Abstract
Introduction
Individuals with Parkinson's disease (PD) have to deal with several aspects of voice and speech decline and thus alteration of communication ability during the course of the disease. Among these communication impairments, 3 major challenges include: (1) dysarthria, consisting of orofacial motor dysfunction and dysprosody, which is linked to the neurodegenerative processes; (2) effects of the pharmacological treatment, which vary according to the disease stage; and (3) particular speech modifications that may be language-specific, that is, dependent on the language spoken by the patients. The main objective of the FraLusoPark project is to provide a thorough evaluation of changes in PD speech as a result of pharmacological treatment and disease duration in 2 different languages (French vs European Portuguese).
Methods and analysis
Individuals with PD are enrolled in the study in France (N=60) and Portugal (N=60). Their global motor disability and orofacial motor functions is assessed with specific clinical rating scales, without (OFF) and with (ON) pharmacological treatment. 2 groups of 60 healthy age-matched volunteers provide the reference for between-group comparisons. Along with the clinical examinations, several speech tasks are recorded to obtain acoustic and perceptual measures. Patient-reported outcome measures are used to assess the psychosocial impact of dysarthria on quality of life.
Ethics and dissemination
The study has been approved by the local responsible committees on human experimentation and is conducted in accordance with the ethical standards. A valuable large-scale database of speech recordings and metadata from patients with PD in France and Portugal will be constructed. Results will be disseminated in several articles in peer-reviewed journals and in conference presentations. Recommendations on how to assess speech and voice disorders in individuals with PD to monitor the progression and management of symptoms will be provided.
Trial registration number
NCT02753192, Pre-results.
Keywords: Parkinson’s disease, Dysarthria, Speech, Cross-language, Disease progression, Pharmacological treatment
Strengths and limitations of this study.
A multicentre (binational), cross-sectional, case-controlled study.
A cross-linguistic, multiparametric and holistic study of speech in Parkinson's disease (PD).
An interdisciplinary approach bringing together data analyses from the speech sciences and neurosciences.
A clinically reasonable number of individuals with PD.
No analysis of phonetic alterations and so far, not a longitudinal study: unable to address individuals' speech deterioration with time.
Introduction
Dysarthria in Parkinson's disease (PD)
Dysarthria denotes a motor speech disorder resulting from a lesion of the peripheral or central nervous system.1–3 Dysarthria and the psychosocial aspects of communication impairments are particularly disabling for individuals with PD. During the progression of the disease, between 70% and 79% of individuals with PD mention that speech4 5 and functional communication are impaired,6 7 contributing to social isolation8 and degradation of social interactions.9 These speech and communication disorders worsen along with the aggravation of other non-motor symptoms such as self-perception, depression10 and cognitive impairment.11 In addition to the alteration of speech intelligibility, signally a motor-driven speech deficit, it is also important to consider the importance of cognitive impairment on everyday communication in individuals with PD.12 Dysarthria can appear at any stage of PD, and usually worsens as the disease progresses,13 which suggests that it is also linked to the evolution of the pathological processes and non-dopaminergic brain circuits.14–16 The main deficits of PD speech are: loss of intensity (hypophonia), monotony of pitch and loudness, reduced stress, inappropriate silences, short rushes of speech, variable rate, imprecise consonant articulation and dysphonia (harsh and breathy voice).1 2 17 Previous studies have shown that treatments in PD have variable effects on these voice and speech symptoms.18 19
Although behavioural treatments mainly focus on two key indices of PD speech, pitch and intensity, dysprosody has been understudied. Yet prosody deficits represent an acoustic hallmark of dysarthria. First, perceptual and acoustic investigations of PD speech have reported alterations on fundamental frequency (F0), as part of speakers' reduced phonatory capacity, and thus the reduction of the frequency range is an indicator of dysarthria in PD.20 In particular, individuals with PD show a loss of the upper part of the frequency range.21 Degradation of prosody has been found to impact speech intelligibility and communication (eg, see ref. 22). Second, the temporal organisation of speech in PD has been addressed in reading tasks.23–25 In French, for example, speech rate tends to be slower in PD, which in turn seems to be correlated with longer pause times. Average durations of pauses are found to be longer in individuals with PD than in healthy individuals, while the average duration of sound sequences are similar.24 25 Studying dysprosody is thus important for differential diagnosis, identifying severity and the need and focus of treatment.20
Remaining challenges to assess dysarthria in PD: the rationale of the FraLusoPark study
Individuals with PD have to cope with several issues that contribute to voice and speech decline and thus to the alteration of communication ability during the course of the disease. Among these communication impairments, three major challenges include: (1) dysarthria, consisting of orofacial motor dysfunction and dysprosody, which is linked to the neurodegenerative processes; (2) the effects of the pharmacological treatment, which vary according to the disease stage and (3) the particular speech modifications that may be language-specific, that is, dependent on the language spoken by the patients. The main objective of the FraLusoPark project is to provide an extensive evaluation of dysarthric speech in PD as a result of pharmacological treatment and disease duration, using acoustic parameters (voice and prosody), perceptual markers (intelligibility) and patient-reported outcome measures (PROMs; psychosocial impact on quality of life) in speakers of two different languages (French and European Portuguese). Based on a large-scale binational collaboration, the interdisciplinary FraLusoPark project aims to address these issues by providing important insights in the domains of neurodegenerative disorders, speech sciences, neuropsychology, clinical research and patient rehabilitation.
