Abstract
To examine the analgesic effect and safety of single-dose intra-articular (IA) magnesium (Mg) after arthroscopic surgery. Pubmed, Embase and Cochrane library were searched through in January 2016. Eight RCTs and eight experimental studies were included. The IA Mg exhibited a significantly lower pain score when compared with placebo (MD, −0.41, 95% CI, −0.78 to −0.05, p = 0.03). There was no significant difference between Mg and bupivacaine in terms of pain relief and the time to first analgesic request. Furthermore, statistically significant differences both in pain score (MD, −0.62, 95% CI, −0.81 to −0.42, p < 0.00001) and time to first analgesic request (MD, 6.25, 95% CI, 5.22 to 7.29, p < 0.00001) were observed between Mg plus bupivacaine and bupivacaine alone. There was no statistically significant difference among the various groups with respect to adverse reactions. Most of the included in vitro studies reported the chondrocyte protective effect of Mg supplementation. There were also two in vivo studies showing the cartilage protective effect of IA Mg. The single-dose IA Mg following arthroscopic surgery was effective in pain relief without increasing adverse reactions, and it could also enhance the analgesic effect of bupivacaine. In addition, Mg seemed to possess the cartilage or chondrocyte protective effect based on experimental studies.
Post-operative pain after arthroscopic surgery, which is commonly performed as a day case, is one of the obstacles delaying hospital discharge and hindering early rehabilitation1,2. The single-dose intra-articular (IA) analgesic agents has been widely used for pain relief after arthroscopic surgery as a simple and economical approach. Previous meta-analyses have demonstrated that the single-dose IA morphine3,4, bupivacaine5,6,7, ropivacaine8, clonidine9 or the combination of morphine and bupivacaine10,11 were effective in pain relief. However, it is commonly known that IA local anesthetics must be used cautiously due to the concern of chondrotoxicity12, especially for bupivacaine13,14,15,16,17,18,19, levobupivacaine13,14,19, ropivacaine13,14,16,17, mepivacaine17 and lidocaine13,16,18,20.
Magnesium (Mg), a physiologic N-methyl-D-aspartate (NMDA) receptor antagonist, could be effective in postoperative analgesia21,22. More importantly, Mg has been proved to be able to enhance the chondrocyte viability either alone or in combination with a local anesthetic (rather than a local anesthetic alone), and it was therefore proposed to be a potential alternative IA analgesic agent due to its effect on chondrotoxicity reduction13,14. However, it remains to be a controversial issue whether the single-dose IA Mg after arthroscopic surgery is effective in pain relief when compared with placebo, or whether the IA Mg exhibits a comparable effect when compared with bupivacaine, which is the most widely used local analgesic at present23,24,25,26,27,28,29,30. In addition, there is also a great significance for clinical practice to explore whether IA Mg can amplify the analgesic effect of bupivacaine and reduce the chondrotoxicity of local anesthetics. Furthermore, some broader issues related to the analgesic ability and chondrocyte protective effect of IA Mg in other settings, such as osteoarthritis, are to be addressed.
Therefore, the objectives of this systematic review and meta-analysis were to investigate the analgesic effect of single-dose IA based on the following comparisons: (1) Mg versus placebo, (2) Mg versus bupivacaine and (3) Mg plus bupivacaine versus bupivacaine alone, after arthroscopic surgery. Another objective was to assess the protective effect of Mg on chondrocyte or cartilage based on randomized controlled trials (RCTs), in vitro and in vivo studies.
Methods
Literature search
This meta-analysis was in accord with the Preferred Reporting Items for Systematic review and Meta-analyses statement31. The electronic databases of Pubmed, Embase and Cochrane library were searched through in January 2016 using a series of logic combination of keywords and text words related to arthroscopic knee surgery, magnesium and randomized controlled trials (RCTs) (Appendix 1). The Pubmed and Embase databases were also searched through in January 2016 to retrieve in vitro and in vivo experimental studies of Mg supplementation (Appendix 1). No restriction was imposed, and the references of the retrieved articles and reviews were evaluated.
Study selection
Two researchers reviewed all the retrieved titles, abstracts and full texts independently. Disagreements were resolved through discussions and/or consultations to a thrid researcher. All of the eligible trials must meet the following criteria: (1) patients undergone arthroscopic surgery; (2) including a treatment group of single-dose IA Mg or Mg plus bupivacaine for postoperative pain relief; (3) including a controlled group of IA placebo or bupivacaine alone, (4) other interventions should be balanced between comparative groups, (5) RCTs. The exclusion criteria for this study were: (1) case series, reviews, protocols or vitro studies; (2) non-RCTs; (3) abstract or full text was not available; (4) no IA injection; (5) meeting abstract.
Data extraction
The available information and outcomes of each included study were extracted by two independent researchers. The retained data included the first author, year of publication, size of each group, doses of intervention, follow-up time points, type of operation, type of anesthesia and injection time. The Cochrane risk of bias table was used to assess the methodological quality of the included RCTs. A total of seven potential risks of bias were evaluated: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias (mainly including the conflict of interests)32. Each risk item was evaluated based on a three-level rating system: low risk, unclear risk and high risk. Studies with three or more unclear or high risk of bias were considered as poor methodological quality.
The primary outcome of interests for this study was the effects of IA Mg and Mg plus bupivacaine on postoperative pain control. If a study reported multiple pain scales, the highest one on the hierarchy of pain scale related to the outcomes was adopted, as described by Jüni and colleagues33. The secondary outcomes of interest were the time interval to the first request of analgesia and the side effects. If data was presented in figures, the GetData software would be used (http://getdata-graph-digitizer.com/index.php) to extract data from the figures. If the outcomes were reported in terms of the median and the range or interquartile range, the median would be considered as mean and the standard deviation would be estimated by the range or interquartile range32.
