Abstract
Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality worldwide and diet is an important contributor to CVD risk. Thus, several food derivatives are being investigated for their beneficial impact on reducing cardiometabolic risk factors, either in risk groups or in healthy population as a preventive measure. Wheat germ is a food by-product with high nutritional value, especially as a concentrated source of dietary fibre and essential fatty acids, but its incorporation into the diet has been rare up to now. Previous studies do not clarify the hypothesised potential causal relationship between the consumption of wheat germ and benefits for human health.
Methods and analysis
We are conducting a randomised, double-blinded, crossover, placebo-controlled clinical trial designed to assess the physiological effects of daily consumption of wheat germ-enriched bread (containing 6 g of wheat germ) compared with non-enriched bread, over a 4-week period with a 15-week follow-up, in a healthy human population. A total of 55 participants (healthy volunteers, aged 18–60) have been recruited from the Porto metropolitan area in northern Portugal. Our aim is to evaluate the health effects of wheat germ on blood cholesterol and triglycerides, postprandial glycaemic response, gastrointestinal function and discomfort, and changes in intestinal microbiota and insulin resistance as secondary outcomes. The study follows the best practices for evaluating health claims in food according to the European Food Safety Authority (EFSA) scientific opinion, namely random allocation, double blinding, reporting methods to measure and maximise compliance, and validated outcomes with beneficial physiological effects as recommended by EFSA.
Ethics and dissemination
The study has been approved by the Health Ethics Committee of São João Hospital Centre (156-15) and the Ethics Committee of Faculty of Medicine of the University of Porto (PCEDCSS-FMUP07/2015). Results will be disseminated through peer-reviewed publications and presentations at international scientific meetings.
Trial registration number
NCT02405507; pre-results.
Keywords: wheat germ, health claims, randomized controlled trial, cardiovascular risk, gastrointestinal discomfort
Strengths and limitations of this study.
The outcomes measured in this clinical trial are considered to be beneficial physiological effects, according to the European Food Safety Authority (EFSA) scientific opinion.
The study follows the best practices for evaluating health claims in food, namely random allocation, double blinding, reporting methods to measure and maximise compliance, and validated outcomes.
The proposed trial will clarify the role of wheat germ as a functional ingredient, namely whether there is any benefit to including it in a normal diet to reduce the symptoms associated with gastrointestinal discomfort and the management of hyperlipidaemia and glucose metabolism.
Any statistically significant beneficial health could contribute to providing the scientific evidence needed for validation of health claims related to wheat germ.
Compliance with self-reported questionnaires could decline over the 15-week follow-up.
Introduction
In recent decades, epidemiological studies and clinical trials have established an association between unhealthy dietary behaviours and chronic diseases such as cardiovascular diseases (CVD)1–3 and type 2 diabetes,4–6 which are leading causes of death in high-income countries. In turn, some studies have shown that populations can benefit from the incorporation of functional ingredients into their diets, proposing that functional food should be an integral part of public health programmes.7 8 Accordingly, two recent systematic reviews confirmed that whole grain intake is associated with a reduced risk of CVD.9 10
At the same time, governmental authorities have limited health claims on food, allowing only those based on solid scientific evidence. Examples of authorised health claims are the causal relationship between consumption of β-glucans from oats and blood cholesterol levels,11 12 and a cause–effect relationship between walnut ingestion and improvement of endothelium-dependent vasodilation.13
Wheat germ is the main by-product of the flour milling industry, and although it is considered an excellent source of minerals, vitamins, fibre and essential fatty acids, it has been underused. Incorporation of wheat germ in the diet is almost exclusively restricted to animal feeding.14–16 Even so, ingestion of raw wheat germ seems to reduce cholesterol and triglycerides (TG) in rats17 18 and in human subjects with hypercholesterolaemia.19 20 In turn, Matteuzzi et al21 showed that ingestion of commercial wheat germ modifies the human colon microflora by lowering Gram-negative bacteria such as coliforms, while increasing potentially health-promoting bacteria, such as bifidobacteria and lactobacilli. However, existing studies reporting wheat germ are not sufficient to validate a causal relationship, especially due to the limited number of participants included.
Study aims
Our aim is to evaluate the impact of bread supplemented with wheat germ on blood cholesterol and TG, postprandial glycaemic response and insulin resistance; gastrointestinal discomfort will be evaluated in parallel. These outcomes are considered beneficial physiological effects by the European Food Safety Authority (EFSA), and therefore this randomised crossover controlled trial has been designed to provide scientific substantiation for health claims related to wheat germ. Bread was selected as the vehicle for wheat germ since it is a widely consumed food product and is therefore the ideal product to deliver functionality. Moreover, bread itself is the main contributor to the glycaemic index (GI) of the human diet22 and since it is regularly consumed, a small change in its GI has been shown to have beneficial effects on health.22–24 The percentage of wheat germ present in the enriched bread is 6%, a figure chosen because it offers the best ratio in terms of bread texture, volume and flavour.
