Abstract
We report a case of interhemispheric and bifrontal cortical superficial siderosis in association with two intracranial aneurysms. The patient had no clinical history suggestive of aneurysm rupture, no feature of amyloid angiopathy or other apparent etiology for cortical siderosis. We performed high resolution brain MRI with dark blood T1 sequences before and after IV contrast injection. An anterior communicating aneurysm showed partial wall enhancement on the posterior wall whereas a left posterior communicating aneurysm did not. In the light of recent reports of the association of wall enhancement with unstable aneurysms, we considered wall enhancement to be a marker of inflammation and remodeling of the aneurysm wall, resulting in chronic hemorrhagic suffusion in the subarachnoid spaces. To our knowledge, this is the first report offering proof for a possible link between apparently unruptured aneurysms and cortical siderosis.
Keywords: Aneurysm, MRI, Vessel Wall
Background
Aneurysmal rupture is one of the several documented causes of cortical superficial siderosis.1 However, it is unclear if unruptured aneurysms can be associated with cortical superficial siderosis as a source of small intermittent bleeding in the subarachnoid spaces.
In recent years, newly developed MRI techniques have made it possible to image the walls of intracranial arteries using ‘dark blood’ T1 fat saturation sequences. Initial experiences suggest that the presence of aneurysm wall enhancement using these techniques could help identify ‘unstable’ aneurysms (ruptured, symptomatic or undergoing morphological modification).2 3
We report the case of a patient with anterior interhemispheric siderosis associated with two unruptured aneurysms, of which an anterior communicating aneurysm was shown to have partial wall enhancement on post-contrast MRI.
Case presentation
A 69 year-old-man was admitted to our stroke unit after acute onset of left lower limb weakness on the vascular surgery ward. Two weeks before he had undergone emergency surgery for type B aortic dissection with reimplantation of the subclavian artery on the left common carotid artery and stenting of the descending thoracic aorta. Previous medical history included unexplained progressive sensorineural hearing loss for at least 5 years, depression and mild cognitive impairment.
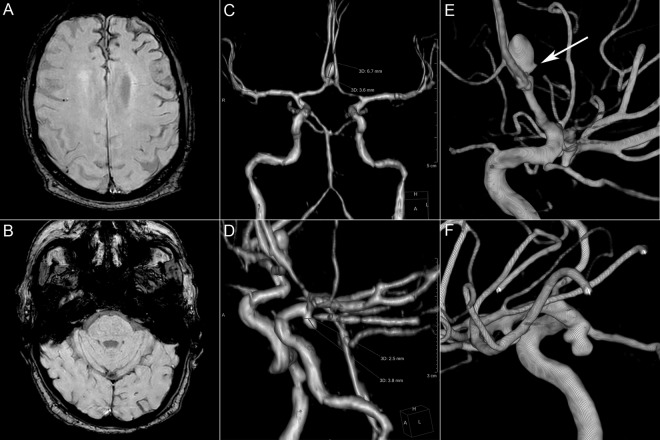
Spinal cord and brain MRI excluded spinal and brain ischemia but revealed superficial cortical siderosis in both frontal lobes and two aneurysms (anterior communicating and left posterior communicating; figure 1). There was no history of cranial trauma, brain or spine surgery, subarachnoid hemorrhage, thunderclap headache or hemorrhagic stroke.
Figure 1.
Conventional MRI sequences and digital subtraction angiography (DSA). (A, B) Susceptibility-weighted imaging demonstrating superficial cortical siderosis in the interhemispheric fissure and left frontal convexity. No siderosis is observed in the posterior fossa. (C) Anterior communicating artery aneurysm. (D) Left posterior communicating artery aneurysm. (E, F) Volume rendered reconstructions of rotational DSA of the right and left internal carotid, respectively. A spicule is noted on the posterior wall of the anterior communicating aneurysm (white arrow).