Statistical analyses
The outcome measures investigated in this meta-analysis were postoperative pain intensity and the time to first request of analgesia. Due to the low incidence of adverse reactions, only qualitative analysis were conducted on relevant adverse effects. Firstly, we calculated the overall mean difference (MD) and its corresponding 95% confidence interval (CI) between comparative groups for postoperative pain intensity at last follow up. Then a subgroup analysis were conducted by calculating the MD at different time points of follow up. If all included trials reported the pain intensity at a same postoperative time point, an overall MD between comparative groups for pain intensity was calculated at this time point of follow up. We also calculated the overall MD and its related 95% CI for the time to first request of analgesia. The heterogeneity of effect size across trials was tested by Q statistics (p < 0.05 was considered heterogeneous). If there was a significant heterogeneity among the studies, the random-effects model was used; otherwise, the fixed effects model was acceptable. We also examined the I2 statistic, which measures the percentage of the total variation across studies due to heterogeneity (I2 > 50 was considered heterogeneous). We further conducted sensitivity analysis to explore possible explanations for heterogeneity and to examine the influence of various exclusion criteria on the overall MD.
Begg’s tests34 and funnel plots were performed to assess the publication bias. Statistical analyses were performed using Review Manager 5 software (RevMan 5, The Cochrane Collaboration, Oxford, UK) and STATA version 11.0 (StataCorp LP, College Station, Texas). A p value less than 0.05 was considered to be statistically significant.
Results
Search results and selected articles
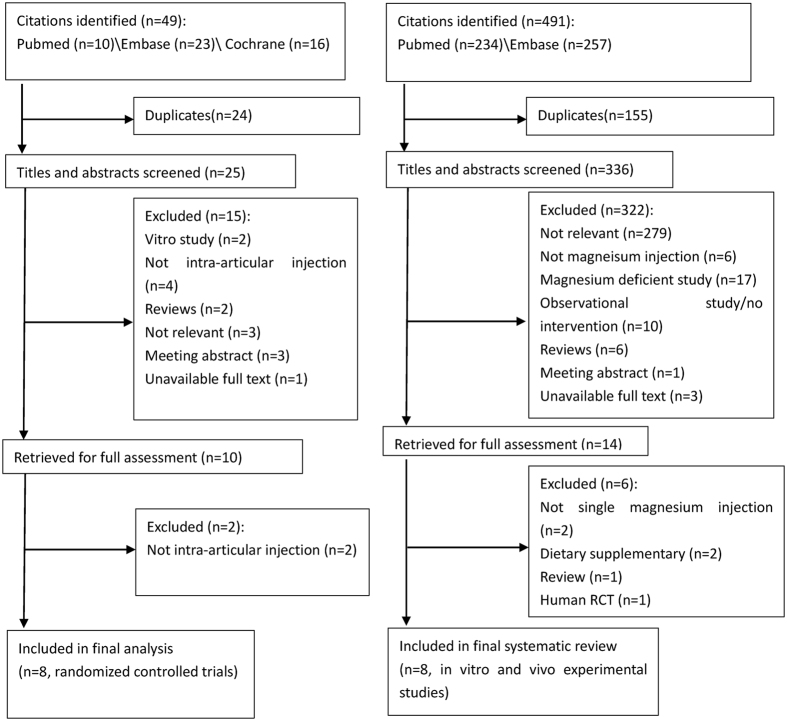
The literature search retrieved a total of 49 citations for potential RCTs examining the analgesic effect of single-dose IA Mg after arthroscopic surgery and 491 citations for potential in vitro and in vivo experimental studies of Mg supplementation, respectively. Two evaluators screened the titles/abstracts and full-texts of all eligible articles. Eventually, 8 RCTs23,24,25,26,27,28 (513 patients) and 8 experimental studies13,14,29,30,35,36,37,38,39,40 (6 in vitro and 2 in vivo studies) were qualified for final analysis (Fig. 1). The characteristics of the included RCTs are presented in Table 1. The characteristics and results of the included in vitro and in vivo experimental studies are listed in Table 2. According to the Cochrane risk of bias table (Appendix 2), three trials25,27,29 did not describe their random sequence generation (high risk of selection bias); six trials24,25,26,28,29,30 did not design a clear allocation concealment plan (unclear or high risk of selection bias); all trials adopted the double-blind method except one27 which did not describe the blinding method of participants (high risk of performance bias); one study30 was rated as unclear risk for both incomplete data and selective reporting; six studies23,24,25,26,27,28 did not present a competing interest statement (unclear risk of other bias). In addition, three trials25,27,30 were rated as low methodological quality for having three or more unclear or high risk of bias.
Figure 1. Flow diagram of included studies.
Table 1. Characteristics of the included 8 randomized controlled trials.
| Study | Number | Dosage | Time | Type of operation | Type of anesthesia | Injection time |
|---|---|---|---|---|---|---|
| Bondok23 | Mg: 30 | 500 mg (10 ml) | 1, 2, 6, 8, 12, 18, 24 h | Arthroscopic knee meniscectomy | General | At the end of the surgery |
| Placebo: 30 | (10 ml) | |||||
| Elshamouby24 | Mg: 27 | 1000 mg (20 ml) | 1, 2, 4, 6, 8, 12, 24 h | Arthroscopic knee meniscectomy | General | At the end of the surgery |
| Placebo: 27 | (20 ml) | |||||
| B: 27 | 0.25% (20 ml) | |||||
| Mg plus B: 27 | 1000 mg plus 0.25% (20 ml) | |||||
| Farouk25 | Mg plus B: 20 | 150 mg plus 0.25% (20 ml) | 1, 2, 24 h | Arthroscopic knee meniscectomy | General | At the end of the surgery |
| B: 20 | 0.25% (20 ml) | |||||
| Suhrita30 | Mg plus B: 30 | 500 mg plus 0.25% (20 ml) | 1, 2, 6, 10, 14, 18 h | Arthroscopic knee meniscectomy and ligament repair | General | At the end of the surgery |
| B: 30 | 0.25% (20 ml) | |||||
| Koltka26 | Mg: 30 | 500 mg (20 ml) | 1, 2, 4, 12, 24, 48 h | Arthroscopic knee meniscectomy | General | At the end of the surgery |
| Placebo: 30 | (20 ml) | |||||
| LB: 30 | 0.5% (20 ml) | |||||
| Radwan27 | Mg: 20 | 800 mg (20 ml) | 1, 2, 4, 6, 12, 24 h | Arthroscopic knee meniscectomy | General | At the end of the surgery |
| B: 20 | 0.5% (20 ml) | |||||
| Saritas28 | Mg: 30 | 100 mg/ml (10 ml) | 1, 2, 6, 8, 12, 18, 24 h | Arthroscopic rotator cuff repair | General | At the end of the surgery |
| Placebo: 30 | (10 ml) | |||||
| Abdulatif29 | Mg: 28 | 1000 mg (20 ml) | 2, 4, 6, 12, 24 h | Arthroscopic ACL reconstruction | General | At the end of the surgery |
| Placebo: 27 | (20 ml) |
Mg, magnesium sulphate; B, bupivacaine; M, morphine; LB, levobupivacaine; h, hour; ACL, anterior cruciate ligament.
