Abstract
Background
Ischaemic mitral regurgitation (IMR) is a detrimental complication of ST elevation myocardial infarction (STEMI).
Objective
We sought to determine patient characteristics and outcomes of patients with IMR with focus on anterior or inferior location of STEMI.
Methods
All patients presenting with STEMI complicated by IMR to our centre who underwent primary percutaneous coronary intervention within the first 12 hours of presentation from 1995 to 2014 were included. IMR was graded from 1+ to 4+ within 3 days of index myocardial infarction by echocardiography, divided into 2 groups based on infarct location and outcomes were compared.
Results
Overall, 805 patients were included. There were 302 (17.8%) patients with mitral regurgitation (MR) out of the 1700 patients with anterior STEMI while 503 (21.8%) had MR out of the 2305 patients with inferior STEMI. There was no significant difference between both groups in comorbidities, clinical presentation or door-to-balloon time (DBT; median 104 vs 106 min, p=0.5). 30-day and 1-year mortality were higher in anterior STEMI compared with inferior STEMI (14.9% vs 6.8% and 26.4% vs 14.3%, respectively, p<0.001 both), as well as 5-year mortality (39.7% vs 24.8%, p<0.01). When analysis was performed for each grade of IMR, anterior was associated with worse outcomes in every grade. On multivariate cox survival analysis, after adjustment for age, gender, comorbidities, grade of IMR, ejection fraction and DBT, anterior STEMI was still associated with worse outcomes (HR 1.62 (95% CI 1.23 to 2.12), p<0.001).
Conclusions
Although IMR occurs more frequently with inferior infarction, outcomes are worse following anterior infarction.
Key questions.
What is already known about this subject?
In patients with myocardial infarction, ischaemic mitral regurgitation (IMR) is associated with worse outcomes. However, most of the data about prognosis of IMR are from studies with thrombolytics as the main method of revascularisation, or late percutaneous coronary intervention (PCI). Further, none of the studies in the past compared prognosis of IMR based on infarction location.
What does this study add?
Although IMR is more severe and more common in inferior ST elevation myocardial infarction (STEMI), in an era of timed primary PCI within 12 hours of onset of chest pain, it is associated with worse prognosis with anterior STEMI compared with inferior STEMI after adjusting for all known variables.
How might this impact on clinical practice?
This will help with risk stratification of patients presenting with acute STEMI. It will also help in deciding trial designs in the future to adjust for such worse prognosis.
Introduction
Ischaemic mitral regurgitation (IMR) in the setting of acute myocardial infarction (MI) is a well-recognised clinical entity and its presence has been associated with worse clinical outcomes.1–8 Effects of IMR on outcomes have been evaluated in prior studies. However, the comparison was mainly between grades of severity of IMR. Patients in these studies were from a wide spectrum of presentations, including ST elevation myocardial infarction (STEMI), non-STEMI or all acute coronary syndromes. Time of evaluation for IMR was also variable ranging between few days after the infarction to few weeks. Furthermore, intervention was not uniform in most of studied patients, ranging from thrombolysis, late percutaneous coronary intervention (PCI), primary PCI (PPCI) to no intervention.1–27
On the other hand, it is also widely recognised that the mechanism producing IMR is different in anterior compared with inferior STEMI.28 IMR occurs with a higher incidence and is more severe in inferior STEMI despite greater left ventricular (LV) remodelling and global LV dysfunction in anterior STEMI. This is likely due to the increased tethering force of the posteromedial papillary muscle near the site of the infarction in inferior STEMI.29–32 In this study, we evaluate the incidence and impact of IMR based on index infarction location (anterior vs inferior) in an all-comers population of STEMI patients all treated with PPCI within 12 hours of presentation.
Methods
Patient population
In this observational study, we included all patients who presented to the Cleveland Clinic from January 1995 to December 2014 with acute STEMI complicated with IMR who underwent PPCI within the first 12 hours of presentation. We included patients with inferior STEMI, including inferolateral, inferoposterior and inferoposterolateral STEMI. We also included patient with anterior STEMI, including anteroseptal, anterolateral and strict anterior STEMI. ST elevation was defined as the presence of ≥1 mm ST segment elevation in two or more anatomical contiguous leads. All procedures and data collection were approved and monitored by the Cleveland Clinic Institutional Review Board (IRB) with waiver of individual informed consent.
Echocardiography data
The diagnosis of IMR was based on a comprehensive two-dimensional (2D) echocardiograms performed within 3 days of admission to the hospital with the MI. All images were reviewed and read by experienced cardiologists in the imaging section of our institution. LV ejection fraction was measured by modified Simpson's method, and severity of IMR was graded from 1+ to 4+ (mild, moderate, moderately severe and severe) summing all criteria of severity according to the American Society of Echocardiography guidelines.33 IMR was defined as new or worsening degree of mitral regurgitation (MR) compared with an echocardiogram prior to the MI. If there was no previous echocardiogram, the MR was considered ischaemic if the mechanism identified in the echocardiogram was ischaemic in nature. These mechanisms included restricted motion of a valve leaflet due to wall motion abnormality, papillary muscle dysfunction or papillary muscle rupture.
