Abstract
In order to find new natural products with anti-inflammatory activity, chemical investigation of a 3000-meter deep-sea sediment derived bacteria Bacillus subtilis B5 was carried out. A new macrolactin derivative was isolated and identified as 7,13-epoxyl-macrolactin A (1). Owing to the existence of the epoxy ring, 1 exhibited a significant inhibitory effect on the expression of inducible nitric oxide and cytokines, compared with previously isolated known macrolactins (2–5). Real-time Polymerase Chain Reaction (PCR) analysis showed that the new compound significantly inhibited the mRNA expressions of inducible nitric oxide synthase (iNOS), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Reverse transcription-PCR analysis demonstrated that the new compound reduced the mRNA expression level of IL-1β in a concentration-dependent manner.
Keywords: Bacillus subtilis B5; 7,13-epoxyl-macrolactin A; macrolactins; anti-inflammatory
1. Introduction
The process of inflammation is the result of immune system activation which coordinates the normal defense mechanism of our body in response to microbial infection. Uncontrolled inflammation is believed to play crucial roles in the pathogenesis of various diseases, such as cardiovascular diseases [1], inflammatory bowel disease [2], cancer [3], diabetes [4], asthma [5], and Alzheimer’s disease [6]. During the inflammatory process, biochemical parameters, such as expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and 5-lipoxygenase [7,8], and levels of tumor necrosis factor-α (TNF-α), interferon γ (IFN-γ), interleukin-1 (IL-1), and interleukin-6 [9,10] are overexpressed during inflammation. Thus, inhibition of the production of these inflammatory mediators is an important target in the treatment of inflammatory diseases [11].
Several types of drugs are used to treat inflammatory disorders, such as biological, steroidal, and nonsteroidal anti-inflammatory drugs. However, they cause adverse side effects, and biological treatment is expensive. Natural products are alternatives to these drugs and offer hope for discovering bioactive lead compounds that may be developed into drugs for treatment of inflammatory disorders [12]. Plenty of unique marine natural products and their derivatives, such as sesquiterpenoid, polysaccharide, steroid/sterol and alkaloid, are found to manifest an anti-inflammatory action [13,14,15,16,17,18].
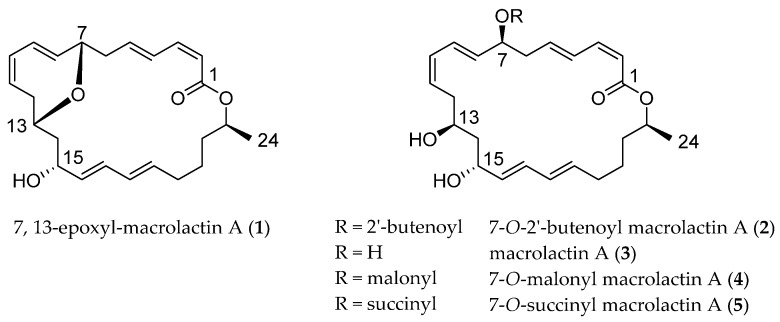
Macrolactins, an important 24-membered macrolactones, are mainly produced by Bacillus [19,20,21,22]. In our previous study [19], a new macrolactin (2) and three known ones (3–5), were isolated from Bacillus subtilis B5, a bacteria derived from the 3000-meter deep-sea sediment of the Pacific Ocean. 7-O-2′E-Butenoyl macrolactin A (2) exhibited antifungal activity against tea pathogenic fungi Pestalotiopsis theae and Colletotrichum gloeosporioides. Continuous investigations on the chemical ingredients of B. subtilis B5 led to the isolation of another new macrolactin, 7,13-epoxyl-macrolactin A (1) (Figure 1). Studies on the anti-inflammatory activity of these macrolactins revealed that the new compound (1) exhibited potent activity, owing to the existence of an epoxy ring. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfonyl)-2H-tetrazolium (MTS) assay demonstrated that these macrolactins did not exhibit obvious cytotoxic effect at the employed concentrations (1–40 μM) on RAW 264.7 cells. Compound 1 significantly inhibited the mRNA expressions of iNOS, IL-1β, and IL-6, while other known macrolactins showed fewer inhibitory effects in LPS-stimulated RAW 264.7 macrophages. Additionally, reverse transcription-PCR analysis demonstrated that the new compound inhibited the mRNA expression level of IL-1β in a concentration-dependent manner.