Table 2. Adverse reactions reported in the included randomized controlled trials.
| Studies | Groups (number) | Adverse reactions (person-time) |
|---|---|---|
| Koltka26 | Mg (30) | Knee effusion (1) |
| Placebo (30) | Knee effusion (1) | |
| LB (30) | Knee effusion (1) | |
| Radwan27 | Mg (20) | Nausea (3), vomiting (2), flushing (2) |
| B (20) | Nausea (2), vomiting (2) | |
| Saritas28 | Mg (30) | Shivering (12) |
| Placebo (30) | Shivering (10) | |
| Abdulatif29 | Mg (28) | Hypotension (1), bradycardia (1), |
| Placebo (27) | Hypotension (1), bradycardia (2), drowsiness (1) |
Mg, magnesium; LB, levobupivacaine; B, bupivacaine.
Effects of pain relief
Mg versus placebo
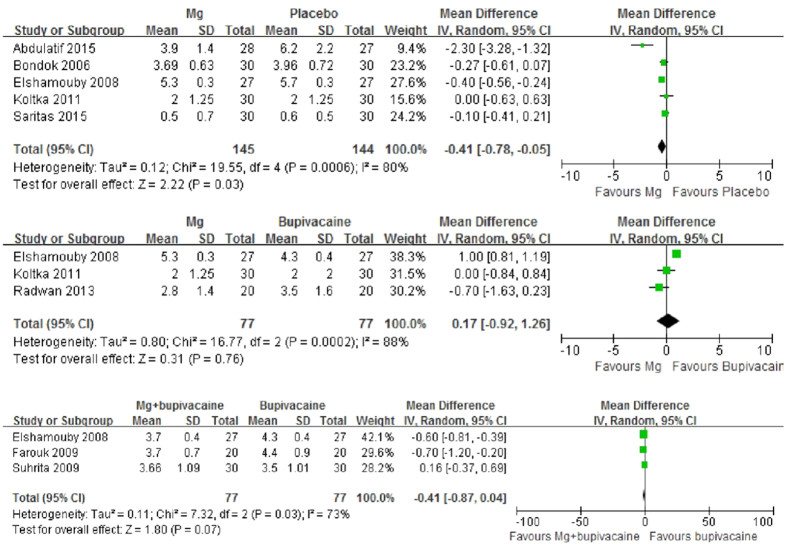
Five trials23,24,27,29,30 were eligible for assessing the postoperative pain score of IA Mg versus placebo after arthroscopic surgery at the last follow-up time point (24 or 48 hours). The combined results showed a significantly lower pain score of Mg (MD, −0.41, 95% CI, −0.78 to −0.05, p = 0.03). Substantial heterogeneity was observed (p = 0.0006, I2 = 80%) (Fig. 2). Sensitivity analyses after excluding studies with poor methodological quality and studies involving femoral nerve block in each group or involving other types of surgery rather than single knee meniscectomy all showed positive results with reduced heterogeneity (p > 0.05) (Appendix 3). One of the studies reported data with median and range was further excluded in sensitivity analysis, and the result was similar. Meanwhile, the positive results of subgroup analyses remained unchanged at different follow-up time points (2, 12 or 24 hours) (Appendix 4). Begg’s rank correlation test suggested no evidence of publication bias among included studies (p = 0.806).
Figure 2. Forest plot of pain intensity at the last follow-up time point.
Mg versus bupivacaine
Three trials were24,27,28 eligible for assessing the postoperative pain score of IA Mg versus bupivacaine (including levobupivacaine) after arthroscopic surgery at the last follow-up time point (24 or 48 hours). The combined results showed no significant difference between Mg and bupivacaine (MD, 0.17, 95% CI, −0.92 to 1.26, p = 0.76). Substantial heterogeneity was observed (p = 0.0002, I2 = 88%) (Fig. 2). Sensitivity analyses after excluding studies with poor methodological quality, or studies involving levobupivacaine rather than bupivacaine, or data reported by median and range showed the same results (Appendix 3). Meanwhile, the results of subgroup analyses remained to be insignificant at different follow-up time points (1, 2, 4, 12 or 24 hours) (Appendix 4). Begg’s rank correlation test suggested no evidence of publication bias among included studies (p = 0.296).
Mg plus bupivacaine versus bupivacaine alone
Three trials24,25,26 were eligible for assessing the postoperative pain score of IA Mg plus bupivacaine versus bupivacaine alone after arthroscopic surgery at the last follow-up time point (18 or 24 hours). The combined results showed that the effect of pain relief of Mg plus bupivacaine approached to the significant level when compared with bupivacaine alone (MD, −0.41, 95% CI, −0.87 to 0.04, p = 0.07). Substantial heterogeneity was observed (p = 0.03, I2 = 73%) (Fig. 2). Sensitivity analyses after excluding studies involving other types of surgery rather than single knee meniscectomy reached a significant result (MD, −0.62, 95% CI, −0.81 to −0.42, p < 0.00001) while heterogeneity disappeared (p = 0.72, I2 = 0%) (Appendix 3). The results of subgroup analyses remained to be significant at different follow-up time points (1 or 2 hours) (Appendix 4). Begg’s rank correlation test suggested no evidence of publication bias among included studies (p = 0.296).
