Abstract
Recumbent cycling exercise achieved by functional electrical stimulation (FES) of the paralyzed leg muscles is effective for cardiopulmonary and musculoskeletal conditioning after spinal cord injury, but its full potential has not yet been realized. Mechanical power output and efficiency is very low and endurance is limited due to early onset of muscle fatigue. The aim of this work was to compare stochastic modulation of the inter-pulse interval (IPI) to constant-frequency stimulation during an isokinetic leg extension task similar to FES-cycling. Seven able-bodied subjects participated: both quadriceps muscles were stimulated (n = 14) with two activation patterns (P1-constant frequency, P2-stochastic IPI). There was significantly higher power output with P2 during the first 30 s (p = 0.0092), the last 30 s (p = 0.018) and overall (p = 0.0057), but there was no overall effect on fatiguability when stimulation frequency was randomly modulated.
Key Words: functional electrical stimulation, fes cycling, interpulse interval, stochastic randomization
Functional electrical stimulation (FES) is commonly used to perform a functional task by artificially activating paralyzed skeletal muscles. Similar to other applications of this technology, recumbent cycling exercise achieved by functional electrical stimulation (FES) of the paralysed leg muscles is effective for cardiopulmonary1 and musculoskeletal2-4 conditioning after spinal cord injury, but its full potential has not yet been realised. Various studies highlighted current limitations of this technology: mechanical power output and efficiency are very low5-9 and endurance is limited due to early onset of muscle fatigue.10
Modulation of neuromuscular stimulation parameters (pulse amplitude, pulse width and frequency) as well as electrode positioning affects the muscle response to stimulation. The effect of pulse amplitude and duration on muscle fatigue is not as predominant as the effects of frequency.11 Hence, different stimulation patterns (constant-frequency trains, initial doublet or triplet trains, doublet trains, and variable-frequency trains) have been studied with the goal of maximizing force and minimizing fatigue.12–15 Various studies examined the performance of different muscle activation strategies applied to single muscle groups during the simplified task of non-isometric dynamometry. Increased force was obtained in non-fatigued and fatigued muscle using variable-frequency trains, in comparison to constant-frequency trains. 16 Other studies examined the effects of stochastic modulation of inter-pulse interval,17-19 which is equivalent to stochastically modulating the pulse frequency.18 It was reported that the amount of time that a leg could be extended against gravity was significantly increased when the inter-pulse interval (IPI) was varied (compared to constant frequency stimulation), but the particular method employed of random modulation of amplitude and pulse width did not appear to have a significant effect on the fatigue rate and the force response of isometric contractions of the quadriceps.19 During voluntary muscle contractions, trains of action potentials are asynchronous in time and some stochastic modulation of the spacing between the action potentials exists. FES, in contrast, usually employs synchronous stimulation and causes the muscle fibres to contract simultaneously.20 Hence, the idea of stochastically modulating the IPI deserves more attention.
The aim of this work was to compare stochastic modulation of the IPI to constant IPI stimulation during an isokinetic leg extension task mimicking FES-cycling with respect to mechanical power output and fatiguability.
Materials and Methods
Short-term performance was compared using repeated, randomised application of different trains during a single experimental session. In order to eliminate the possible confounding effects of timing and possible co-contraction of other muscle groups, stimulation trains were applied repetitively to one key muscle group in isolation (quadriceps).
Subjects
Nine able-bodied subjects were recruited (seven males and two females, age 25-36 years). Subjects were required to abstain from intense physical activity involving the lower limbs during the 24 h prior to each test. Two subjects were not included in the data analysis because no observable response could be elicited from the quadriceps during stimulation. Thus, data were analyzed for an equivalent of 14 muscle groups (n = 14). The study was approved by the Ethics Committee of the Canton of Bern, Switzerland. All participants gave written, informed consent.
Experimental setup
A seated leg extension/curl bench (RLE-382, Tuffstuff Fitness International, USA) was modified and equipped with sensors/actuators to perform an isokinetic knee extension task (Fig. 1a). Subjects were seated on the dynamometer with the shank securely attached to the rotating lever arm just below the knee. A brushless motor (EC45, Maxon Motor AG, Switzerland) was used to rotate the lever arm and the isokinetic knee joint torque was measured by a magnetostrictive torque sensor (S-2220-75, NCTE AG, Germany). A load cell (LCB130, Me-MessSysteme GmbH, Germany) was used to measure the force generated and to cross-calibrate the torque sensor.
