Abstract
Functional Electrical Stimulation assisted cycling (FES-Cycling) is increasingly becoming an alternative option recommended to people with spinal cord injury struggling with paraplegia and interested in practicing sports. In order to propose preconditions to guide FES-Cycling recommendation, we aimed to investigate some features and their potential relationships with responsiveness to Neuromuscular Electrical Stimulation (NMES). Fourteen volunteers attended a public recruitment forum to be assessed about their responsiveness through the 16-sessions of NMES. Volunteers were separated in two groups (responsive and non-responsive to NMES) which were investigated in the light of some personal, clinical, structural and functional features. Fifty seven percent of the initial sample responded to electrical stimulation with a visual contraction. This responsive group was predominantly composed by subjects presenting traumatic spinal cord injuries above T12 vertebral level. Only two subjects became responsive at the 3rd and 16th sessions. Among the observed features, the etiology and level of injuries seems to be more associated to responsiveness. Our observations seem to indicate that subjects with traumatic spinal cord injury above T12 level were the best potential candidates for FES-cycling.
Key Words: electrical stimulation, bicycling, parathletics, spinal cord
Several research studies have proposed ergometers for Functional Electrical Stimulation (FES) as an option to provide active lower limbs involvement in alternative therapy1, and to develop locomotion devices for daily and leisure activities in cases of paraplegia2. Among such alternatives, we have also found FES assisted cycling (FES-Cycling), may be also perceived as a potential opportunity for people living with spinal cord injuries and interested in practicing sports3. In these different contexts, we could find detailed reports about technical advances and benefits arising out of the FES-Cycling training by which natural and artificial muscular recruitment is triggered by sophisticated system, in contrast, we could hardly find information of what would be the preconditions and the parameters to recommend and prepare a FES-Cycling training in the cases of paraplegia. In order to explore the characteristics of the concerned public and get insights to guide protocols to recommend FES-Cycling, we aimed to investigate some personal, clinical, structural and functional characteristics of people with paraplegia interested in sports, assessing their responsiveness to neuromuscular electrical stimulation (NMES) and the potential aspects linked to this issue. We describe how the target persons were recruited and assessed to compose responsiveness and non-responsiveness groups for analysis.
Materials and Methods
Recruitment
In order to identify paraplegics who may be interested in participating in this experimental protocol, we launched a public recruitment forum offering FES-Cycling training. No elective criterions were considered, since we wanted to explore the responsiveness to electrical stimulation of persons of all backgrounds interested in attending FES-Cycling.
After clarification was provided an informed consent was signed (CAAE 50337215.1.0000.0030, approval number 1.413.934, local ethical committee), a screening visit was scheduled with volunteers to register information about their personal (age, gender and sports practice) and health conditions (history of spinal cord injury, time since injury and clinical features). During the same visit, the international standards for the Classification of Spinal Cord Injury were applied according to recommendations from the American Spinal Injury Association (ASIA)4, followed by physical assessment in order to record some structural and functional variables. At the end of the physical assessment, an interview guided by the Functional Independence Measures (FIM) was performed5. On completion of the screening visit, the participants followed a 16-sessions protocol of NMES in order to investigate the responsiveness to electrical stimulation among the subjects recruited.
Protocol
The participants were enrolled in a 16-sessions protocol starting with a knee extension program via surface NMES applied on quadriceps muscle and progressing to other muscle groups as the responsiveness was positively visualized by means of contractions strength classified as grade 3/5 (movement possible against gravity). A 16-session protocol was applied because the most responsive participant had responded to NMES at the first session (NMES1) and got a stimulation sequence of progression through the muscular groups starting from quadriceps to hamstrings, glutei, tibialis anterior and triceps sural muscles in this period of time. Muscles strength was rated using the Medical Research Council (MRC) scale of 0/5 to 5/5 in which 0/5 represented no contraction; 1/5 muscle flicker, but no movement; 2/5 movement possible, but not against gravity when the join was tested in its horizontal plane; 3/5 movement possible against gravity, but not against resistance by the examiner; 4/5 movement possible against some resistance by the examiner and 5/5 normal strength. The 16-sessions of NMES training involved, at the beginning, repeated series of isometric contractions in which the subjects had their legs extended and feet fixed. The sessions were numbered from NMES1 up to NMES16. When the subjects started to respond with contractions reaching the grade 3/5, the series with free joint were initialized. As contractions progressed to higher grades (4/5 or 5/5), more functional exercises simulating real situations were introduced (i.e. sit-to-stand transfer, cycling, etc.). Each session lasted one hour maximum with variable durations determined by fatigue observation. One to three days of rest between sessions was scheduled. The NMES unit, a four channel stimulator providing rectangular biphasic current (Dualpex 071 Quark®) was used. During effective stimulation, frequency of 50Hz, intensity ranging from 0 to 69 mA and pulse width of 800μs were used. The intensity was increased from 0 up to a visual contraction (1/5) or 50 mA, following the protocol FES training employed. Electrodes (5x9 cm2) were placed on the skin at a location in the quadriceps muscle where the better muscular response was found.
