Abstract
Chemotherapy-induced cardiomyopathy is one of the major possible hazards that can result from potential cardiotoxic agents while treating cancer. Prognostic risk factors include the rate of drug administration, history of hypertension, female gender, extremes of age, previous history of mediastinal irradiation, cumulative dose and pre-existing heart disease. Close monitoring of the patients, timely diagnosis, use of well-known biomarkers including cardiac troponins, NT-ProBNP and imaging studies like 2D Echo or cardiac MRI are essential. Emerging biomarkers include carbonyl reductases (CBR1 and CBR3), aldo-keto reductases (AKR, type 1A1, 1C3, 7A2) and topoisomerase2β (Top2β). β blockers and ACE inhibitors have not only been shown to slow down the progression of cardiac dysfunction but also produce symptomatic improvement. Our case report describes a patient with acute myeloblastic leukaemia who developed severe cardiomyopathy acutely after starting the anthracycline-based regimen. Nevertheless, with timely intervention her symptoms improved and subsequently she successfully received allogeneic stem cell transplantation.
Background
Chemotherapy-related cardiac complications are some of the leading causes of morbidity and mortality among cancer survivors. Chemotherapy can cause ventricular contractile dysfunction, arrhythmias, pericarditis, hypertension and thromboembolism. Chemotherapy-induced cardiomyopathy and heart failure (HF) are commonly encountered adverse effects.1 The rate of drug administration, advanced age, cumulative dose, female gender, mediastinal radiation and cardiac risk factors such as hypertension and pre-existing heart disease are the major risk factors for cardiac damage. Ewer and Lippman2 proposed two distinct types of cardiomyopathy. Type 1 cardiomyopathy is irreversible and causes permanent myocyte injury, while type 2 is mostly reversible after the removal of the inciting agent from the therapeutic regimen. Type 1 cardiomyopathy results in histopathological changes in cardiac myocytes due to the production of oxygen-derived free radicals resulting in increased oxidative stress3 and can also cause myocyte necrosis in higher doses. Free radicals result in the intracellular influx of calcium by peroxidation of myocytes. Intracellular iron accumulation causing increased oxidative stress is another proposed mechanism of type 1 cardiomyopathy.4 Early recognition and prompt treatment of type 1 cardiotoxicity may prevent progression to HF. However, any delay in the diagnosis and management can result in an irreversible damage to the myocardial tissue. Since type 2 cardiomyopathy does not cause any permanent ultrastructural changes, baseline cardiac function returns to normal once the drug is discontinued. Cardiomyopathy can be classified as acute (during or shortly after initiating therapy), subacute (within days or weeks of starting therapy) and chronic (within weeks to months of starting therapy).5 Chemotherapies vary in their potential to induce cardiomyopathy (table 1). The incidence is high with certain agents such as doxorubicin (DOX), trastuzumab (TRZ) and sunitinib but relatively low with bevacizumab, imatinib and lapatinib.1 Anthracycline-induced cardiomyopathy (AIC) has a poor prognosis and can even be worse than idiopathic dilated cardiomyopathies.6
Table 1.
Incidence of chemotherapeutic agents associated cardiomyopathy
| Agent | Mechanism | Incidence | Time course | Prognosis |
|---|---|---|---|---|
| Anthracyclines | ||||
| Doxorubicin | Free radical damage Induced apoptosis DNA damage Alteration in ATP |
5% at cumulative dose 400 mg/m2 26% at 550 mg/m2 48% at 700 mg/m2 |
Acute early-onset chronic progressive (<1 year) Late-onset chronic progressive (>1 year) |
Typically, not reversible |
| Epirubicin | 0.9–3.3% | |||
| Idarubicin | 5–18% | |||
| Liposomal doxorubicin | 2% | |||
| Monoclonal antibodies | ||||
| Trastuzumab | Antibody-mediated cardiac myocyte damage via ErbB2 inhibition in cardiomyocytes | 3–8% given alone up to 27% when given concurrently with doxorubicin | Not well defined, but typically within months | Reversible, mean recovery 1.5 months, most within 6 months |
| Bevacizumab | Antibody-mediated cardiac myocyte damage via VEGF inhibition | 1.6% | * | * |
| Alkylating agents | ||||
| Cyclophosphamide | Haemorrhagic myocardial necrosis possibly due to free radical damage | 7–28% dose related | Acute (days to weeks) | Variable, median recover 78 days |
| Ifosfamide | 17% in high-dose ifosfamide | Acute (mean 12 days) | Variable | |
| Protein kinase inhibitors | ||||
| Sunitinib | Induction of myocardial apoptosis via multifactorial effects of tyrosine kinase inhibition | 4.1–28% | Mean onset 22 days | Reversible |
| Sorafenib | * | * | Reversible | |
| Lapatinib | 0.2% | Mean onset 13 weeks | Reversible, mean recovery 7.3 weeks | |
| Imatinib | Induced myocyte death via mitochondrial damage | Rare, 1.7% in pooled analysis | * | * |
From ‘Chemotherapy-induced cardiomyopathy’ by AY Higgins et al.1
Copyright 2015. Reprinted with permission.
