Abstract
The effect of three electrical stimulation (ES) frequencies (10, 35, and 50 Hz) on two muscle groups with different proportions of fast and slow twitch fibers (abductor pollicis brevis (APB) and vastus lateralis (VL)) was explored. We evaluated the acute muscles’ responses individually and during hybrid activations (ES superimposed by voluntary activations). Surface electromyography (sEMG) and force measurements were evaluated as outcomes. Ten healthy adults (mean age: 24.4 ± 2.5 years) participated after signing an informed consent form approved by the university Institutional Review Board. Protocols were developed to: 1) compare EMG activities during each frequency for each muscle when generating 25% Maximum Voluntary Contraction (MVC) force, and 2) compare EMG activities during each frequency when additional voluntary activation was superimposed over ES-induced 25% MVC to reach 50% and 75% MVC. Empirical mode decomposition (EMD) was utilized to separate ES artifacts from voluntary muscle activation. For both muscles, higher stimulation frequency (35 and 50Hz) induced higher electrical output detected at 25% of MVC, suggesting more recruitment with higher frequencies. Hybrid activation generated proportionally less electrical activity than ES alone. ES and voluntary activations appear to generate two different modes of muscle recruitment. ES may provoke muscle strength by activating more fatiguing fast acting fibers, but voluntary activation elicits more muscle coordination. Therefore, during the hybrid activation, less electrical activity may be detected due to recruitment of more fatigue-resistant deeper muscle fibers, not reachable by surface EMG.
Key Words: Muscle, rehabilitation, surface electromyography (sEMG)
Electrical Stimulation (ES) has been used in many clinical and research interventions to improve injured tissues or to modify muscle function by inducing contractions. In a clinical setting, clinicians typically use different ES frequencies with the anticipation of obtaining the optimal response from the muscle without causing pain or injury to the tissue. Muscle response to different levels of ES frequency has been examined in terms of variation in muscle thickness1 and change in muscle performance during fatigue.2 However, muscle activity generated with different frequencies of ES in a non-fatiguing protocol has never been explored. Furthermore, when ES/electromyography (EMG) biofeedback is used in clinical settings, a combination of ES and voluntary activations are often used to improve muscle recovery. Studies have shown that differences in muscle fiber recruitment patterns and functions elicited by voluntary or combination of voluntary and ES-activation are different and do not follow the same recruitment principle and patterns. Therefore, voluntary and ES-induced muscle activation behavior can be unpredictable due to possible differences in activation mechanisms.3,4 A normal voluntary contraction follows a size-progressive recruitment pattern which typically involves the recruitment of small, slower motor units followed by larger sized, faster motor units becoming engaged in the task.5,6 During ES, a change occurs in the distribution of motor unit recruitment in which larger, more fatigable units are recruited first, inducing a large demand on muscle metabolism and may induce greater physiological change. In addition, ES only stimulates the muscles on which electrodes are located, and voluntary movement implies activation from several synergistic and stabilizing muscles. One of the major challenges for evaluation of stimulated muscle has been removing the stimulus artifact from an EMG signal at higher frequencies. In many EMG analyses involving ES, lower frequencies have been used because removal of the artifact is easier when the muscle is activated at lower frequencies and the stimulation peak does not overlap with the muscle activation recording in the time domain. Due to the difficulty in isolating muscle response from ES-activated muscle’s EMG signals, the recruitment mechanism for fibers activated by ES is not clear. In this study we used Empirical Mode Decomposition (EMD), a technique that involves the decomposition of a signal into intrinsic mode functions, to isolate the muscular components from the stimulation artifacts7,8
The purpose of this investigation was to 1) compare EMG activities during three frequency rates (10, 35, and 50 Hz) for two muscles with different fiber characteristics and firing rates when generating 25% MVC force, and 2) compare muscle activation patterns and differences during each frequency, when additional voluntary activation was superimposed over ES-induced 25%MVC to reach 50% and 75%MVC. We hypothesize that muscles with different size and fiber type will respond differently to ES activation and since ES activation does not follow the same size progression normally seen in voluntary activation, the muscle response signals generated from ES will be different than the signals generated by voluntary activation.