Results
Fourteen volunteers (3 males and 11 females) aging between 23 and 56 years old living with paraplegia from 2 up to 50 months responded to the call. Out of these (n=14), eight (57%) presented a visible muscular contraction triggered by the NMES (graded 1/5) at the first electrical stimulation session (NMES1) taking part in the responsive group (Table 1). The participants without visible contraction at the NMES1 (graded 0/5) composed the non-responsive group. The total sample, considering responsiveness and non-responsiveness, presented predominantly complete spinal cord injury (64%, ASIA by completeness A classified under medical diagnosis) with people practicing from zero to four different types of sports simultaneously.
Table 1.
Clusters of quantitative and qualitative variables recorded by their responsiveness and non-responsiveness
| Clusters of variables | Responsiveness | Non-responsiveness | |
|---|---|---|---|
| Quantitive/ qualitative | measures (units) classes | ||
| Size | 8 [57%] | 6 [43%] | |
| Personal factors | |||
| Age | birthday (years) | 23,00 ├32,50┤ 47,00 | 28,00├29,00┤ 56,00 |
| Gender | male | 3 [38%] | 2 [33%] |
| female | 11 [63%] | 12 [67%] | |
| Sports practice | sports played (amount) | 0,00 ├2,00┤ 3,00 | 2,00 ├2,50┤ 4,00 |
| Health condition | |||
| Damage by etiology | traumatic | 8 [100%] | 4 [67%] |
| infeccious | 0 [0%] | 2 [33%] | |
| Time since injury | day of the event (years) | 2,00 ├11,00┤ 26,00 | 3,00 ├14,00┤ 50,00 |
| Level of injury | above T12 | 8 [100%] | 3 [50%] |
| inside bellow T12 | 0 [0%] | 3 [50%] | |
| ASIA by completeness | A | 5 [63%] | 4 [67%] |
| B, C, D e E | 3 [37%] | 2 [33%] | |
| Structural and functional domain | |||
| ASIA by subscores | Motor right (sum) | 15,00 ├25,00┤ 27,00 | 25,00 ├25,50┤ 26,00 |
| left (sum) | 15,00 ├25,00┤ 28,00 | 25,00 ├25,50┤ 26,00 | |
| Sensory right (sum) | 15,00 ├29,50┤ 80,00 | 22,00 ├56,50┤ 78,00 | |
| left (sum) | 18,00 ├28,50┤ 78,00 | 22,00 ├56,50┤ 78,00 | |
| BMI | kg/m2 | 18,26 ├23,18┤ 32,77 | 19,04 ├24,35┤ 27,85 |
| Circumferences | Thigh level right (cm) | 40,00 ├4S,00┤ 53,00 | 32,00 ├49,00┤ 56,00 |
| left (cm) | 36,00 ├46,50┤ 53,00 | 31,00 ├50,75┤ 60,00 | |
| Calf leveal right (cm) | 24,00 ├28,25┤ 35,50 | 22,00 ├28,00┤ 31,00 | |
| left (cm) | 26,00 ├31,00┤ 35,00 | 23,00 ├27,75┤ 28,00 | |
| Chest expansion | Axillary level (cm) | 4,00 ├6,00┤ 8,00 | 4,00 ├6,00┤ 6,00 |
| Mamary level (cm) | 2,00 ├4,00┤ 6,00 | 3,00 ├5,50┤ 6,00 | |
| Xiphoid level (cm) | 2,00 ├3,50┤ 8,00 | 2,00 ├4,00┤ 7,00 | |
| Hemodinamic stability | SBP at rest (mmHg) | 90,00 ├100,00┤ 150,00 | 110,00 ├125,00┤ 140,00* |
| DBP at rest(nnHg) | 60,00 ├68,00┤ 110,00 | 70,00 ├80,00┤ 90,00 | |
| HR at rest (bmp) | 50,00 ├67,50┤ 74,00 | 52,00 ├63,00┤ 69,00 | |
| Activity and participation | |||
| FTM | total score | 70,00 ├112,00┤ 118,00 | 101,00 ├114,50┤ 119,00 |
Comparative analysis
Once the volunteers were separated into responsive or non-responsive groups, they were compared to each other and/or to the total sample (n=14). In the table 1, values from quantitative variables are presented by median between minimum and maximum intervals (min ├median┤ max), considering a non-Gaussian distribution; in turn, qualitative variables are shown by absolute (n) and relative (%) frequencies distributed by each class. Each quantitative/qualitative variable has its unit/classes indicated in the column “measures units/classes”. Differences between proportions observed by responsive behavior (responsiveness or non-responsiveness) were detected by comparisons between groups, taking as a basis the proportion expected in the total sample