*Insufficient data.
Case presentation
A 60-year-old Hispanic woman presented to us in May 2014 with symptoms of frequent nose bleeds, easy bruising from multiple sites and dizziness. After a complete workup, she was diagnosed with acute myeloblastic leukaemia (AML). Bone marrow biopsy was positive for 40% myeloblasts and cellularity of 80%. Cytogenetics showed 46 XX, normal fluorescent in situ hybridisation (FISH) and no molecular mutations. 2D echocardiography before the start of induction chemotherapy showed left ventricular ejection fraction (LVEF) of 57% and normal right ventricular systolic pressure. She was started on induction chemotherapy with daunorubicin/cytarabine. Treatment course was complicated by symptoms of acute systolic HF within 2–3 weeks of starting the treatment, possibly related to anthracycline use. Cardiac MRI within 3 weeks of starting chemotherapy revealed decreased LVEF (23%) with severe global hypokinesis of the left ventricle. Troponin I level was 0.37 (normal 0.01–0.02), and the patient developed signs of fluid overload consistent with congestive HF including pitting lower extremity oedema and paroxysmal nocturnal dyspnea (PND). Physical examination revealed bibasilar crackles at lungs bases. Chest CT angiogram was performed which ruled out pulmonary embolism. To rule out infectious aetiologies causing HF, PCR for Trypanosoma cruzi and Coxsackie virus serology were carried out and found out to be negative.
Daunorubicin was discontinued, and she was initially managed with Lasix and lisinopril. Owing to a generalised skin eruption on trunk, both of the drugs were discontinued. She was then started on bumetanide, spironolactone and losartan and responded well to these medications. Her troponin I level was also improved from 0.37 in June to 0.06 in July. After she became euvolemic, carvedilol was added to her medication list. Repeat echocardiography in September 2014 revealed a significant improvement in her global LV systolic function with EF of 40%. Pedal oedema and PND also resolved completely with improvement in LVEF. She declined treatment with intensive chemotherapy. Azacitidine was added in August 2014 for AML and background myelodysplastic syndrome (MDS). Repeat echocardiography in January 2015 revealed further improvement in LVEF to 44% and by early June 2015 it increased to 52% (figure 1). Drugs used to manage her cardiomyopathy included losartan, spironolactone, bumetanide and carvedilol. The patient later on received fludarabine and melphalan-based reduced intensity conditioning. She underwent allogenic peripheral blood stem cell transplantation (PBSCT) with donor being her HLA-matched brother in October 2015.
Figure 1.
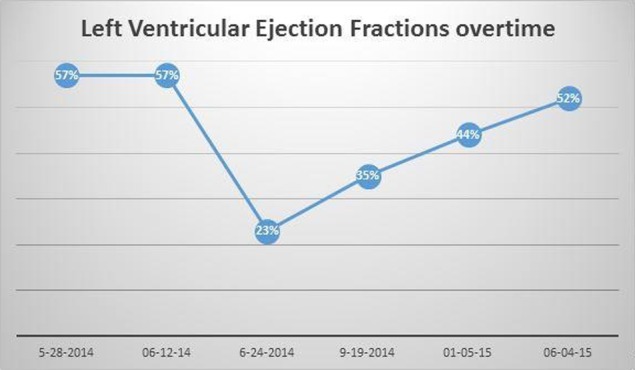
LVEF at base line and follow-up over 1 year after acute onset, type II cardiomyopathy. LVEF, left ventricular ejection fraction.
Outcome and follow-up
At 6-month follow-up of our patient is being managed with low dose carvedilol and losartan and continues to do well without any cardiac symptoms.