Figure 1.
Structures of compounds 1–5 isolated from an extract of Bacillus subtilis B5.
2. Results
2.1. Structural Identification of 7,13-Epoxyl-macrolactin A (1)
Compound 1 (7,13-epoxyl-macrolactin A) was isolated as a yellow amorphous powder. The molecular formula of C24H32O4, which gave 9 unsaturation degrees, was established by the HR-ESI-MS ion peak at m/z 407.2190 [M + Na] +. The IR spectrum showed the presence of OH groups (3464 cm−1), olefinic protons (1450 cm−1) and carbonyls (1664 cm−1). The UV maximum absorption wave length at λmax (log ε): 233 (3.88) nm indicating the presence of conjugated carbonyls. The 1H and 13C NMR spectra, including DEPT, clearly showed two carbonyl carbons and 12 olefinic methines belonging to 6 ethylenic bonds, in the sp2 low field region. The sp3 high field region showed the existence of a methyl, four oxygenated methines, and six methylenes.
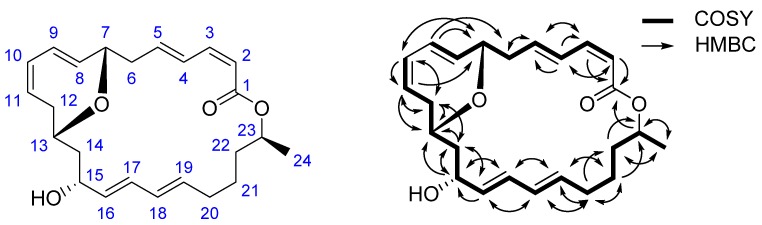
Most of the 1D NMR spectral data of 1 approach to those of macrolactin A (3), a typical macrolactin isolated from culture of a deep-sea marine bacterium [23], indicating the presence of a macrolactin nucleus in 1. The molecular formula of 1, when compared with that of 3, found that an H2O unit was lost, suggesting the existence of an epoxy moiety. The 13C NMR signals assigned to C-8 and C-12 of macrolactin A were up-field shifted from δ 137.9 to δ 130.6 and from δ 36.0 to δ 31.3 respectively in 1, indicating that the epoxy moiety was formed by the condensation between 7-OH and 13-OH. The chemical shifting effect was also observed in 1H NMR spectra, in which the H-7 signal of macrolactin A was down-field shifted from δ 4.19 to δ 4.56 in 1, while 7-OH and 13-OH signal at δ 5.05 and δ 4.60 of macrolactin A were missing. Comprehensive 1H–1H COSY and HMBC analysis allowed the complete assignment of the proton and carbon signals for 1 (Table 1 and Figure 2). As a result, the structure of 1 was elucidated as 7,13-epoxyl-macrolactin A. It was a newly isolated compound produced by deep-sea sediment of the Pacific Ocean. Many isolated macrolactins including epoxyl-macrolactin that have been isolated [19,20,21,22] and all macrolactins contain three separate diene structure elements.
Table 1.