Material and Methods
Participants
Ten healthy participants (males and females, mean age: 24.4 ± 2.5 years) with no history of musculoskeletal, cardiovascular, or orthopedic problems were recruited for this study. Participants with a known allergy to Ag-AgCl surface electrodes or adhesive were excluded. The protocol was reviewed and approved by an Institutional Review Board (IRB) and written consent was obtained from all participants prior to participation.
Instruments
Isometric force data was collected with a hand-held dynamometer (MicroFET2 HHD, Hoggan Health Industries, West Jordan, Utah), validated previously 9. MVC information was collected with the dynamometer at maximal force and then used as an indication tool to determine when the participant reached 25%, 50%, and 75% MVC force.
EMG data was collected continuously with a physiological modeling system (Nexus-10, MindMedia B.V., Netherlands) at a sampling rate of 2048 Hz. Ag-AgCl surface electrodes were placed on the muscle belly parallel to the muscle fibers directly over the ‘motor’ points of the muscles to be activated to collect muscle response data with the EMG. These locations were determined using Surface Electro-Myo-Graphy for the Non-Invasive Assessment of Muscles (SENIAM) electrode placement guidelines.
The electrical stimulation was applied using Respond Select® neuromuscular electrical stimulation system (Empi, Inc., St. Paul, Minnesota) and biopolar surface electrodes.
Experimental Set-Up
Protocols were developed for the abductor pollicis brevis (APB) and vastus lateralis (VL) to ensure stabilization and isolation of the related muscles during testing. For VL testing, participants sat on an upright chair so that their feet were not touching the floor and the back of their knee joints positioned at the edge of the chair seat. 9 For the thumb muscle (APB), participants were required to sit upright with the shoulder and outside of the arm pressed against the wall at a 90-degree angle.
Procedure
Part One: Voluntary muscle contraction
Participants were asked to undergo knee extensions and thumb abductions voluntarily. sEMG data was collected while participants completed a maximal voluntary contraction (MVC), held for four seconds and then released back to rest. The mean of three force values from the dynamometer at maximal force was recorded and then 25, 50, and 75%MVC thresholds were calculated (± 10%) and sEMG data recorded at each force level (four seconds each). Participants rested between each contraction for at least one minute. A non-fatiguing protocol was used to minimize muscle activity changes caused by fatigue.
Part Two: Electrically-elicited muscle contraction
Randomizations between muscles (APB and VL) and frequencies (10, 35, and 50 Hz) were done between subjects to prevent learning and cross-over effects. sEMG was recorded as electrical stimulation was delivered starting at 0 and increasing intensity until reaching 25%MVC (+/- 10%). Once reaching the desired force threshold of 25%MVC, the stimulation was held at a constant intensity for four seconds. Participants were then asked to voluntarily contract their related muscles (VL or APB) to reach 50% and then 75% of the MVC. Data were collected continuously and 50% and 75% were labeled on EMG recordings.
Signal Analysis
The recorded EMG signal was first cleaned and filtered (Butterworth, cutoff: 20-500Hz). For data recorded during ES, Empirical Mode Decomposition (EMD), a technique that involves the decomposition of a signal into intrinsic mode functions and a residual using sifting, was used to isolate the muscular components from the stimulation artifacts.7,8 EMD processing breaks the signal into separate intrinsic mode functions and a residual using sifting amplitudes, eliminates modes with stimulation artifact, and reconstructs the remaining components. The full process has been discussed previously.7
Statistical Analysis
Post-EMD processing, three data sets were evaluated for statistical significance using ANOVAs and Tukey post-hocs: 1) stimulation intensities (mA) required to reach 25% MVC, 2) the muscle activation patterns (uV) at 25% MVC, and 3) muscle activity (uV) during superimposed voluntary activation at 50 and 75%MVC. For each of these outputs, tests of normality were completed.