Medication effects along disease progression
Early studies assessing the effect of the levodopa (L-dopa) on PD speech found favourable results, arguing for a beneficial effect, as for limb impairments.26–29 However, the long-term use of L-dopa is associated with motor complications which occur in up to 80% of patients.30 31 This may be the reason why the following studies reported no improvement32 33 and/or detrimental effects of L-dopa on speech.34 35 More recent studies face similar problems: beneficial effects of L-dopa can be observed in advanced patients with PD,36 37 whereas a lack of improvement is reported for speech parameters in early stage patients with PD.38 It is also commonly accepted that in the later stages of PD, non-motor symptoms (dementia, psychosis, depression and apathy) are a major source of disability together with axial symptoms (eg, alteration of gait, balance, posture, speech).39 Thus, both clinicians and researchers have to dissociate various intermingled effects. For example, when individuals with PD respond to L-dopa at an early stage of the disease, in time they are likely to experience speech decline that may be the result of the degeneration of non-dopaminergic structures and/or adverse effects of L-dopa (ie, dyskinesia). Despite the large number of recent studies that have focused on the effect of L-dopa on speech in PD,36–44 the question of disease evaluation still remains a matter of debate.
Language specificities of prosody
One missing component in the description of PD speech deficits is language-specific aspects, in particular of dysprosody. Prosodic information, including intonation, tempo, stress and rhythm, serves many functions for the listener and speaker: it helps to segment the continuous flow of spoken language into words, groups these words into phrases for interpretation, and indicates the relative importance and function of the interpreted meanings.45–48 Each language has its own prosodic structure. For example, although they are sister languages, French and European Portuguese (both Romance languages) differ prosodically in a number of important ways. European Portuguese has contrastive lexical stress: each content word (noun, adjective, verb, etc) has one syllable that is particularly salient or stressed, and changing the position of the lexical stress can change the meaning of a word.47 49–51 Stressed syllables may be accompanied by a pitch accent, realised as a modulation in F0 (eg, a rise or a fall) and aligned in language-specific ways with the syllable. In contrast, French has a fixed stress, characterised by a systematic F0 rise on the last syllable in a word.52 53 In French, stress is not a property of the word, but of a larger unit called the accentual phrase that can include one or more content words and any preceding function words (articles, prepositions, etc), often realised with F0 rises at its right and left edges. French listeners use these F0 rises as cues to word segmentation, finding the beginning and ends of words in the speech stream, and to lexical access, retrieving words from the mental lexicon.48 54
Such differences across languages make the comparison of prosodic deficits in individuals with PD particularly interesting. Very few studies of PD dysprosody have looked beyond global measures to examine the extent to which linguistically important, language-specific patterns are affected.55 56 Therefore, studying speech in individuals with PD whose languages include different prosodic modulations is essential in determining the role of prosody in patients' speech intelligibility and quality of life. Does a Portuguese patient experience different communication impairments compared with a French patient? If this is the case, could this difference be related, for example, to the fact that in Portuguese stress is distinctive and varies in position? Finally, how do these differences relate to disease duration and pharmacological treatment? Our project presents a novel approach to these questions and is characterised by the collection of cross-linguistic data in a single cohort.
Methods and analysis
FraLusoPark is a binational (data collection is performed in two countries: France and Portugal), cross-sectional (data are collected once for each participant) and case-controlled (both individuals with PD and control subjects are recruited) study, carried out in two different languages (French and European Portuguese).
Aims and hypotheses
The main objectives of our project are to evaluate modulations in voice/speech acoustics parameters (acoustics and prosody), perceptual markers (intelligibility) and PROMs (psychosocial impact of dysarthria in PD) across two different languages (French and European Portuguese).
Our three a priori hypotheses are the following: (1) global acoustic features are altered similarly in French and Portuguese individuals with PD; (2) language-specific prosodic patterns are altered differently in French and Portuguese individuals with PD and (3) the impact of speech disorders on intelligibility and quality of life depends on the cultural and linguistic environment. In addition, the FraLusoPark project will allow for a better understanding of the progression of speech symptoms and their response to pharmacological treatment, which is important for pathophysiological aspects and clinical management.
Participants
Two groups of 60 healthy volunteers (one in France and one in Portugal) are age-matched and sex-matched with the individuals with PD to provide control references for the obtained performance measures. Individuals with PD are recruited in France (N=60; Neurology Department, Centre Hospitalier du Pays d'Aix, Aix-en-Provence, France) and in Portugal (N=60; Movement Disorders Unit, Hospital de Santa Maria, Lisbon, and Campus Neurológico Sénior (CNS), Torres Vedras, Portugal) and correspond to the UK Parkinson's Disease Brain Bank Criteria57 for the diagnosis of idiopathic PD. Individuals with PD and healthy controls are all native speakers of French or European Portuguese speakers (French–European Portuguese bilinguals were excluded) and right-handed (Handedness Edinburgh test >80%).58 Inclusion and exclusion criteria of patients with PD and healthy controls are summarised in table 1. To assess the effects of L-dopa at various stages of the disease, we consider three subgroups of patients (N=20 patients each): subgroup 1, early, with a disease duration between 0 and 3 years and no motor fluctuations; subgroup 2, medium, with a disease duration between 4 and 9 years, or between 0 and 3 years and experiencing motor fluctuations; subgroup 3, advanced, with a disease duration of over 10 years.
Table 1.
Inclusion and exclusion criteria
| All participants | ||
| Inclusion criteria | ||
| Age between 35 and 85 years | ||
| Good cooperation | ||
| Ability to understand the information sheet | ||
| Given signed consent | ||
| Enrolled in a medical insurance plan | ||
| Other stable medical problems not interfering with the proposed study | ||
| Idiopathic Parkinson's disease* | ||
| Absence of any neurological, psychiatric or behavioural pathology† | ||
| Exclusion criteria | ||
| Illiteracy | ||
| French/Portuguese not native language, or bilingual | ||
| Participant under tutorship or guardianship, or any other administrative or legal dependence | ||
| No cooperation or consent withdrawn | ||
| Cognitive deficits, severe depression, dementia, psychosis (including medication-induced) or behavioural, neurological, medical, psychological disorders that may interfere with evaluations | ||
| Non-idiopathic Parkinson's disease* | ||
| Deep brain stimulation* | ||
| Severe motor impairment impeding participation in the study* | ||
*Patients with Parkinson's disease.