by the Fisher’s exact test (p<0.05). The tables cells highlighted in boxes in the “responsiveness” and “non-responsiveness” columns were used to indicate significant differences (p<0.05) between observed (groups) and expected proportions (sample). As observed in the same table, all subjects from the responsive group had their damage caused by traumatic accident while in the non-responsive group it was found that some damages were also caused by infections (Transverse myelitis). Both proportions highlighted by the boxes were significantly different from the total sample which presented 86% traumatic and 14% infectious causes. Concerning the level of injury, above or inside/bellow the twelfth thoracic level (T12), we observed, also highlighted in table 1, a sub-sample totally composed by subjects with injuries above T12 level in the responsive group, while in the non-responsive group subjects with injuries above and inside/bellow T12 level were present in equal proportions. Both proportions observed were different from the expected in the total sample (79% above and 21% inside/bellow T12). The Mann Whitney test applied in the comparisons between responsiveness and non-responsiveness (Table 1) only detected significant differences (p<0.05) for SBP at rest, showing that all participants, in the non-responsiveness group, presented systolic blood pressure (SBP) at rest higher than 110 mmHg while responsiveness group presented fifty percent (50%) of their subjects recording SBP at rest lower than 100 mmHg at rest.
Responsiveness through the 16-sessions of NMES
Not all participants had the same sequence of responsiveness to NMES (Figure 1). We had two subjects who only responded to electrical stimulation with contractions scored with 1/5 contraction strength at the third (NMES3) and sixteenth sessions (NMES16). The stimulation was just applied to the other muscle groups in the progression after have obtained a 1/5 contraction in the previous muscle group of the established sequence of progression. These two mentioned volunteers increased the responsiveness size from 57% to 64% at NMES3 and to 71% at the NMES16. At the NMES16 the proportion observed was significantly different from the expected (NMES1) as detected by the Fisher’s exact test (p<0.05).
Fig 1.
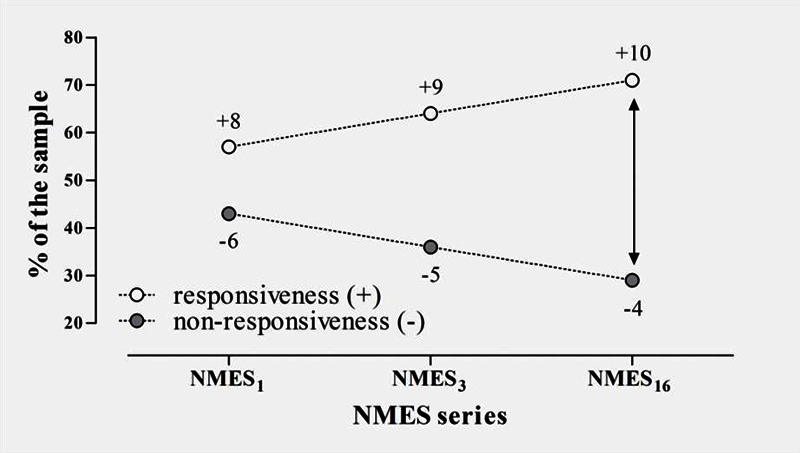
Sample proportion presenting at least 1/5 contraction during the 16-sessions protocol. Progressive increase into responsiveness may be observed.
Responsiveness and level of injury
All volunteers who responded to NMES1 had spinal cord injuries above T12 (responsive group) while among them who did not respond to NMES1 we found half above and half inside/below T12. At NMES3, a volunteer who had a T5 level spinal cord injury started to respond with a 1/5 contraction, changing non-responsiveness proportion to 40% above and 60% inside/below T12 level (Figure 2). When reaching the NMES16, another volunteer who had spinal cord injury at the T12 level started to respond with a 1/5 contraction, returning the non-responsiveness proportion to 50% above and 50% inside/below T12 level and the responsiveness group came into possession a subject with spinal cord injury inside/below T12 (Figure 2). It was interesting to point out that when we removed infectious cause from the level of injury analysis, as observed in the pairs of groups indicated by subjects having only traumatic spinal cord injuries remained in the distributions in the figure 2, we could observe, with the exception of only one subject (T12 level); that all volunteer in the non-responsive group had spinal cord injuries inside or below T12 level.