To isolate the voluntary component from the hybrid activation at 50% and 75% MVC, the ES-only muscle response was subtracted using equation 1. Mean muscle activity (in uV) from 50% and 75%MVC signals are considered outputs from hybrid activation, YHybrid. Y25%MVC represents the mean electrical activity (uV) detected at 25%MVC (ES-only), and Y Isolated VR represents the isolated voluntary response.
| YHybrid – Y25%MVC = YIsolatedVR. | (1) |
ANOVA and Tukey post-hocs were run to determine if there were differences between stimulation frequencies and muscle types.
For all tests, p-values were set at p<0.05 to determine significance and values reported in terms of mean ± SE. SAS V9.4 was used for all statistical analysis.
Results
Required Intensity to reach 25%MVC
In general, the APB required less intensity for activation compared to the VL (mean APB: 17.73±0.40mA, mean VL: 52.40±2.70mA, p<0.001) for all frequencies. For VL, stimulation with 10Hz required higher intensities (p<0.05) to reach 25% MVC, however, there was no difference between 35 and 50Hz (Figure 1).
Fig 1.
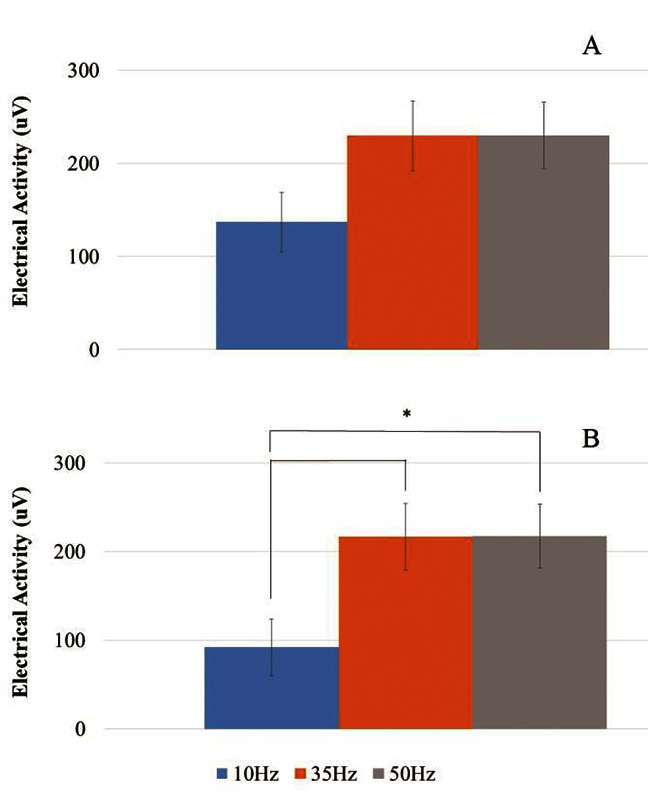
Activity at 25% MVC for (A) APB and (B) VL.
ES-induced Muscle Response at 25%MVC
Electrical stimulation generating 25% MVC force under three separate frequencies showed that there was a significant difference between APB and VL (p<0.05). For VL, significant differences were seen between high and low frequency rates, as there were differences between 10Hz and 35 and 50Hz (p<0.05), but not between the two higher rates.
Hybrid activation muscle response at 50% and 75% MVC
There were significant differences in electrical activity at different stimulation levels (p<0.05). The trend between increasing contraction levels (% MVC) and electrical activity (uV) output from the muscles was positive for all stimulation frequencies (10, 35, and 50 Hz) (Figure 2), indicating more muscle activation for increased muscular contraction.
Fig 2.