†Control subjects.
Study design
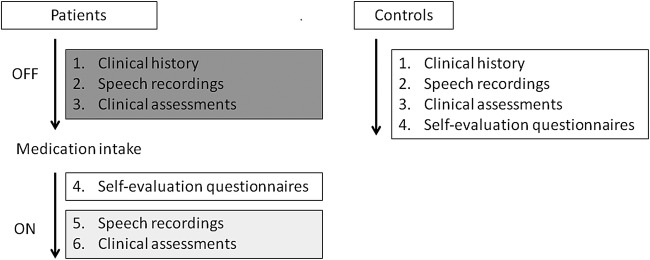
Healthy control participants undergo the same non-invasive assessments and examinations as individuals with PD. The only difference for patients is that they are evaluated twice, in the OFF and ON L-dopa states. This entails: (1) at least 12 hours after withdrawal of all anti-Parkinsonian drugs and (2) following at least 1 hour after the administration of the usual medication. The full study design is illustrated in figure 1.
Figure 1.
Overview of the FraLusoPark study design. Patient OFF-medication assessments (clinical history, speech recordings and clinical assessments, without medication) are shown in dark grey. ON-medication assessments (speech recordings and clinical assessments after medication are effective) are shown in light grey.
Speech recordings
In a quiet room, specialised speech recording equipment (EVA2 system, SQLab, Aix-en-Provence, France; http://www.sqlab.fr/; Marantz PMD661 MKII recorder, USA) is used for the speech/voice recordings. Participants are recorded while performing several speech production tasks with increasing complexity in a fixed order: (1) steady vowel /a/phonation (at a comfortable pitch and loudness) repeated three times; (2) maximum phonation time (vowel /a/sustained as long as possible on one deep breath at a comfortable pitch and loudness), repeated twice; (3) oral diadochokinesia (repetition of the pseudoword pataka at a fast rate for 30 s); (4) reading aloud of 10 words and 10 sentences created by adapting the intelligibility part of V.2 of the Frenchay Dysarthria Assessment (FDA-2);59 (5) reading aloud of a short text (‘The North Wind and the Sun’, French and European Portuguese adaptations);50 60 (6) storytelling speech guided by visual stimuli (pictures from the wordless story ‘Frog, Where are you?’,61 for the rationale of using this procedure and this book, see ref. 62); (7) reading aloud of a set of sentences with specific language-dependent prosodic properties (31 sentences in French, 20 in European Portuguese); and (8) free conversation for 3 min.
Acoustic measures
The acoustic measures characterise dimensions of aero-phonatory control.63 For the steady vowel /a/phonation, two kinds of measures will be extracted: first, for a macro analysis, F0 (Hz) and F0 variation (%); and second, for a micro analysis, perturbation measures such as jitter factor (%), absolute shimmer (dB) and harmonics-to-noise ratio (HNR, %). For the maximal phonation time, the longest duration (in seconds) of the sustained vowel /a/will be extracted. For the oral diadochokinesia task, the extracted measures will be the following: (1) the number of breath groups, that is, the period during which the pseudoword was repeated in a single expiration, (2) the ratio between the cumulated speech duration of the breath groups and the total duration of the session, that is, the proportion of speech, (3) the articulatory rate (syllables/second), (4) the pause-to-sound ratio (%) and (5) the speech proportion per number of breath groups. As an initial step to investigate global prosodic aspects of PD speech compared with the speech of healthy controls, we will extract the F0 curve of one sentence selected from the short text (‘The North Wind and the Sun’). This sentence is selected to be comparable across French and European Portuguese in terms of semantics and syllable length. This will provide a global phrasal pattern of F0 and intensity for patients and controls within and across languages (see below in the discussion for subsequent studies…). A summary of the acoustic measures that will be analysed is listed in table 2.
Table 2.
Acoustic measures
| Speech tasks | Function assessed | Acoustic measures |
|---|---|---|
| Steady vowel /a/ phonation | Phonation | Mean F0 (Hz) F0 variation (SD, in Hz) Shimmer (%)—cycle-to-cycle F0 variation Jitter (%)—cycle-to-cycle intensity variation HNR (%) |
| Maximal phonation time of the vowel /a/ | Aero-phonatory control | Longest duration (in seconds) |
| Oral diadochokinesia | Supralaryngeal articulatory control | Number of breath groups Proportion of breath groups (%) Articulatory rate (in syllables/second) Pause-to-sound ratio (%) Speech proportion ratio (%) |
| Reading aloud of text | Prosody | F0 range (Hz) Intensity (dB) |
F0, fundamental frequency; HNR, harmonics-to-noise ratio.
Clinical assessments
The neurological assessment is the Unified Parkinson's Disease Rating Scale,64 using the revised version provided by the Movement Disorders Society (MDS-UPDRS).65 The FDA-2 is used to assess the functions of the speech organs,59 reflecting the state of the muscular effectors involved in speech production. The original FDA-2, in English, includes an evaluation of intelligibility through 10 words and 10 short sentences. Using the same methodology, we developed a set of cross-linguistically adapted words and sentences in French and European Portuguese that will be used for the intelligibility assessment in each language. During the OFF medication state, individuals with PD will be administered the FDA-2 and the motor part (part 3) of the MDS-UPDRS. During the ON medication state, these two assessments are performed together with the non-motor (part 1.A) and motor complication (part 4) part of the MDS-UPDRS. During the ON medication state, the participants' cognitive abilities are evaluated using the Montreal Cognitive Assessment (MoCA),66 and the Clinical Global Impression (CGI) is also reported.67 For healthy controls, the assessment is similar to that of the patients with PD during ON medication (except part 4 of the MDS-UPDRS). A summary of the clinical assessments is given in table 3.
Table 3.