1H, 13C NMR data and 1H–1H COSY, HMBC correlations of compound 1.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC | |
|---|---|---|---|---|---|
| 1 | 166.0 | C | |||
| 2 | 5.59 br.d (11.4) | 117.8 | CH | H-3 | C-1,4 |
| 3 | 6.69 br.t (11.4) | 143.4 | CH | H-2,4 | C-1,5 |
| 4 | 7.11 br.dd (14.7, 11.7) | 128.1 | CH | H-3,5 | C-2,3,5 |
| 5 | 6.17 dt (14.9, 7.4) | 142.1 | CH | H-4,6 | C-3,6 |
| 6 | 2.91 m | 36.0 | CH2 | H-5 | C-5 |
| 7 | 4.56 br.s | 72.2 | CH | H-8 | n. o. a |
| 8 | 5.48 overlapped | 130.6 | CH | H-7,9 | C-6 |
| 9 | 5.54 m | 129.7 | CH | H-8 | C-5,6,7 |
| 10 | 5.71 br.d (10.5) | 128.4 | CH | H-11 | C-7 |
| 11 | 5.81 m | 125.2 | CH | H-10,12 | C-12 |
| 12 | 1.98 m and 1.87 m | 31.3 | CH2 | H-11,13 | C-8,10,13,14 |
| 13 | 3.50 overlapped | 65.0 | CH | H-12,14 | n. o. |
| 14 | 1.68 m and 1.61 m | 44.1 | CH2 | H-13,15 | C-15,16 |
| 15 | 3.93 br.s | 69.4 | CH | H-14,16 | C-14 |
| 16 | 5.48 overlapped | 135.3 | CH | H-15,17 | C-14,15 |
| 17 | 6.06 br.dd (14.9, 10.5) | 130.1 | CH | H-16,18 | C-15 |
| 18 | 5.97 br.dd (14.9, 10.6) | 130.3 | CH | H-17,19 | C-16,20 |
| 19 | 5.67 m | 133.9 | CH | H-18,20 | C-20,21 |
| 20 | 2.13 m and 2.00 m | 32.1 | CH2 | H-19,21 | C-19,21,22 |
| 21 | 1.44 m | 25.4 | CH2 | H-20,22 | C-19,20,22 |
| 22 | 1.59 m | 35.3 | CH2 | H-21,23 | C-20,21,24 |
| 23 | 4.96 m | 70.8 | CH | H-22,24 | C-1,21,22,24 |
| 24 | 1.22 d (6.2) | 20.4 | CH3 | H-23 | C-22,23 |
| 15-OH | 4.65 br.s | ||||
a n. o. is not observed.
Figure 2.
Structure and key 1H–1H COSY, HMBC correlations of 1.
2.2. Anti-Inflamatory Activity of Macrolactins
2.2.1. Cytotoxicity
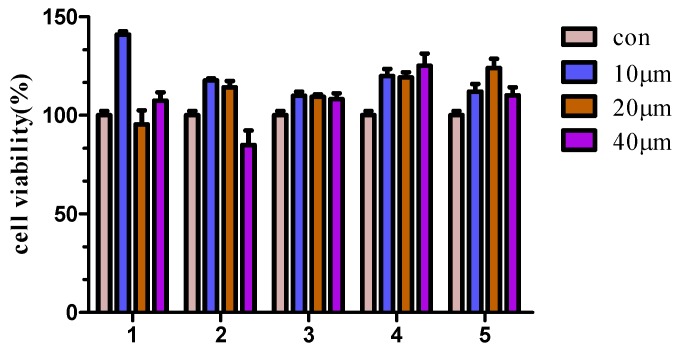
Cytotoxicity of compounds 1–5 in RAW 264.7 cells were tested by using the MTS assay. The result showed that compounds 1–5 did not exhibit obvious cytotoxic effect at the employed concentrations (10–40 μM) (Figure 3). Interestingly, Compound 1 can promote cell proliferation under the low concentration 10 μM. This may be because, in this case, it activates the cell survival. The detailed mechanism still needs further research.
Figure 3.
Compounds cytotoxicity at different concentrations on the viability of RAW 264.7 cells. Cells (1 × 104 cells/well) plated on 96-well plates were treated with compounds 1–5 at concentrations of 10–40 μM at 37 °C for 24 h. Cytotoxicity was assessed by MTS assay. Values are expressed as mean ± SD, n = 5.