Discussion
AIC can be generally divided into three subtypes based on the onset of cardiac dysfunction and physical symptoms. Acute cardiac toxicity occurs during or immediately following the drug administration and is relatively less common with an incidence of ∼11%.7 Subacute cardiomyopathy usually occurs up to 8 months after the final dose, with symptoms peaking at around 3 months. Late-onset chronic cardiomyopathy presents five or more years after the causative therapy with an estimated incidence of ∼1.7%.8 Subacute and late-onset presentations are progressive and usually irreversible.9 Table 2 summarises the chemotherapeutic drugs that can lead to the development of HF. The clinical presentation of cardiac toxicity usually includes symptoms of HF, chest pain due to myocardial inflammatory changes, palpitations due to sinus tachycardia, premature atrial or ventricular beats and paroxysmal nonsustained supraventricular tachycardia.
Table 2.
Chemotherapeutic drug frequently associated with cardiomyopathy
| Drugs class | Examples |
|---|---|
| Anthracyclines | Dauxorubicin, daunorubicin, epirubicin, idarubicin |
| Anthraquinolones | Mitaxantrone |
| Alkylating agents | Cyclophosphamide, cisplatin, busulphan, ifosfamide |
| Antimetabolites | 5-Fluorouracil |
| Antimicrotubules | Piclataxel, vinca alkaloids |
| Tyrosine kinase inhibitors | Imitanib, lipatinib, sunitinib, sorafenib |
Timely diagnosis of this potentially fatal cardiac condition is the cornerstone to managing the problem effectively. Monitoring purely based on symptoms might miss the chance to detect cardiotoxicity early and may eventually lead to an irreversible cardiac failure. Cardiac biomarkers and dynamic imaging studies such as 2D echocardiography can help in the early detection of AIC.10 Dobutamine stress Echo, nuclear ventriculography and cardiac MRI are other effective tools to assess for cardiac damage in the setting of ongoing chemotherapy but relatively time-consuming.11
Patients at high risk of AIC can be identified at an early stage with an efficient N-terminal prohormone brain natriuretic peptide (NT-proBNP),12 which is deemed to be relatively better than the commonly employed cardiac troponin I.13 14 Other AIC biomarkers, including growth differentiating factors-15 (GDF-15), CBR1, AKR1A1 and AKR7A2 protein, and topoisomerase2β (Top2β) were studied in different populations and can also be used.15 16 In childhood cancer survivors GDF-15, a member of TGF superfamily, can be used as a marker of AIC.17 Metabolism of anthracyclines is mediated predominantly by enzymes like carbonyl reductases (CBR) and aldo-keto reductases (AKR1A1, AKR7A2). Growing evidence suggests that AKRs superfamily (AKR1A1, AKR7A2 and AKR1C3) and two monomeric carbonyl reductases, ie CBR1 and CBR3, produce alcohol metabolites (eg, daunorubicinol and doxorubicinol) derived from anthracyclines and therefore can be used to predict the synthesis of daunorubicin in heart.18 Top2β level in the peripheral blood leucocytes has also been investigated as a potential biomarker to determine individual susceptibility to AIC. A study compared levels of Top2b between anthracycline-sensitive and anthracycline-resistant patients. Twenty-one anthracycline-sensitive patients who received DOX at a cumulative dose of ≤250 mg/m2 showed a decline in LVEF of >10% and overall LVEF <50%. In contrast, 15 anthracycline-resistant patients maintained LVEF >50% despite receiving a cumulative DOX dose of 450 mg/m2. Top2b levels were found to be substantially higher in the former group of patients. Although large-scale prospective studies are lacking, these results suggest that Top2b levels might be quite useful to stratify patients at risk of developing cardiomyopathy from anthracyclines.19
In untreated patients, mortality due to AIC can be as high as 40% over 5 years, but the institution of timely medical therapy improves the prognosis of patients dramatically.20 Successful ways to prevent cardiotoxic effects include altering the chemical structure, adjusting the dosages of anthracycline and using efficient cardioprotective agents.21 Alteration of the tissue distribution and pharmacokinetics explain the mechanism of cardioprotective effect associated with the liposomal delivery system. This mode of delivery increases the blood circulation time and also prolongs the elimination time with a half-life of ∼55 hours. The encapsulated drug molecules are confined in a vascular system such as heart but easily penetrate into the immature vessels supplying the tumour.22 23 The result of a meta-analysis showed that the use of liposomal DOX instead of conventional DOX significantly decreased the risk of clinical (OR 0.18, 95% CI 0.08 to 0.38) and subclinical cardiotoxicity (relative risk (RR) 0.31, 95% CI 0.20 to 0.48).24 However, the results of one trial showed an insignificant difference between free epirubicin and liposomal DOX in terms of causing cardiomyopathy.
