotella aurantium. Tetrahedron Lett. 2001;42:9273–9276. doi: 10.1016/S0040-4039(01)01993-1. [DOI] [Google Scholar]
- 24.Kita M., Gise B., Kawamura A., Kigoshi H. Stylissatin A, a cyclic peptide that inhibits nitric oxide production from the marine sponge Stylissa massa. Tetrahedron Lett. 2013;54:6826–6828. doi: 10.1016/j.tetlet.2013.10.003. [DOI] [Google Scholar]
- 25.Zhang H.J., Yi Y.H., Yang G.J., Hu M.Y., Cao G.D., Yang F., Lin H.W. Proline-containing cyclopeptides from the marine sponge Phakellia fusca. J. Nat. Prod. 2010;73:650–655. doi: 10.1021/np9008267. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt E.W., Raventos-Suarez C., Bifano M., Menendez A.T., Fairchild C.R., Faulkner D.J. Scleritodermin A, a cytotoxic cyclic peptide from the lithistid sponge Scleritoderma nodosum. J. Nat. Prod. 2004;67:475–478. doi: 10.1021/np034035z. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez R., Omar S., Feliz M., Quinoa E., Riguera R. Malaysiatin, the first cyclic heptapeptide from a marine sponge. Tetrahedron Lett. 1992;33:6017–6020. doi: 10.1016/S0040-4039(00)61115-2. [DOI] [Google Scholar]
- 28.Erickson K.L., Gustafson K.R., Milanowski D.J., Pannell L.K., Klose J.R., Boyd M.R. Myriastramides A–C, new modified cyclic peptides from the Phillipines marine sponge Myriastra clavosa. Tetrahedron. 2003;59:10231–10238. doi: 10.1016/j.tet.2003.10.060. [DOI] [Google Scholar]
- 29.Brennan M.R., Costello C.E., Maleknia S.D., Pettit G.R., Erickson K.L. Stylopeptide 2, a proline-rich cyclodecapeptide from the sponge Stylotella sp. J. Nat. Prod. 2008;71:453–436. doi: 10.1021/np0704856. [DOI] [PubMed] [Google Scholar]
- 30.Pettit G.R., Gao F., Schmidt J.M., Cerny R. Isolation and structure of axinastatin 5 from a Republic of Comoros marine sponge. Bioorg. Med. Chem. Lett. 1994;4:2935–2940. doi: 10.1016/S0960-894X(01)80843-X. [DOI] [Google Scholar]
- 31.Kobayashi J., Tsuda M., Nakamura T., Mikami Y., Shigemori H. Hymenamides A and B, new proline-rich cyclic heptapeptides from the okinawan marine sponge hymeniacidon sp. Tetrahedron. 1993;49:2391–2402. doi: 10.1016/S0040-4020(01)86318-3. [DOI] [Google Scholar]
- 32.Pettit G.R., Cichacz Z., Barkoczy J., Dorsaz A.C., Herald D.L., Williams M.D., Doubek D.L., Schmidt J.M., Tackett L.P., Brune D.C., et al. Isolation and structure of the marine sponge cell growth inhibitory cyclic peptide phakellistatin 1. J. Nat. Prod. 1993;56:260–267. doi: 10.1021/np50092a011. [DOI] [PubMed] [Google Scholar]
- 33.Arai M., Yamano Y., Fujita M., Setiawan A., Kobayashi M. Stylissamide X, a new proline-rich cyclic octapeptide as an inhibitor of cell migration, from an Indonesian marine sponge of Stylissa sp. Bioorg. Med. Chem. Lett. 2012;22:1818–1821. doi: 10.1016/j.bmcl.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Afifi A.H., El-Desoky A.H., Kato H., Mangindaan R.E.P., de Voogd N.J., Ammar N.M., Hifnawy M.S., Tsukamoto S. Carteritins A and B, cyclic heptapeptides from the marine sponge Stylissa carteri. Tetrahedron Lett. 2016;57:1285–1288. doi: 10.1016/j.tetlet.2016.02.031. [DOI] [Google Scholar]
- 35.Pettit G.R., Clewlow P.J., Dufrense C., Doubek D.L., Cerny R.L., Rutzler K. Antineoplastic agents. 193. Isolation and structure of the cyclic peptide hymenistatin 1. Can. J. Chem. 1990;68:708–711. doi: 10.1139/v90-110. [DOI] [Google Scholar]