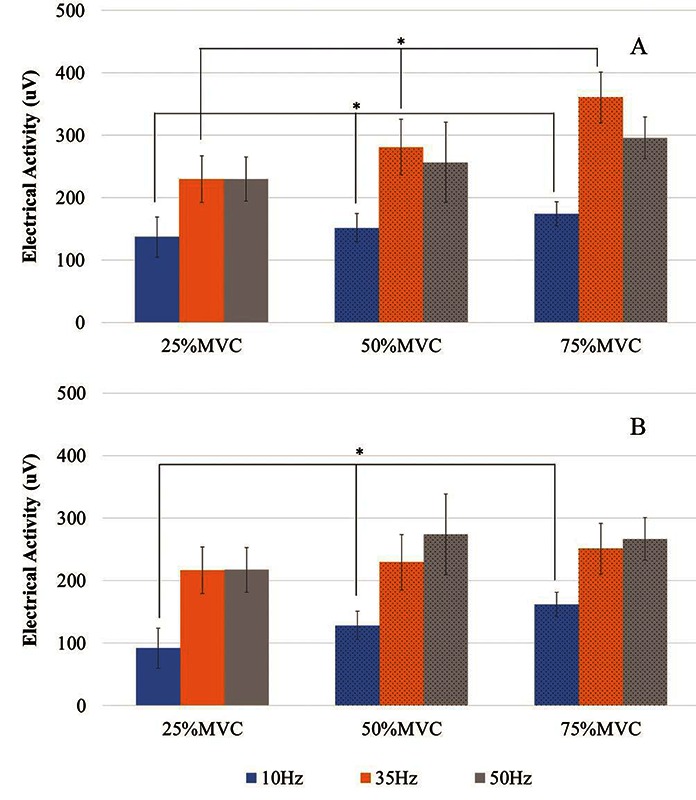
Activity generated at 25% Maximal Voluntary Contraction (MVC) (ES), 50% MVC (Hybrid), and 75% MVC (Hybrid) for (A) APB and (B) VL
For APB, there were significant differences between increasing contraction levels at the lower frequency levels: 10 and 35 Hz (p<0.05). For VL, electrical activity (uV) in terms of increasing contraction levels (% MVC) was significant only when stimulation was delivered at 10 Hz (p<0.05) (Figure 2).
Hybrid activation contains ES-induced and voluntary components, and so the voluntary component was isolated by subtracting the output from 25% MVC (pure ES-elicited response). Overall, significantly less electrical output was seen when adding voluntary contraction to supplement the stimulation contractions than the initial activity generated from 0-25% MVC (pure ES-elicited response) for all frequencies (p<0.05).
Discussion
The VL required significantly higher ES intensity (mA) to reach the 25% MVC force threshold. This is most likely due to its size and greater total force output by this muscle. According to the S-shaped relationship between frequency and force output reported by Binder-Macleod et al.,10 normalized force generated by the muscle increases with higher frequencies. In fact, the progressive relationship between increasing stimulation intensity and force output has been reported previously by Frigo,11 who used frequencies of 16.67 and 25 Hz.
In our study, post-EMD processed data also depicted differences in muscular activity output (uV) between low and higher frequencies of stimulation when generating the same force in the muscle (25% MVC). For the VL, there were significant differences in electrical activity measured between 10Hz and higher rates of 35 and 50 Hz (p<0.05). APB showed a similar relationship between frequency rates, but the trend was not significant. Overall, greater muscle activation occurs when the muscle is subject to faster stimulation rates.
Consistent with our results for both muscles, a recent study exploring the effects of single motor unit stimulation of the human tibial nerve found that higher stimulation frequencies recruited more units at shorter latencies than lower frequencies.12 Motor units are recruited as their motor axons are depolarized and then relax after the action potential has fired and the axon is repolarized.13 However, if activation is delivered so frequently it does not allow for full depolarization, tetanic, or fused contraction to occur. This may create a cumulative effect involving multiple stimuli, causing greater muscle recruitment and higher EMG amplitudes. As a result, higher stimulation frequencies such as 35 and 50Hz at high duty cycles may cause tetanic contractions, forcing additional motor units to be recruited to complete the task.