2.2.2. Inhibitory Effect of Compounds on LPS-Induced iNOS, IL-1β and IL-6 mRNA Expression
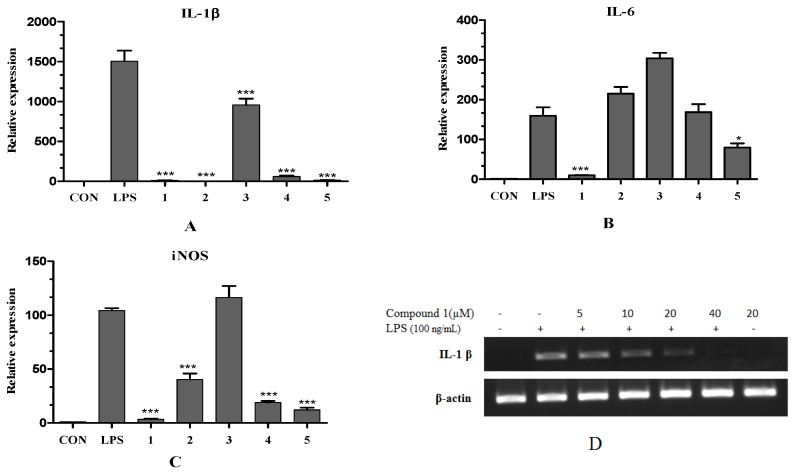
LPS can evoke innate immune response by stimulating the expression of several factors such as nitric oxide (NO) and pro-inflammatory cytokines, known to be involved in the immune response in macrophages. Compared with controls, upon LPS stimulation, macrophages strongly expressed the mRNA of iNOS, IL-1β and IL-6. Herein, compounds 1–5 were tested for in vitro anti-inflammatory activity and were found to suppress the mRNA expressions of iNOS, IL-1β and IL-6 in LPS-stimulated RAW 264.7 macrophages.
As shown in Figure 4A–C, pretreatment of LPS activated cells with compounds 1 and 5 resulted in significant reduction of the mRNA expression of IL-1β, IL-6 and iNOS. Compounds 2 and 4 reduced the production of IL-1β and iNOS but had little effect on the expression level of IL-6. Compound 3 only slightly reduced the mRNA expression of IL-1β.
Figure 4.
Effects of compounds 1–5 on the mRNA expression of LPS-induced interleukin-1β (IL-1β) (A), interleukin-6 (IL-6) (B) and nitric oxide synthase (iNOS) (C) of RAW 264.7 cells at the concentration of 40 μM by Real-Time Polymerase Chain Reaction Analysis. (D) Effects of 1 on IL-1β mRNA expressions at concentrations of 5–40 μM by Reverse Transcription-Polymerase Chain Reaction Analysis. The β-actin was used as a loading control. The cells in a six-well were pretreated with compounds for 1.5 h and then stimulated with LPS (100 ng/mL) for another 12 h. LPS-induced mRNA expression of iNOS and IL-6 was reduced by 96.6% and 93.8%, respectively, when cells were cotreated with compound 1. Total RNAs were isolated from cells and analyzed. The p values were assessed by Student’s t test. A p value less than 0.05 was considered statistically significant (* p < 0.05; *** p < 0.001). (Data represent the mean ± SD of different experiments.
Based on the above results, we further investigated the effects of 1 on IL-1β mRNA expressions in LPS-stimulated RAW 264.7 macrophages by Reverse Transcription-PCR analysis. As shown in Figure 4D, compound 1 inhibited IL-1β mRNA expression in a concentration-dependent manner, while the house-keeping β-actin mRNA expression was unchanged by compound 1 under the same condition.
3. Materials and Methods
3.1. General Experimental Procedures
The HR-ESI-MS analysis was performed on the Thermo Q-Exactive Orbitrap Mass spectrometer (Thermo Fisher Scientific Corporation, Waltham, MA, USA) equipped with electrospray ionization source (ESI). The preparative HPLC was performed with Varian binary gradient LC system (Varian Inc. Corporate, Santa Clara, CA, USA) containing two solvent deliver modules (PrepStar 218), a photodiode array detector (ProStar 335) and a fraction collector (ProStar 704), using an the preparative Cosmosil ODS column (250 mm × 20.0 mm i.d., 5 μm, Cosmosil, Nakalai Tesque Co. Ltd., Kyoto, Japan). UV spectra were recorded on a Shimadzu UV-260 spectrometer (Shimadzu Corporation, Tokyo, Japan). IR spectra were determined on a Perkin-Elmer 683 infrared spectrometer (PerkinElmer, Inc., Waltham, MA, USA) in KBr pellets. Optical rotations were measured using a JASCO P-200 polarimeter (JASCO Corporation, Tokyo, Japan) with a 5-cm cell. The NMR spectra were taken with TMS as the internal standard on a Brucker Avance III 600 FT NMR spectrometer (Bruker Corporation, Billerica, MA, USA). Column chromatography was performed on silica gel (Yantai Chemical Industry Research Institute, Yantai, China) and Cosmosil 75 C18-OPN (75 μm, Nakalai Tesque Co. Ltd., Kyoto, Japan).