Time to first analgesic request
Mg versus placebo
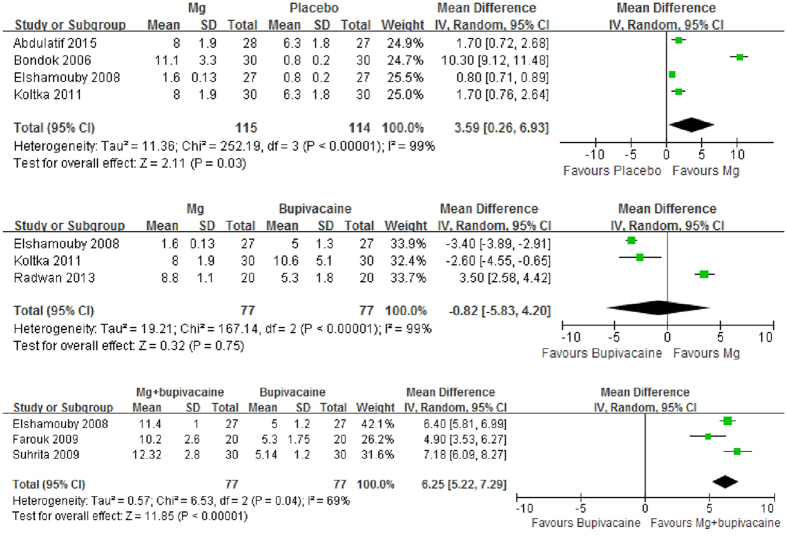
Four trials23,24,27,30 were eligible for assessing the time interval before the first request for analgesic medication of IA Mg versus placebo after arthroscopic surgery. The combined results showed a significantly longer time interval of Mg (MD, 3.59, 95% CI, 0.26 to 6.93, p = 0.03). Substantial heterogeneity was observed (p < 0.00001, I2 = 99%) (Fig. 3). However, sensitivity analyses after excluding studies with poor methodological quality and studies involving femoral nerve block in each group or involving other types of surgery rather than single knee meniscectomy all denied such positive results (p > 0.05) (Appendix 3). Begg’s rank correlation test suggested no evidence of publication bias among included studies (p = 0.308).
Figure 3. Forest plot of time to first analgesic request.
Mg versus bupivacaine
Three trials24,27,28 were eligible for assessing the time interval before the first request for analgesic medication of IA Mg versus bupivaciane after arthroscopic surgery. The combined results showed no significant difference between Mg and bupivaciane (MD, −0.82, 95% CI, −5.83 to 4.20, p = 0.75). Substantial heterogeneity was observed (p < 0.00001, I2 = 99%) (Fig. 3). Sensitivity analyses after excluding studies with poor methodological quality or studies involving levobupivacaine rather than bupivacaine reached the same results (p > 0.05) (Appendix 3). Begg’s rank correlation test suggested no evidence of publication bias among included studies (p = 1.000).
Mg plus bupivacaine versus bupivacaine alone
Four trials24,25,26 were eligible for assessing the time interval before the first request for analgesic medication of IA Mg plus bupivacaine versus bupivacaine alone after arthroscopic surgery. The combined results showed a significantly longer time interval of Mg plus bupivacaine (MD, 6.25, 95% CI, 5.22 to 7.29, p < 0.00001). Substantial heterogeneity was observed (p = 0.04, I2 = 69%) (Fig. 3). Sensitivity analyses after excluding studies with poor methodological quality reached the same positive results with reduced heterogeneity (p = 0.22, I2 = 34%) (Appendix 3). Begg’s rank correlation test suggested no evidence of publication bias among included studies (p = 1.000).
Safety
Adverse reactions reported in RCTs
Only four included RCTs27,28,29,30 reported adverse reactions (including knee effusion, nausea, vomiting, flushing, shivering, hypotension, bradycardia and drowsiness), as illustrated in Table 2. There was no statistically significant difference between comparable groups (including IA Mg versus placebo) in each trial.
Results of in vitro and in vivo experimental studies of Mg supplementation
The characteristics and analysis results of the included experimental studies were reported in Table 3. In comparison with local analgesics, Mg-free or low-level Mg medium, Mg exhibited a clear chondrocyte protective effect, which is exerted by increasing the number of attached chondrocytes35, enhancing chondrocyte proliferation and redifferentiation36, and reducing chondrocyte toxicity (increasing viability)13,14. However, there are two studies37,38 suggesting that high level of Mg (50 and 100 mM) or extra-high concentration of Mg could decrease the extracellular matrix protein, such as collagen and glycosaminoglycan. Only two in vivo studies39,40 further revealed that IA Mg attenuated the development of osteoarthritis in the rat model of collagenase-induced osteoarthritis and promoted cartilage formation of synovial mesenchymal stem cells in the rabbit osteochondral defect model. It should be highlighted that the limited number of in vivo studies retrieved in this area may affect the reliability of any conclusion obtained in this respect.
