Abstract
As a neuroprosthetic technique, functional electrical stimulation (FES) can restore lost motor performance of impaired patients. Through delivering electrical pulses to target muscles, the joint movement can be eventually elicited. This work presents a real-time FES system which is able to deal with two neuroprosthetic missions: one is estimating FES-induced joint torque with evoked electromyograph (eEMG), and the other is artificially controlling muscle activation with such eEMG feedback. The clinical experiment results on spinal cord injured (SCI) patients and healthy subjects show promising performance of the proposed FES system.
Key Words: Functional electrical stimulation (FES), Torque prediction, Muscle activation control, Hybrid stimulation system
Functional electrical stimulation (FES) is a neuroprosthetic technique aiming at assisting restoring motor functions of impaired patients who survive from spinal cord injury (SCI) or stroke, through delivery of electrical short pulses to target muscles. As a useful neuroprosthetic technique, FES has been widely applied among patients who suffer motor injury and neural disorder on motor restoring/retraining tasks, such as grasping or lifting objects, correcting drop foot during ambulation. Current FES systems are designed based on open-loop principle and have been widely used in clinical environment. Such open-loop based FES systems manipulate stimulation parameters (e.g., pulse width) manually without considering adaptive modeling and control between stimulation and muscle biofeedback and joint mechanical output. Such open-loop systems lack feedback information to adjust model parameters and control configuration adaptively, which may degrade performances and limit application domains. To remedy disadvantages of open-loop FES systems, closed loop FES systems are to be developed for clinical environment1-3. Closed-loop FES systems possess better adaptivity in modeling and control but have not yet been widely applied for clinical uses. Evoked electromyography (eEMG) is considered as a direct image of electrical muscle activity under stimulation: it has been frequently used as biofeedback for control and estimation purposes in FES4-6. In this work, we present a hybrid real-time closed-loop FES system which both can control muscle activations and predict FES-induced torque based on eEMG. Such FES system was tested on able-bodied and SCI subjects, and promising joint torque prediction and muscle activation results demonstrate its feasibility and efficiency.
Materials and Methods
System Architecture and Experiment Setup
The hybrid FES system consisted of two functional parts. The first one controls muscle activation with eEMG feedback, and the second one estimates/predicts FES-induced joint torque with eEMG. The whole functionality of the system is shown on Fig. 1. Model predictive controller is designed in the control part and Kalman filter/recurrent neural network (RNN) is used in the torque estimation part6.
Fig 1.
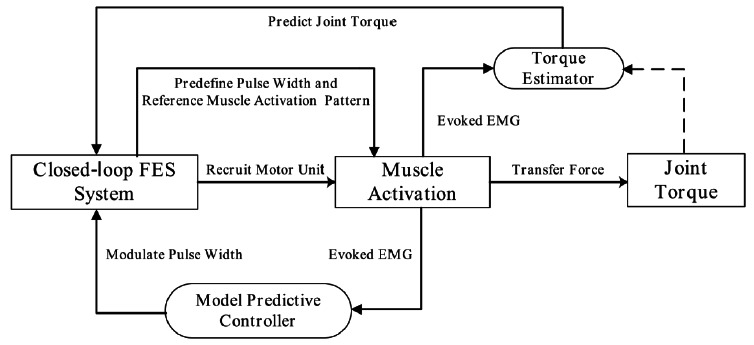
The architecture diagram of the hybrid FES system. The system contains two functionality parts: one is to control muscle activations with eEMG feedback and the other is to predict FES-induced torque based on eEMG.
Subjects
The system tests were conducted on able-bodied subjects and SCI patients upon their consent with a protocol approved by Nimes Ethics Committee, France, 2013.
System
The system consists of a wireless stimulator7 (emanating from a transfer of our technology to Vivaltis Inc., Montpellier, France), eEMG (MP100, Biopac Systems Inc., Santa Barbara, CA, USA), torque acquisition devices (Biodex 3, Shirley Corp., NY, USA), and a laptop computer with the MATLAB (version 2012a) interface for remote control of wireless stimulator and real-time data acquisition/processing. The wireless stimulator is a regulated current dual-channel stimulator. The main features of the wireless stimulator are as follows: maximal current is 100mA with 0mA to 1mA step on a maximum load of 1kW, stimulation frequency ranges from 1Hz to 1kHz, onset pulse-width is 50ms with 1ms step, and electrical polarity can be configured according as required. Pulse width and Intensity are dynamically and remotely adjustable. The maximum pulse width for the experiment is limited to 450ms, the stimulation intensity is chosen between 25mA and 35mA, and the stimulation frequency is chosen between 30Hz–40Hz to ensure a good fusion of muscle’s contraction.