Clinical assessments and patient-reported outcome measures
| Description | Subsections | Minimum–maximum scores (worst values in bold) |
|---|---|---|
| A. Clinical assessments | ||
| Movement Disorders Society—Unified Parkinson's Disease Rating Scale (MDS-UPDRS)—Assessment of motor and non-motor features of Parkinson's disease65 | Non-motor experience of daily living—motor experience of daily living—motor examination—motor complications | 0–260 |
| Frenchay Dysarthria Assessment, second edition (FDA-2)—Assessment of speech and voice organs59 | Reflexes—Respiration—Lips—Palate—Larynx—Tongue—Intelligibility | 0–104 |
| Montreal Cognitive Assessment (MoCA)—Global assessment of cognitive functions66 | Visuospatial—Naming—Memory—Attention—Verbal fluency—Abstraction—Orientation | 0–30 |
| Clinical Global Impression (CGI)—Global impression of the clinician for the symptom67 | Speech | 1–7 |
| B. Patient-reported outcome measures (PROMs) | ||
| 39-Item Parkinson's Disease Questionnaire (PDQ-39)—Quality of life in Parkinson's disease71 | Mobility—Daily living activities—Emotional well-being—Stigma—Social support—Cognition—Communication—Body discomfort | 0–156 |
| Voice Handicap Index (VHI)72 | Physical—Functional—Emotional | 0–120 |
| Dysarthria Impact Profile (DIP)—Psychosocial impact of speech deficits68 | Effect of dysarthria on me—Accepting my dysarthria—How I feel others react to my speech—How dysarthria affects my communication—Dysarthria relative to other worries and concerns | 48–240 |
| Patient Global Impression (PGI)—Global impression of the patient on the dysfunction79 | Speech | 1–7 |
| Beck Depression Inventory (BDI)—Global assessment of the depression profile80 | 0–84 | |
Patient-reported outcome measures
PROMs, such as the Dysarthria Impact Profile (DIP),68 are used to obtain self-reported information about the functional impact of an individual's speech/communication impairment.69 Additional self-assessments focus on the patients' perception of their quality of life (the 39-Item Parkinson’s Disease Questionnaire (PDQ-39))70 71 and on how voice/speech impairment may induce a handicap (Voice Handicap Index, VHI).72 The French73 and European Portuguese adapted DIP, VHI74 75 and PDQ-3976–78 are used in our study. The Patient Global Impression (PGI) scoring79 and the Beck Depression Inventory (BDI)80 are also administered. The MDS-UPDRS also includes a patient self-assessment (parts 1.B and 2), which is administered together with the other questionnaires in the ON condition. For healthy controls, the assessment is the same as for individuals with PD. A summary of the PROMs from the self-administered questionnaires are listed in table 3.
Statistical analyses
The analyses of the data (acoustic, clinical measures and PROMs) will be performed with linear mixed-effects models that account for the variability across individuals, using the latest version of the statistical software R (R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2014. ISBN 3-900051-07-0, http://www.R-project.org/). For each performance measure, the between-group factors group (patients vs controls), disease duration (early vs medium vs advanced) and language (French vs European Portuguese) will be investigated. In addition, we will explore the effects of the within-patient factor medication (OFF vs ON) for all measures. Further relevant participant-related measures such as age, gender and education level will also be taken into account in the analyses.
Discussion
The present study will provide a unique, thorough and reliable assessment of PD voice, speech and prosody disorders and an evaluation of the impact of these aspects on the quality of life of individuals with PD.
Main and subsequent analyses of the FraLusoPark study
Acoustic and prosodic measurements (table 2), clinical assessment and PROMs (table 3) are the dependent variables to be analysed according to the statistical plan. These findings will be reported in the primary analysis as the main results of the project. However, the FraLusoPark investigation protocol will allow us to conduct additional analyses focusing on specific subdimensions of speech and voice deficits. There are at least four such subsequent analyses, exploring important aspects of PD speech and communication in more detail.
First, the intelligibility part of the FDA-259 requires the words and sentences produced by the patient to be perceptually rated by the clinician in charge of the assessment. Since these speech productions are recorded during the FraLusoPark protocol, an evaluation of speech intelligibility by panels of auditory judges in France and Portugal will be run. This will allow an additional and unbiased judgement of speech and voice disorders beyond that of the speech therapists and experts involved in the study. This approach further complements the global assessment of dysarthric speech in patients with PD.
Second, further prosodic analysis will be conducted on the defined sets of sentences, which manipulate language-general and language-specific details of prosody. One particular focus will be the analysis of tonal alignment in the F0 curve, that is, the temporal coordination of high and low tones with specific syllables in the sentences.81–83 Tonal alignment is likely to be a relevant factor in the study of PD dysprosody since it relies on the precise coordination of glottal and supraglottal articulatory gestures required to achieve language-specific temporal patterns for pitch accents and boundary tones.
Third, taking into consideration patients' personal feelings with respect to the physical, psychological and social domains has received increasing interest over the past decade. Individuals with PD are affected by voice and speech disorders, which contribute to an impairment of general communication abilities. Individuals with PD are therefore less likely to participate in conversations or social interactions.6 9 Several studies suggest that a growing discomfort in verbal communication during the progression of the disease leads to an important negative impact on social life.70 84 85 Taken together, these studies argue for experimental designs that include different types of speech assessments (clinical, perceptual, instrumental and psychosocial), as in the current protocol, and that explore the relationships between these different measures. Further analyses will therefore focus on linking the different dimensions of voice and speech description (eg, acoustic measures, FDA-2) with the contributions of various participant-related measures such as intelligibility, cognition and functional communication.
Fourth, production and prosodic parameters from the three different speech tasks (ie, short text reading, orientated picture description and conversation) will be compared. This will allow us to compare speech and voicing disorders in increasingly more complex communication contexts. These comparisons are of interest since communication abilities in PD are quite different in the presence of external cueing, such as during reading, compared with spontaneous speech, which involves more complex speech planning strategies.