We divided the patients who had any degree of IMR at time of presentation into two groups—anterior and inferior STEMI. Baseline demographic characteristics, clinical presentation, procedural details, and short-term and long-term outcomes were compared. We then performed analysis for each grade of IMR between anterior and inferior STEMI.
Follow-up and outcomes
The date of the STEMI presentation and PPCI at our institution was defined as the beginning of the observational period for the patient. Follow-up was ascertained by chart review and we recorded the date at which events occurred. Mortality data were obtained from medical records or from US Social Security Death Index database (last inquiry in October 2015). Primary outcome was 30-day and 1-year all-cause mortality.
Statistical analysis
Continuous variables are expressed as mean±SD, or median and IQRs for skewed distributions, and compared using the Student's t-test or analysis of variance (for normally distributed variables) or the Mann-Whitney test (for non-normally distributed variables). Categorical data are expressed as percentage and compared using χ2 test or Fisher's exact test, as appropriate. To assess outcomes, multivariate Cox proportional hazards analysis was performed to assess independent predictors of outcome (using a p value cut-off of <0.05 for statistical significance). Univariable analysis was performed initially for available variables to test association with mortality and factors with p<0.2 were considered for the multivariate cox analysis. Clinical factors known to be associated with mortality or worse outcomes were included in the multivariate model regardless of the univariate analysis results. HRs with 95% CIs were calculated and reported. Cumulative proportion of events as a function over time was obtained by the Kaplan-Meier method to do the survival analysis. Statistical analysis was performed using JMP pro V.10.0.
Results
Baseline characteristics and clinical presentation
There were 302 (17.8%) patients with IMR out of the 1700 patients with anterior STEMI while 503 (21.8%) had IMR out of the 2305 patients with inferior STEMI. Baseline characteristics of the patients in the two groups are shown in table 1. There was no significant difference between both groups at baseline regarding risk factors and different comorbidities. Distribution of grades of MR was similar between both groups. Clinical presentation in the two groups is shown in table 1. There was no difference between the two groups regarding medications, baseline haemoglobin, peak CK level, peak Creatine Kinase Myocardial Band (CK-MB) level, functional status (New York Heart Association(NYHA) class) or clinical shock on presentation.
Table 1.
Baseline characteristics, clinical presentation and medications in patients IMR and anterior or inferior STEMI
| Variable | Anterior STEMI | Inferior STEMI | p Value |
|---|---|---|---|
| Number | 302 | 503 | <0.01 |
| Grades of MR | 1+57% 2+30.5% 3+11.3% 4+1.3% |
1+50.7% 2+33.4% 3+11.3% 4+4.6% |
0.5 |
| Age (year) | 66.6±13 | 65±12.6 | 0.8 |
| Sex (male, %) | 53.6% | 60.2% | 0.7 |
| Diabetes mellitus | 30.8% | 27.8% | 0.4 |
| Hypertension | 74.6% | 73.6% | 0.8 |
| Renal impairment | 10.6% | 7.6% | 0.2 |
| Atrial fibrillation | 21.5% | 25.8% | 0.2 |
| COPD | 9.5% | 11.3% | 0.5 |
| PAD | 9.6% | 10.2% | 0.8 |
| BMI (kg/m2) | 27.8±6 | 28.3±6 | 0.3 |
| Obese (BMI>30 kg/m2) | 28% | 30.1% | 0.5 |
| Stroke | 11.7% | 8.6% | 0.2 |
| Current smoker | 28.5% | 27.4% | 0.7 |
| Prior MI | 35.3% | 35.8% | 0.9 |
| History of CABG | 10.3% | 19.3% | 0.007* |
| Heart rate | 85±17 | 79±19 | 0.07 |
| Systolic blood pressure | 122±27 | 123±24 | 0.4 |
| NYHA | I 60.3% II 6.6% III 6.3% IV 26.8% |
I 62.2% II 2.8% III 5.7% IV 29.3% |
0.1 |
| Shock | 15.9% | 11.1% | 0.06 |
| IABP | 27.5% | 17.3% | 0.0009* |
| Number of diseased vessels | 1=56.5% 2=24.3% 3=19.3% |
1=60.7% 2=19% 3=20.4% |
0.2 |
| HB at baseline: median (IQR) | 13 (12–15) | 13.6 (11.7–14.8) | 0.9 |
| Anaemia (HB<12) | 25% | 27.7% | 0.5 |
| Peak of total CK: median (IQR) | 1319 (5 022 947) | 1417 (6 702 633) | 0.9 |
| Peak of CK-MB: median (IQR) | 136 (39 306) | 139 (57 281) | 0.9 |
| ACE inhibitor (before admission) | 12.3% | 16.8% | 0.1 |
| Aspirin (before admission) | 83.2% | 84.5% | 0.7 |
| β-blocker (before admission) | 27.1% | 29.7% | 0.5 |
*values have reached statistical significance.
BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; HB, haemoglobin; IABP, intra-aortic balloon pump; IMR, ischaemic mitral regurgitation; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association class; PAD, peripheral arterial disease; STEMI, ST elevation myocardial infarction.
Echocardiography data
Echocardiography parameters for the study population are presented in table 2. Patients with anterior STEMI had significantly lower ejection fraction compared with patients with inferior STEMI (36±14% vs 44±12%, p<0.001). There was no significant difference between the two groups regarding LV dimensions; however, left atrium was more dilated in the anterior STEMI group. There was no difference in right ventricular systolic pressure, or severity of tricuspid regurgitation (TR). Majority of the patients in anterior STEMI had a centrally directed MR jet followed by anteriorly, while in inferior STEMI majority had posteriorly directed jet followed by centrally.
Table 2.
Echocardiographic characteristics of study population
| Variable | Anterior STEMI | Inferior STEMI | p Value |
|---|---|---|---|
| Ejection fraction (%) | 36.3±14 | 44.6±12.3 | <0.01 |
| End diastolic volume (mL) | 154±27 | 150±22 | 0.1 |
| End systolic volume (mL) | 87±12.2 | 82±15.5 | 0.01 |
| LVID diastolic (cm) | 4.7±0.8 | 4.7±0.7 | 0.4 |
| LVID systolic (cm) | 3.1±0.9 | 3.3±0.8 | 0.08 |
| LA diameter (cm) | 4.5±0.5 | 3.8±0.7 | 0.03 |
| Direction of jet | |||
| Posteriorly Anteriorly Central |
3.4 7.8 88.8 |
70.9 2.2 26.9 |
<0.001 |
| RVSP (mm Hg) | 33±11 | 31±10 | 0.3 |
| Tricuspid regurgitation | |||
| Trivial 1+ 2+ 3+ 4+ |
69.2 18.7 9.4 2.2 0.6 |
70.3 18.6 7.7 2.6 0.8 |
0.2 |
LA, left atrial; LVID, left ventricular internal dimension; RVSP, right ventricular systolic pressure; STEMI, ST elevation myocardial infarction.
Coronary intervention
Procedural details of the two groups are shown in table 3. There were no differences between the two groups in door-to-balloon time (DBT; median, 104 vs 106 min in anterior vs inferior STEMI, p=0.5), type of intervention, radial access, number of diseased vessels on coronary angiogram, glycoprotein IIb/IIIa use, blood transfusion, puncture site complications or acute kidney injury. Median length of hospital stay was longer for anterior STEMI compared with inferior. However, there was no difference between both groups regarding medical therapy on discharge (table 3).
Table 3.
Procedural details, medications and clinical outcomes for patients with ischaemic MR presenting with anterior and inferior STEMI