- 36.Vicente J., Vera B., Rodriguez A.D., Rodriguez-Escudero I., Raptis R.G. Euryjanicin A: A new cycloheptapeptide from the Caribbean marine sponge Prosuberites laughlini. Tetrahedron Lett. 2009;50:4571–4574. doi: 10.1016/j.tetlet.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung B.K.S., Nakao Y., Kinnel R.B., Carney J.R., Yoshida W.Y., Scheuer P.J., Kelly-Borges M. The Kapakahines, cyclic peptides from the marine sponge Cribrochalina olemda. J. Org. Chem. 1996;61:7168–7173. doi: 10.1021/jo960725e. [DOI] [PubMed] [Google Scholar]
- 38.Rashid M.A., Gustafson K.R., Cartner L.K., Shigematsu N., Pannell L.K., Boyd M.R. Microspinosamide, a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J. Nat. Prod. 2001;64:117–121. doi: 10.1021/np0002379. [DOI] [PubMed] [Google Scholar]
- 39.Pettit G.R., Gao F., Cerny R.L., Doubek D.L., Tackett L.P., Schmidt J.M., Chapuis J.C. Antineoplastic agents. 278. Isolation and structure of axinastatins 2 and 3 from a western Caroline Island marine sponge. J. Med. Chem. 1994;37:1165–1168. doi: 10.1021/jm00034a014. [DOI] [PubMed] [Google Scholar]
- 40.Carroll A.R., Bowden B.F., Coll J.C., Hockless D.C.R., Skelton B.W., White A.H. Studies of australian ascidians. IV. Mollamide, a cytotoxic cyclic heptapeptide from the compound ascidian Didemnum molle. Aust. J. Chem. 1994;47:61–69. doi: 10.1071/CH9940061. [DOI] [Google Scholar]
- 41.Tan K.O., Wakimoto T., Takada K., Ohtsuki T., Uchiyama N., Goda Y., Abe I. Cycloforskamide, a cytotoxic macrocyclic peptide from the sea slug Pleurobranchus forskalii. J. Nat. Prod. 2013;76:1388–1391. doi: 10.1021/np400404r. [DOI] [PubMed] [Google Scholar]
- 42.Whitson E.L., Ratnayake A.S., Bugni T.S., Harper M.K., Treland C.M. Isolation, structure elucidation and synthesis of eudistomides A and B, lipopeptides from a fijian ascidian Eudistoma sp. J. Org. Chem. 2009;74:1156–1162. doi: 10.1021/jo8022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinehart K.L., Jr., Gloer J.B., Cook J.C., Jr., Mizsak S.A., Scahill T.A. Structures of the didemnins, antiviral and cytotoxic depsipeptides from a Caribbean tunicate. J. Am. Chem. Soc. 1981;103:1857–1859. doi: 10.1021/ja00397a055. [DOI] [Google Scholar]
- 44.Vervoort H., Fenical W. Tamandarins A and B: New cytotoxic depsipeptides from a Brazilian ascidian of the family Didemnidae. J. Org. Chem. 2000;65:782–792. doi: 10.1021/jo991425a. [DOI] [PubMed] [Google Scholar]
- 45.Mercader A.G., Duchowicz P.R., Sivakumar P.M. Chemometrics Applications and Research: QSAR in Medicinal Chemistry. Apple Academic Press, Inc.; Oakville, ON, Canada: 2016. p. 278. [Google Scholar]
- 46.Han B., Goeger D., Maier C.S., Gerwick W.H. The Wewakpeptins, cyclic depsipeptides from a papua new guinea collection of the marine cyanobacterium Lyngbya semiplena. J. Org. Chem. 2005;70:3133–3139. doi: 10.1021/jo0478858. [DOI] [PubMed] [Google Scholar]
- 47.Lopez J.A.V., Al-Lihaibi S.S., Alarif W.M., Abdel-Lateff A., Nogata Y., Washio K., Morikawa M., Okino T. Wewakazole B, a cytotoxic cyanobactin from the cyanobacterium Moorea producens collected in the red sea. J. Nat. Prod. 2016;79:1213–1218. doi: 10.1021/acs.jnatprod.6b00051. [DOI] [PubMed] [Google Scholar]
- 48.An T., Kumar T.K., Wang M., Liu L., Lay J.O., Jr., Liyanage R., Berry J., Gantar M., Marks V., Gawley R.E., et al. Structures of pahayokolides A and B, cyclic peptides from a Lyngbya sp. J. Nat. Prod. 2007;70:730–735. doi: 10.1021/np060389p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo S., Krunic A., Kang H.S., Chen W.L., Woodard J.L., Fuchs J.R., Swanson S.M., Orjala J. Trichormamides A and B with antiproliferative activity from the cultured freshwater cyanobacterium Trichormus sp. UIC 10339. J. Nat. Prod. 2014;77:1871–1880. doi: 10.1021/np5003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujii K., Sivonen K., Kashiwagi T., Hirayama K., Harada K.I. Nostophycin, a novel cyclic peptide from the toxic cyanobacterium Nostoc sp. 152. J. Org. Chem. 1999;64:5777–5782. doi: 10.1021/jo982306i. [DOI] [Google Scholar]