Finally, the FraLusoPark study might provide us the opportunity to address the deterioration of the speech of individuals with PD over time. In a longitudinal follow-up study, we would recruit a subgroup of patients from group 1 (disease duration up to 4-year) and group 2 (disease duration between 4 and 10 years). These patients would be evaluated again at a later point in time (about 5 years after the original recordings). This would allow us to describe the precise progression of speech deficits associated with PD within the same individual for French and Portuguese speakers.
Ethics and dissemination
This study has been approved by the local responsible committees on human experimentation (France: Comité de Protection des Personnes, Sud Méditerranée 1, project reference number 13-84, approval date 9 January 2014; Portugal: Ethics Committee of the Lisbon Academic Medical Centre, project reference number 239-14, approval date 1 June 2014). The study is conducted in accordance with the ethical standards of the Declaration of Helsinki.86 The patients are included in the study after providing their written informed consent. The FraLusoPark trial is registered under the reference NCT02753192 (26 April 2016) on https://clinicaltrials.gov/.
Results of the FraLusoPark project will be disseminated at several research conferences at the national and international levels and published as articles in peer-reviewed journals and clinical magazines. The publication strategy is based on one principal article reporting the main results of the project and several subsequent articles deriving from it, including more detailed analyses of specific subdimensions of the speech and voice deficits.
Owing to inter-speaker variability, any generalisation drawn from speech parameters in the clinical population requires data from a large number of speakers.87 The FraLusoPark project is in line with this idea in that it builds a large-scale corpus of PD speech recordings and includes a large set of metadata (clinical examinations, speech measurements, linguistic features, patient-based indices). This allows a more accurate description of PD dysarthria, documenting the evolution of the symptoms and their response to pharmacological treatment. Both in medical (eg, http://www.mrc.ac.uk/research/research-policy-ethics/data-sharing/data-sharing-population-and-patient-studies/) and linguistic (http://sldr.org/) domains, data sharing is important to maximise the lifetime value of human health data. It is our intention to contribute to this practice by archiving our data for long-term preservation and making them accessible after the completion of our analyses.
Furthermore, an important recommendation from the International Classification of Functioning, Disability and Health88 is to improve quality of healthcare and to encourage clinicians to adopt a more holistic approach to the assessment and treatment of patients. Research in the field of speech sciences needs to incorporate this vantage point when studying pathological speech. The FraLusoPark project is in line with this perspective and will provide important recommendations for speech and voice assessments in patients with PD. This will be helpful to health practitioners and clinicians when monitoring the progression of symptoms and their management, and will also advance our understanding of dysarthria in PD within a cross-linguistic and cross-cultural context.
Footnotes
Contributors: SP and JJF are the principal investigators of the FraLusoPark study. They designed the study and ensure the good performance of the study. JJF and FV are the neurologists in charge of patient recruitment and neurological assessments. RC, JS, HS, CM, JC, FV and SP perform data acquisition and other clinical examinations. RC, JS, PO and IG are in charge of the pre-processing and analyses of acoustic measurements. CA-C and AL are in charge of the analyses of the PROMs and clinical assessments. PW, PO, MD, MC, SF and MV are the linguistic experts in charge of the prosody evaluations. IPM is the neurobehaviour, language and cognition expert. SP wrote the draft of the present article. All coauthors commented and revised it critically for important intellectual content, and approved the final version to be published.
Funding: This study is supported by a bilateral transnational funding between France and Portugal: support from the French government, through the French National Agency for Research (ANR—Agence Nationale de la Recherche—grant number ANR-13-ISH2-0001-01) and from the Portuguese government, through the Portuguese National Foundation for Science and Technology (FCT—Fundação para a Ciência e a Tecnologia—grant number FCT-ANR/NEU-SCC/0005/2013). CA-C wishes to thank his PhD grant scheme co-funders: PACA Regional Council and Orthomalin (http://www.orthomalin.com/).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: France: Comité de Protection des Personnes, Sud Méditerranée 1, project reference number 13-84, approval date 09/01/2014; Portugal: Ethics Committee of the Lisbon Academic Medical Centre, project reference number 239-14, approval date 12/06/2014.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We addressed this point in the “Ethics and dissemination” section of the article, as follows: Both in medical (eg, http://www.mrc.ac.uk/research/research-policy-ethics/data-sharing/data-sharing-population-and-patient-studies/) and linguistic (http://sldr.org/) domains, data sharing is important to maximise the lifetime value of human health data. It is our intention to contribute to this practice by archiving our data for long-term preservation and making them accessible after the completion of our analyses.