The cumulative dose of DOX is another important determinant of HF with the majority of cases occurring at a cumulative dose of ≥550 mg/m2. Chances of developing HF can be significantly lowered by using smaller and divided doses. The majority of the patients are asymptomatic, and a cumulative dose of ≥350 mg/m2 can cause a dose-dependent decrease in LVEF. However, LVEF can stabilise when anthracycline therapy is discontinued provided that the cardiotoxicity was moderate, ie absolute LVEF of 30–45% and a decline in LVEF ≥15%.25 26 Results of three controlled trials reported that 26% of total 630 patients, who received 550 mg/m2 cumulative dose of DOX, developed HF.27 Keeping these findings in the view, generally recommended dose in adults is in the range of 450–500 mg/m2. However, considerable variation in sensitivity has been noted among different patients, with some showing tolerance to doses as high as 1000 mg/m2 while others developing toxicity to much lower doses of 300 mg/m2.28 What exactly determines the differences in sensitivity among different individuals to the toxic effects of anthracyclines is yet to be explained.
A wide range of antioxidants has been used to minimise the cardiotoxic effects of free reactive oxygen species (ROS) as shown in table 3. Among the leading treatment options for AIC, dexrazoxane is considered to be the first choice and has been approved for such use. It has also been proved to be protective against AIC in patients breast cancer and stands out among other choices due to its better cardiac and haematological safety profile.29 Selenium acts as a cofactor for the enzyme glutathione peroxidase and catalyses the reaction of hydrogen peroxide reduction by using glutathione. In children with cardiac failure and raised Pro-BNP levels, selenium has shown cardioprotective effects.30 In animal studies conducted by Walker et al, mice prophylactically managed with probucol after treatment with DOX and TRZ showed preserved systolic function.
Table 3.
Cardioprotective agents
| Drugs | Actions | References |
|---|---|---|
| Dexrazoxane | Chelates iron and prevents superoxide radical formation from anthracyclines | 29 |
| Selenium | Antioxidant agent as a component of peroxidase enzymes | 30 |
| Probucol | Antioxidant agent with antilipidemic effects | 31 |
| Ranolazine | Selectively inhibits cardiomyocyte late inward sodium current (INaL), with anti-ischemic effects | 32 |
| Statins | Inhibits Ras-homologous GTPase Rac1 | 33 |
| β blockers | Antioxidant plus antiapoptotic effects | 34 |
Pretreatment with probucol substantially decreased mortality to 40% at day 10 after being treated with DOX+TRZ compared to around 80% at day 5 of combination regimen administration without prophylactic probucol. This finding is further supported by the reduced extent of apoptosis and cardiac tissue damage in mice receiving pretreatment with probucol before being given a combination of DOX+TRZ.31 A novel agent ranolazine is another addition to the cardioprotective agents against AIC and in turn reduces late inward sodium current (INaL) in cardiac tissue susceptible to ischaemic damage and apoptosis.32 HMG-CoA-reductase inhibitors target Ras-related C3 botulinum toxin 1 and in turn decrease the extent of topo II poison-induced DNA damage.33 One prospective and randomised trial conducted in 80 female patients with breast cancer showed that administration of carvedilol during anthracycline therapy could prevent AIC.34
Once patients present with chemotherapy-related cardiomyopathy, the mainstay of management comprises the same combination of drugs used for treating non-AIC congestive HF. ACE inhibitors and β blockers, together with loop diuretics for the fluid overload, have shown promising results to treat AIC. According to the results of a recent study, management with a combination of carvedilol and enalapril led to improvement in 42% of the patients who experienced a decrease in LVEF of ≤45% in response to AIC.35 In another prospective study, seven out of eight women diagnosed with breast cancer and treated with epirubicin experienced ≥15% increase in LVEF after administration of ACE inhibitors. In contrast, the same response was noted in only 1 of 33 cases who were treated with a combination of digoxin and diuretic agent; however, no deaths were reported in that study.36 This observation also hints towards the common pathophysiological basis of systolic HF between AIC and non-AIC congestive HF.
Learning points.
Anthracyclines and other drugs implicated as a cause of cardiomyopathy include anthraquinolones, tyrosine kinase inhibitors (eg, sunitinib, sorafenib, lapatinib), trastuzumab, 5-florouracil and antimicrotubule agents such as paclitaxel. Clinical manifestations of cardiac toxicity usually include symptoms of heart failure, chest pain and palpitations.