- 51.Davies-Coleman M.T., Dzeha T.M., Gray C.A., Hess S., Pannell L.K., Hendricks D.T., Arendse C.E. Isolation of homodolastatin 16, a new cyclic depsipeptide from a Kenyan collection of Lyngbya majuscula. J. Nat. Prod. 2003;66:712–715. doi: 10.1021/np030014t. [DOI] [PubMed] [Google Scholar]
- 52.Nogle L.M., Gerwick W.H. Isolation of four new cyclic depsipeptides, antanapeptins A–D, and dolastatin 16 from a madagascan collection of Lyngbya majuscula. J. Nat. Prod. 2002;65:21–24. doi: 10.1021/np010348n. [DOI] [PubMed] [Google Scholar]
- 53.Dahiya R. Cyclopolypeptides with antifungal interest. Coll. Pharm. Commun. 2013;1:1–15. [Google Scholar]
- 54.Dahiya R., Gautam H. Synthesis, characterization and biological evaluation of cyclomontanin D. Afr. J. Pharm. Pharmacol. 2011;5:447–453. doi: 10.5897/AJPP10.384. [DOI] [Google Scholar]
- 55.Dahiya R., Gautam H. Synthetic and pharmacological studies on a natural cyclopeptide from Gypsophila arabica. J. Med. Plant Res. 2010;4:1960–1966. [Google Scholar]
- 56.Dahiya R., Singh S. Synthesis, characterization and biological screening of diandrine A. Acta Pol. Pharm. 2016 submitted. [PubMed] [Google Scholar]
- 57.Dahiya R., Gautam H. Solution phase synthesis and bioevaluation of cordyheptapeptide B. Bull. Pharm. Res. 2011;1:1–10. [Google Scholar]
- 58.Dahiya R. Synthesis of a phenylalanine-rich peptide as potential anthelmintic and cytotoxic agent. Acta Pol. Pharm. 2007;64:509–516. [PubMed] [Google Scholar]
- 59.Dahiya R., Gautam H. Toward the first total synthesis of gypsin D: A natural cyclopolypeptide from Gypsophila arabica. Am. J. Sci. Res. 2010;11:150–158. [Google Scholar]
- 60.Dahiya R., Kaur K. Synthesis and pharmacological investigation of segetalin C as a novel antifungal and cytotoxic agent. Arzneimittelforschung. 2008;58:29–34. doi: 10.1055/s-0031-1296463. [DOI] [PubMed] [Google Scholar]
- 61.Dahiya R. Synthetic and pharmacological studies on longicalycinin A. Pak. J. Pharm. Sci. 2007;20:317–323. [PubMed] [Google Scholar]
- 62.Dahiya R., Kumar A. Synthetic and biological studies on a cyclopolypeptide of plant origin. J. Zhejiang Univ. Sci. B. 2008;9:391–400. doi: 10.1631/jzus.B0720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahiya R., Gautam H. Synthesis and pharmacological studies on a cyclooligopeptide from marine bacteria. Chin. J. Chem. 2011;29:1911–1916. [Google Scholar]
- 64.Dahiya R. Synthesis, characterization and biological evaluation of a glycine-rich peptide—Cherimolacyclopeptide E. J. Chil. Chem. Soc. 2007;52:1224–1229. doi: 10.4067/S0717-97072007000300006. [DOI] [Google Scholar]
- 65.Dahiya R., Gautam H. Toward the synthesis and biological screening of a cyclotetrapeptide from marine bacteria. Mar. Drugs. 2011;9:71–81. doi: 10.3390/md9010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dahiya R., Maheshwari M., Yadav R. Synthetic and cytotoxic and antimicrobial activity studies on annomuricatin B. Z. Naturforsch. 2009;64:237–244. doi: 10.1515/znb-2009-0215. [DOI] [Google Scholar]