Methods
Design
This study is a consortium-initiated, randomised, double-blinded, crossover, placebo-controlled clinical trial designed to assess the physiological effects of daily consumption of wheat germ-enriched bread in a healthy human population. The trial has been implemented at the Faculty of Medicine of the University of Porto (Portugal)'s Centre for Health Technology and Services Research (CINTESIS).
Partners
The study is funded by the National Strategic Reference Framework (Portugal), and is part of the VALORINTEGRADOR project, jointly promoted by the Portuguese agro-food industry and three different Portuguese Research and Development (R&D) institutions (the Centre for Biological Engineering at the University of Minho, the College of Biotechnology at the Portuguese Catholic University and CINTESIS). The wheat bread supplemented with wheat germ was formulated by the two latter R&D institutions in partnership with GERM, South Africa, an agro-food company within the ambit of VALORINTEGRADOR.
Recruitment, randomisation and blinding
Volunteers were recruited through public advertisements in the university and faculty websites, and in online newspapers. After an initial phone contact, volunteers were invited to visit CINTESIS for a physical examination and a brief questionnaire about their medical history in order to check their eligibility to participate in the study. Study inclusion and exclusion criteria are described in table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Adult men or women | 1. Participant under prescription for medication for digestive symptoms such as antispasmodic, laxatives and antidiarrhoeic drugs or other digestive auxiliaries |
| 2. Age 18–60 years | 2. Relevant history, presence of any medical disorder or intake of medication/dietary supplements, potentially interfering with this trial at screening |
| 3. Healthy volunteers free of chronic diseases with relevant effect on the gastrointestinal system or on visceral motility | 3. Participants with stool frequency of ≤1 stool every 7 days |
| 4. Without a diagnosis of any digestive disease including functional bowel disorders such as IBS | 4. Participants not willing to avoid prebiotics and probiotics for the duration of the study |
| 5. Non-diabetic, no gastric bypass surgery | 5. Intake of antibiotics in the past 4 weeks and laxatives in the past 2 weeks |
| 6. Fasting plasma glucose (finger-stick) <100 mg/dL (<5.5 mmol/L) | 6. Current use of medication for lowering blood cholesterol or glucose |
| 7. Non-smoker | 7. Change of dietary habits within the 4 weeks prior to screening (for instance, start of a diet high in fibre) |
| 8. Willing and able to provide written informed consent | 8. Pregnant participant or participant planning to become pregnant during the study; breastfeeding participant |
| 9. Participants with a history of drug, alcohol or other substance abuse, or other factors limiting their ability to cooperate during the study | |
| 10. Participants anticipating a change in their lifestyle or physical activity levels since this may also influence the results | |
| 11. Known food intolerance or allergy | |
| 12. Participant involved in any clinical or food study within the preceding month |
IBS, irritable bowel syndrome.
The full study protocol has been explained in depth to the participants, who were instructed not to change their normal diet during the study. A diary pack containing self-reported questionnaires and stool sampling kits is being delivered to each participant on enrolment.
Eligible volunteers have been randomised into two intervention groups (ratio 1:1) using a computer-generated allocation sequence, and concealed through sequentially numbered, opaque, sealed envelopes. The allocation sequence was generated by a statistician who had no involvement in recruitment and intervention delivery. Groups 1 and 2 will take, daily, wheat bread (100 g) named as A and B, respectively. Half of them will start with A and the other half with B. A and B are the intervention and placebo breads but the matching code will be blinded during the trial, in all stages until data analysis can be done. The participants, the principal investigator and the entire research team are blinded to the bread code; only the company contracted to produce the bread for the trial is aware of this code.
In this regard, we performed several tests and trials to define the final bread formula. Accordingly, we tested the best formula for white bread preparation that masks wheat germ supplementation regarding texture and colour. Subsequently, different bread preparations in which the percentage of wheat germ varied were tested. We found that the bread enriched with 6% of wheat germ has the best ratio in terms of bread texture, volume and flavour, being indistinguishable from the placebo. This finding was validated by a group of experts belonging to the VALORINTEGRADOR project. Moreover, the bread (A or B) has been delivered to each participant in opaque bags.