Increased muscle activation has been linked to progress toward recovery14,15 and greater muscle hypertrophy. However, muscle fatigue is a limiting factor for continuous stimulation at high frequencies. It has been well-established that muscle fatigue occurs faster with ES than with voluntarily-activated muscle,16 higher ES frequencies lead to greater muscle fatigue17 and the optimal frequency depends on the type of muscle.18 Preliminary investigations showed ES at lower frequencies (below 40-50 Hz) excited more slow-twitch, fatigue resistant fibers, and higher frequencies elicited fast-twitch, fatigable units.19,20 When considering applying ES for muscle recovery, clinicians should be aware of the potential influences of ES frequency rates on muscle recruitment capacity and fatigue. Typically, during voluntary activation of a muscle, as muscle force generation increases there is a proportional increase in muscle fiber recruitment (from slow, less fatiguing to fast, fatigable motor units) activity to meet the demand and reach the desired force. After EMD processing, our data indicated that when voluntary activation was superimposed on already-activated muscle at 25% MVC by ES to increase the force to 50% and 75%, significantly less electrical output was seen.
One probable explanation for the differences between the ES-elicited and combined activations could be based on a physiological hierarchy that may stimulate more fibers during ES. The voluntary superimposition may not generate proportionally higher muscle activation due to saturated electrical charges from ES. Furthermore, when voluntary activation is commanded to achieve 50 or 75% MVC force levels, enough fibers may be already activated with ES to achieve the movement, reducing the need for additional fibers to be recruited voluntarily. Additionally, if ES activates fibers non-preferentially near the stimulating electrode, there are differences in mechanical fiber properties that could affect the fiber recruitment. Moreover, a voluntary movement will activate synergistic and stabilizer muscles to complete a task, an arrangement not replicated by ES.21 Therefore, ES cannot complete muscle movements with equivalent strength, as intermuscular coordination is not employed. Finally, another possibility for this marked reduction in electrical activity measured with the EMG could be due to fiber regionalization in the muscle. EMG surface electrodes detect signals of the closest muscle fibers to the surface. If all the fibers closest to the surface of the skin were already recruited by the ES, it is possible deeper motor units then needed to be called upon to engage the muscle up to 50 or 75% MVC. In this case, the preferential recruitment caused by voluntary activation would still occur with the fibers that were available (the deeper units), but the far location from the recording electrode would cause less electrical detection by the EMG. Only small amounts of increased activity were measured at 50 and 75% MVC, although the muscle output two to three times the amount of force, supporting this theory. Because of the many favorable outcomes with hybrid activation, it is crucial to first evaluate the effectiveness of the combined therapy. A combined treatment, including voluntary and electrically-activated components, restores strength post-surgery, particularly in the early phases of rehabilitation.22,23 We were able to successfully activate muscles with electrical stimulation and then supplement with voluntary activation to employ high recruitment levels up to 75% MVC. In conclusion, ES and voluntary activations appear to generate two different modes of muscle recruitment. ES may provoke muscle strength by activating more fatiguing fast acting fibers, but voluntary activation elicits more muscle coordination. Therefore during hybrid activation, less electrical activity may be generated due to recruitment of more fatigue-resistant, deeper muscle fibers unreachable by ES-alone. ES-activated muscle demonstrated several characteristics of non-preferential, proximal-based fiber recruitment. Fibers closest to the electrode appear to be recruited first, regardless of their type, and so electrical sEMG outputs were highest from muscles such as the APL, with many small, Type I fibers located superficially. Future analysis should include a larger investigation with greater number of participants to increase the power of the study.
Acknowledgment
We would like to thank Dr. Kamyar Momeni and Maria Vromans for their help with data collection and Dr. Tania Huedo-Medina for her statistical advice.
This work is supported in part by the National Science Foundation under EFRI Grant 1332329.