3.2. Bacteria and Fermentation
The bacterial strain B5 was isolated from deep-sea sediments which were collected at water depths of 3000 m Pacific Ocean by the Third Institute of Oceanography of China, and was identified as Bacillus subtilis B5 by complete 16S rRNA gene sequence. This bacterium was cultivated on 160 L scale using 250 mL shake flasks containing 100 mL of the seed medium (tryptone 1%, yeast extract 0.5%, NaCl 1%, pH 7.4) cultured at 37 °C for 18 h at 200 rpm. One percent of the seed culture was inoculated into 100 L fermentors containing 80 L of fermentation medium consisting of soluble starch 0.5%, yeast extract 1%, K2HPO4 1%, NaNO3 0.5%, pH 6.8–7.0 for 2 days at the same condition mentioned above, and fermentations were conducted twice.
3.3. Extraction and Isolation
HPD-100 macroporous adsorption resin (Cangzhou Bonchem Co., Ltd., Changzhou, China) was used to deal with the fermentation broth (160 L). After being eluted with water, the 95% ethanol eluents were collected. After removal of ethanol under vacuum, the water suspension (1.5 L) was extracted with ethyl acetate (v/v 1:1) three times. The organic phase was evaporated under reduced pressure to afford a residue (76 g). The residue was divided into 11 fractions (Fr. 1~11) over silica gel column eluted with petroleum ether-ethyl acetate (v/v) (50:1; 20:1; 10:1; 5:1; 2:1; 1:1) and chloroform-methyl alcohol (v/v) (50:1; 20:1; 10:1; 5;1; 2:1). Fr. 5 (1.2 g) was subjected to ODS chromatography and eluted with MeOH–H2O (30%–100%) to give a subfraction Fr. 5.6. Then Fr. 5.6 was purified by preparative reversed-phase HPLC using a C18 column and isocratic eluted with acetonitrile-H2O (62:38) to obtain compound 1 (9 mg).
7,13-epoxyl-macrolactin A (1): Amorphous white powder; mp: 92–96 °C; −34° (c = 0.1, MeOH), IR (KBr) (νmax): 3465, 3268, 1664, 1450 cm−1. UV (MeOH) λmax (log ε): 233 (3.88) nm. 13C NMR (125 MHz, DMSO-d6) and 1H NMR (600 MHz, DMSO-d6) spectral data were listed in Table 1; HR-ESI-MS: m/z 407.2190 (calcd. for C16H11N2O3BrNa, 407.2193).
3.4. Cell Cultivation
The murine macrophage cell line RAW 264.7 was purchased from ATCC (Rockville, MD, USA). RAW 264.7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, UT, USA) supplemented with 1% penicillin-streptomycin (100 U/mL penicillin and 100 μg/mL streptomycin in 0.85% NaCl) and 10% of fetal bovine serum (FBS, Hyclone, Logan, UT, USA). The cells were incubated in a humidified atmosphere of 5% CO2 at 37 °C.
3.5. Cytotoxicity Assay
The cytotoxicity of compounds 1–5 were assessed by CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI, USA). RAW 264.7 cells (1 × 104 cells/well) plated on 96-well plates were treated with different concentrations of samples (1–40 μM in DMSO) for 24 at 37 °C in 5% CO2. With a pipet, we put 20 μL of CellTiter 96® AQueous One Solution Reagent (MTS) into each well of the 96-well assay plate containing the samples in 100 μL of culture medium. Then, the plate was incubated at 37 °C for 3 h in a humidified, 5% CO2 atmosphere and then the absorbance was recorded at 490 nm with a 96-well plate Microplate reader MK3 (Thermo Fisher Scientific Corporation, Waltham, MA, USA). The optical density of formazan formed in control cells (without treatment with samples) was taken as 100% viability.