- 67.Aneiros A., Garateix A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004;803:41–53. doi: 10.1016/j.jchromb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Silver F.H. Mechanosensing and Mechanochemical Transduction in Extracellular Matrix. Biochemical, Chemical, Engineering, and Physiological Aspects. Macromolecular Structures in Tissues. Volume XVI. Springer; Berlin/Heidelberg, Germany: 2006. p. 33. [Google Scholar]
- 69.Pandey A.K., Naduthambi D., Thomas K.M., Zondlo N.J. Proline editing: A general and practical approach to the synthesis of functionally and structurally diverse peptides. Analysis of steric versus stereoelectronic effects of 4-substituted prolines on conformation within peptides. J. Am. Chem. Soc. 2013;135:4333–4363. doi: 10.1021/ja3109664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roxin A., Zheng G. Flexible or fixed: A comparative review of linear and cyclic cancer-targeting peptides. Future Med. Chem. 2012;4:1601–1618. doi: 10.4155/fmc.12.75. [DOI] [PubMed] [Google Scholar]
- 71.Goodwin D., Simerska P., Toth I. Peptides as therapeutics with enhanced bioactivity. Curr. Med. Chem. 2012;19:4451–4461. doi: 10.2174/092986712803251548. [DOI] [PubMed] [Google Scholar]
- 72.Jensen J.E., Mobli M., Brust A., Alewood P.F., King G.F., Rash L.D. Cyclisation increases the stability of the sea anemone peptide APETx2 but decreases its activity at acid-sensing ion channel 3. Mar. Drugs. 2012;10:1511–1527. doi: 10.3390/md10071511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roxin A. Ph.D. Thesis. Graduate Department of Pharmaceutical Sciences, University of Toronto; Toronto, ON, Canada: 2014. Towards Targeted Photodynamic Therapy: Synthesis and Characterization of Aziridine Aldehyde-Cyclized Cancertargeting Peptides and Bacteriochlorin Photosensitizers. [Google Scholar]
- 74.Fosgerau K., Hoffmann T. Peptide therapeutics: Current status and future directions. Drug Discov. Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Shanmugam S., Kumar S.T., Selvam K.P. Laboratory Handbook on Biochemistry. 1st ed. Prentice-Hall of India Private Limited; New Delhi, India: 2010. [Google Scholar]
- 76.Pettit G.R., Gao F., Cerny R. Isolation and structure of axinastatin 4 from the western indian ocean marine sponge Axinella cf. carteri. Heterocycles. 1993;35:711–718. doi: 10.3987/COM-93-S(T)137. [DOI] [Google Scholar]
- 77.Kawagishi H., Somoto A., Kuranari J., Kimura A., Chiba S. A novel cyclotetrapeptide produced by Lactobacillus helveticus as a tyrosinase inhibitor. Tetrahedron Lett. 1993;34:3439–3440. doi: 10.1016/S0040-4039(00)79177-5. [DOI] [Google Scholar]
- 78.Pergament I., Carmeli S. Schizotrin A; a novel antimicrobial cyclic peptide from a cyanobacterium. Tetrahedron Lett. 1994;35:8473–8476. doi: 10.1016/S0040-4039(00)74436-4. [DOI] [Google Scholar]
- 79.Pettit G.R., Srirangam J.K., Herald D.L., Xu J.P., Boyd M.R., Cichacz Z., Kamano Y., Schmidt J.M., Erickson K.L. Isolation and crystal structure of stylopeptide 1, a new marine porifera cycloheptapeptide. J. Org. Chem. 1995;60:8257–8261. doi: 10.1021/jo00130a027. [DOI] [Google Scholar]
- 80.Carroll A.R., Coll J.C., Bourne J.C., MacLeod J.K., Zanriskie T.M., Ireland C.M., Bowden B.F. Patellins 1-6 and Trunkamide A: Novel cyclic hexa-, hepta- and octa-peptides from colonial ascidians, Lissoclinurn sp. Aust. J. Chem. 1996;49:659–667. [Google Scholar]
- 81.Kobayashi J., Nakamura T., Tsuda M. Hymenamide F, new cyclic heptapeptide from marine sponge Hymeniacidon sp. Tetrahedron. 1996;52:6355–6360. doi: 10.1016/0040-4020(96)00281-5. [DOI] [Google Scholar]