References
- 1.Cho HK, Jung GS, Kim EH, et al. The effects of neuromuscular electrical stimulation at different frequencies on the activations of deep abdominal stabilizing muscles. Journal of back and musculoskeletal rehabilitation. 2015:1-7. [DOI] [PubMed] [Google Scholar]
- 2.Kesar T, Binder-Macleod S. Effect of frequency and pulse duration on human muscle fatigue during repetitive electrical stimulation. Exp Physiol 2006;91:967-76. [DOI] [PubMed] [Google Scholar]
- 3.Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: A critical appraisal. Eur J Appl Physiol 2011;111:2399-407. [DOI] [PubMed] [Google Scholar]
- 4.Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol 2010;110:223-34. [DOI] [PubMed] [Google Scholar]
- 5.Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol (Lond) 1973;230:359-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabre JF, Spellman NT. The demonstration of the size principle in humans using macro electromyography and precision decomposition. Muscle Nerve 1996;19:338-41. [DOI] [PubMed] [Google Scholar]
- 7.Pilkar RB, Yarossi M, Forrest G. Empirical mode decomposition as a tool to remove the function electrical stimulation artifact from surface electromyograms: Preliminary investigation. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1847-50. doi: 10.1109/EMBC.2012.6346311. [DOI] [PubMed] [Google Scholar]
- 8.Huang NE, Shen Z, Long SR, et al. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. 1998;454(1971):903-95. [Google Scholar]
- 9.Bohannon RW, Kindig J, Sabo G, et al. Isometric knee extension force measured using a handheld dynamometer with and without belt-stabilization. Physiother Theory Pract 2012;28:562-8. [DOI] [PubMed] [Google Scholar]
- 10.Binder-Macleod SA, Halden EE, Jungles KA. Effects of stimulation intensity on the physiological responses of human motor units. Med Sci Sports Exerc 1995;27:556-65. [PubMed] [Google Scholar]
- 11.Frigo C, Ferrarin M, Frasson W, et al. EMG signals detection and processing for on-line control of functional electrical stimulation. J Electromyogr Kinesiol 2000;10:351-60. [DOI] [PubMed] [Google Scholar]
- 12.Dean JC, Clair-Auger JM, Lagerquist O, et al. Asynchronous recruitment of low-threshold motor units during repetitive, low-current stimulation of the human tibial nerve. Front Hum Neurosci 2014. Dec 16;8:1002 doi: 10.3389/fnhum.2014.01002. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mortimer JT. Motor prostheses. Comprehensive Physiology. 1981. [Google Scholar]
- 14.Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am 2009;91:966-78. [DOI] [PubMed] [Google Scholar]
- 15.Andersen LL, Jorgensen MB, Blangsted AK, et al. A randomized controlled intervention trial to relieve and prevent neck/shoulder pain. Med Sci Sports Exerc 2008;40:983. [DOI] [PubMed] [Google Scholar]
- 16.Marsolais EB, Edwards BG. Energy costs of walking and standing with functional neuromuscular stimulation and long leg braces. Arch Phys Med Rehabil 1988;69:243-9. [PubMed] [Google Scholar]
- 17.Dreibati B, Lavet C, Pinti A, Poumarat G. Influence of electrical stimulation frequency on skeletal muscle force and fatigue. Ann Phys Rehabil Med 2010;53:266-77. [DOI] [PubMed] [Google Scholar]
- 18.Ferry B, Poumarat G. Effects of frequency on muscular force induced by electric stimulation. Arch Int Physiol Biochim Biophys 1994;102:319-24. [DOI] [PubMed] [Google Scholar]
- 19.Benton LA. Functional electrical stimulation: A practical clinical guide. Rancho Los Amigos Rehabilitation Engineering Center, Rancho Los Amigos Hospital; 1980. [Google Scholar]
- 20.Jones D, Bigland-Ritchie B, Edwards R. Excitation frequency and muscle fatigue: Mechanical responses during voluntary and stimulated contractions. Exp Neurol 1979;64:401-13. [DOI] [PubMed] [Google Scholar]
- 21.Hamada T, Kimura T, Moritani T. Selective fatigue of fast motor units after electrically elicited muscle contractions. J Electromyogr Kinesiol 2004;14:531-8. [DOI] [PubMed] [Google Scholar]
- 22.Wigerstad-Lossing I, Grimby G, Jonsson T, et al. Effects of electrical muscle stimulation combined with voluntary contractions after knee ligament surgery. Med Sci Sports Exerc 1988;20:93-8. [DOI] [PubMed] [Google Scholar]
- 23.Snyder-Mackler L, Binder-Macleod SA, Williams PR. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med Sci Sports Exerc 1993;25:783-9. [DOI] [PubMed] [Google Scholar]