3.6. Total RNA Isolation
RAW 264.7 cells were plated onto 6-well plates at a density of 1 × 106 cells/well and incubated with compounds at the indicated concentration for 1.5 h prior to LPS (100 ng/mL) stimulation. After 12 h, cells were lysed and total RNA extraction was performed by using Trizol reagent (Life Technologies, Carlsbad, MA, USA). Cells were homogenized in 200 μL of Trizol reagent, and then samples were left to rest at room temperature for 5 min. After that, 40 μL of chloroform was added and the tubes were vigorously shaken for 15 s and allowed to rest at room temperature for 5 min. Tubes were then centrifuged at 12,000× g (Eppendorf Centrifuge 5424 R, Eppendorf Instruments, Hamburg, Germany) 4 °C for 15 min. The aqueous phase was transferred to a new tube. Isopropyl alcohol (100 μL) was added to the aqueous phase: the tube was then gently mixed and incubated at room temperature for 10 min. After incubation, samples were centrifuged at 12,000× g, 4 °C for 10 min. The supernatant was poured out and the pellet was washed by 200 μL of 75% ethanol and centrifuged at 12,000× g, 4 °C for 5 min. The washing step and centrifuge were repeated. The final supernatant was removed and the pellet was dried until it was colorless. Total RNA was then dissolved in 20 μL of DEPC H2O, incubated at 65 °C for 5 min, and stored at −80 °C until used.
3.7. Real-Time PCR Analysis of iNOS, IL-1β and IL-6 mRNA
The inhibitory effect of compounds 1–5 on the mRNA expression of LPS-induced IL-1β, IL-6 and iNOS of RAW 264.7 cells at the concentration of 40 μM in DMSO was analyzed by Real-Time PCR. The expression of mRNA transcripts of iNOS (forward: 5′-GTTCTCAGCCCAACAATACAAGA-3′, reverse: 5′-GTGGACGGGTCGATGTCAC-3′), IL-1β (forward: 5′-GAAATGCCACCTTTTGACAGTG-3′, reverse: 5′-TGGATGCTCTCATCAGGACAG-3′), IL-6 (forward: 5′-CCAGAGATACAAAGAAATGATGG-3′, reverse: 5′-ACTCCAGAAGACCAGAGGAAAT-3′) and β-actin (forward: 5′-GGCTGTATTCCCCTCCATCG-3′, reverse: 5′-CCAGTTGGTAACAATGCCATGT-3′) was determined by real-time RT-PCR. The cDNA was synthesized from total RNA using oligo (dT)18 primers. FastStart Universal SYBR Green Master (Rox, Roche, Basel, Switzerland) and Stepone Real-Time PCR Detection System (Applied Biosystems, Foster, CA, USA) were used for Real-time PCR analysis. Levels of all mRNAs were normalized to that of GAPDH mRNA.
3.8. Reverse Transcription-PCR Analysis of IL-1β mRNA
The inhibitory effect of compound 1 at the indicated concentrations on IL-1β mRNA expressions was determined by Reverse Transcription-PCR. Primers used were as follows: IL-1β (forward: 5′-GGGCCTCAAAGGAAAGAATC-3′, reverse: 5′-TACCAGTTGGGGAACTCTGC-3′), β-actin (forward: 5′-CCACAGCTGAGAGGGAAATC-3′, reverse: 5′-AAGGAAGGCTGGAAAAGAGC-3′). The total RNA was converted to cDNA and analyzed. PCR amplifications were performed on an Eppendorf traditional PCR machine (Eppendorf Instruments, Hamburg, Germany). The cDNA was amplified with 30 cycles of 95 °C for 45 s, 60 °C for 15 s, and 72 °C for 45 s. After amplification, the PCR products were separated on 1.5% agarose gel for 30 min at 100 V.