| Variable | Anterior STEMI | Inferior STEMI | p Value |
|---|---|---|---|
| Intervention | Plain old ballon angioplasty (POBA) 29.5% Bare metal stent (BMS) 49.7% Drug eluting stent (DES) 21.8% |
POBA 22% BMS 59.3% DES 18.2% |
0.02* |
| DBT: median (IQR) | 104 (37 184) | 106 (40 168) | 0.5 |
| Radial access | 0.3% | 0.3% | 0.8 |
| GP IIB/IIIA inhibitors | 67.8% | 72.3% | 0.2 |
| TIMI III result | 87.2% | 91.3% | 0.07 |
| Residual stenosis >90% | 6.7% | 5% | 0.4 |
| Blood transfusion | 24.2% | 20.1% | 0.2 |
| RP bleed | 1.3% | 0.4% | 0.2 |
| Haematoma | 5.7% | 3.8% | 0.2 |
| Pseudoaneurysm | 1.8% | 0.9% | 0.3 |
| AV fistula | 0.4% | 0.4% | 0.9 |
| Acute kidney injury | 10.1% | 8.9% | 0.6 |
| Emergency CABG | 2.7% | 3% | 0.8 |
| LOS (days): median (IQR) | 5.3 (3.3–10.2) | 4 (3–8.6) | 0.005* |
| Aspirin (ASA) at discharge | 97.5% | 98.2% | 0.3 |
| Statin at discharge | 88.2% | 87.6% | 0.4 |
| β-blocker at discharge | 79.5% | 78.8% | 0.7 |
| ACE inhibitors at discharge | 71.2% | 68% | 0.1 |
| 30-day mortality | 14.9% | 6.8% | 0.001* |
| 1-year mortality | 26.4% | 14.3% | <0.0001* |
| 3-year mortality | 39.7% | 24.8% | <0.0001* |
*values have reached statistical significance
ASA, Aspirin; AV, arteriovenous; BMS, Bare metal stent; CABG, coronary artery bypass graft; DBT, door-to-balloon time; DES, Drug eluting stent; GP, glycoprotein; LOS, length of hospital stay; MR, mitral regurgitation; POBA, Plain old ballon angioplasty; RP, retroperitoneal; STEMI, ST elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Short-term and long-term outcomes
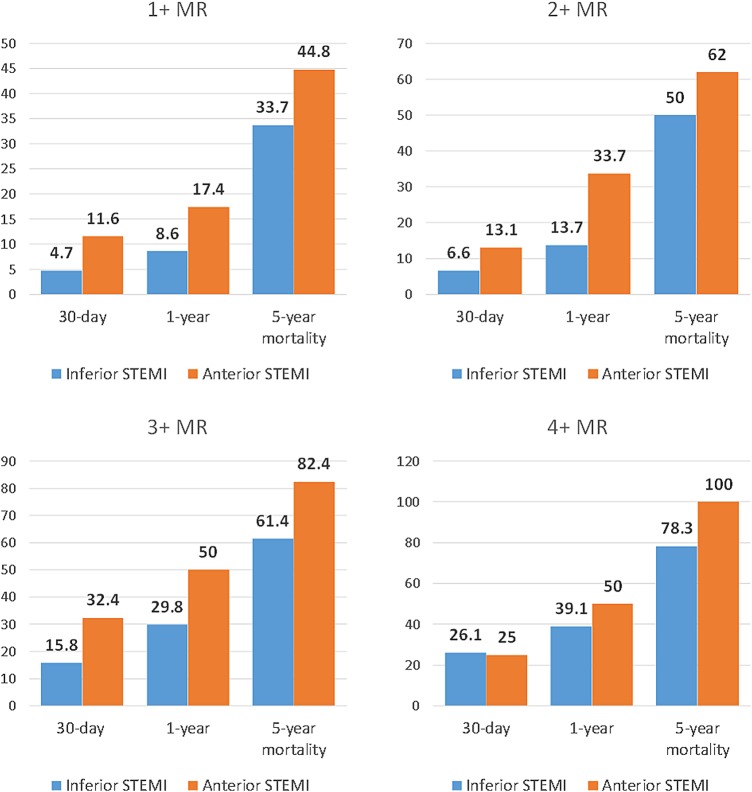
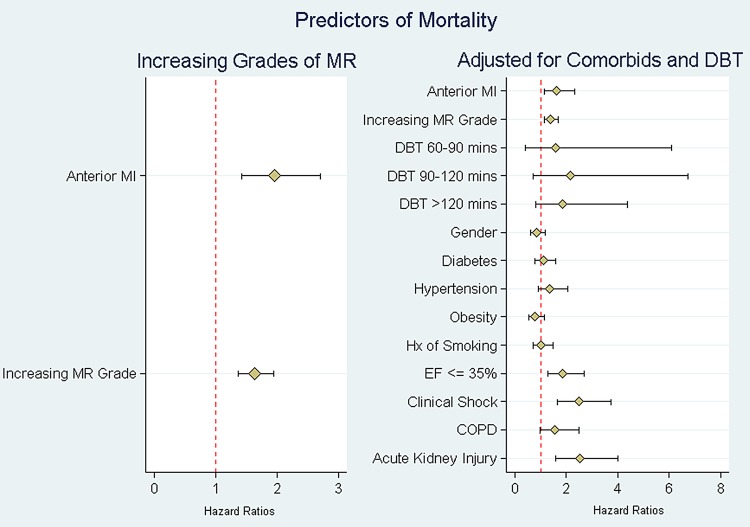
Mean follow-up was 5.9±2.1 years. At 5 years, 40 patients (8%) and 31 patients (10%) were lost to follow-up in the inferior and anterior STEMI groups, respectively. Thirty-day, 1-year and 5-year mortality rates were higher in group 1 (anterior STEMI) compared with group 2 (inferior STEMI; 14.9% vs 6.8%, 26.4% vs 14.3% and 39.7% vs 24.8%, respectively, p<0.001 for all). When the analysis was performed for each grade of IMR between anterior and inferior STEMI, anterior was associated with worse outcomes on every grade of IMR compared with inferior STEMI (figure 1). Kaplan-Meier curves showing long-term survival for the two groups are shown in figure 2. Multivariate Cox survival analysis for predictors of long-term mortality is shown in table 4 and figure 3. After adjusting for age, gender, comorbidities, ejection fraction, grade of IMR and DBT, anterior STEMI was associated with worse outcomes (HR 1.62 (95% CI 1.23 to 2.12), p<0.001).