Intervention
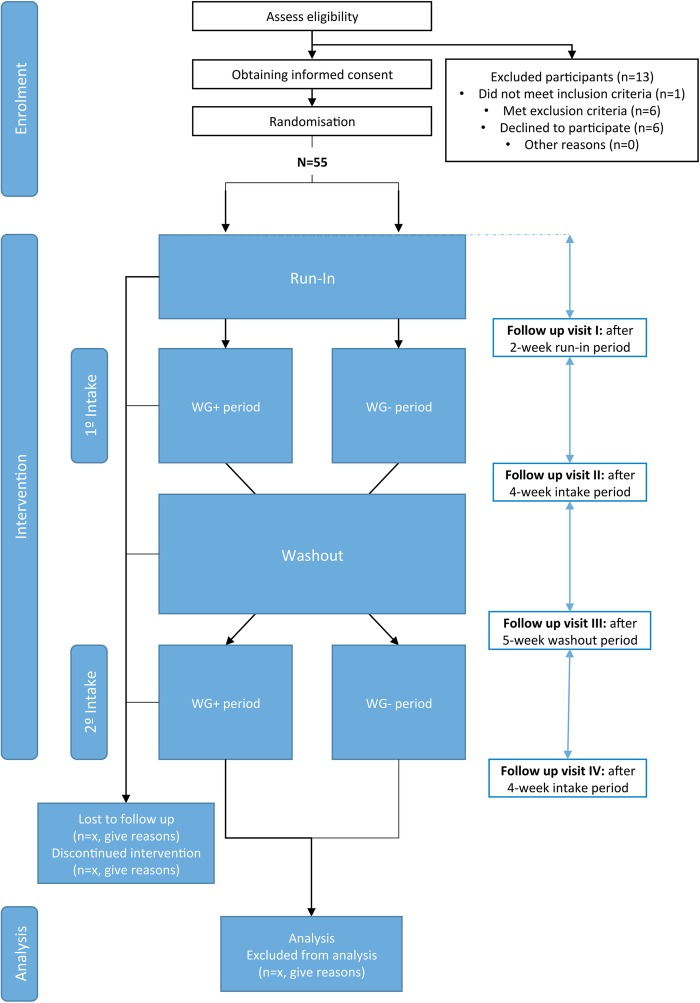
An overview of the proposed study protocol is shown in figure 1. This is a 15-week parallel group randomised crossover trial. In the first stage, eligible participants will undergo a 2-week run-in period. During this period, all participants will fill out a daily questionnaire to evaluate defaecation frequency and stool consistency (using the Bristol stool scale), as well as any gastrointestinal discomfort. During the run-in phase, participants do not consume any study product. The second stage corresponds to the randomised crossover phase. In this phase, participants are randomised to take daily wheat bread (100 g) supplemented with wheat germ (6 g/day; the intervention arm) or to consume daily wheat bread (100 g) without any supplementation (placebo; the control arm) for a 4-week period. This will be followed by a 5-week washout phase after which the participants will be crossed over for a second 4-week phase.
Figure 1.
Trial flow chart. WG+ is wheat bread with wheat germ supplementation. WG− is wheat bread without wheat germ.
The participants will fill out a daily questionnaire to determine compliance to the study protocol (daily consumption of bread) and any changes in defaecation frequency and stool consistency. We will use diaries to assess compliance because there is no biomarker for wheat germ intake. Participants have been instructed to register and divulge any medication prescribed (not declared during recruitment) or adverse events during the study.
Follow-up and data collection
The evaluation will be performed at four different time points: initial visit (T1, at the end of the 2-week run-in period), intervention visit (T2, 4 weeks after T1), washout (T3, at the end of the 5-week washout phase), and final visit (T4; at the end of the intervention stage, after crossover). At each follow-up visit, a venous blood sample will be collected in fasting state from which capillary glycaemia will be measured using a glucose metre at 0, 30, 60 and 120 min after wheat bread (supplemented with wheat germ or placebo) intake. In addition, the participant will provide one stool sample from up to 72 hours beforehand, using the stool sampling kit provided by the research team. The self-reported questionnaires to score gastrointestinal discomfort and constipation-related quality of life, and to evaluate perceived stress during the run-in and intervention stages will be delivered on follow-up. Table 2 shows the schedule of visits and the variables under investigation in the present study.
Table 2.
Schedule of assessments
| Follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|
| Run-in |
1st intake |
Washout |
2nd intake |
|||||
| Variables | 1 week | 2 weeks | 4 weeks | 6 weeks | 9 weeks | 11 weeks | 13 weeks | 15 weeks |
| Visit | x | x | x | x | ||||
| Blood sample | ||||||||
| Total cholesterol | xf | xf | xf | xf | ||||
| Triglycerides | xf | xf | xf | xf | ||||
| HDL cholesterol | xf | xf | xf | xf | ||||
| LDL cholesterol | xf | xf | xf | xf | ||||
| C reactive protein | xf | xf | xf | xf | ||||
| Insulin | xf | xf | xf | xf | ||||
| Glucose | xf | xf | xf | xf | ||||
| Capillary blood glucose | xg | xg | xg | xg | ||||
| Stool sample | ||||||||
| Intestinal microbiota | x | x | x | x | ||||
| Self-reported questionnaire | ||||||||
| Compliance (consumption of bread) | xd | xd | xd | xd | xd | xd | xd | xd |
| Stool frequency and consistency | xd | xd | xd | xd | xd | xd | xd | xd |
| PAC-SYM | x | x | x | x | x | x | x | |
| PAC-QOL | x | x | x | x | x | x | x | |
| PSS | x | x | x | x | ||||
HDL, high-density lipoprotein; LDL, low-density lipoprotein; PAC-QOL, Patient Assessment of Constipation Quality of Life; PAC-SYM, Patient Assessment of Constipation Symptoms; PSS, Perceived Stress Scale; xd, daily questionnaire; xf, fasting venous blood sample; xg, capillary blood glucose at 0, 30, 60 and 120 min after wheat bread (supplemented with wheat germ or placebo) intake.