- 82.Shin H.J., Matsuda H., Murakami M., Yamaguchi K. Agardhipeptins A and B, two new cyclic hepta- and octapeptide, from the cyanobacterium Oscillatoria agardhii (NIES-204) Tetrahedron. 1996;52:13129–13136. doi: 10.1016/0040-4020(96)00775-2. [DOI] [Google Scholar]
- 83.Belofsky G.N., Gloer J.B., Wicklow D.T., Dowd P.F. Shearamide A: A new cyclic peptide from the ascostromata of Eupenicillium shearii. Tetrahedron Lett. 1998;39:5497–5500. doi: 10.1016/S0040-4039(98)01161-7. [DOI] [Google Scholar]
- 84.Murakami M., Itou Y., Ishida K., Shin H.J. Prenylagaramides A and B, new cyclic peptides from two strains of Oscillatoria agardhii. J. Nat. Prod. 1999;62:752–755. doi: 10.1021/np980396g. [DOI] [PubMed] [Google Scholar]
- 85.Milanowski D.J., Rashid M.A., Gustafson K.R., O’Keefe B.R., Nawrocki J.P., Pannell L.K., Boyd M.R. Cyclonellin, a new cyclic octapeptide from the marine sponge Axinella carteri. J. Nat. Prod. 2004;67:441–444. doi: 10.1021/np030336x. [DOI] [PubMed] [Google Scholar]
- 86.Leikoski N., Fewer D.P., Jokela J., Wahlsten M., Rouhiainen L., Sivonen K. Highly diverse cyanobactins in strains of the genus Anabaena. Appl. Environ. Microbiol. 2010;76:701–709. doi: 10.1128/AEM.01061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng Y.X., Zhou L.L., Yan Y.M., Chen K.X., Hou F.F. Diabetic nephropathy-related active cyclic peptides from the roots of Brachystemma calycinum. Bioorg. Med. Chem. Lett. 2011;21:7334–7439. doi: 10.1016/j.bmcl.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Aviles E., Rodriguez A.D. Euryjanicins E–G, poly-phenylalanine and poly-proline cyclic heptapeptides from the Caribbean sponge Prosuberites laughlini. Tetrahedron. 2013;69:10797–10804. doi: 10.1016/j.tet.2013.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pettil G.R., Tan R., Williams M.D., Tackett L., Schmidt J.M., Cerny R.L., Hooper J.N.A. Isolation and structure of phakellistatin 2 from the eastern indian ocean marine sponge phakellia carteri. Bioorg. Med. Chem. Lett. 1993;3:2869–2874. doi: 10.1016/S0960-894X(01)80781-2. [DOI] [Google Scholar]
- 90.Tsuda M., Sasaki T., Kobayashi J. Hymenamides G, H, J, and K, four new cyclic octapeptides from the Okinawan marine sponge Hymeniacidon sp. Tetrahedron. 1994;50:4667–4680. doi: 10.1016/S0040-4020(01)85006-7. [DOI] [Google Scholar]
- 91.Pettit G.R., Tan R., Ichihara Y., Williams M.D., Doubek D.L., Tackett L.P., Schmidt J.M., Cerny R.L., Boyd M.R., Hooper J.N. Antineoplastic agents, 325. Isolation and structure of the human cancer cell growth inhibitory cyclic octapeptides phakellistatin 10 and 11 from Phakellia sp. J. Nat. Prod. 1995;58:961–965. doi: 10.1021/np50120a025. [DOI] [PubMed] [Google Scholar]
- 92.Rashid M.A., Gustafson K.R., Boswell J.L., Boyd M.R. Haligramides A and B, two new cytotoxic hexapeptides from the marine sponge Haliclona nigra. J. Nat. Prod. 2000;63:956–959. doi: 10.1021/np000051+. [DOI] [PubMed] [Google Scholar]
- 93.Guan L.L., Sera Y., Adachi K., Nishida F., Shizuri Y. Isolation and evaluation of nonsiderophore cyclic peptides from marine sponges. Biochem. Biophy. Res. Commun. 2001;283:976–981. doi: 10.1006/bbrc.2001.4890. [DOI] [PubMed] [Google Scholar]
- 94.Tabudravu J.N., Morris L.A., Kettenes-van den Bosch J.J., Jaspars M. Axinellin C, a proline-rich cyclic octapeptide isolated from the Fijian marine sponge Stylotella aurantium. Tetrahedron. 2002;58:7863–7868. doi: 10.1016/S0040-4020(02)00898-0. [DOI] [Google Scholar]
- 95.Sera Y., Adachi K., Fujii K., Shizuri Y. Isolation of haliclonamides: New peptides as antifouling substances from a marine sponge species, Haliclona. Mar. Biotechnol. 2002;4:441–446. doi: 10.1007/s10126-001-0082-6. [DOI] [PubMed] [Google Scholar]