Figure 1.
Thirty-day, 1-year and 5-year mortality rates in anterior versus inferior STEMI across different grades of MR. MR, mitral regurgitation; STEMI, ST elevation myocardial infarction.
Figure 2.
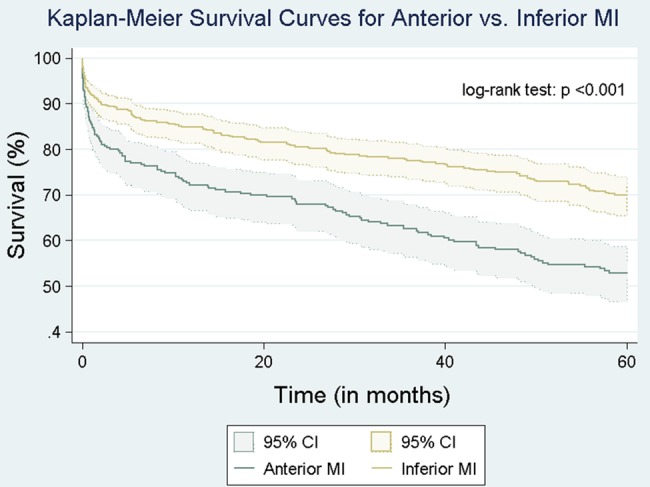
Kaplan-Meier curve showing long-term survival in patients with ischaemic MR presenting with anterior STEMI and inferior STEMI. MI, myocardial infarction; MR, mitral regurgitation; STEMI, ST elevation myocardial infarction.
Table 4.
Multivariate analysis with proportional hazards of 1-year mortality. Predictors of mortality by location of STEMI adjusted for comorbidities and DBT
| Variable | HR | SE | Z value | p Value | 95% CI |
|---|---|---|---|---|---|
| Anterior STEMI | 1.62 | 0.22 | 3.49 | <0.001 | 1.23 to 2.12 |
| Rising grade of IMR | 1.39 | 0.10 | 4.53 | <0.001 | 1.21 to 1.61 |
| DBT (compared with 60 min) | |||||
| 60–90 min | 1.57 | 0.83 | 0.40 | 0.56 to 4.41 | |
| 90–120 min | 2.15 | 0.95 | 0.08 | 0.91 to 5.12 | |
| >120 min | 1.86 | 0.62 | 0.06 | 0.97 to 3.57 | |
| Male gender | 0.84 | 0.11 | −1.31 | 0.189 | 0.65 to 1.09 |
| Diabetes mellitus | 1.10 | 0.16 | 0.69 | 0.493 | 0.84 to 1.46 |
| Hypertension | 1.35 | 0.22 | 1.86 | 0.062 | 0.98 to 1.85 |
| Obesity | 0.77 | 0.12 | −1.69 | 0.090 | 0.58 to 1.04 |
| Smoking | 1.02 | 0.14 | 0.17 | 0.866 | 0.77 to 1.35 |
| Ejection fraction ≤35% | 1.84 | 0.27 | 4.21 | <0.001 | 1.38 to 2.45 |
| Clinical shock | 2.48 | 0.39 | 5.69 | <0.001 | 1.81 to 3.38 |
| COPD | 1.56 | 0.29 | 2.41 | 0.016 | 1.09 to 2.23 |
| Acute kidney injury | 2.52 | 0.45 | 5.15 | <0.001 | 1.77 to 3.58 |
COPD, chronic obstructive pulmonary disease; DBT, door-to-balloon time; IMR, ischaemic mitral regurgitation; STEMI, ST elevation myocardial infarction.
Figure 3.
Multivariate Cox survival analysis with predictors of mortality after adjusting for comorbidities and DBT. COPD, chronic obstructive pulmonary disease; DBT, door-to-balloon time; EF, ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; STEMI, ST elevation myocardial infarction.
Discussion
In this study, we demonstrate that overall incidence of IMR by transthoracic 2D echocardiography in patients presenting with acute STEMI who undergo PPCI is 17.8% in anterior STEMI compared with 21.8% in inferior STEMI. We also demonstrate that most common direction of jet in anterior STEMI is central while in inferior STEMI is posterior then central. In addition, IMR in anterior STEMI is associated with worse short-term and long-term outcomes than inferior STEMI across all grades of MR even after adjusting for age, gender, comorbidities, grade of IMR, ejection fraction and DBT.
Higher incidence of IMR in inferior than anterior STEMI was shown in previous studies;8 20 28 32 however, overall numbers in these studies were much higher. This could be explained by the fact that in these studies, many of the patients got thrombolytic therapy or late PCI rather than PPCI, and it has been previously reported that PPCI lowers the incidence of MR in STEMI patients.21 Also, patients with trace MR were not included in our study; had these been included, this might have increased our reported incidence.