For eEMG acquisition, the bipolar AgCl EMG electrodes were positioned over the muscle belly in the direction of muscle fiber with 20mm interelectrode spacing. The reference electrode was placed on the patella of contra lateral leg. Electrical current pulses were delivered to the Tibialis (TA) or Medial Gastrocnemius (MG) muscle group with surface electrodes placed. The raw eEMG signals are acquired at a sampling frequency fs = 4096Hz through Biopac 100 system in order to capture the full range samplings of M-wave. The real-time identification and control are performed between 2 stimulation pulses so that the update and control frequency is the same as the stimulation frequency. The computation window for the eEMGis thus of the length of the period between 2 pulses i.e. around Nwin = fs/fstim) where fs and fstim denote the eEMG sampling frequency and stimulation frequency respectively. Stimulation artifact suppression and mean-absolute operations using eEMG signals are performed.
The blanking template operation is used to suppress the stimulation artifacts8, and such blanking operation is performed in every Nwin samplings of eEMG at a frequency fstim. The mean absolute values (MAV) of the Nwin samplings eEMG are computed at a frequency fstim, and then the muscle activations under every stimulation loop are obtained through normalization of the maximum MAV of eEMG against their maximum. For joint torque estimation computations, the subject needed to be seated on the chair with the ankle at 90 degrees, while the joint center was aligned with the axis of a calibrated dynamometer. The shank was adjusted with the knee joint at 40 degrees. The foot was strapped to the pedal to transmit ankle torque to the dynamometer and to allow the optimal recording of isometric/non-isometric ankle torque. Raw eEMG of TA or MG muscle group and ankle joint torque were recorded, amplified (gain 1000) and sampled at a frequency fsamp = 4096Hz through the Biopac amplifier and a 16-bit A/D national instrument card.
Results
FES-induced torque prediction with eEMG
The stimulation frequency fstim was set between 30 and 40Hz leading to the loop execution time between 25ms and 33ms. The suitable stimulation intensities were found to be from 20mA to 50mA specific to each patient. Then the stimulation intensity is fixed and PW modulation is performed to have different levels of muscle contractions to induce regular joint movement. The test session included 2 phases: identification and prediction phases. Each sequence contained trapezoidal trains consisting of 2s stimulation (0.5s ramp up, 1s plateau and 0.5s ramp-down) and 2s rest. During identification phase that lasts between 33 and 40s, the plateau stimulation PW of each trapezoidal train was increased gradually with step size of from 40% to 100% of the maximum PW. After identification, the plateau stimulation PW was randomly determined within 50% to 100% of maximum PW in the prediction phase. The stimulation artifacts in the raw eEMG were removed by blanking with a window size at 10ms. Both the stimulus-activation model and activation-torque model are identified during the same identification phase. Fig. 2 shows the ankle joint torque prediction results based on RNN which was proposed6 for one SCI patient. Observed from the figure, we can see the joint torque prediction seems promising on the SCI patient, with root mean square error (RMSE) being 0.24Nm and variance accounted for (VAF) being 78.75% respectively.
Fig 2.
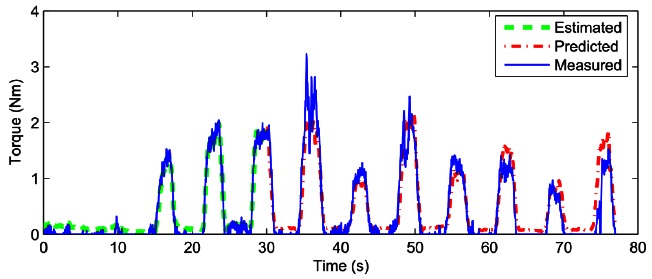
Prediction of FES-induced ankle joint torque with eEMG for a single SCI patient.
Muscle Activation Control with eEMG
Afterwards, the model predictive controller is computing the PW level with PW-NMAV model updated at every stimulation loop and send the PW value to the wireless stimulator to perform the tracking control. Four different reference patterns of muscle activation under FES were tested and part of these results are presented: Every control loop is executed within 20ms, which does not exceed the maximum allowable time period for every stimulation loop k. In this paper, the desired muscle activation for the realtime control by FES is generated artificially following two patterns: 1) One kind of natural patterns with two continuous sinusoidal contraction9 for drop foot correction; 2) Actual muscle contraction pattern measured by EMG envelope from the able-bodied subject during a whole cycle of actual walking test. Fig. 3 shows the control results on one SCI patient, we could see from this figure that the muscle activation controlled by the FES controller can track the desired reference quite well.
Fig 3.