Table 3. Characteristics and results of the included 8 in vitro and vivo experimental studies of magnesium supplementation.
| Study | Mg supplementation | Control group | Chondrocyte or animal model | Results of Mg supplementation |
|---|---|---|---|---|
| In vitro studies | ||||
| Egerbacher35 | MgCl22 (0.0612 mg/ml) | Mg-free medium | Quinolone-treated horse and dog chondrocytes | The number of attached cells increased to 40–70% that of control group (threefold dose led to better results); Cell proliferation did not increase |
| MgSO4 (0.0488 mg.ml) | ||||
| MgCl2 (0.0612*3 mg/ml) | ||||
| MgSO4 (0.0488*3 mg/ml) | ||||
| Feyerabend36 | MgSO4 (1, 2, 5, 10, 15, 20, 25, 30 mM) | Without adding MgSO4 | Human articular chondrocytes | Enhanced chondrocyte proliferation and redifferentiation (dosage dependent); Increased growth factor effectiveness |
| Baker12 | MgSO4 (10%, 20%, 50%) | Placebo | Normal human chondrocytes | MgSO4 alone was no more toxic than placebo; MgSO4 in combination with a local anesthetic reduced chondrocyte toxicity compared with a local anesthetic alone |
| Lidocaine (2%) plus MgSO4 (10%, 20%, 50%) | Lidocaine (2%) | |||
| Levobupivacaine (0.5%) plus MgSO4 (10%, 20%, 50%) | Levobupivacaine (0.5%) | |||
| Bupivacaine (0.5%) plus MgSO4 (10%, 20%, 50%) | Bupivacaine (0.5%) | |||
| Ropivacaine (0.75%) plus MgSO4 (10%, 20%, 50%) | Ropivacaine (0.75%) | |||
| Baker13 | MgSO4 (10%) | Levobupivacaine (0.13%, 0.25%, 0.5%) | Normal human chondrocytes | No significant difference in chondrocyte viability between MgSO4 and placebo; With the exception of 0.13% levobupivacaine, all local anesthetics showed significantly greater toxic effects than MgSO4 |
| Bupivacaine (0.13%, 0.25%, 0.5%) | ||||
| Ropivacaine (0.19%, 0.38%, 0.75%) | ||||
| placebo | ||||
| Hagandora37 | MgCl2 (20, 50, 100 mM) | Baseline Mg concentration (0.8 mM) | Goat costal chondrocytes (scaffoldless approach) | Collagen and glycosaminoglycan content of the 50 and 100 mM MgCl2 and MgSO4 constructs was significantly lower than the control |
| MgSO4 (20, 50, 100 mM) in addition to the baseline Mg concentration (0.8 mM) | ||||
| Dou38 | MgCl2 (10, 20, 30 mM) | Blank control | Knee chondrocytes of Wuzhishan miniature pigs | In 2D culture, low concentrations of Mg ions enhanced excretion of extracellular matrix, whereas extra-high concentration of Mg inhibited the gene expression |
| In vivo studies | ||||
| Lee39 | MgSO4 (500 μg/0.1 ml) twice a week for 5 weeks | Placebo | Rat model of collagenase-induced osteoarthritis | Local intra-articular MgSO4 attenuates the development of osteoarthritis and reduces nociception |
| Shimaya40 | Synovial mesenchymal stem cells inμl PBS with 1 or 10 mM Mg | Synovial mesenchymal stem cells inμl PBS | Rabbit osteochondral defect model | Mg promoted cartilage formation of synovial mesenchymal stem cells |
Mg, magnesium; MgCl2, magnesium chloride; MgSO4, magnesium sulfate; mM, millimole; PBS, phosphate buffered saline.
Discussion
This systematic review and meta-analysis was performed on a total of 8 RCTs (published 2006 to 2015) and 8 in vitro and in vivo experimental studies. The most important finding of the present study is that the administration of single-dose IA Mg at the end of arthroscopic surgery was effective in pain relief without increasing adverse reactions when compared with placebo, and exhibited a comparable analgesic effect in comparison with bupivacaine. In addition, IA Mg could enhance the analgesic effect of bupivacaine. Another important finding is that Mg seemed to possess cartilage or chondrocyte protective effects according to the included experimental studies. Thus, IA Mg should perhaps be considered as an alternative to local anesthetics for pain relief after arthroscopic surgery. However, the optimal concentration and dosage of IA Mg still needs to be further explored.
Mg is a physiological antagonist of N-methyl-D-aspartate (NMDA) receptor, which is essential to the development and functionality of the nervous system; it serves as a target for the treatment of cognitive impairment, depression, schizophrenia and pain41,42,43,44,45. Mg could be effective in pain relief by exerting the antinociceptive effect46, inhibiting TNF-α47, and modulating hypesthesia and hyperalgesia48,49, by blocking the NMDA receptor. The pain relief effect of Mg has been illustrated by several studies in many cases, such as postoperative sore throat50, tourniquet pain51, major non-laparoscopic gastrointestinal surgery52, diabetic neuropathic pain53, cardiac surgery54, and cancer-related neuropathic pain55. The present study also demonstrated that the single-dose IA Mg was effective in pain relief after arthroscopic surgery.
Several studies, including our previous surveys, have revealed that dietary and serum Mg were negatively associated with the prevalence of knee osteoarthritis56,57,58,59, indicating that Mg could possess the cartilage or chondrocyte protective effects. In addition to the included experimental studies (Table 3), Mg was reported to be able to regulate the level of sex determining region Y-box 9, which plays a vital role of cartilage growth plates and is required in the successive steps of chondrogenesis60. Furthermore, calcium is critical to the regulation of many cellular physiological functions; the overload of calcium is detrimental to the mitochondrial function61. Thus, the inhibition of NMDA receptor by Mg could also decrease the entry of extra-cellular calcium into cells, and thereby exhibits the chondrocyte protective effects. In combination with the findings of the present study, it seems that IA Mg not only can be regarded as an alternative to local analgesics relying on its potential chondrotoxicity, but also shows the cartilage and chondrocyte protective effects. Previous in vitro studies have indicated that local anesthetics such as bupivacaine, lidocaine and ropivacaine exert chondrotoxicity by reducing the chondrocyte viability13,14. The cell death or IA crystal formation caused by mitochondrial DNA damage in chondrocytes could possibly explain the potential toxicity of local anesthetics62. However, the exact mechanisms still needs to be further explored.
Strengths
The present study has several strengths. Firstly, this is the first meta-analysis that examined the analgesic effects of single-dose IA Mg after arthroscopic surgery and demonstrated its effectiveness and safety. Secondly, this study included both in vitro and in vivo experimental studies to elucidate the potential cartilage and chondrocyte protective effects of Mg supplementation. Thirdly, a comprehensive literature search was performed in several major databases to cover as many eligible RCTs or experimental studies as possible. Thus, the chance of missing any relevant study was fairly low. Lastly, the sensitivity and subgroup analyses supported the robustness of most findings.