It is known that there are two major types of mitral valve apparatus changes that can happen after acute MI, the first is the asymmetric remodelling, where posterior tethering of both mitral leaflets occurs, resulting in posterior bending of the posterior leaflet with over-ride of the anterior leaflet, the resulting IMR jet is usually directed posteriorly. The second is the symmetric remodelling where there is apical tethering of both leaflets with restricted motion of anterior leaflet resulting in apical tenting with apically displaced coaptation line. This results in centrally directed IMR jet. The former occurs mostly in inferior MI while the latter occurs more commonly in anterior MI.34 35 This is similar to what was found regarding direction of IMR jet our study.
MI can cause MR by different mechanisms such as changes in LV geometry, mitral valve annulus dilation, LV dysfunction reducing systolic closing pressure, or functional disruption of the posteromedial papillary muscle by displacement increasing tethering forces (tenting). The interplay between these different factors determines the severity of the MR.29–32 36 37 Inferior MI causes distortion to the mitral valve apparatus more frequently resulting in incompetent valve compared with anterior STEMI. Anterior MI causes MR due to apical tethering of the valve, a mechanism that requires LV dilation. Therefore, MR in anterior STEMI is frequently associated with more dysfunctional LV compared with inferior STEMI. Further, while apical tethering of the leaflets and Mitral valve (MV) annular deformation and dilation are seen in inferior and anterior MI, it has been shown that the severity of these deformities is greater in patients with anterior MI.38 39 This is an important distinction as the systolic tenting area of the mitral valve, mitral annular area and spherical index are all independent determinants of regurgitant orifice area.40 Whether it is these differences that result in the difference in outcomes between both types of STEMI is not known, but our novel finding here that IMR has worse outcomes with anterior compared with inferior STEMI may provide a rationale to explore these mechanistic differences in future studies.
Associations of increased 30-day mortality10 and 1-year mortality1 3–5 10 23 with IMR have been previously reported. Comparisons were carried out between two groups (non-significant vs significant IMR) or three groups (no MR, mild MR and moderate/severe MR). Our study is the first to compare between IMR regarding index STEMI location. This might be because of the large sample size of our study which provided enough power to perform this comparison. Outcomes of anterior STEMI overall is known to be worse than inferior STEMI, but in our study, there was no difference between both groups in all risk factors, clinical presentation, comorbidities and coronary intervention. Even after adjusting for known factors associated with more mortality, still IMR in anterior STEMI showed worse prognosis.
To the best of our knowledge, our study is the first to date to compare outcomes of IMR by STEMI location in a large population of patients >800 patients who underwent PPCI within the first 12 hours of presentation and had a mean follow-up of 5.9 years.
Study limitations
As with any retrospective, single-centre analysis, our study may be limited by selection bias and the results might not be generalisable to other centres or hospitals. A second limitation is that our patient population spans a 20-year period, over which advances in coronary intervention and cardiac care were tremendous. A third limitation is that we did not have grades of MR in follow-up echocardiography, as several studies before reported that the severity of MR changes over time in the subacute phase of the MI.17 24 In addition, we did not have data on clinical follow-up such as heart failure readmissions, or number of patients who received surgical intervention for the MR in follow-up, and this might have affected noticed outcomes. Another limitation is that patients who presented with STEMI and died before performing an echocardiogram were not accounted for and the presence of IMR in these patients was not known.
Conclusion
IMR occurs more commonly with inferior compared with anterior STEMI. However, IMR with anterior had much worse outcomes in every grade of regurgitation compared with inferior STEMI.
Footnotes
Competing interests: None declared.