Fig 1.
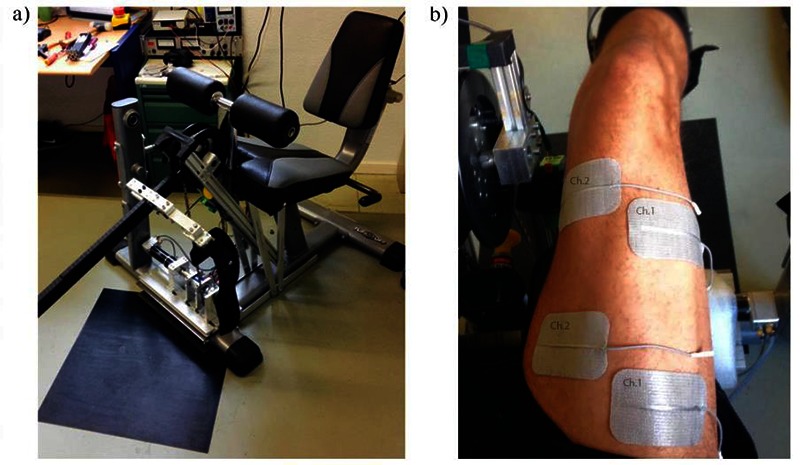
(a) The Knee Dynamometer designed to measure isokinetic knee joint torque. (b) Electrode setup on the right leg.
A PC-controlled stimulator was used (Rehastim, Hasomed GmbH) which delivers biphasic current-controlled rectangular pulses through surface electrodes (Axelgaard, Pals Platinum, USA). Sensor data was fed into a data acquisition card (PCI-6221, National Instruments, USA) at 1 kHz sampling rate. Device control and data acquisition was implemented with Matlab/Simulink and the Real-Time Workshop (Mathworks, USA). A graphical user interface was also implemented to set the desired values of stimulation parameters and/or trains.
Experimental Protocol
For each subject, the experiment consisted of two sessions where the left and right quadriceps were stimulated for a total of 6 minutes: 3 minutes with a constant frequency pattern denoted as P1 and 3 minutes with stochastically-varied IPI denoted as pattern P2; there were short periods of motion but no stimulation before, between and after stimulation phases (Fig. 2). The design was counterbalanced by randomizing the order of presentation of P1 and P2, i.e. P1 then P2 vs. P2 then P1. The range of motion and angular velocity were set to mimic cycling at 50 rpm. The maximum stimulation pulse width was found for each subject before the first session in a familiarization by gradually increasing the pulse width until the pain threshold and the maximally tolerated pain were reached. Then 80% of this maximum was used for the individual pulse width during the experiments. For each stimulation pattern (P1 and P2), pulse amplitude was kept constant at 40 mA. Each muscle was stimulated with 2 channels and the electrodes (Fig. 1b). were placed on the vastus lateralis muscle motor point (MPvl) and vastus medialis motor point (MPvm) to improve the effectiveness of stimulation.21 Muscle motor points were first detected with a stimulation pen (Motor Point Pen, Compex, Switzerland).
Fig 2.
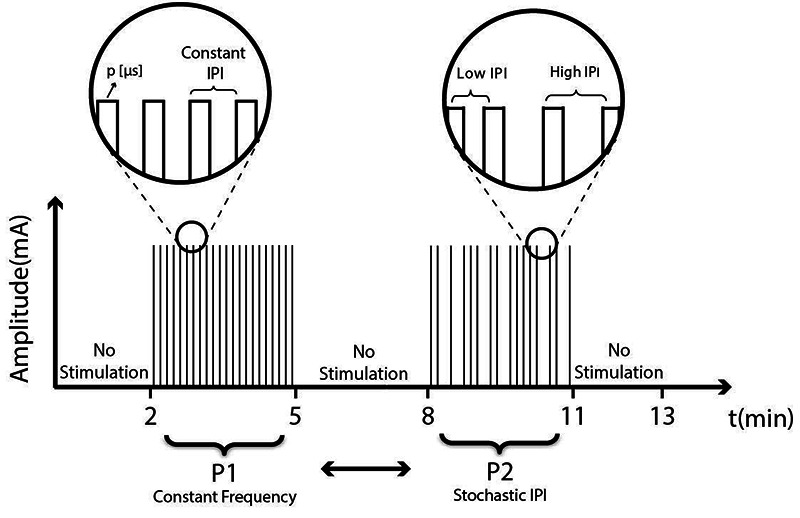
Test protocol. The order of presentation of P1 and P2 was randomly selected for each leg.