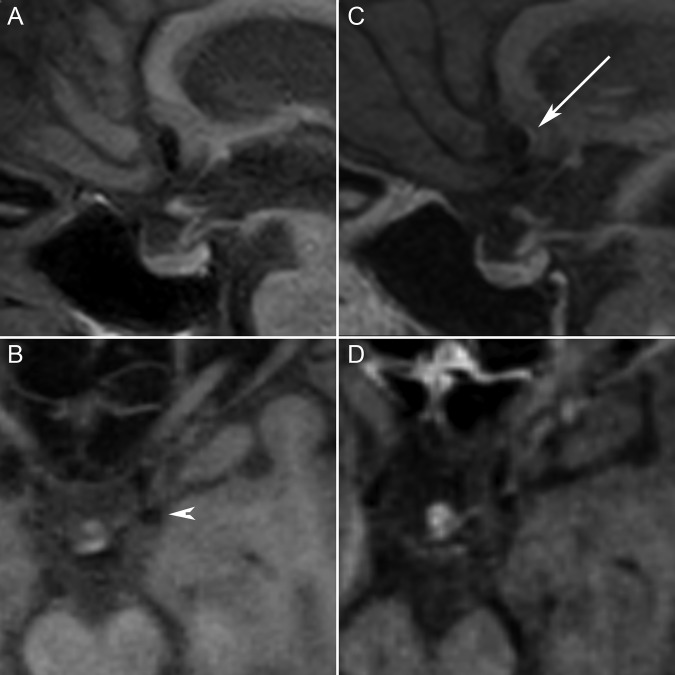
We performed aneurysmal wall imaging using a 1.5 T MRI machine (Philips Ingenia, Best, The Netherlands). A 3D T1 turbo-spin-echo with selective fat suppression (TSE SPIR) sequence was acquired before and after IV contrast administration (field of view 23×23×16 cm3; repetition time/echo time 450/23 ms; matrix 288×286; spatial resolution 0.51×0.51×0.9 mm) pre- and post-gadolinium (10 mL gadoteric acid; Dotarem, Guerbet, France) and showed partial arterial wall enhancement in the anterior communicating aneurysm but not in the left posterior communicating aneurysm (figure 2).
Figure 2.
Dark blood T1 fat saturation sequences. Multiplanar reconstructions of T1 turbo-spin-echo with selective fat suppression sequence before (A, B) and after (C, D) IV contrast injection. Partial wall enhancement is observed on the posterior wall of the anterior communicating aneurysm (white arrow); there is no wall enhancement in the posterior communicating aneurysm (arrow head).
Treatment
The anterior communicating aneurysm was embolized by balloon-assisted coiling with no procedural complications.
Outcome and follow-up
At 3 months the patient was neurologically stable.
Discussion
The classically described pattern of superficial siderosis of the CNS primarily affects the infratentorial regions and typically presents with slowly progressive sensorineural hearing impairment, cerebellar ataxia and corticospinal tract signs.4 The source of repeated subarachnoid bleeding can be identified in 50–65% of cases,5 6 including intracranial or spinal vascular malformations, head trauma, tumors, chronic subdural hematomas, avulsion of brachial plexus or history of head/brain surgery.
More recently, several authors have described another pattern of brain siderosis restricted to the supratentorial compartment. The most commonly employed term is cortical superficial siderosis.4 7 More specific causes other than those previously mentioned include primary CNS angiitis, reversible vasoconstriction syndrome and cortical venous thrombosis. Amyloid angiopathy is considered the most frequent etiology in patients over 60 years of age.7 8
In the presented case there were no clinical or anamnestic features for any of the aforementioned etiologies. In particular, the criteria for amyloid angiopathy were not met as there was no history of parenchymal hematoma and no parenchymal microbleeds on MRI. Cortical siderosis is frequently seen after aneurysmal subarachnoid hemorrhage.9 Although there was no clinical history of aneurysm rupture, the pattern of frontal and anterior interhemispheric siderosis in our case was highly suggestive of anterior communicating aneurysm rupture.
We identified a single specific report in the literature for the association of CNS siderosis and an unruptured aneurysm;10 they were considered unrelated, based on the pattern of bleeding and results of surgical exploration. However, based on the highly suggestive siderosis pattern in our case, we sought to evaluate signs of instability in the anterior communicating aneurysm.
Intracranial vessel wall imaging was initially developed to aid in the diagnosis of CNS angiitis; subsequently, it was used for intracranial atherosclerosis, Moyamoya disease and intracranial aneurysms.3 Several papers have reported partial or circumferential wall enhancement in ruptured aneurysms but also in aneurysms exerting mass effect, partial thrombosis and evolving size/morphology.2 3 In all situations, enhancement is considered to be a sign of wall inflammation. Indeed, at least one paper3 has confirmed inflammatory infiltrates in pathology pieces of unruptured aneurysms obtained after surgical treatment; these infiltrates corresponded to areas of enhancement on MRI.