Biomarkers for diagnosis include BNP, cardiac troponin I (cTnI) and NT-proBNP, whereas for paediatric population growth differentiating factors-15 (GDF-15) can be used. Carbonyl reductases (CBR1 and CBR3) and aldo-keto reductases (AKR, type 1A1, 1C3, 7A2) catalyse the synthesis of cardiotoxic C-13 anthracycline-derived metabolites and might be used to predict the synthesis of cardiotoxic daunorubicin in heart. Peripheral blood leucocyte Top2β can act as a biomarker for individual susceptibility. Additionally, imaging with 2D Echo, radionuclide ventriculography and cardiac MRI is very helpful for diagnosis and monitoring for recovery.
Antioxidants such as dexrazoxane, selenium along with probucol, ranolazine, β blockers with free radicals scavenging effect and ACE inhibitors can limit the AIC to a great extent. Mainstay of treatment includes β blockers such as carvedilol, ACE inhibitors, angiotensin receptors blockers and diuretics for symptom control.
Footnotes
Contributors: MAS, RI, UZ and FA designed the study and searched the published literature for the review. All authors performed the study, contributed to data extraction, analyzed the data and wrote the paper.
Funding: The study was supported by National Cancer Institute (P30 CA023074).
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Higgins AY, O'Halloran TD, Chang JD. Chemotherapy-induced cardiomyopathy. Heart Fail Rev 2015;20:721–30.1 10.1007/s10741-015-9502-y [DOI] [PubMed] [Google Scholar]
- 2.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005;23:2900–2. 10.1200/JCO.2005.05.827 [DOI] [PubMed] [Google Scholar]
- 3.Singal PK, Li T, Kumar D et al. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem 2000;207:77–86. 10.1023/A:1007094214460 [DOI] [PubMed] [Google Scholar]
- 4.Kwok JC, Richardson DR. Anthracyclines induce accumulation of iron in ferritin in myocardial and neoplastic cells: inhibition of the ferritin iron mobilization pathway. Mol Pharmacol 2003;63:849–61. 10.1124/mol.63.4.849 [DOI] [PubMed] [Google Scholar]
- 5.Shakir DK, Rasul KI. Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res 2009;13:8–12. 10.4021/jocmr2009.02.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira AL, Matsubara LS, Matsubara BB. Anthracycline-induced cardiotoxicity. Cardiovasc Hematol Agents Med Chem 2008;6:278–81. 10.2174/187152508785909474 [DOI] [PubMed] [Google Scholar]
- 7.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 2007;49:330–52. 10.1016/j.pcad.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Von Hoff DD, Layard MW, Basa P et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;01:710–17. 10.7326/0003-4819-91-5-710 [DOI] [PubMed] [Google Scholar]
- 9.Ng R, Better N, Green MD. Anticancer agents and cardiotoxicity. Semin Oncol 2006;33:2–14. [DOI] [PubMed] [Google Scholar]
- 10.Oztarhan K, Guler S, Aktas B et al. The value of echocardiography versus cardiactroponin I levels in the early detection of anthracyclinecardiotoxicity in childhood acute leukemia: prospective evaluation of a 7-year-long clinical follow-up. Pediatr Hematol Oncol 2011;28:380–94. 10.3109/08880018.2011.563772 [DOI] [PubMed] [Google Scholar]
- 11.Basar EZ, Corapcioglu F, Babaoglu K et al. Are cardiac magnetic resonance imaging and radionuclide ventriculography good options against echocardiography for evaluation of anthracycline induced chronic cardiotoxicity in childhood cancer survivors? Pediatr Hematol Oncol 2014;31:237–52. 10.3109/08880018.2013.851753 [DOI] [PubMed] [Google Scholar]
- 12.Sherief LM, Kamal AG, Khalek EA et al. Biomarkers and early detection of late onset anthracycline-induced cardiotoxicity in children. Hematology 2012;17:151–6. 10.1179/102453312X13376952196412 [DOI] [PubMed] [Google Scholar]
- 13.Romano S, Fratini S, Ricevuto E et al. Serial measurements of NT-proBNP are predictive of not-high-dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer 2011;105:1663–8. 10.1038/bjc.2011.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stachowiak P, Kornacewicz-Jach Z, Safranow K. Prognostic role of troponin and natriuretic peptides as biomarkers for deterioration of left ventricular ejection fraction after chemotherapy. Arch Med Sci 2014;10:1007–18. 10.5114/aoms.2013.34987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quiñones-Lombraña A, Ferguson D, Hageman Blair R et al. Interindividual variability in the cardiac expression of anthracycline reductases in donors with and without Down syndrome. Pharmaceut Res 2014;31:1644–55. 10.1007/s11095-013-1267-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokiniwa H, Horiguchi J, Takata D et al. Topoisomerase II alpha expression and the Ki-67 labeling index correlate with prognostic factors in estrogen receptor-positive and human epidermal growth factor type-2-negative breast cancer. Breast Cancer 2012;19:309–14. 10.1007/s12282-011-0291-4 [DOI] [PubMed] [Google Scholar]