In order to increase retention and complete follow-up, participants will receive a phone call 1-week before each visit, and a reminder text message on the day before. Self-collected and nurse-collected biological samples are labelled with the participant's study code assigned at enrolment. The samples will be used only for the purposes of this study, as stated in the protocol, and will be destroyed at the end. The diary packages containing self-reported questionnaires are also anonymised. Indeed, all collected data will be linked to the participant code and only one member of the research team has access to participant information. Moreover, only the research coordinators will have access to all trial data.
Outcomes
Rationale for outcome measured
This randomised crossover controlled trial has been designed to provide scientific substantiation of health claims related to wheat germ, and therefore the primary outcomes measured can be considered beneficial physiological effects by the EFSA.
The outcomes measured are grouped into two categories: (1) risk factors for CVD and (2) gastrointestinal function. For cardiovascular health, changes in the blood lipid profile will be investigated by measuring low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and TG as primary outcomes. LDL cholesterol is a biomarker for CVD risk25 and modification of one of these markers (LDL, HDL or TG) is considered a beneficial physiological effect by the EFSA.26 Blood concentration of C reactive protein (CRP) will be also evaluated as a primary outcome since CRP could be related to long-term risk of CVD.27 The EFSA considers that the maintenance of low plasma concentrations of CRP might be beneficial to human health.28
The hypoglycaemic effect of wheat germ will be also investigated. This food by-product
has a high content of dietary fibre (∼18%),29 which has been demonstrated to reduce blood glucose response after carbohydrate intake (postprandial).30 The intake of cereal fibre and whole grains as part of a diet with a low GI is a valuable strategy in the prevention of type 2 diabetes mellitus and CVD in the general population.31 Besides the postprandial glucose response, the concentration of fasting plasma glucose and insulin will be measured as a secondary outcome to calculate homeostasis model assessment of insulin resistance (HOMA-IR). Several studies have demonstrated an association between insulin resistance, type 2 diabetes and CVD,32 33 while wheat germ is rich in α-linolenic acid (nearly 0.5%),29 a dietary precursor of omega-3, which has been reported to have beneficial effects on insulin resistance.34
Gastrointestinal discomfort will be assessed in the context of the maintenance or improvement of digestive function. Wheat germ modifies colonic microbiota21 and it has previously been documented that these microbiota affect gut health in healthy individuals.35 36 Gastrointestinal discomfort affects 16% of the healthy population and a reduction in its symptoms is considered to be an indicator of improved gastrointestinal function.37 38 Patient-reported outcomes by means of self-reported validated questionnaires will be used for evaluation of gastrointestinal discomfort in this study. The use of these questionnaires as outcome variables for the scientific substantiation of health claims is accepted by the EFSA.39 At the same time, the composition of colonic bacterial microbiota will be analysed as a secondary outcome. Decreasing potentially pathogenic gastrointestinal microorganisms might be a beneficial physiological effect, according to experts at the EFSA.40 41
Primary outcomes
The primary outcomes in this trial will be to demonstrate the difference (change) between wheat bread supplemented with wheat germ (6 g) and placebo in (1) serum TG and total cholesterol as well as LDL and HDL, (2) CRP, (3) postprandial blood glucose and (4) gastrointestinal discomfort associated with constipation.
Analysis of TG; total, LDL and HDL cholesterol; and CRP will be performed by an accredited outsourced laboratory. Constipation symptoms will be assessed using the validated Patient Assessment of Constipation Symptoms (PAC-SYM) questionnaire.42 43 This self-reported questionnaire includes 12 constipation-related symptoms grouped into three subscales related to abdominal, stool and rectal symptoms. PAC-SYM is rated on a five-point scale, from 0 (no symptom) to 4 (very severe), aiming to measure the severity of gastrointestinal discomfort over the previous 2 weeks. A reduction in score (total or subscale) will reflect an improvement in symptoms. Cultural adaptation and linguistic validation of the PAC-SYM for Portugal was performed by the Mapi Research Trust (France).44
The change in blood glucose response will be calculated by computing the difference between the blood glucose concentration at 30, 60 and 120 min after bread intake and the baseline (t=0). Capillary finger-stick blood samples will be measured using a glucometre (Glucocard MX, ARKRAY Factory). The total blood glucose response will be expressed as the incremental area under curve (AUC), ignoring the area under the baseline, and will be calculated geometrically using the trapezoidal rule.45
Secondary outcomes
The secondary outcomes in this trial will be to demonstrate the difference (change) between wheat bread supplemented with wheat germ (6 g) and placebo in (1) self-reported quality of life related to constipation, (2) stool frequency and consistency, (3) intestinal microbiota and (4) insulin resistance.