In our case, we identified enhancement in the posterior wall of the anterior communicating aneurysm but not for the posterior communicating aneurysm. Wall inflammation in the area of enhancement associating with presumed small repeated subarachnoid bleeding was intuitively deemed highly compatible with the pattern of interhemispheric siderosis. Moreover, the presence of a spicule at rotational digital subtraction angiography (figure 1) was considered an additional criterion for fragility of the posterior wall, corroborating the MRI findings. This assisted us in the decision to electively treat the anterior communicating aneurysm.
However, the hypothesis of small repeated aneurysmal bleeding and its association with wall enhancement needs to be confirmed in larger series, ideally involving pathological specimens. Not all unruptured aneurysms with wall enhancement are unstable or symptomatic. In a recently published study,3 eight out of 24 unruptured aneurysms showed wall enhancement: four giant aneurysms with mass effect, one aneurysm with progressive growth over 4 years, one mirror aneurysm to a contralateral ruptured aneurysm; in the last two cases the aneurysms were stable and asymptomatic. Moreover, it is still unclear whether wall inflammation precedes rupture/microrupture or whether it is simply a secondary event. In our case the follow-up period was too short to determine if the treatment prevented the development of further symptoms.
Conclusion
We report a case of cortical superficial siderosis in which the use of the newly developed intracranial vessel wall MRI sequences revealed wall enhancement in an apparently unruptured aneurysm. To our knowledge, this is the first report that offers objective proof for a possible link between apparently unruptured intracranial aneurysms and superficial cortical siderosis.
Learning points.
Our case suggests that chronic bleeding from an apparently unruptured aneurysm can lead to cortical superficial siderosis.
Pre- and post-contrast T1 dark blood sequences can detect aneurysm wall enhancement, which is associated with unstable aneurysms.
In other papers, inflammatory infiltrates have been confirmed on pathology specimens in the areas of wall enhancement.
Footnotes
Contributors: BY: manuscript drafting, diagnosis, clinical care. RP: manuscript drafting and review, acquisition and interpretation of imaging, embolisation procedure. IZ, MD, SC, VW: manuscript review, clinical care. MM, RB: manuscript review, interpretation of imaging, embolization procedure.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hughes JT, Oppenheimer DR. Superficial siderosis of the central nervous system. A report on nine cases with autopsy. Acta Neuropathol 1969;13:56–74. 10.1007/BF00686141 [DOI] [PubMed] [Google Scholar]
- 2.Edjlali M, Gentric JC, Regent-Rodriguez C et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke 2014;45:3704–6. 10.1161/STROKEAHA.114.006626 [DOI] [PubMed] [Google Scholar]
- 3.Hu P, Yang Q, Wang DD et al. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology. Published Online First: 20 July 2016. [DOI] [PubMed] [Google Scholar]
- 4.Charidimou A, Linn J, Vernooij MW et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015;138(Pt 8):2126–39. 10.1093/brain/awv162 [DOI] [PubMed] [Google Scholar]
- 5.Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain 1995;118(Pt 4):1051–66. 10.1093/brain/118.4.1051 [DOI] [PubMed] [Google Scholar]
- 6.Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neurol 2007;3:54–8; quiz 9 10.1038/ncpneuro0356 [DOI] [PubMed] [Google Scholar]
- 7.Linn J, Herms J, Dichgans M et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol 2008;29:184–6. 10.3174/ajnr.A0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khurram A, Kleinig T, Leyden J. Clinical associations and causes of convexity subarachnoid hemorrhage. Stroke 2014;45:1151–3. 10.1161/STROKEAHA.113.004298 [DOI] [PubMed] [Google Scholar]
- 9.Bendel P, Koivisto T, Kononen M et al. MR imaging of the brain 1 year after aneurysmal subarachnoid hemorrhage: randomized study comparing surgical with endovascular treatment. Radiology 2008;246:543–52. 10.1148/radiol.2461061915 [DOI] [PubMed] [Google Scholar]
- 10.Lai MT, Ohmichi T, Yuen K et al. Superficial siderosis of the central nervous system: a case with an unruptured intracranial aneurysm. J Laryngol Otol 1995;109:549–52. [DOI] [PubMed] [Google Scholar]