- 96.Nogle L.M., Marquez B.L., Gerwick W.H. Wewakazole, a novel cyclic dodecapeptide from a papua new guinea Lyngbya majuscule. Org. Lett. 2003;5:3–6. doi: 10.1021/ol026811k. [DOI] [PubMed] [Google Scholar]
- 97.Sun J., Cheng W., de Voogd N.J., Proksch P., Lin W. Stylissatins B–D, cycloheptapeptides from the marine sponge Stylissa massa. Tetrahedron Lett. 2016 in press. [Google Scholar]
- 98.Wieland T., Luben G., Ottenheym H., Faesel D.C.J., de Vries J.X., Prox A., Schmid D.C.J. The discovery, isolation, elucidation of structure, and synthesis of antamanide. Angew. Chem. Int. Ed. 1968;7:204–208. doi: 10.1002/anie.196802041. [DOI] [PubMed] [Google Scholar]
- 99.Ibrahim S.R.M., Edrada-Ebel R.A., Mohamed G.A., Youssef D.T.A., Wray V., Proksch P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. ARKIVOC Arch. Org. Chem. 2008;2008:164–171. [Google Scholar]
- 100.Pettit G.R., Tan R., Herald D.L., Cerny R.L., Williams M.D. Antineoplastic agents. 277. Isolation and structure of phakellistatin 3 and isophakellistatin 3 from a republic of Comoros marine sponge. J. Org. Chem. 1994;59:1593–1595. doi: 10.1021/jo00086a001. [DOI] [Google Scholar]
- 101.Martins J., Vasconcelos V. Cyanobactins from cyanobacteria: Current genetic and chemical state of knowledge. Mar. Drugs. 2015;13:6910–6946. doi: 10.3390/md13116910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Donia M.S., Ravel J., Schmidt E.W. A global assembly line to cyanobactins. Nat. Chem. Biol. 2008;4:341–343. doi: 10.1038/nchembio.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mojsoska B., Jenssen H. Peptides and peptidomimetics for antimicrobial drug design. Pharmaceuticals. 2015;8:366–415. doi: 10.3390/ph8030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wedemeyer W.J., Welker E., Scheraga H.A. Proline cis-trans isomerization and protein folding. Biochemistry. 2002;41:14637–14644. doi: 10.1021/bi020574b. [DOI] [PubMed] [Google Scholar]
- 105.Sarkar P., Reichman C., Saleh T., Birge R.B., Kalodimos C.G. Proline cis-trans isomerization controls autoinhibition of a signaling protein. Mol. Cell. 2007;25:413–426. doi: 10.1016/j.molcel.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vitagliano L., Berisio R., Mastrangelo A., Mazzarella L., Zagari A. Preferred proline puckerings in cis and trans peptide groups: Implications for collagen stability. Protein Sci. 2001;10:2627–2632. doi: 10.1110/ps.ps.26601a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhushan R., Bruckner H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids. 2004;27:231–247. doi: 10.1007/s00726-004-0118-0. [DOI] [PubMed] [Google Scholar]
- 108.Anand M., Alagar M., Ranjitha J., Selvaraj V. Total synthesis and anticancer activity of a cyclic heptapeptide from marine sponge using water soluble peptide coupling agent EDC. Arab. J. Chem. 2016 in press. [Google Scholar]
- 109.Shinde N.V., Himaja M., Bhosale S.K., Ramana M.V., Sakarkar D.M. Synthesis and biological evaluation of delavayin-C. Indian J. Pharm. Sci. 2008;70:827–831. doi: 10.4103/0250-474X.49137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dahiya R. Synthesis, spectroscopic and biological investigation of cyclic octapeptide: Cherimolacyclopeptide G. Turk. J. Chem. 2008;32:205–215. [Google Scholar]
- 111.Dahiya R. Total synthesis and biological potential of psammosilenin A. Arch. Pharm. Chem. Life Sci. 2008;341:502–509. doi: 10.1002/ardp.200800006. [DOI] [PubMed] [Google Scholar]