Participant's quality of life will be assessed using the Patient Assessment of Constipation Quality of Life (PAC-QOL) questionnaire.46 47 The validated PAC-QOL is composed of 28 items grouped into four subscales related to physical discomfort, psychosocial discomfort, worries and concerns, and satisfaction. This self-reported questionnaire is rated on a five-point scale, from 0 to 4 (where 0=at no time/not at all, 4=all the time/extremely), and is used over a 2-week recall period. Cultural adaptation and linguistic validation of the PAC-QOL for Portugal was performed by the Mapi Research Trust (France).44
Secondary outcomes for stool frequency and consistency will be evaluated by means of self-reported questionnaires, while intestinal microbiota will be analysed by real-time quantitative PCR based on DNA extracted from stool samples.
HOMA-IR will be calculated to determine the degree of insulin resistance. The following formula will be used: HOMA-IR=(glucose×insulin)/405, where glucose is fasting glucose (mg/dL) and insulin is fasting insulin (mU/mL).48
Confounding variables
In addition to the randomisation protocol, the effect of wheat germ on gastrointestinal discomfort will be controlled for psychological stress, a confounding variable known to cause gastrointestinal symptoms. A psychometric assessment will be carried out using the Perceived Stress Scale (PSS). This 13-item self-reported questionnaire is designed to measure the degree to which situations in a person's life are appraised as stressful. Cultural adaptation and linguistic validation of the PSS for Portugal was implemented by Pais-Ribeiro and Marques.49
Sample size estimation
Sample size calculations have taken into account all the primary outcome variables. The sample size calculations determined that 40 participants were required to allow for an 80% power and 95% confidence level. Based on previous studies, the assumed difference in mean change from baseline and the respective SD were 5.0 and 11.0 mg/dL for total cholesterol;50 51 2.0 and 4.0 mg/dL for HDL cholesterol;50 51 5.0 and 11.0 mg/dL for LDL cholesterol;50 51 9.0 and 20.0 mg/dL for TG;50 51 0.035 and 0.080 mg/dL for CRP;52 35.0 and 80.0 mmol min/L for the incremental AUC of postprandial blood glucose;53 54 and 0.35 and 0.75 for the PAC-SYM score.55 Our aim was to enrol at least 50 participants to allow for dropouts.
Statistical analysis
Statistical analysis will be performed using SPSS V.23 software (SPSS, Chicago, Illinois, USA). Non-parametric tests will be used to analyse changes in PAC-SYM and PAC-QOL scores, since these data are not normally distributed. All other outcome parameters will be analysed using parametric approaches. Student's two-tailed unpaired t-tests will be used to compare baseline characteristics between participants. A Student's two-tailed paired t-test or repeated measures analysis of variance (ANOVA) will be used to analyse wheat germ supplementation effects within groups. The difference in the mean values of outcome variables between groups (intervention and control) will be measured using a Student's two-tailed unpaired t-test. All reported p values will be two-sided and tests will be performed with a 5% level of significance. Results will be summarised as descriptive statistics for baseline, and after 2-week and 4-week intake periods. Changes from baseline will be also evaluated for weeks 2 and 4.
The crossover design is only feasible in a setting where the conditions of interest remain stable during the study. If the conditions change during the study, they will affect the results, so it is very important to test for period effect.56 Moreover, the study design includes a washout period between the two treatment periods. This washout period aims to minimise the risk of a carry-over effect from the intervention in period 1 into period 2. Therefore, to check for any residual carry-over effect, a test of interaction between treatment and period is also recommended.56
The statistical analysis will be performed applying an intention-to-treat (ITT) approach, Chichester, UK: John Wiley & Sons, with all randomised participants included in the analysis. It will make two main assumptions (in addition to the normality assumption for Student's paired and unpaired t-tests): no period effect and no carry-over effect (assessed by the treatment–period interaction). These two assumptions will be tested using the methods recommended by Pocock.56 If the period effect is significant, the analysis will be adjusted in order to take the period effect into consideration.56 Moreover, if the carry-over effect (interaction between treatment and period) is significant, only the first period will be used in the analysis, also following Pocock's recommendations.56
The PAC-SYM and PAC-QOL total score and subscale scores will be computed based on non-missing item responses. If more than 50% of items in the total scale/subscale are missing, the total scale/subscale score is not calculated and will be designated as ‘missing’. For these previously described parameters, non-parametric tests will be used, as these data are not normally distributed. Changes within and between groups will be checked with the Wilcoxon matched-pairs signed-rank test or the Mann-Whitney test, as appropriate.