Each experiment started with a rest phase (2 min) where the lever arm moved the subject’s leg at a constant angular velocity without any stimulation. After the rest phase, stimulation starts with P1 or P2. If the subject’s right leg was stimulated first with P1, the left leg was stimulated starting with P2. It has been stated that even 10-min of rest is insufficient,18 but in order to examine the recovery effect compared with voluntary activation in a short-term protocol, a 3-min rest time was administered between each activation pattern.22 For P2, the interpulse interval was stochastically modulated by setting the stimulation frequency as a normal distribution, f ~ N(¯f = 35 Hz, σf = 15 Hz). Here, ¯f is the mean frequency and σf the standard deviation. For P1, a constant frequency of 35 Hz was used.
Data evaluation
Power output (P) was assessed as the product of angular velocity and torque during knee extension. The mean power output in the first (PF30) and last 30 s (PL30) as well as total mean power (Pm) of each phase were evaluated. Differences between these outcomes between P1 and P2 were examined using paired t-tests (data normality was checked using a Kolmogorov-Smirnov test). The significance level was set to α = 0.05. Mean differences (MD) and 95% confidence intervals were also calculated.
Fatigue was measured for power output values for each stimulation pattern and is shown as the percentage power output loss: Ploss = 100% × (PF30 – PL30)/PF30 of each stimulation phase.4 All statistical analysis was carried out using the Matlab Statistics Toolbox (Mathworks Inc, USA).
Results
There was significantly higher power output with the stochastically-modulated pattern P2 during the first 30 s (PF30 11.96 ± 3.40 W vs. 15.66 ± 6.24 W, P1 vs. P2, p = 0.0092), the last 30 s (PL30 8.98 ± 2.56 W vs. 11.32 ± 3.88 W, p = 0.018) and overall (Pm 10.66 ± 2.46 W vs. 13.95 ± 4.79 W, p = 0.0057), cf. Tab. 1 and Fig. 3. There was no significant difference between the patterns with regards to fatiguability: Ploss 22.8 ± 22.9 % vs.
Table 1.
P rimary outcomes for paired comparisons and p-values for comparison of means
| Mean (SD) |
MD (95% CI) |
p-value | |||
|---|---|---|---|---|---|
| P1 | P2 | P2-P1 | |||
| PF30 | [W] | 11.96 (3.40) |
15.66 (6.24) |
3.71 (1.09,6.32) |
0.009 |
| PL30 | [W] | 8.98 (2.56) |
11.32 (3.88) |
2.34 (0.46,4.22) |
0.018 |
| Pm | [W] | 10.66 (2.46) |
13.95 (4.79) |
3.29 (1.14,5.44) |
0.005 |
| Ploss | [%] | 22.8% (22.9) |
23.5% (17.4) |
0.6 (-15.05,16.25) |
0.93 |
n = 14, MD: Mean Difference, SD: Standard Deviation, CI: Confidence Interval
Fig 3.

Power output samples for P1 and P2, sample differences (D = P2-P1), mean difference (MD) and 95% confidence intervals. (a) First 30 s, PF30. (b) Last 30 s, PL30. (c) Overall, Pm. The red horizontal bars are mean values.