Limitations
Limitations of the present study should also be acknowledged. Firstly, the number of retrieved RCTs was limited and the sample size of each trial was relatively small. This may bias the results. Secondly, a variety of factors may contribute to the heterogeneity of some indexes and affect the results, including the differences in the types of surgery, the dosage of Mg, the setting of follow-up time point, the ages of patients, the severity of pain and the unequal indications for arthroscopy. Especially, the selected dosages of IA Mg were ranged from 500 to 1000 mg, which required to be further investigated to determine the optimal dosage and to demonstrate the safety as the dosage increases. After all, two studies37,38 suggested that high level of Mg (50 and 100 mM) or extra-high concentration of Mg may not always be beneficial. Thirdly, because of the limited number of included RCTs, this study was unable to compare IA Mg with other local analgesics such as morphine and ropivacaine, which had been demonstrated effective after arthroscopic surgery by our previous meta-analyses3,8. However, other relevant studies suggested that Mg exhibited the similar analgesic effects when compared with morphine and amplified the effect of morphine or ropivacaine22,63,64,65,66,67. Finally, the limited retrieval of in vivo studies (one was about the collagen induced OA model, which is a severe model; the other one was related to stem cells) in the related area may limit the conclusion that Mg supplementation possess the chondroprotective effect.
Conclusion
Single-dose IA Mg at the end of arthroscopic surgery was effective in pain relief without increasing adverse reactions, and it could also enhance the analgesic effect of bupivacaine. In addition, Mg seemed to exhibit the cartilage or chondrocyte protective effect according to the experimental studies. Perhaps IA Mg should be considered as an alternative to local anesthetics after arthroscopic surgery. However, the optimal concentration and dosage of IA Mg still needs to be explored in further fully powered RCTs, to fully address this point.
Additional Information
How to cite this article: Zeng, C. et al. Analgesic effect and safety of single-dose intra-articular magnesium after arthroscopic surgery: a systematic review and meta-analysis. Sci. Rep. 6, 38024; doi: 10.1038/srep38024 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the Hunan Provincial Innovation Foundation for Postgraduate (CX2014A005), the National Natural Science Foundation of China (No. 81201420, 81272034, 81472130, 81501923), the Provincial Science Foundation of Hunan (No. 14JJ3032), the Scientific Research Project of the Development and Reform Commission of Hunan Province ([2013]1199), the Scientific Research Project of Science and Technology Office of Hunan Province (2013SK2018), the Doctoral Scientific Fund Project of the Ministry of Education of China (20120162110036).
Footnotes
Author Contributions All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. G.H.L., C.Z. and Y.S.L. conceived the study. G.H.L., C.Z. and Y.S.L. were responsible for conception and design of the study and drafted the manuscript. J.W., D.X.X., X.X. and L.J.L. contributed to data collection. J.W. contributed to preparation and data analysis. S.G.G., W.L., Y.L.X. and W.F.X. contributed to study retrieval. G.H.L. contributed to revision of the manuscript. All the authors contributed to the interpretation of the data and critically reviewed the manuscript for publication.
References
- Gelici Oral E., Hanci A., Ulufer Sivrikaya G., Dobrucali H. & Turkoglu Kilinc L. The analgesic effects of morphine and tramadol added to intra-articular Levobupivacaine-Tenoxicam combination for arthroscopic knee surgery on postoperative pain; A randomized clinical trial. Anesth Pain Med. 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura E. C. et al. Minimum effective concentration of bupivacaine in Ultrasound-Guided femoral nerve block after arthroscopic knee meniscectomy: A randomized, Double-Blind, controlled trial. Pain Physician. 19, E79–E86 (2016). [PubMed] [Google Scholar]
- Zeng C. et al. Single-Dose Intra-Articular morphine after arthroscopic knee surgery: A Meta-Analysis of randomized Placebo-Controlled studies. Arthroscopy. 29, 1450–1458 (2013). [DOI] [PubMed] [Google Scholar]
- Wei J. et al. Single-dose intra-articular bupivacaine versus morphine after arthroscopic knee surgery. Clin J Pain. 1 (2014). [DOI] [PubMed] [Google Scholar]
- Wei J., Yang H. B., Qin J. B., Kong F. J. & Yang T. B. Single-dose intra-articular bupivacaine after knee arthroscopic surgery: A meta-analysis of randomized placebo-controlled studies. Knee Surg Sports Traumatol Arthrosc. 22, 1517–1528 (2014). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Single-dose intra-articular bupivacaine plus morphine versus bupivacaine alone after arthroscopic knee surgery: A meta-analysis of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc (2015). [DOI] [PubMed] [Google Scholar]
- Sun Q., Liu S., Meng Q., Qu H. & Zhang Z. Single administration of intra-articular bupivacaine in arthroscopic knee surgery: A systematic review and meta-analysis. BMC Musculoskel. Dis. 16, 21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. et al. Single-dose intra-articular ropivacaine after arthroscopic knee surgery decreases post-operative pain without increasing side effects: A systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc (2015). [DOI] [PubMed] [Google Scholar]