References
- 1.Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. J Speech Hear Res 1969;12:462–96. 10.1044/jshr.1203.462 [DOI] [PubMed] [Google Scholar]
- 2.Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res 1969;12:246–69. 10.1044/jshr.1202.246 [DOI] [PubMed] [Google Scholar]
- 3.Duffy JR. Defining, understanding, and categorizing motor speech disorders. In: Duffy JR, edr. Motor speech disorders—substrates, differential diagnosis, and management. 3rd edn Saint Louis: Elsevier Mosby, 2013:3–13. [Google Scholar]
- 4.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson's disease and multiple sclerosis: a survey. Folia Phoniatr Logop 1994;46:9–17. 10.1159/000266286 [DOI] [PubMed] [Google Scholar]
- 5.Miller N, Deane KHO, Jones D et al. . National survey of speech and language therapy provision for people with Parkinson's disease in the United Kingdom: therapists’ practices. Int J Lang Commun Disord 2011;46:189–201. 10.3109/13682822.2010.484849 [DOI] [PubMed] [Google Scholar]
- 6.Miller N, Noble E, Jones D et al. . Life with communication changes in Parkinson's disease. Age Ageing 2006;35:235–9. 10.1093/ageing/afj053 [DOI] [PubMed] [Google Scholar]
- 7.Ramig LO, Bonitati CM, Lemke JH et al. . Voice treatment for patients with Parkinson's disease. Development of an approach and preliminary efficacy data. J Med Speech Lang Pathol 1994;2:191–209. [Google Scholar]
- 8.Karlsen KH, Tandberg E, Arsland D et al. . Health related quality of life in Parkinson's disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatr 2000;69:584–9. 10.1136/jnnp.69.5.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox C, Ramig L. Vocal sound pressure level and self-perception of speech and voice in men and women with idiopathic Parkinson disease. Am J Speech Lang Pathol 1997;2:29–42. [Google Scholar]
- 10.Miller N, Noble E, Jones D et al. . How do I sound to me? Perceived changes in communication in Parkinson's disease. Clin Rehabil 2008;22:14–22. [DOI] [PubMed] [Google Scholar]
- 11.Barnish MS, Whibley D, Horton SMC et al. . Roles of cognitive status and intelligibility in everyday communication in people Parkinson's disease: a systematic review. J Parkinsons Dis 2016;6:453–62. 10.3233/JPD-150757 [DOI] [PubMed] [Google Scholar]
- 12.Schneider JS, Sendek S, Yang C. Relationship between motor symptoms, cognition, and demographic characteristics in treated mild/moderate Parkinson's disease. PLoS ONE 2015;10:e0123231 10.1371/journal.pone.0123231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klawans HL. Individual manifestations of Parkinson's disease after ten or more years of levodopa. Mov Disord 1986;1:187–92. 10.1002/mds.870010304 [DOI] [PubMed] [Google Scholar]
- 14.Agid Y, Graybiel AM, Ruberg M et al. . The efficacy of levodopa treatment declines in the course of Parkinson's disease: do nondopaminergic lesions play a role? Adv Neurol 1990;53:83–100. [PubMed] [Google Scholar]
- 15.Braak H, Braak E, Yilmazer D et al. . Nigral and extranigral pathology in Parkinson's disease. J Neural Transm Suppl 1995;46:15–31. [PubMed] [Google Scholar]
- 16.Halliday G, Lees A, Stern M. Milestones in Parkinson's disease—clinical and pathologic features. Mov Disord 2011;26:1015–21. 10.1002/mds.23669 [DOI] [PubMed] [Google Scholar]
- 17.Ho AK, Bradshaw JL, Iansek R et al. . Speech volume regulation in Parkinson's disease: effects of implicit cues and explicit instructions. Neuropsychologia 1999;37:1453–60. 10.1016/S0028-3932(99)00067-6 [DOI] [PubMed] [Google Scholar]
- 18.Pinto S, Ozsancak C, Tripoliti E et al. . Treatments for dysarthria in Parkinson's disease. Lancet Neurol 2004;3:547–56. 10.1016/S1474-4422(04)00854-3 [DOI] [PubMed] [Google Scholar]
- 19.Atkinson-Clement C, Sadat J, Pinto S. Behavioral treatments for speech in Parkinson's disease: meta-analyses and review of the literature. Neurodegener Dis Manag 2015;5:233–48. 10.2217/nmt.15.16 [DOI] [PubMed] [Google Scholar]
- 20.Patel R. Assessment of prosody. In: Lowit A, Kent R, eds. Assessment of motor speech disorders. San Diego: Plural Publishing, 2011:75–96. [Google Scholar]
- 21.Viallet F, Meynadier Y, Lagrue B et al. . The reductions of tonal range and of average pitch during speech production in “off” parkinsonians are restored by L-DOPA. Mov Disord 2000;15:S131. [Google Scholar]
- 22.Cheang HS, Pell MD. An acoustic investigation of Parkinsonian speech in linguistic and emotional contexts. J Neurolinguistics 2007;20:221–41. 10.1016/j.jneuroling.2006.07.001 [DOI] [Google Scholar]
- 23.Hammen VL, Yorkston KM. Speech and pause characteristics following speech rate reduction in hypokinetic dysarthria. J Comm Disord 1996;29:429–44. 10.1016/0021-9924(95)00037-2 [DOI] [PubMed] [Google Scholar]
- 24.Duez D. Organisation temporelle de la parole et dysarthrie parkinsonienne. In: Ozsancak C, Auzou P, eds. Les troubles de la parole et de la déglutition dans la maladie de Parkinson. Marseille: Solal, 2005:195–211. [Google Scholar]
- 25.Duez D. Segmental duration in Parkinsonian French speech. Folia Phoniatr Logop 2009;61:239–46. 10.1159/000228001 [DOI] [PubMed] [Google Scholar]
- 26.Rigrodsky S, Morrison EB. Speech changes in parkinsonism during L-dopa therapy: preliminary findings. J Am Geriatr Soc 1970;18:142–51. 10.1111/j.1532-5415.1970.tb02111.x [DOI] [PubMed] [Google Scholar]
- 27.Mawdsley C, Gamsu CV. Periodicity of speech in Parkinsonism. Nature 1971;231:315–16. 10.1038/231315a0 [DOI] [PubMed] [Google Scholar]