Discussion
This work set out to compare stochastic modulation of the IPI to constant frequency stimulation during an isokinetic leg extension task similar to FES-cycling. Crude control of muscle groups is one of the main factors responsible for low power outputs achieved with FES.8 This study demonstrated significantly higher power output with the stochastically-modulated P2 compared to stimulation with constant frequency P1. The results showed no overall effect on reducing fatigability (Ploss) when stimulation frequency was randomly modulated. Previous research indicates that constant high frequency stimulation protocols produce more fatigue than constant low frequency stimulation protocols.13,23 Further investigation should be carried out in progressive randomized modulation of IPIs (40 Hz < f < 60 Hz and 20Hz < f < 30Hz). Significantly lower rates of muscle fatigue observed in a previous study could have been the result of recruiting more muscle fibres at higher frequencies (f > 50 Hz).17 Marsden et al.24 have shown that the degree of muscle fatigue is directly related to the number of pulses received by the muscle. Therefore, in general, the greater the pulse frequency, the more rapidly fatigue develops. Further investigation is needed to compare the number of pulses received by a muscle during short term experiments in order to analyse fatigue rate in detail.
Although previous studies indicate that repeatable results have been achieved using at least 10-min rest time,18,23,25,26 in our experiments, a 3-min rest time did not show any layover effect. This could be due to the short-term (2 x 3 minutes) stimulation protocol and the intermittent stimulation protocol (no stimulation during knee flexion, similar to FES-cycling).
These observations motivate further examination of different randomization strategies for maximum mechanical advantage: in contrast to constant-frequency stimulation, where motor units of different type are recruited in a non-selective, spatially fixed, and temporally synchronous pattern,27 stochastic modulation of IPI is more akin to natural stimulation which has varying discharge patterns employing non-synchronous, selective recruitment and which exploits high-frequency bursts and the catch-like property.28 In conclusion, this study showed that stimulation strategies that use randomized modulation of IPIs can improve the ability of functional electrical stimulation applications to perform repetitive, non-isometric contractions with significantly higher power output in short term tasks.
Acknowledgement
The authors thank Prof. Dr. Robert Riener of ETH Zurich for contributions to the protocol development.
Research supported by the Swiss National Science Foundation (SNF-Nr.: 320030_150128/1).
Contributor Information
Marco Laubacher, Email: lhm5@bfh.ch.
Stuart Binder-Macleod, Email: sbinder@udel.edu).
Kenneth J. Hunt, Email: kenneth.hunt@bfh.ch.
References
- 1.Berry HR, Perret C, Saunders BA, et al. Cardiorespiratory and power adaptations to stimulated cycle training in paraplegia. Med Sci Sports Exerc. 2008;40(9):1573-1580. [DOI] [PubMed] [Google Scholar]
- 2.Frotzler A, Coupaud S, Perret C, et al. Effect of detraining on bone and muscle tissue in subjects with chronic spinal cord injury after a period of electrically-stimulated cycling: a small cohort study. J Rehabil Med 2009;41:282-5. [DOI] [PubMed] [Google Scholar]
- 3.Frotzler A, Coupaud S, Perret C, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 2008;43:169-76. [DOI] [PubMed] [Google Scholar]
- 4.Duffell LD, Donaldson NDN, Perkins TA, et al. Long-term intensive electrically stimulated cycling by spinal cord-injured people: effect on muscle properties and their relation to power output. Muscle Nerve 2008;38:1304-11. [DOI] [PubMed] [Google Scholar]
- 5.Hunt KJ, Hosmann D, Grob M, et al. Metabolic efficiency of volitional and electrically stimulated cycling in able-bodied subjects. Med Eng Phys 2013;35:919-25. [DOI] [PubMed] [Google Scholar]
- 6.McRae CGA, Johnston TE, Hunt KJ, et al. Work Efficiency and Stimulation Cost during FES-cycling by Children with a Spinal Cord Injury. 13th Ann Conf Int FES Soc, Freiburg, Ger Sept. 2008. [Google Scholar]