- 17.Arslan D, Cihan T, Kose D et al. Growth-differentiation factor-15 and tissue Doppler ımaging in detection of asymptomatic anthracycline cardiomyopathy in childhood cancer survivors. Clin Biochem 2013;46:1239–43. 10.1016/j.clinbiochem.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 18.Bains OS, Grigliatti TA, Reid RE et al. Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin. J Pharmacol Exp Ther 2010;335:533–45. 10.1124/jpet.110.173179 [DOI] [PubMed] [Google Scholar]
- 19.Vejpongsa P, Massey MR, Acholonu SA et al. Topoisomerase 2ß expression in peripheral blood predicts susceptibility to anthracycline-induced cardiomyopathy. Circulation 2013;128:A11619. [Google Scholar]
- 20.Sutter TM, Mier B. Detection of anthracycline-induced cardiotoxicity: is there light at the end of the tunnel? Ann Oncol 2002;13:647–9. 10.1093/annonc/mdf231 [DOI] [PubMed] [Google Scholar]
- 21.Vejpongsa P, Yeh ET. Topoisomerase 2β: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther 2014;95:45–52. 10.1038/clpt.2013.201 [DOI] [PubMed] [Google Scholar]
- 22.Ewer MS, Martin FJ, Henderson C et al. Cardiac safety of liposomal anthracyclines. Semin Oncol 2004;31(Suppl. 13):161–81. 10.1053/j.seminoncol.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 23.Calabresi L, Rossoni G, Gomaraschi M et al. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ Res 2003;92:330–7. 10.1161/01.RES.0000054201.60308.1A [DOI] [PubMed] [Google Scholar]
- 24.Smith LA, Cornelius VR, Plummer CJ et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010;10:337 10.1186/1471-2407-10-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander J, Dainiak N, Berger HJ et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979;300:278–83. 10.1056/NEJM197902083000603 [DOI] [PubMed] [Google Scholar]
- 26.Buzdar AU, Marcus C, Smith TL et al. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer 1985;55:2761–5. [DOI] [PubMed] [Google Scholar]
- 27.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003;97:2869–79. 10.1002/cncr.11407 [DOI] [PubMed] [Google Scholar]
- 28.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med 1996;125:47–58. 10.7326/0003-4819-125-1-199607010-00008 [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Zhang S, Zhang XB et al. Protective effect of dexrazoxane on cardiotoxicity in breast cancer patients who received anthracycline-containing chemotherapy. ZhonghuaZhong Liu ZaZhi 2013;35:135–9. 10.3760/cma.j.issn.0253-3766.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 30.Tacyildiz N, Ozyoruk D, Ozelci Kavas G et al. Selenium in the prevention of anthracycline-induced cardiac toxicity in children with cancer. J Oncol 2012;2012:651630 10.1155/2012/651630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker JR, Sharma A, Lytwyn M et al. The cardioprotective role of probucol against anthracycline and trastuzumab-mediated cardiotoxicity. J Am Soc Echocardiogr 2011;24:699–705. 10.1016/j.echo.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 32.Corradi F, Paolini L, De Caterina R. Ranolazine in the prevention of anthracycline cardiotoxicity. Pharmacol Res 2014;79:88–102. 10.1016/j.phrs.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 33.Huelsenbeck J, Henninger C, Schad A et al. Inhibition of Rac1 signaling by lovastatin protects against anthracycline-induced cardiac toxicity. Cell Death Dis 2011;2:e190 10.1038/cddis.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elitok A, Oz F, Cizgici AY et al. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: a prospective randomized controlled study with six-month follow-up. Cardiol J 2014;21:509–15. 10.5603/CJ.a2013.0150 [DOI] [PubMed] [Google Scholar]
- 35.Cardinale D, Colombo A, Lamantia G et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–20. 10.1016/j.jacc.2009.03.095 [DOI] [PubMed] [Google Scholar]
- 36.Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol 2002;13:699–709. 10.1093/annonc/mdf132 [DOI] [PubMed] [Google Scholar]