ETHICS AND Dissemination
All participants provided written informed consent. Any major amendments to the approved protocol will be resubmitted to the Ethics Committees. The trial has been conducted in accordance with the ethical principles of the Declaration of Helsinki, international law and Good Clinical Practice guidelines. Data monitoring has been performed by research coordinators along the study recruitment and follow-up an independent data monitoring committee has not been established for this study due to its minimal risk for human health. This trial was registered in ClinicalTrials.gov Database, reference NCT02405507, on 11 March 2015. The results from this study will be disseminated through peer-reviewed publications and presentations at international scientific meetings.
Discussion
There is some evidence to suggest that wheat germ has a beneficial physiological effect in maintaining normal cholesterol levels19 20 and in decreasing potentially pathogenic gastrointestinal microorganisms.21 However, existing studies are scarce and have relevant design limitations, namely small sample size and insufficient information on study design (eg, sample size calculation, allocation concealment and blinding/masking). Thus, they are still inconclusive regarding the potential causal relationship between wheat germ intake and its alleged health benefits. Moreover, all these studies have used wheat germ as a supplement. Given that bread is one of the most widely consumed food products57 and that the majority of consumers prefer whiter and softer bread formulations,23 58 the aim was to create a wheat germ-enriched bread that preserved the taste and texture of refined white bread. Consequently, we expect that even a small health benefit effect, if observed, could lead to a major impact on public health.
To the best of our knowledge, this is the first clinical trial to assess the impact of wheat germ-enriched bread intake in healthy individuals. Furthermore, this study has used outcomes which are considered to be beneficial physiological effects, according to the EFSA. Hence, this randomised crossover controlled trial could have an important contribution to make for the research community, mainly for those who are facing challenges in providing scientific evidence for the physiological benefits of food derivatives for human health.
Acknowledgments
The authors are grateful to all participants for their participation in this study. They thank Padaria Ribeiro for the production of bread used in the trial. They thank R Shackleford for proofreading this protocol manuscript.
Footnotes
Contributors: AMR was partially responsible for study design, statistical analysis plan, carrying out the trial, protocol manuscript writing and final revision. HP was partially responsible for study design, carrying out the trial, manuscript writing and final revision. CC was responsible for the general coordination of the project, study design, protocol manuscript writing and final revision. LFA was responsible for the general coordination of the project, study design, statistical analysis plan, protocol manuscript writing and final revision.
Funding: This work was supported by FEDER through the operations FCOMP-01-0202-FEDER-038861 and POCI-01-0145-FEDER-007746 funded by the Programa Operacional Competitividade e Internacionalização – COMPETE2020 and by National Funds through FCT - Fundação para a Ciência e a Tecnologia within CINTESIS, R&D Unit (reference UID/IC/4255/2013).
Disclaimer: The funder had no role in study design and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests: None declared.
Ethics approval: Health Ethics Committee of São João Hospital Centre (reference 156-15) and the Ethics Committee of the Faculty of Medicine of the University of Porto (reference PCEDCSS-FMUP 07/2015).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.McGee DL, Reed DM, Yano K et al. . Ten-year incidence of coronary heart disease in the Honolulu Heart Program. Relationship to nutrient intake. Am J Epidemiol 1984;119:667–76. [DOI] [PubMed] [Google Scholar]
- 2.Michas G, Micha R, Zampelas A. Dietary fats and cardiovascular disease: putting together the pieces of a complicated puzzle. Atherosclerosis 2014;234:320–8. 10.1016/j.atherosclerosis.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 3.Schwingshackl L, Hoffmann G. Dietary fatty acids in the secondary prevention of coronary heart disease: a systematic review, meta-analysis and meta-regression. BMJ Open 2014;4:e004487 10.1136/bmjopen-2013-004487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 2002;76:274S–80S. [DOI] [PubMed] [Google Scholar]
- 5.Hauner H, Bechthold A, Boeing H et al. . Evidence-based guideline of the German Nutrition Society: carbohydrate intake and prevention of nutrition-related diseases. Ann Nutr Metab 2012;60(Suppl 1):1–58. 10.1159/000335326 [DOI] [PubMed] [Google Scholar]
- 6.Esposito K, Maiorino MI, Bellastella G et al. . A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open 2015;5:e008222 10.1136/bmjopen-2015-008222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong N, Hoendervangers CT, Bleeker JK et al. . The opinion of Dutch dietitians about functional foods. J Hum Nutr Diet 2004;17:55–62. 10.1046/j.1365-277X.2003.00498.x [DOI] [PubMed] [Google Scholar]
- 8.Vella MN, Stratton LM, Sheeshka J et al. . Functional food awareness and perceptions in relation to information sources in older adults. Nutr J 2014;13:44 10.1186/1475-2891-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong G, Gao A, Hu FB et al. . Whole grain intake and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Circulation 2016;133:2370–80. 10.1161/CIRCULATIONAHA.115.021101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aune D, Keum N, Giovannucci E et al. . Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 2016;353:i2716 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehead A, Beck EJ, Tosh S et al. . Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;100:1413–21. 10.3945/ajcn.114.086108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to beta glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2009; 7(9):1254. http://www.efsa.europa.eu/en/efsajournal/pub/1254 (accessed 13 Jan 2016).