- Sun R. et al. Intra-articular clonidine for post-operative analgesia following arthroscopic knee surgery: A systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 22, 2076–2084 (2014). [DOI] [PubMed] [Google Scholar]
- Wang Y. L. et al. Single-dose intra-articular bupivacaine plus morphine after knee arthroscopic surgery: A meta-analysis of randomised placebo-controlled studies. BMJ Open. 5, e6815 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D. et al. A Single-Dose Intra-Articular Morphine plus Bupivacaine versus Morphine Alone following Knee Arthroscopy: A Systematic Review and Meta-Analysis. PLoS One. 10, e140512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper S., Kramer J., Kim H. & Feeley B. Effects of local anesthetics on articular cartilage. Am J Sports Med. 39, 2245–2253 (2011). [DOI] [PubMed] [Google Scholar]
- Baker J. F., Byrne D. P., Walsh P. M. & Mulhall K. J. Human chondrocyte viability after treatment with local anesthetic and/or magnesium: Results from an in vitro study. Arthroscopy. 27, 213–217 (2011). [DOI] [PubMed] [Google Scholar]
- Baker J. F., Walsh P. M., Byrne D. P. & Mulhall K. J. In vitro assessment of human chondrocyte viability after treatment with local anaesthetic, magnesium sulphate or normal saline. Knee Surg Sports Traumatol Arthrosc. 19, 1043–1046 (2011). [DOI] [PubMed] [Google Scholar]
- Piper S. L. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 90, 986 (2008). [DOI] [PubMed] [Google Scholar]
- Grishko V., Xu M., Wilson G. & Pearsall A. T. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 92, 609–618 (2010). [DOI] [PubMed] [Google Scholar]
- Breu A., Rosenmeier K., Kujat R., Angele P. & Zink W. The cytotoxicity of bupivacaine, ropivacaine, and mepivacaine on human chondrocytes and cartilage. Anesth Analg. 117, 514–522 (2013). [DOI] [PubMed] [Google Scholar]
- Rao A., Johnston T., Harris A., Smith R. & Costouros J. Inhibition of chondrocyte and synovial cell death after exposure to commonly used anesthetics: Chondrocyte apoptosis after anesthetics. Am J Sports Med. 42, 50–58 (2014). [DOI] [PubMed] [Google Scholar]
- Cobo-Molinos J., Poncela-Garcia M., Marchal-Corrales J. A. & Delgado-Martinez A. D. Effect of levobupivacaine on articular chondrocytes. Eur. J. Anaesth. 31, 635–639 (2014). [DOI] [PubMed] [Google Scholar]
- Dragoo J. L., Braun H. J., Kim H. J., Phan H. D. & Golish S. R. The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med. 40, 794–799 (2012). [DOI] [PubMed] [Google Scholar]
- Tramer M. R., Schneider J., Marti R. A. & Rifat K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology. 84, 340–347 (1996). [DOI] [PubMed] [Google Scholar]
- Begon S., Pickering G., Eschalier A. & Dubray C. Magnesium increases morphine analgesic effect in different experimental models of pain. Anesthesiology. 96, 627–632 (2002). [DOI] [PubMed] [Google Scholar]
- Bondok R. S. Intra-articular magnesium is effective for postoperative analgesia in arthroscopic knee surgery. Brit. J. Anaesth. 97, 389–392 (2006). [DOI] [PubMed] [Google Scholar]
- Elsharmouby N. M., Eid H. E., Abou Elezz N. F. & Moharram A. N. Intraarticular injection of magnesium sulphate and/or bupivacaine for postoperative analgesia after arthroscopic knee surgery. Anesth Analg. 106, 1548–1552 (2008). [DOI] [PubMed] [Google Scholar]
- Farouk S. & Aly A. A comparison of intra-articular magnesium and/or morphine with bupivacaine for postoperative analgesia after arthroscopic knee surgery. J. Anesth. 23, 508–512 (2009). [DOI] [PubMed] [Google Scholar]
- Koltka K., Koknel-Talu G., Asik M. & Ozyalcin S. Comparison of efficacy of intraarticular application of magnesium, levobupivacaine and lornoxicam with placebo in arthroscopic surgery. Knee Surg Sports Traumatol Arthrosc. 19, 1884–1889 (2011). [DOI] [PubMed] [Google Scholar]
- Radwan Y. A., Alfeky A. A. & Faramawi M. F. Analgesic effect of intra-articular magnesium sulphate compared with bupivacaine after knee arthroscopic menisectomy. J Adv Res. 4, 355–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saritas T. B., Borazan H., Okesli S., Yel M. & Otelcioglu S. Is intra-articular magnesium effective for postoperative analgesia in arthroscopic shoulder surgery? Pain Res Manag. 20, 35–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulatif M., Amin S. M. M., Aboul-Ela A., Samuel E. W. M. & Abdel-Hakim S. M. A. Intra-articular versus intravenous magnesium-sulfate as adjuvant to femoral nerve block in arthroscopic knee surgery under general anesthesia: Randomized controlled trial. Egyptian Journal of Anaesthesia. 31, 239–246 (2015). [Google Scholar]
- Suhrita P., Prasad B., Ghosh S., Dawn S. & Niloy C. Postoperative analgesia for arthroscopic knee surgery: A comparison between intra-articular bupivacaine alone or in combination with magnesium sulphate or clonidine. Pharmacologyonline. 3, 938–943 (2009). [Google Scholar]
- Liberati A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 62, e1–e34 (2009). [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available at: www.cochrane-handbook.org. (Date of access: 01/03/2015).