- 28.Leanderson R, Meyerson BA, Persson A. Effect of L-dopa on speech in Parkinsonism. An EMG study of labial articulatory function. J Neurol Neurosurg Psychiatry 1971;34:679–81. 10.1136/jnnp.34.6.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano KK, Zubick H, Tyler HR. Speech defects of parkinsonian patients. Effects of levodopa therapy on speech intelligibility. Neurology 1973;23:865–70. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira JJ, Rascol O, Poewe W et al. . A double-blind, randomized, placebo and active-controlled study of nebicapone for the treatment of motor fluctuations in Parkinson's disease. CNS Neurosci Ther 2010;16:337–47. 10.1111/j.1755-5949.2010.00145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mestre T, Ferreira JJ. Pharmacotherapy in Parkinson's disease: case studies. Ther Adv Neurol Disord 2010;3:117–26. 10.1177/1756285609352366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe VI, Garvin JS, Bacon M et al. . Speech changes in Parkinson's disease during treatment with L-dopa. J Commun Disord 1975;8:271–9. 10.1016/0021-9924(75)90019-2 [DOI] [PubMed] [Google Scholar]
- 33.Quaglieri CE, Celesia GG. Effect of thalamotomy and levodopa therapy on the speech of Parkinson patients. Eur Neurol 1977;15:34–9. 10.1159/000114786 [DOI] [PubMed] [Google Scholar]
- 34.Marsden CD, Parkes JD. “On-off” effects in patients with Parkinson's disease on chronic levodopa therapy. Lancet 1976;1:292–6. 10.1016/S0140-6736(76)91416-1 [DOI] [PubMed] [Google Scholar]
- 35.Critchley EM. Letter: peak-dose dysphonia in parkinsonism. Lancet 1976;1:544 10.1016/S0140-6736(76)90337-8 [DOI] [PubMed] [Google Scholar]
- 36.De Letter M, Santens P, De Bodt M et al. . The effect of levodopa on respiration and word intelligibility in people with advanced Parkinson's disease. Clin Neurol Neurosurg 2007;109:495–500. 10.1016/j.clineuro.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 37.De Letter M, Santens P, Estercam I et al. . Levodopa-induced modifications of prosody and comprehensibility in advanced Parkinson's disease as perceived by professional listeners. Clin Linguist Phon 2007;21:783–91. 10.1080/02699200701538181 [DOI] [PubMed] [Google Scholar]
- 38.Skodda S, Visser W, Schlegel U. Short- and long-term dopaminergic effects on dysarthria in early Parkinson's disease. J Neural Transm (Vienna) 2010;117:197–205. 10.1007/s00702-009-0351-5 [DOI] [PubMed] [Google Scholar]
- 39.Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol 2012;8:435–42. 10.1038/nrneurol.2012.126 [DOI] [PubMed] [Google Scholar]
- 40.Goberman A, Coelho C, Robb M. Phonatory characteristics of parkinsonian speech before and after morning medication: the ON and OFF states. J Commun Disord 2002;35:217–39. 10.1016/S0021-9924(01)00072-7 [DOI] [PubMed] [Google Scholar]
- 41.Goberman AM, Blomgren M. Parkinsonian speech disfluencies: effects of L-dopa-related fluctuations. J Fluency Disord 2003;28:55–70. 10.1016/S0094-730X(03)00005-6 [DOI] [PubMed] [Google Scholar]
- 42.Pinto S, Gentil M, Krack P et al. . Changes induced by levodopa and subthalamic nucleus stimulation on parkinsonian speech. Mov Disord 2005;20:1507–15. 10.1002/mds.20601 [DOI] [PubMed] [Google Scholar]
- 43.Skodda S, Flasskamp A, Schlegel U. Instability of syllable repetition in Parkinson's disease—influence of levodopa and deep brain stimulation. Mov Disord 2011;26:728–30. 10.1002/mds.23439 [DOI] [PubMed] [Google Scholar]
- 44.Skodda S, Grönheit W, Schlegel U. Intonation and speech rate in Parkinson's disease: general and dynamic aspects and responsiveness to levodopa admission. J Voice 2011;25:e199–205. 10.1016/j.jvoice.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 45.Ladd R. Intonational phonology. Cambridge: Cambridge University Press, 1996. [Google Scholar]
- 46.Frota S. Nuclear falls and rises in European Portuguese: a phonological analysis of declarative and question intonation. Probus 2002;14:113–46. 10.1515/prbs.2002.001 [DOI] [Google Scholar]
- 47.Frota S. The intonational phonology of European Portuguese. In: Jun SA, edr. Prosodic typology II. The phonology of intonation and phrasing, chapter 2. Oxford: Oxford University Press, 2014:6–42. [Google Scholar]
- 48.Welby P. The role of early fundamental frequency rises and elbows in French word segmentation. Speech Commun 2007;49:28–48. 10.1016/j.specom.2006.10.005 [DOI] [Google Scholar]
- 49.Cruz-Ferreira M. Intonation in European Portuguese. In: Hirst D, Di Cristo A, eds. Intonation systems. a survey of twenty languages. Cambridge: Cambridge University Press, 1998:167–78. [Google Scholar]
- 50.Cruz-Ferreira M. Part 2: Illustrations of the IPA. Portuguese (European). In: Handbook of the International Phonetic Association. A guide to the use of the International Phonetic Alphabet. Cambridge: Cambridge University Press, 1999:126–30. [Google Scholar]
- 51.Frota S. Prosody and focus in European Portuguese. Phonological phrasing and intonation. New York: Garland Publishing, 2000. [Google Scholar]
- 52.Jun SA, Fougeron C. Realizations of accentual phrase in French. Probus 2002;14:147–72. [Google Scholar]
- 53.Welby P. French intonational structure: evidence from tonal alignment. J Phonetics 2006;34:343–71. 10.1016/j.wocn.2005.09.001 [DOI] [Google Scholar]
- 54.Spinelli E, Grimault N, Meunier F et al. . An intonational cue to word segmentation in phonemically identical sequences. Atten Percept Psychophys 2010;72:775–87. 10.3758/APP.72.3.775 [DOI] [PubMed] [Google Scholar]
- 55.Ma JK, Whitehill TL, So SY. Intonation contrast in Cantonese speakers with hypokinetic dysarthria associated with Parkinson's disease. J Speech Lang Hear Res 2010;53:836–49. 10.1044/1092-4388(2009/08-0216) [DOI] [PubMed] [Google Scholar]