Fig 2.
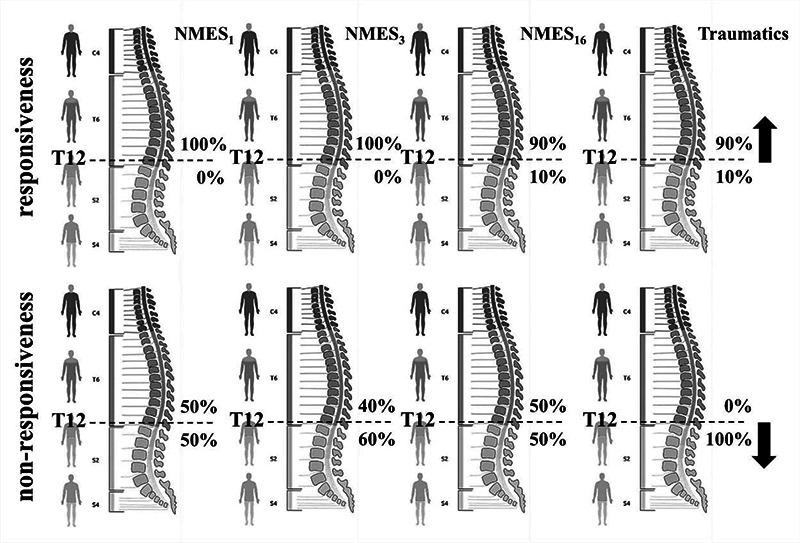
Adjustments in the course of the 16-sessions of NMES on the proportions founded for the volunteers who had spinal cord injuries above or inside/below T12 level by groups (responsiveness and non-responsiveness).
Discussion
Our preliminary public recruitment towards parameters and protocols to recommend FES-Cycling for people with paraplegia interested in sports reveals to be important to consider features related to etiology (physical trauma) and level of the spinal cord injury. Before the analysis of the responsiveness observed in our sample, we had hypothesized that time since injury could be a feature responsible to non-responsiveness to NMES. Our initial hypothesis found basis in the evidences showing substantial tissue atrophies followed the paraplegia acquisition which drastically reduces physical activity and motor/vascular functions in the lower limbs as long as time goes on6. However, no significant difference in terms of time since injury was found between groups which could justify more inactivity in the lower limbs generating structural atrophies and motor/vascular dysfunctions enhanced by the time in non-responsive group. Maybe, the lack of difference could be explained by the fact that our sample was composed by active subjects who practiced different types of sports. Other features not related to time since injury must be considered in order to analyze responsiveness, as aspects related the nature of the damage and its impact. To explain our results, we hypothesized that after spinal cord injury, the individuals in the responsive group present signals and symptoms related to the disconnection syndrome (i.e. damage in the descending motor fibers from the motoneurons situated in the motor cortex to the motoneurons in the spinal cord)7 and the individuals in the non-responsive group present damage of the peripheral motor pathways (i.e. located at nerve roots and/or the axons of the motoneurons)8. Unfortunately we did not record variables related to disconnection syndrome, a limitation to confirm our hypothesis. Nevertheless, it is interesting to notice that no subject whom damage cause was infectious responded to NMES, even above T12 level and after 16-sessions protocol. The two subjects presented diagnostic of transverse myelitis caused by infection, had neurologic impairment in ventral column of the cord9 with damage in specific motoneurons inside and below their levels of injury. How the mechanism of action of the NMES involves electrical stimulation exciting the motor nerve going to muscle and not muscle itself10, we believe that the peripheral motor pathways were damaged in the two subjects diagnosed with transverse myelitis, justifying the non-responsiveness. In general, the subjects with traumatic spinal cord injuriy inside/below T12 level did not respond to NMES because the lumbosacral spinal cord is almost entirely contained in the segments inside the vertebral T12 level where all motoneurons to innervate the hip and legs coming from11. However, we had a participant whom level of injury was T12 and, in the NMES16, he began to respond. In this case is important to consider that the levels were recorded from the medical diagnoses, not examining voluntary motor or conscious sensory function below the injury site (ASIA recommendations). The clinical examination of the level, extent, severity8 and motor impairment (disconnection syndrome or peripheral motor pathways) could be useful to explain his responsiveness at the NMES16. The systolic blood pressure (SBP) was the unique variable in the functional domain of this study to present a significant difference between groups. The non-responsive group manifested SBP significant higher than the responsive group. It could be interesting to investigate more detailed this behavior once the non-responsive group was mostly formed by low level of injury volunteers who had more intact sympathetic nervous system. However, the SBP was only measured before the protocol application, a limitation to discuss this point. After having explored the characteristics of the people with spinal cord injury interested in practicing sports, searching for insights to guide protocols to recommend FES-Cycling, we conclude that a little more than half part of our subject sample (57%) responded to the NMES at the first application, increasing to 71% at the end of our 16-sessions knee extension protocol training. Among the observed features, the etiology and level of injuries seems to be more associated to responsiveness, showing that the subjects with traumatic spinal cord injury above T12 level are the best potential candidates for FES-cycling.