- 7.Berry HR, Kakebeeke TH, Donaldson N, et al. Energetics of paraplegic cycling: Adaptations to 12 months of high volume training. Technol Heal Care 2012;20:73-84. [DOI] [PubMed] [Google Scholar]
- 8.Hunt KJ, Fang J, Saengsuwan J, et al. On the efficiency of FES cycling: A framework and systematic review. Technol Heal Care 2012;20:395-422. [DOI] [PubMed] [Google Scholar]
- 9.Hunt KJ, Saunders BA, Perret C, et al. Energetics of paraplegic cycling: a new theoretical framework and efficiency characterisation for untrained subjects. Eur J Appl Physiol 2007;101:277-85. [DOI] [PubMed] [Google Scholar]
- 10.Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol 2011;111:2399-2407. [DOI] [PubMed] [Google Scholar]
- 11.Antonio D-A, Aikaterini K, Elisabeth B-E, et al. A comparison of customized strategies to manage muscle fatigue in isometric artificially elicited muscle contractions for incomplete SCI subjects. J Autom Control 2013;21:19-25. [Google Scholar]
- 12.Binder-Macleod SA, Lee SC, Russ DW, et al. Effects of activation pattern on human skeletal muscle fatigue. Muscle Nerve 1998;21:1145-52. [DOI] [PubMed] [Google Scholar]
- 13.Kebaetse MB, Lee SC, Johnston TE, et al. Strategies that improve paralyzed human quadriceps femoris muscle performance during repetitive, nonisometric contractions. Arch Phys Med Rehabil 2005;86:2157-64. [DOI] [PubMed] [Google Scholar]
- 14.Scott WB, Lee SCK, Johnston TE, et al. Switching stimulation patterns improves performance of paralyzed human quadriceps muscle. Muscle Nerve 2005;31:581-8. [DOI] [PubMed] [Google Scholar]
- 15.Chou L, Kesar TM, Binder-Macleod SA. Using Customized Rate-Coding and Recruitment Strategies to Maintain Forces During Repetitive Activation of Human Muscles. Phys Ther 2008;88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott WB, Lee SCK, Johnston TE, et al. Effect of electrical stimulation pattern on the force responses of paralyzed human quadriceps muscles. Muscle and Nerve 2007;35:471-8. [DOI] [PubMed] [Google Scholar]
- 17.Graupe D, Suliga P, Prudian C, et al. Stochastically-modulated stimulation to slow down muscle fatigue at stimulated sites in paraplegics using functional electrical stimulation for leg extension. Neurol Res 2000;22:703-4. [DOI] [PubMed] [Google Scholar]
- 18.Thrasher A, Graham GM, Popovic MR. Reducing muscle fatigue due to functional electrical stimulation using random modulation of stimulation parameters. Artif Organs. 2005;29:453-8. [DOI] [PubMed] [Google Scholar]
- 19.Graham GM, Thrasher TA, Popovic MR. The Effect of Random Modulation of Functional Electrical Stimulation Parameters on Muscle Fatigue. IEEE Trans NEURAL Syst Rehabil Eng 2006;14:38-45. [DOI] [PubMed] [Google Scholar]
- 20.Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol 1987;253(5 Pt 2):H993-H1004. [DOI] [PubMed] [Google Scholar]
- 21.Gobbo M, Maffiuletti NA, Orizio C, et al. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J Neuroeng Rehabil 2014;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bigland-Ritchie BR, Dawson NJ, Johansson RS, et al. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol 1986;379:451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kebaetse MB, Turner AE, Binder-Macleod SA. Effects of stimulation frequencies and patterns on performance of repetitive, nonisometric tasks. J Appl Physiol 2002;92:109-16. [DOI] [PubMed] [Google Scholar]
- 24.Marsden CD, Meadows JC, Merton PA. “Muscular wisdom” that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. Adv Neurol 1983;39:169-211. [PubMed] [Google Scholar]
- 25.Bigland-Ritchie BR, Zijdewind I, Thomas CK. Muscle fatigue induced by stimulation with and without doublets. Muscle Nerve 2000;23:1348-55. [DOI] [PubMed] [Google Scholar]
- 26.Thomas CK, Griffin L, Godfrey S, et al. Fatigue of paralyzed and control thenar muscles induced by variable or constant frequency stimulation. J Neurophysiol 2003;89:2055-64. [DOI] [PubMed] [Google Scholar]
- 27.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther 2005;85:358-64. [PubMed] [Google Scholar]
- 28.Maladen RD, Perumal R, Wexler AS, et al. Effects of activation pattern on nonisometric human skeletal muscle performance. J Appl Physiol 2007;102:1985-91. [DOI] [PubMed] [Google Scholar]