- 13.European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to walnuts and maintenance of normal blood LDL-cholesterol concentrations (ID 1156, 1158) and improvement of endothelium-dependent vasodilation (ID 1155, 1157) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(4):2074. http://www.efsa.europa.eu/en/efsajournal/pub/2074 (accessed 13 Jan 2016).
- 14.Brandolini A, Hidalgo A. Wheat germ: not only a by-product. Int J Food Sci Nutr 2012;63(Suppl 1):71–4. 10.3109/09637486.2011.633898 [DOI] [PubMed] [Google Scholar]
- 15.Ge Y, Sun A, Ni Y et al. . Some nutritional and functional properties of defatted wheat germ protein. J Agric Food Chem 2000;48:6215–18. 10.1021/jf000478m [DOI] [PubMed] [Google Scholar]
- 16.Zhou C, Ma H, Yu X et al. . Pretreatment of defatted wheat germ proteins (by-products of flour mill industry) using ultrasonic horn and bath reactors: effect on structure and preparation of ACE-inhibitory peptides. Ultrason Sonochem 2013;20:1390–400. 10.1016/j.ultsonch.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 17.Lairon D, Lacombe C, Borel P et al. . Beneficial effect of wheat germ on circulating lipoproteins and tissue lipids in rats fed a high fat, cholesterol-containing diet. J Nutr 1987;117:838–45. [DOI] [PubMed] [Google Scholar]
- 18.Cara L, Borel P, Armand M et al. . Effects of increasing levels of raw or defatted wheat germ on liver, feces and plasma lipids and lipoproteins in the rat. Nutrition Res 1991;11:907–16. 10.1016/S0271-5317(05)80618-9 [DOI] [Google Scholar]
- 19.Cara L, Borel P, Armand M et al. . Plasma lipid lowering effects of wheat germ in hypercholesterolemic subjects. Plant Foods Hum Nutr 1991;41:135–50. 10.1007/BF02194082 [DOI] [PubMed] [Google Scholar]
- 20.Cara L, Armand M, Borel P et al. . Long-term wheat germ intake beneficially affects plasma lipids and lipoproteins in hypercholesterolemic human subjects. J Nutr 1992;122:317–26. [DOI] [PubMed] [Google Scholar]
- 21.Matteuzzi D, Swennen E, Rossi M et al. . Prebiotic effects of a wheat germ preparation in human healthy subjects. Food Microbiology 2004;21:119–24. 10.1016/S0740-0020(03)00009-1 [DOI] [Google Scholar]
- 22.van Bakel MM, Kaaks R, Feskens EJ et al. . Dietary glycaemic index and glycaemic load in the European prospective investigation into cancer and nutrition. Eur J Clin Nutr 2009;63(Suppl 4):S188–205. 10.1038/ejcn.2009.81 [DOI] [PubMed] [Google Scholar]
- 23.Burton PM, Monro JA, Alvarez L et al. . Glycemic impact and health: new horizons in white bread formulations. Crit Rev Food Sci Nutr 2011;51:965–82. 10.1080/10408398.2010.491584 [DOI] [PubMed] [Google Scholar]
- 24.Brand-Miller J, Hayne S, Petocz P et al. . Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7. 10.2337/diacare.26.8.2261 [DOI] [PubMed] [Google Scholar]
- 25.Stein EA, Raal FJ. Targeting LDL: is lower better and is it safe? Best Pract Res Clin Endocrinol Metab 2014;28:309–24. 10.1016/j.beem.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 26.European Food Safety Authority. Guidance on the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA Journal 2011;9(12):2474. http://www.efsa.europa.eu/en/efsajournal/pub/2474 (accessed 13 Jan 2016).