4. Conclusions
A new macrolactins derivatives 7,13-epoxyl-macrolactin A (1), was isolated from a marine-derived bacterial strain Bacillus subtilis B5. Evaluation of its anti-inflammatory effect indicated that the new compound inhibited the expression of proinflammatory cytokines IL-1β, IL-6 and iNOS induced by LPS in RAW 264.7 macrophages, similar to the effect of 7-O-succinyl macrolactin A (5). 7-O-2′E-butenoyl macrolactin A (2) and 7-O-malonyl macrolactin A (4) reduced the production of IL-1β and iNOS but had no effect the level of IL-6. Macrolactin A (3) only slightly reduced the production of IL-1β. Additionally, 1 inhibited IL-1β mRNA expressions in in a concentration-dependent manner. Based on the anti-inflammatory effects of compounds 1–5 in LPS-stimulated RAW 264.7 macrophages, it could be suggested that different substituents at the position 7 might be key structural features for the anti-inflammatory activity. The presence of the epoxy moiety was special in the structure of the new compound and might greatly affect the anti-inflammatory activity.
Acknowledgments
The project was supported by the International S & T Cooperation Program of China (No. 2015DFA20500), National Basic Research Program of China (973 Program) (No. 2015CB755901). The authors would also like to acknowledge the financial supports from National Natural Science Foundation of China (No. 81273400) and Xiamen Ocean Research and Development Institute project. The authors would also like to acknowledge the financial supports from Fujian Key Science and Technology Program (No. 2013N0019), Natural Science Foundation of Fujian Province (No. 2013J05121), Xiamen Science and Technology Program (No. 3502Z20142009), National Natural Science Foundation of China (No. 81301705), Xiamen Oceanic Administration (No. 14PYY051SF04), and Xiamen South Marine Center project (13GZP002NF08).
Author Contributions
X.-X.T. and Z.-W.Y. supplied and identified the Bacteria. Y.-X.Z. performed the anti-inflammatory activity assay. Y.-K.Q. and F.-Q.J. supervised the project. X.Y. and X.-X.L. isolated the compounds. M.-J.F. and Z.W. was responsible for structural elucidation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Libby P., Ridker P.M., Hansson G.K. Inflammation in atherosclerosisfrom pathophysiology to practice. J. Am. Coll. Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto R., Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J. Gastroenterol. 2016;51:11–21. doi: 10.1007/s00535-015-1098-4. [DOI] [PubMed] [Google Scholar]
- 3.Crusz S.M., Balkwill F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 4.Pickup J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 5.Kurakula K., Vos M., Logiantara A., Roelofs J.J., Nieuwenhuis M.A., Koppelman G.H., Postma D.S., van Rijt L.S., de Vries C.J.M. Nuclear receptor Nur77 attenuates airway inflammation in mice by suppressing NF-κB activity in lung epithelial cells. J. Immunol. 2015;195:1388–1398. doi: 10.4049/jimmunol.1401714. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S., Banerjee S., Sil P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Jean Y.-H., Chen W.-F., Duh C.-Y., Huang S.-Y., Hsu C.-H., Lin C.-S., Sung C.-S., Chen I.M., Wen Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from formosan soft coral lemnalia cervicorni. Eur. J. Pharmacol. 2008;578:323–331. doi: 10.1016/j.ejphar.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Yan W.-X., Zhang J.-H., Zhang Y., Meng D.-L., Yan D. Anti-inflammatory activity studies on the stems and roots of Jasminum lanceolarium Roxb. J. Ethnopharmacol. 2015;171:335–341. doi: 10.1016/j.jep.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 9.Deng J.-S., Huang S.-S., Lin T.-H., Lee M.-M., Kuo C.-C., Sung P.-J., Hou W.-C., Huang G.-J., Kuo Y.-H. Analgesic and anti-inflammatory bioactivities of eburicoic acid and dehydroeburicoic acid isolated from antrodia camphorata on the inflammatory mediator expression in mice. J. Agric. Food Chem. 2013;61:5064–5071. doi: 10.1021/jf303820k. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Y.Y., Zhu J.X., Bian T., Gao F., Qian X.F., Du Q., Yuan M.Y., Sun H., Shi L.Z., Yu M.H. Protective effects of astragaloside IV against ovalbumin-induced lung inflammation are regulated/mediated by T-bet/GATA-3. Pharmacology. 2014;94:51–59. doi: 10.1159/000362843. [DOI] [PubMed] [Google Scholar]