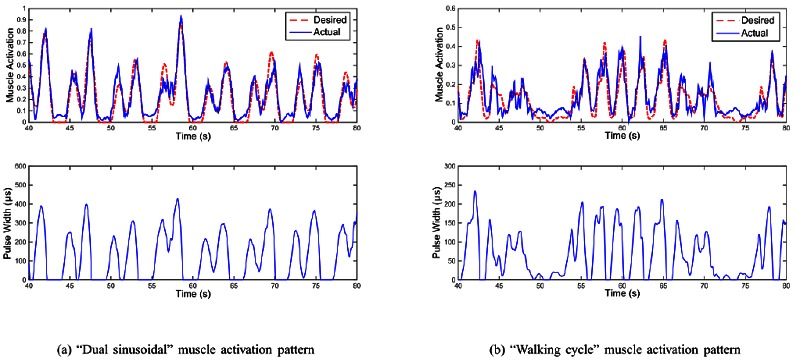
(a) “Dual sinusoidal” muscle activation pattern; (b) “Walking cycle” muscle activation pattern. Real-time control results on one SCI patient. Upper of subfigure: real-time control performance of muscle activation with desired muscle activation patterns (red dash line is desired muscle activation trajectory and blue solid line is the measured muscle activation under the muscle activation control by FES). Lower of subfigure: the corresponding computed stimulation pulse width, which was systematically generated by the predictive FES controller.
Discussion
This paper presents a hybrid FES system which can control muscle activation and predict joint torque with eEMG. Clinical experiment results on SCI subjects verify its feasibility and efficiency. Future work could be extended to applying such FES system on estimation/control issues of multiple muscles/joints with muscle/joint synergy considered10, and comparison of M-wave during dynamic contractions and during isometric contractions is also worth investigating.
Acknowledgement
The authors wish to thank all PROPARA therapists and the patients. This paper was prepared for the IFESS Conference 2016.
Contributor Information
Zhan Li, Email: zhan.li@uestc.edu.cn.
David Guiraud, Email: guiraud@lirmm.fr.
David Andreu, Email: andreu@lirmm.fr.
Charles Fattal, Email: cfattal@cos-asso.org.
Anthony Gelis, Email: A.GELIS@propara.fr.
References
- 1.Li Z, Hayashibe M, Andreu D, Guiraud D. Real-time closed-loop fes control of muscle activation with evoked emg feedback, in Neural Engineering (NER), 2015 7th International IEEE/EMBS Conference 2015;623–626. [Google Scholar]
- 2.Jezernik S, Wassink R, Keller T. Sliding mode closed-loop control of fes controlling the shank movement, Biomedical Engineering, IEEE Transactions 2004; 51:263–272. [DOI] [PubMed] [Google Scholar]
- 3.Azevedo C, Sijobert B, Froger J, Fattal C. Preliminary developments towards closed-loop fes-assistance of posture and gait, IFAC PapersOnLine 2015;48:333 – 337. [Google Scholar]
- 4.Chen J, Yu NY. The validity of stimulus-evoked emg for studying muscle fatigue characteristics of paraplegic subjects during dynamic cycling movement, Rehabilitation Engineering, IEEE Transactions 1997;5:170–178. [DOI] [PubMed] [Google Scholar]
- 5.Erfanian A, Chizeck H, Hashemi R. Using evoked emg as a synthetic force sensor of isometric electrically stimulated muscle. Biomedical Engineering, IEEE Transactions 1998;45:188–202. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Hayashibe M, Fattal C, Guiraud D. Muscle fatigue tracking with evoked emg via recurrent neural network: Toward personalized neuroprosthetics. Computational Intelligence Magazine IEEE 2014;9: 38–46. [Google Scholar]
- 7.Toussaint M, Andreu D, Fraisse P, Guiraud D. Wireless distributed architecture for therapeutic functional electrical stimulation: a technology to design network-based muscle control. Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE 2010;6218–6221. [DOI] [PubMed] [Google Scholar]
- 8.Frigo C, Ferrarin M, Frasson W, Pavan E, Thorsen R. Emg signals detection and processing for on-line control of functional electrical stimulation,” J Electromyogr Kinesiol 2000;10:351-360. [DOI] [PubMed] [Google Scholar]
- 9.O’Keeffe DT, Donnelly AE, Lyons GM. The development of a potential optimized stimulation intensity envelope for drop foot applications, Neural Systems and Rehabilitation Engineering, IEEE Transactions 2003;11:249–56. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Guiraud D, Hayashibe M. Inverse estimation of multiple muscle activations from joint moment with muscle synergy extraction. IEEE J Biomed Health Inform. 2015;19:64-73. doi: 10.1109/JBHI.2014. 2342274. Epub 2014 Jul 23. [DOI] [PubMed] [Google Scholar]