- 112.Dahiya R., Pathak D., Himaja M., Bhatt S. First total synthesis and biological screening of hymenamide E. Acta Pharm. 2006;56:399–415. [PubMed] [Google Scholar]
- 113.Dahiya R., Kumar A., Gupta R. Synthesis, cytotoxic and antimicrobial screening of a proline-rich cyclopolypeptide. Chem. Pharm. Bull. (Tokyo) 2009;57:214–217. doi: 10.1248/cpb.57.214. [DOI] [PubMed] [Google Scholar]
- 114.Dahiya R., Gautam H. Total synthesis and antimicrobial activity of a natural cycloheptapeptide of marine origin. Mar. Drugs. 2010;8:2384–2394. doi: 10.3390/md8082384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poojary B., Belagali S.L. Synthetic studies on cyclic octapeptides: Yunnanin F and hymenistatin. Eur. J. Med. Chem. 2005;40:407–412. doi: 10.1016/j.ejmech.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 116.Poojary B., Kumar K.H., Belagali S.L. Synthesis and biological evaluation of pseudostellarin B. Pharmaco. 2001;56:331–334. doi: 10.1016/S0014-827X(01)01031-X. [DOI] [PubMed] [Google Scholar]
- 117.Dahiya R., Kaur K. Synthetic and biological studies on natural cyclic heptapeptide: Segetalin E. Arch. Pharm. Res. 2007;30:1380–1386. doi: 10.1007/BF02977360. [DOI] [PubMed] [Google Scholar]
- 118.El Khatib M., Elagawany M., Caliskan E., Davis E.F., Faidallah H.M., El-Feky S.A., Katritzky A.R. Total synthesis of cyclic heptapeptide rolloamide B. Chem. Commun. (Camb.) 2013;49:2631–2633. doi: 10.1039/c3cc39291k. [DOI] [PubMed] [Google Scholar]
- 119.Poojary B., Kumar K.H., Belagali S.L. Synthesis of a new cyclic peptide, pseudostellarin G. Z. Naturforsch. B. 2004;59:817–820. doi: 10.1002/chin.200447167. [DOI] [Google Scholar]
- 120.Zhang C.M., Guo J.X., Wang L., Chai X.Y., Hu H.G., Wu Q.Y. Total synthesis of cyclic heptapeptide euryjanicin B. Chin. Chem. Lett. 2011;22:631–634. doi: 10.1016/j.cclet.2010.12.039. [DOI] [Google Scholar]
- 121.McKeever B., Pattenden G. Total synthesis of mollamide, a reverse prenyl substituted cytotoxic cyclic peptide from Didemnum molle. Tetrahedron Lett. 1999;40:9317–9320. doi: 10.1016/S0040-4039(99)01955-3. [DOI] [Google Scholar]
- 122.Dellai A., Maricic I., Kumar V., Arutyunyan S., Bouraoui A., Nefzi A. Parallel synthesis and anti-inflammatory activity of cyclic peptides cyclosquamosin D and Met-cherimolacyclopeptide B and their analogs. Bioorg. Med. Chem. Lett. 2010;20:5653–5657. doi: 10.1016/j.bmcl.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fairweather K.A., Sayyadi N., Roussakis C., Jolliffi K.A. Synthesis of the cyclic heptapeptide axinellin A. Tetrahedron. 2010;66:935–939. doi: 10.1016/j.tet.2009.11.090. [DOI] [Google Scholar]
- 124.Napolitano A., Bruno I., Riccio R., Gomez-Paloma L. Synthesis, structure, and biological aspects of cyclopeptides related to marine phakellistatins 7–9. Tetrahedron. 2005;61:6808–6815. doi: 10.1016/j.tet.2005.04.067. [DOI] [Google Scholar]
- 125.Ali L., Musharraf S.G., Shaheen F. Solid-phase total synthesis of cyclic decapeptide phakellistatin 12. J. Nat. Prod. 2008;71:1059–1062. doi: 10.1021/np070648q. [DOI] [PubMed] [Google Scholar]
- 126.Sleebs M.M., Scanlon D., Karas J., Maharani R., Hughes A.B. Total synthesis of the antifungal depsipeptide petriellin A. J. Org. Chem. 2011;76:6686–6693. doi: 10.1021/jo201017w. [DOI] [PubMed] [Google Scholar]
- 127.Napolitano A., Bruno I., Rovero P., Lucas R., Peris M.P., Gomez-Paloma L., Riccio R. Synthesis, structural aspects and bioactivity of the marine cyclopeptide hymenamide C. Tetrahedron. 2001;57:6249–6255. doi: 10.1016/S0040-4020(01)00579-8. [DOI] [Google Scholar]