Contributions
JAG: recruitment of volunteers, experimental and clinical setup implementation, clinical data acquisition; LOdF: experimental and technical setup implementation, technical data acquisition; CCdS-C-P: coordination of the clinical and theoretical aspects of the study, protocol design; APLB: coordination of the technical aspects of the study, technical expertise in stimulator programming; CF: study design, expertise in FES applied to SCI rehabilitation; CA-C: protocol and setup design expertise; EF-M: protocol design, processing of data, advisor and coordination of the project with rehabilitation expertise.
Acknowledgement
We thank professor U. Araujo who provided us an excellent work environment and a network of collaboration between CETEFE – Associação Centro de Treinamento de Educação Física Especial and the NTAAI – Núcleo de Tecnologia Assistiva, Acessibilidade e Inovação from FCE-UnB. We also thank the financial support to CACAO by INRIA and FAPDF, CAPES and CNPq and the participants who give us motivation to continue this work.
Contributor Information
Juliana Araujo Guimarães, Email: juliana16grey@gmail.com.
Lucas Oliveira da Fonseca, Email: lucasfonseca27@gmail.com.
Clarissa Cardoso dos Santos-Couto-Paz, Email: clarissacardoso@yahoo.com.br.
Antônio Padilha Lanari Bó, Email: apastilha@gmail.com.
Charles Fattal, Email: cfattal@cos-asso.org.
Christine Azevedo-Coste, Email: christine.azevedo@inria.fr..
References
- 1.Gföhler M, Lugner P. Cycling by means of functional electrical stimulation IEEE Transl Rehabil Eng 2000;8:233–43. [DOI] [PubMed] [Google Scholar]
- 2.Perkins TA, Donaldson NN, Hatcher NAC, et al. Control of leg-powered paraplegic cycling using stimulation of the lumbo-sacral anterior spinal nerve roots. IEEE Transl Neur Sys Rehabil Eng 2002;10:158-64. [DOI] [PubMed] [Google Scholar]
- 3.Fornusek C, Davis G. Cardiovascular and metabolic responses during functional electric stimulation cycling at different cadences. Arch Phys Med Rehabil 2008;89:719-25. [DOI] [PubMed] [Google Scholar]
- 4.American Spinal Injury Association (ASIA), International standards for neurological classification of spinal cord injury. www.asiaspinalinjury.org, available in February, 28th 2016. [Google Scholar]
- 5.Riberto M Miyazaki MH Jucá SSH.et al. . Validation of the Brasilian version of Function Independence Measure. Acta Fisiátrica 2004;11:72-6. [Google Scholar]
- 6.Castro MJ, Apple DF, Jr, Staron RS, et al. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86 350-8. [DOI] [PubMed] [Google Scholar]
- 7.Geschwind N. Disconnexion syndromes in animal and man. Brain 1965;88:237–94. [DOI] [PubMed] [Google Scholar]
- 8.Curt A, Dietz V. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal cord 1999;37:157–65. [DOI] [PubMed] [Google Scholar]
- 9.West TW, Hess C, Cree BAC. Acute transverse myelitis: Demyelinating, inflammatory, and infectious myelopathies. Semin Neurol 2012;32:97–113. [DOI] [PubMed] [Google Scholar]
- 10.Newham DJ, Donaldson Nde N. FES cycling. Acta Neurochir 2007;97:395–402. [DOI] [PubMed] [Google Scholar]
- 11.Martin J.H. Neuroanatomy: text and atlas, 2nd ed., New York: Appleton & Lange, 1996, 578. [Google Scholar]