- 11.Yoon W.-J., Heo S.-J., Han S.-C., Lee H.-J., Kang G.-J., Kang H.-K., Hyun J.-W., Koh Y.-S., Yoo E.-S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharm. Res. 2012;35:1421–1430. doi: 10.1007/s12272-012-0812-5. [DOI] [PubMed] [Google Scholar]
- 12.Gautam R., Jachak S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009;29:767–820. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- 13.Cuong N.X., Thao N.P., Luyen B.T.T., Ngan N.T.T., Thuy D.T.T., Song S.B., Nam N.H., Kiem P.V., Kim Y.H., Minh C.V. Cembranoid diterpenes from the soft coral Lobophytum crassum and their anti-inflammatory activities. Chem. Pharm. Bull. 2014;62:203–208. doi: 10.1248/cpb.c13-00805. [DOI] [PubMed] [Google Scholar]
- 14.Kim K.-S., Cui X., Lee D.-S., Sohn J., Yim J., Kim Y.-C., Oh H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-κB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules. 2013;18:13245–13259. doi: 10.3390/molecules181113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.-P., Huang S.-Y., Lin Y.-Y., Wang H.-M., Jean Y.-H., Wu S.-F., Duh C.-Y., Wen Z.-H. Soft coral-derived lemnalol alleviates monosodium urate-induced gouty arthritis in rats by inhibiting leukocyte infiltration and iNOS, COX-2 and c-Fos protein expression. Mar. Drugs. 2013;11:99–113. doi: 10.3390/md11010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira J.G., Mesquita J.X., Aragão K.S., Franco Á.X., Souza M.H.L.P., Brito T.V., Dias J.M., Silva R.O., Medeiros J.-V.R., Oliveira J.S., et al. Polysaccharides isolated from Digenea simplex inhibit inflammatory and nociceptive responses. Carbohydr. Polym. 2014;108:17–25. doi: 10.1016/j.carbpol.2014.01.105. [DOI] [PubMed] [Google Scholar]
- 17.Robertson R., Guihéneuf F., Bahar B., Schmid M., Stengel D., Fitzgerald G., Ross R., Stanton C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs. 2015;13:5402–5424. doi: 10.3390/md13085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin H., Pil G., Heo S.-J., Lee H.-S., Lee J., Lee Y.-J., Lee J., Won H. Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar. Drugs. 2016;14:14. doi: 10.3390/md14010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W., Tang X.-X., Yan X., Wu Z., Yi Z.-W., Fang M.-J., Su X., Qiu Y.-K. A new macrolactin antibiotic from deep sea-derived bacteria Bacillus subtilis B5. Nat. Prod. Res. 2016:1–6. doi: 10.1080/14786419.2016.1155576. [DOI] [PubMed] [Google Scholar]
- 20.Lu X.-L., Xu Q.-Z., Liu X.-Y., Cao X., Ni K.-Y., Jiao B.-H. Marine drugs—Macrolactins. Chem. Biodivers. 2008;5:1669–1674. doi: 10.1002/cbdv.200890155. [DOI] [PubMed] [Google Scholar]
- 21.Mondol M.A.M., Tareq F.S., Kim J.H., Lee M.A., Lee H.-S., Lee Y.-J., Lee J.S., Shin H.J. Cyclic ether-containing macrolactins, antimicrobial 24-membered isomeric macrolactones from a marine Bacillus sp. J. Nat. Prod. 2011;74:2582–2587. doi: 10.1021/np200487k. [DOI] [PubMed] [Google Scholar]
- 22.Tareq F.S., Kim J.H., Lee M.A., Lee H.-S., Lee J.-S., Lee Y.-J., Shin H.J. Antimicrobial gageomacrolactins characterized from the fermentation of the marine-derived bacterium Bacillus subtilis under optimum growth conditions. J. Agric. Food Chem. 2013;61:3428–3434. doi: 10.1021/jf4009229. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson K., Roman M., Fenical W. The macrolactins, a novel class of antiviral and cytotoxic macrolides from a deep-sea marine bacterium. J. Am. Chem. Soc. 1989;111:7519–7524. doi: 10.1021/ja00201a036. [DOI] [Google Scholar]