- 56.Whitehill TL. Studies of Chinese speakers with dysarthria: informing theoretical models. Folia Phoniatr Logop 2010;62:92–6. 10.1159/000287206 [DOI] [PubMed] [Google Scholar]
- 57.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatr 1988;51:745–52. 10.1136/jnnp.51.6.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- 59.Enderby P, Palmer R. Frenchay Dysarthria Assessment. (FDA-2). 2nd edn Austin, TX: Pro-ED, 2007. [Google Scholar]
- 60.Fougeron C, Smith CL. Part 2: illustrations of the IPA. French. In: Handbook of the International Phonetic Association. A guide to the use of the International Phonetic Alphabet. Cambridge: Cambridge University Press, 1999:78–81. [Google Scholar]
- 61.Mayer M. Frog, where are you? Sequel to a boy, a dog and a frog. New-York: Dial books, 1969. [Google Scholar]
- 62.Berman RA, Slobin DI. Relating events in narrative: a crosslinguistic developmental study. Hillsdale, NJ: Lawrence Erlbaum Associates,1994. [Google Scholar]
- 63.Baken RJ, Orlikoff RF. Clinical measurement of speech and voice. San Diego: Singular Publishing Group Inc, 2000. [Google Scholar]
- 64.Fahn S, Elton RL, members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, eds. Recent developments in Parkinson's disease, Vol. 2. Florham Park: MacMillan Health Care Information, 1987:153–64. [Google Scholar]
- 65.Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–50. 10.1002/mds.10473 [DOI] [PubMed] [Google Scholar]
- 66.Nasreddine ZS, Phillips NA, Bédirian V et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 67.Busner J, Targum SD. The Clinical Global Impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 68.Walshe M, Peach RK, Miller N. Dysarthria impact profile: development of a scale to measure psychosocial effects. Int J Lang Com Disord 2009;44:693–715. 10.1080/13682820802317536 [DOI] [PubMed] [Google Scholar]
- 69.Walshe M. The psychosocial impact of acquired motor speech disorders. In: Lowit A, Kent RD, eds. Assessment of motor speech disorders. San Diego: Plural Publishing Inc, 2011:97–122. [Google Scholar]
- 70.Jenkinson C, Peto V, Fitzpatrick R et al. . Self-reported functioning and well-being in patients with Parkinson's disease: comparison of the short-form health survey (SF-36) and the Parkinson's Disease Questionnaire (PDQ-39). Age Ageing 1995;24:505–9. 10.1093/ageing/24.6.505 [DOI] [PubMed] [Google Scholar]
- 71.Peto V, Jenkinson C, Fitzpatrick R et al. . The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 1995;4:241–8. 10.1007/BF02260863 [DOI] [PubMed] [Google Scholar]
- 72.Jacobson BH, Johnson A, Grywalski C et al. . The Voice Handicap Index (VHI), development and validation. Am J Speech Pathol 1997;6:66–70. 10.1044/1058-0360.0603.66 [DOI] [Google Scholar]
- 73.Letanneux A, Viallet F, Walshe M et al. . The Dysarthria Impact Profile: a preliminary French experience with Parkinson's disease. Parkinsons Dis 2013;2013:403680 10.1155/2013/403680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guimarães I, Abberton E. An investigation of the Voice Handicap Index with speakers of Portuguese: preliminary data. J Voice 2004;18:71–82. 10.1016/j.jvoice.2003.07.002 [DOI] [PubMed] [Google Scholar]
- 75.Woisard V, Bodin S, Puech M. [The Voice Handicap Index: impact of the translation in French on the validation]. Rev Laryngol Otol Rhinol 2004;125:307–12. [PubMed] [Google Scholar]
- 76.Auquier P, Sapin C, Ziegler M et al. . [Validation of the French language version of the Parkinson's Disease Questionnaire—PDQ-39]. Rev Neurol (Paris) 2002;158:41–50. [PubMed] [Google Scholar]
- 77.Souza RG, Borges V, Silva SM et al. . Quality of life scale in Parkinson's disease PDQ-39 (Brazilian Portuguese version) to assess patients with and without levodopa motor fluctuation. Arq Neuro Psiq 2007;65:787–91. 10.1590/S0004-282X2007000500010 [DOI] [PubMed] [Google Scholar]
- 78.Vieira EM. Qualidade de vida na doença de Parkinson. Ph.D. thesis. Faculdade de Medicina da Universidade de Coimbra 2008.
- 79.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004;27:26–3. 10.1016/j.jmpt.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 80.Beck AT, Ward CH, Mendelson M et al. . An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 81.D'Imperio M. Prosodic representations (section on tonal alignment). In: Cohn A, Fougeron C, Huffman M, eds. The Oxford Handbook of Laboratory Phonology. Oxford: Oxford University Press, 2011:275–87. [Google Scholar]
- 82.Welby P, Lœvenbruck H. Anchored down in anchorage: syllable structure and segmental anchoring in French. Italian J Ling 2006;18:74–124. [Google Scholar]
- 83.Frota S. Tonal association and target alignment in European Portuguese nuclear falls. In: Gussenhoven C, Warner N, eds. Laboratory phonology 7. Berlin/New York: Mouton de Gruyter, 2002:387–418. [Google Scholar]
- 84.Martínez-Martin P. An introduction to the concept of “quality of life in Parkinson's disease”. J Neurol 1998;245:S2–6. 10.1007/PL00007733 [DOI] [PubMed] [Google Scholar]
- 85.Kuopio AM, Marttila RJ, Helenius H et al. . The quality of life in Parkinson's disease. Mov Disord 2000;15:216–23. [DOI] [PubMed] [Google Scholar]
- 86.World Medical Association. 59th General Assembly. Seoul, Korea: World Medical Association, 2008. [Google Scholar]
- 87.Ghio A, Pouchoulin G, Teston B et al. . How to manage sound, physiological and clinical data of 2500 dysphonic and dysarthric speakers? Speech Commun 2012;54:664–79. 10.1016/j.specom.2011.04.002 [DOI] [Google Scholar]
- 88.World Health Organisation (WHO). International Classification of Functioning (ICF), Disability and Health. Geneva, Switzerland: World Health Organisation, 2001. [Google Scholar]