- Juni P., Reichenbach S. & Dieppe P. Osteoarthritis: Rational approach to treating the individual. Best Pract Res Clin Rheumatol. 20, 721–740 (2006). [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egerbacher M., Wolfesberger B. & Gabler C. In vitro evidence for effects of magnesium supplementation on quinolone-treated horse and dog chondrocytes. Vet Pathol. 38, 143–148 (2001). [DOI] [PubMed] [Google Scholar]
- Feyerabend F., Witte F., Kammal M. & Willumeit R. Unphysiologically high magnesium concentrations support chondrocyte proliferation and redifferentiation. Tissue Eng. 12, 3545–3556 (2006). [DOI] [PubMed] [Google Scholar]
- Hagandora C. K., Tudares M. A. & Almarza A. J. The effect of magnesium ion concentration on the fibrocartilage regeneration potential of goat costal chondrocytes. Ann Biomed Eng. 40, 688–696 (2012). [DOI] [PubMed] [Google Scholar]
- Dou Y., Li N., Zheng Y. & Ge Z. Effects of fluctuant magnesium concentration on phenotype of the primary chondrocytes. J Biomed Mater Res. A. 102, 4455–4463 (2014). [DOI] [PubMed] [Google Scholar]
- Lee C. H. et al. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: Association with attenuation of N-methyl-d-aspartate (NMDA) receptor subunit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthritis Cartilage. 17, 1485–1493 (2009). [DOI] [PubMed] [Google Scholar]
- Shimaya M., Muneta T., Ichinose S., Tsuji K. & Sekiya I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthritis Cartilage. 18, 1300–1309 (2010). [DOI] [PubMed] [Google Scholar]
- Lee C. et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 511, 191–197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti , P., Bellone C. & Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 14, 383–400 (2013). [DOI] [PubMed] [Google Scholar]
- Tramèr M. R. & Glynn C. J. Magnesium bier’s block for treatment of chronic limb pain: A randomised, double-blind, cross-over study. Pain. 99, 235–241 (2002). [DOI] [PubMed] [Google Scholar]
- Takano Y., Sato E., Kaneko T. & Sato I. Antihyperalgesic effects of intrathecally administered magnesium sulfate in rats. Pain. 84, 175–179 (2000). [DOI] [PubMed] [Google Scholar]
- Felsby S., Nielsen J., Arendt-Nielsen L. & Jensen T. S. NMDA receptor blockade in chronic neuropathic pain: A comparison of ketamine and magnesium chloride. Pain. 64, 283–291 (1996). [DOI] [PubMed] [Google Scholar]
- Vuckovic S., Srebro D., Savic V. K. & Prostran M. The antinociceptive effects of magnesium sulfate and MK-801 in visceral inflammatory pain model: The role of NO/cGMP/K(+)ATP pathway. Pharm Biol. 53, 1621–1627 (2015). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Magnesium L-threonate prevents and restores memory deficits associated with neuropathic pain by inhibition of TNF-alpha. Pain Physician. 16, E563–E575 (2013). [PubMed] [Google Scholar]
- Ushida T. et al. Intradermal administration of magnesium sulphate and magnesium chloride produces hypesthesia to mechanical but hyperalgesia to heat stimuli in humans. J Neuroinflammation. 6, 25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begon S. et al. Role of spinal NMDA receptors, protein kinase C and nitric oxide synthase in the hyperalgesia induced by magnesium deficiency in rats. Br J Pharmacol. 134, 1227–1236 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teymourian H., Mohajerani S. A. & Farahbod A. Magnesium and ketamine gargle and postoperative sore throat. Anesth Pain Med. 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsumae T., Yamaguchi H., Inomata S. & Tanaka M. Magnesium sulfate attenuates tourniquet pain in healthy volunteers. J Anesth. 27, 231–235 (2013). [DOI] [PubMed] [Google Scholar]
- Shariat Moharari R. et al. Magnesium can decrease postoperative physiological ileus and postoperative pain in major non laparoscopic gastrointestinal surgeries: A randomized controlled trial. Anesth Pain Med. 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondón L. J. et al. Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain. J Physiol. 588, 4205–4215 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlechner B. Magnesium moderately decreases remifentanil dosage required for pain management after cardiac surgery. Brit J Anaesth. 96, 444–449 (2006). [DOI] [PubMed] [Google Scholar]
- Crosby V., Wilcock A. & Corcoran R. The safety and efficacy of a single dose (500 mg or 1 g) of intravenous magnesium sulfate in neuropathic pain poorly responsive to strong opioid analgesics in patients with cancer. J Pain Symptom Manage. 19, 35–39 (2000). [DOI] [PubMed] [Google Scholar]
- Qin B. et al. Association of dietary magnesium intake with radiographic knee osteoarthritis: Results from a population-based study. Arthrit Care Res. 64, 1306–1311 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. J. Evidence of altered bone turnover, vitamin D and calcium regulation with knee osteoarthritis in female twins. Rheumatology. 42, 1311–1316 (2003). [DOI] [PubMed] [Google Scholar]
- Zeng C. et al. Association between Dietary Magnesium Intake and Radiographic Knee Osteoarthritis. PLoS One. 10, e127666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C. et al. Relationship between Serum Magnesium Concentration and Radiographic Knee Osteoarthritis. J Rheumatol. 42, 1231–1236 (2015). [DOI] [PubMed] [Google Scholar]
- Gruber H. E. et al. Alterations in growth plate and articular cartilage morphology are associated with reduced SOX9 localization in the magnesium-deficient rat. Biotech Histochem. 79, 45–52 (2004). [DOI] [PubMed] [Google Scholar]
- Peng T. I. & Jou M. J. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 1201, 183–188 (2010). [DOI] [PubMed] [Google Scholar]
- Gulihar A., Robati S., Twaij H., Salih A. & Taylor G. J. Articular cartilage and local anaesthetic: A systematic review of the current literature. J Orthop. 12, S200–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrenberg A. et al. Antinociceptive effects of epidural magnesium sulphate alone and in combination with morphine in dogs. Vet Anaesth Analg. 42, 319–328 (2015). [DOI] [PubMed] [Google Scholar]
- Unlugenc H., Ozalevli M., Guler T. & Isik G. Postoperative pain management with intravenous patient-controlled morphine: Comparison of the effect of adding magnesium or ketamine. Eur J Anaesthesiol. 20, 416–421 (2003). [DOI] [PubMed] [Google Scholar]
- Kim E. M. et al. Magnesium as an adjuvant for caudal analgesia in children. Paediatr Anaesth. 24, 1231–1238 (2014). [DOI] [PubMed] [Google Scholar]
- Tauzin-fin P., Sesay M., Svart L., Krol-houdek M. C. & Maurette P. Wound infiltration with magnesium sulphate and ropivacaine mixture reduces postoperative tramadol requirements after radical prostatectomy. Acta Anaesth Scand. 53, 464–469 (2009). [DOI] [PubMed] [Google Scholar]
- Simon M. J. et al. Cardiovascular parameters and liver blood flow after infusion of a colloid solution and epidural administration of ropivacaine 0.75%: The influence of age and level of analgesia. Eur J Anaesthesiol. 26, 166–174 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.