- 128.Garcia-Barrantes P.M., Lindsley C.W. Total synthesis of gombamide A. Org. Lett. 2016;18:3810–3813. doi: 10.1021/acs.orglett.6b01825. [DOI] [PubMed] [Google Scholar]
- 129.Sellanes D., Manta E., Serra G. Toward the total synthesis of scleritodermin A: Preparation of the C1–N15 fragment. Tetrahedron Lett. 2007;48:1827–1830. doi: 10.1016/j.tetlet.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dahiya R., Pathak D. First total synthesis and biological evaluation of halolitoralin A. J. Serb. Chem. Soc. 2007;72:101–107. doi: 10.2298/JSC0702101D. [DOI] [Google Scholar]
- 131.Dahiya R., Maheshwari M., Kumar A. Toward the synthesis and biological evaluation of hirsutide. Monatsh. Chem. 2009;140:121–127. doi: 10.1007/s00706-008-0052-z. [DOI] [Google Scholar]
- 132.Huang T., Zou Y., Wu M.C., Zhao Q.J., Hu H.G. Total synthesis of proline-rich cyclic octapeptide stylissamide X. Chem. Nat. Prod. 2015;51:523–526. doi: 10.1007/s10600-015-1329-1. [DOI] [Google Scholar]
- 133.Santhakumar G., Payne R.J. Total synthesis of polydiscamides B, C, and D via a convergent native chemical ligation-oxidation strategy. Org. Lett. 2014;16:4500–4503. doi: 10.1021/ol502045u. [DOI] [PubMed] [Google Scholar]
- 134.Pettit G.R., Holman J.W., Boland G.M. Synthesis of the cyclic heptapeptides axinastatin 2 and axinastatin 3. J. Chem. Soc. Perkin Trans. 1. 1996:2411–2416. doi: 10.1039/p19960002411. [DOI] [Google Scholar]
- 135.Dahiya R., Pathak D. Cyclic peptides: New hope for antifungal therapy. Egypt. Pharm. J. (NRC) 2006;5:189–199. [Google Scholar]
- 136.Pathak D., Dahiya R. Cyclic peptides as novel antineoplastic agents: A review. J. Sci. Pharm. 2003;4:125–131. [Google Scholar]
- 137.Pettit G.R., Xu J.P., Dorsaz A.C., Williams M.D., Boyd M.R., Cerny R.L. Isolation and structure of the human cancer cell growth inhibitory cyclic decapeptides phakellistatins 7, 8 and 9. Bioorg. Med. Chem. Lett. 1995;5:1339–1344. doi: 10.1016/0960-894X(95)00219-J. [DOI] [Google Scholar]
- 138.Pettit G.R., Tan R. Antineoplastic agents 390. Isolation and structure of phakellistatin 12 from a Chuuk Archipelago marine sponge. Bioorg. Med. Chem. Lett. 2003;13:685–688. doi: 10.1016/S0960-894X(02)01054-5. [DOI] [PubMed] [Google Scholar]
- 139.Li L.H., Timmins L.G., Wallace T.L., Krueger W.C., Prairie M.D., Im W.B. Mechanism of action of didemnin B, a depsipeptide from the sea. Cancer Lett. 1984;23:279–288. doi: 10.1016/0304-3835(84)90095-8. [DOI] [PubMed] [Google Scholar]
- 140.Zheng L.H., Wang Y.J., Sheng J., Wang F., Zheng Y., Lin X.K., Sun M. Antitumor peptides from marine organisms. Mar. Drugs. 2011;9:1840–1859. doi: 10.3390/md9101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Siemion I.Z., Cebrat M., Wieczorek Z. Cyclolinopeptides and their analogs—A new family of peptide immunosuppressants affecting the calcineurin system. Arch. Immunol. Ther. Exp. 1999;47:143–153. [PubMed] [Google Scholar]
- 142.Malaker A., Ahmad S.A.I. Therapeutic potency of anticancer peptides derived from marine organism. Int. J. Eng. Appl. Sci. 2013;2:53–65. [Google Scholar]
- 143.Simmons T.L., Andrianasolo E., McPhail K., Flatt P., Gerwick W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005;4:333–342. [PubMed] [Google Scholar]
- 144.Proksch P., Ebel R., Edrada R.A., Wray V., Steube K. Bioactive natural products from marine invertebrates and associated fungi. Prog. Mol. Subcell. Biol. 2003;37:117–142. doi: 10.1007/978-3-642-55519-0_5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.