Ethics approval: Institutional Review Board (IRB) of Cleveland Clinic.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Barzilai B, Davis VG, Stone PH et al. . Prognostic significance of mitral regurgitation in acute myocardial infarction. The MILIS Study Group. Am J Cardiol 1990;65:1169–75. 10.1016/0002-9149(90)90968-7 [DOI] [PubMed] [Google Scholar]
- 2.Aronson D, Goldsher N, Zukermann R et al. . Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med 2006;166:2362–8. 10.1001/archinte.166.21.2362 [DOI] [PubMed] [Google Scholar]
- 3.Lehmann KG, Francis CK, Dodge HT. Mitral regurgitation in early myocardial infarction. Incidence, clinical detection, and prognostic implications. TIMI Study Group. Ann Intern Med 1992;117:10–17. 10.7326/0003-4819-117-1-10 [DOI] [PubMed] [Google Scholar]
- 4.Feinberg MS, Schwammenthal E, Shlizerman L et al. . Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. Am J Cardiol 2000;86:903–7. 10.1016/S0002-9149(00)01119-X [DOI] [PubMed] [Google Scholar]
- 5.Hillis GS, Møller JE, Pellikka PA et al. . Prognostic significance of echocardiographically defined mitral regurgitation early after acute myocardial infarction. Am Heart J 2005;150:1268–75. 10.1016/j.ahj.2005.01.020 [DOI] [PubMed] [Google Scholar]
- 6.Bursi F, Enriquez-Sarano M, Nkomo VT et al. . Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295–301. 10.1161/01.CIR.0000151097.30779.04 [DOI] [PubMed] [Google Scholar]
- 7.Lamas GA, Mitchell GF, Flaker GC et al. . Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and ventricular enlargement investigators. Circulation 1997;96:827–33. 10.1161/01.CIR.96.3.827 [DOI] [PubMed] [Google Scholar]
- 8.Grigioni F, Enriquez-Sarano M, Zehr KJ et al. . Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759–64. 10.1161/01.CIR.103.13.1759 [DOI] [PubMed] [Google Scholar]
- 9.Lehmann KG, Francis CK, Sheehan FH et al. . Effect of thrombolysis on acute mitral regurgitation during evolving myocardial infarction. Experience from the Thrombolysis in Myocardial Infarction (TIMI) Trial. J Am Coll Cardiol 1993;22:714–19. 10.1016/0735-1097(93)90181-Y [DOI] [PubMed] [Google Scholar]
- 10.Pellizzon GG, Grines CL, Cox DA et al. . Importance of mitral regurgitation in patients undergoing percutaneous coronary intervention for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol 2004;43:1368–74. 10.1016/j.jacc.2003.11.046 [DOI] [PubMed] [Google Scholar]
- 11.Wita K, Berger-Kucza A, Filipecki A et al. . Predictive value of ischemic mitral regurgitation during the acute phase of ST elevation myocardial infarction treated with primary coronary intervention for left ventricular remodeling in long-term follow-up. Coron Artery Dis 2010;21:325–9. 10.1097/MCA.0b013e32833aa6bb [DOI] [PubMed] [Google Scholar]
- 12.Tcheng JE, Jackman JD, Nelson CL et al. . Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med 1992;117:18–24. 10.7326/0003-4819-117-1-18 [DOI] [PubMed] [Google Scholar]
- 13.De Servi S, Vaccari L, Assandri J et al. . Clinical significance of mitral regurgitation in patients with recent myocardial infarction. Eur Heart J 1988;9(Suppl F):5–9. 10.1093/eurheartj/9.suppl_F.5 [DOI] [PubMed] [Google Scholar]
- 14.Hickey MS, Smith LR, Muhlbaier LH et al. . Current prognosis of ischemic mitral regurgitation. Implications for future management. Circulation 1988;78:I51–9. [PubMed] [Google Scholar]
- 15.Chung SY, Lin FC, Chua S et al. . Clinical profile and outcome of first acute myocardial infarction with ischemic mitral regurgitation. Chang Gung Med J 2008;31:268–75. [PubMed] [Google Scholar]
- 16.Barra S, Providência R, Paiva L et al. . Mitral regurgitation during a myocardial infarction—new predictors and prognostic significance at two years of follow-up. Acute Card Care 2012;14:27–33. 10.3109/17482941.2012.655295 [DOI] [PubMed] [Google Scholar]
- 17.Amigoni M, Meris A, Thune JJ et al. . Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: prognostic significance and relation to ventricular size and function. Eur Heart J 2007;28:326–33. 10.1093/eurheartj/ehl464 [DOI] [PubMed] [Google Scholar]
- 18.Li S, Barywani S, Fu M. Prognostic significance of mitral regurgitation in long-term all-cause mortality in patients aged ≥80 years with acute coronary syndrome. Int J Cardiol 2014;176:340–5. 10.1016/j.ijcard.2014.06.084 [DOI] [PubMed] [Google Scholar]
- 19.Perez de Isla L, Zamorano J, Quezada M et al. . Prognostic significance of functional mitral regurgitation after a first non-ST-segment elevation acute coronary syndrome. Eur Heart J 2006;27:2655–60. 10.1093/eurheartj/ehl287 [DOI] [PubMed] [Google Scholar]
- 20.Leor J, Feinberg MS, Vered Z et al. . Effect of thrombolytic therapy on the evolution of significant mitral regurgitation in patients with a first inferior myocardial infarction. J Am Coll Cardiol 1993;21:1661–6. 10.1016/0735-1097(93)90384-D [DOI] [PubMed] [Google Scholar]
- 21.Chua S, Hung J, Chung S-Y et al. . Primary percutaneous coronary intervention lowers the incidence of ischemic mitral regurgitation in patients with acute ST-elevated myocardial infarction. Circ J 2010;74:2386–92. 10.1253/circj.CJ-10-0435 [DOI] [PubMed] [Google Scholar]
- 22.Poh K-K, Lee GK, Lee L-C et al. . Reperfusion therapies reduce ischemic mitral regurgitation following inferoposterior ST-segment elevation myocardial infarction. Coron Artery Dis 2012;23:555–9. 10.1097/MCA.0b013e32835aab65 [DOI] [PubMed] [Google Scholar]
- 23.Engström AE, Vis MM, Bouma BJ et al. . Mitral regurgitation is an independent predictor of 1-year mortality in ST-elevation myocardial infarction patients presenting in cardiogenic shock on admission. Acute Card Care 2010;12:51–7. 10.3109/17482941003802148 [DOI] [PubMed] [Google Scholar]
- 24.López-Pérez M, Estévez-Loureiro R, López-Sainz A et al. . Long-term prognostic value of mitral regurgitation in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol 2014;113:907–12. 10.1016/j.amjcard.2013.11.050 [DOI] [PubMed] [Google Scholar]
- 25.MacHaalany J, Bertrand OF, O'Connor K et al. . Predictors and prognosis of early ischemic mitral regurgitation in the era of primary percutaneous coronary revascularisation. Cardiovasc Ultrasound 2014;12:14 10.1186/1476-7120-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrabba N, Parodi G, Valenti R et al. . Clinical implications of early mitral regurgitation in patients with reperfused acute myocardial infarction. J Card Fail 2008;14:48–54. 10.1016/j.cardfail.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 27.Uddin AM, Henry TD, Hodges JS et al. . The prognostic role of mitral regurgitation after primary percutaneous coronary intervention for acute ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2012;80:779–86. 10.1002/ccd.23400 [DOI] [PubMed] [Google Scholar]
- 28.Izumi S, Miyatake K, Beppu S et al. . Mechanism of mitral regurgitation in patients with myocardial infarction: a study using real-time two-dimensional Doppler flow imaging and echocardiography. Circulation 1987;76:777–85. 10.1161/01.CIR.76.4.777 [DOI] [PubMed] [Google Scholar]
- 29.Ragosta M. What to do about ischemic mitral regurgitation? JACC Cardiovasc Interv 2015;8:364–6. 10.1016/j.jcin.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 30.Silbiger JJ. Mechanistic insights into ischemic mitral regurgitation: echocardiographic and surgical implications. J Am Soc Echocardiogr 2011;24:707–19. 10.1016/j.echo.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 31.Kalra K, Wang Q, McIver BV et al. . Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J Am Coll Cardiol 2014;64:1867–79. 10.1016/j.jacc.2014.07.988 [DOI] [PubMed] [Google Scholar]
- 32.Kumanohoso T, Otsuji Y, Yoshifuku S et al. . Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg 2003;125:135–43. 10.1067/mtc.2003.78 [DOI] [PubMed] [Google Scholar]
- 33.Zoghbi WA, Enriquez-Sarano M, Foster E et al. . Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802. 10.1016/S0894-7317(03)00335-3 [DOI] [PubMed] [Google Scholar]
- 34.Agricola E, Oppizzi M, Maisano F et al. . Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr 2004;5:326–34. 10.1016/j.euje.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 35.Agricola E, Oppizzi M, Pisani M et al. . Ischemic mitral regurgitation: mechanisms and echocardiographic classification. Eur J Echocardiogr 2007;9:207–21. 10.1016/j.euje.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 36.Yosefy C, Beeri R, Guerrero JL et al. . Mitral regurgitation after anteroapical myocardial infarction: new mechanistic insights. Circulation 2011;123:1529–36. 10.1161/CIRCULATIONAHA.110.977843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golba K, Mokrzycki K, Drozdz J et al. . Mechanisms of functional mitral regurgitation in ischemic cardiomyopathy determined by transesophageal echocardiography (from the Surgical Treatment for Ischemic Heart Failure Trial). Am J Cardiol 2013;112:1812–18. 10.1016/j.amjcard.2013.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe N, Ogasawara Y, Yamaura Y et al. . Geometric differences of the mitral valve tenting between anterior and inferior myocardial infarction with significant ischemic mitral regurgitation: quantitation by novel software system with transthoracic real-time three-dimensional echocardiography. J Am Soc Echocardiogr 2006;19:71–5. 10.1016/j.echo.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 39.Watanabe N, Ogasawara Y, Yamaura Y et al. . Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation 2005;112:I458–62. 10.1161/CIRCULATIONAHA.104.524595 [DOI] [PubMed] [Google Scholar]
- 40.Song JM, Qin JX, Kongsaerepong V et al. . Determinants of ischemic mitral regurgitation in patients with chronic anterior wall myocardial infarction: a real time three-dimensional echocardiography study. Echocardiography 2006;23:650–7. 10.1111/j.1540-8175.2006.00284.x [DOI] [PubMed] [Google Scholar]