- 27.Ahmadi-Abhari S, Luben RN, Wareham NJ et al. . Seventeen year risk of all-cause and cause-specific mortality associated with C-reactive protein, fibrinogen and leukocyte count in men and women: the EPIC-Norfolk study. Eur J Epidemiol 2013;28:541–50. 10.1007/s10654-013-9819-6 [DOI] [PubMed] [Google Scholar]
- 28.European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to astaxanthin and maintenance of joints, tendons, and connective tissue (ID 1918, 1978, 3142), protection of DNA, proteins and lipids from oxidative damage (ID 1449, 3141), maintenance of visual acuity (ID 1448), maintenance of blood cholesterol concentrations and maintenance of low plasma concentrations of C-reactive protein (ID 1450) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2009; 7(9):1253. http://www.efsa.europa.eu/en/efsajournal/pub/1253 (accessed 13 Jan 2016).
- 29.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010;23:65–134. 10.1017/S0954422410000041 [DOI] [PubMed] [Google Scholar]
- 30.Scazzina F, Siebenhandl-Ehn S, Pellegrini N. The effect of dietary fibre on reducing the glycaemic index of bread. Br J Nutr 2013;109:1163–74. 10.1017/S0007114513000032 [DOI] [PubMed] [Google Scholar]
- 31.Anand SS, Hawkes C, de Souza RJ et al. . Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol 2015;66:1590–614. 10.1016/j.jacc.2015.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol 2012;32:1754–9. 10.1161/ATVBAHA.111.241885 [DOI] [PubMed] [Google Scholar]
- 33.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014;10:293–302. 10.1038/nrendo.2014.29 [DOI] [PubMed] [Google Scholar]
- 34.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 2009;12:138–46. 10.1097/MCO.0b013e3283218299 [DOI] [PubMed] [Google Scholar]
- 35.Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr 2009;63:1277–89. 10.1038/ejcn.2009.64 [DOI] [PubMed] [Google Scholar]
- 36.Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem 2014;25:270–80. 10.1016/j.jnutbio.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 37.European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to decreasing potentially pathogenic gastro-intestinal microorganisms (ID 785) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA Journal 2011;9(6):2256. http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/2256.pdf (accessed 15 Sep 2015).
- 38.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011;25:3–18. 10.1016/j.bpg.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 39.European Food Safety Authority. General scientific guidance for stakeholders on health claim applications. EFSA Journal 2016;14(1):4367. http://www.efsa.europa.eu/en/efsajournal/pub/4367 (accessed 13 Jan 2016).
- 40.European Food Safety Authority. Scientific opinion on the substantiation of health claims related to various food(s)/food constituents(s) and increasing numbers of gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905), and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(10):1809. http://www.efsa.europa.eu/en/efsajournal/pub/1809 (accessed 13 Jan 2016).
- 41.European Food Safety Authority. Scientific opinion on the substantiation of health claims related to fructo-oligosaccharides (FOS) and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 781) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(6):2222. http://www.efsa.europa.eu/en/efsajournal/pub/2222. (accessed 13 Jan 2016).
- 42.Frank L, Kleinman L, Farup C et al. . Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol 1999;34:870–7. 10.1080/003655299750025327 [DOI] [PubMed] [Google Scholar]
- 43.Slappendel R, Simpson K, Dubois D et al. . Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur J Pain 2006;10:209–17. 10.1016/j.ejpain.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 44.Mapi Research Trust. http://www.mapi-trust.org. (accessed 25 Feb 2015).
- 45.Wolever TMS. The glycaemic index: a physiological classification of dietary carbohydrate. Oxfordshire: CABI, 2006. [Google Scholar]
- 46.Marquis P, De La Loge C, Dubois D et al. . Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540–51. 10.1080/00365520510012208 [DOI] [PubMed] [Google Scholar]
- 47.Dubois D, Gilet H, Viala-Danten M et al. . Psychometric performance and clinical meaningfulness of the Patient Assessment of Constipation-Quality of Life questionnaire in prucalopride (RESOLOR) trials for chronic constipation. Neurogastroenterol Motil 2010;22:e54–63. 10.1111/j.1365-2982.2009.01408.x [DOI] [PubMed] [Google Scholar]
- 48.Ostrowska L, Witczak K, Adamska E. Effect of nutrition and atherogenic index on the occurrence and intensity of insulin resistance. Pol Arch Med Wewn 2013;123:289–96. [DOI] [PubMed] [Google Scholar]
- 49.Pais-Ribeiro J, Marques T. A avaliação do stresse: a propósito de um estudo de adaptação da escala de percepção de stresse. Psicol Saúde Doenças 2009;10:237–48. [Google Scholar]
- 50.Kuriyan R, Kumar DR, R R et al. . An evaluation of the hypolipidemic effect of an extract of Hibiscus Sabdariffa leaves in hyperlipidemic Indians: a double blind, placebo controlled trial. BMC Complement Altern Med 2010;10:27 10.1186/1472-6882-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SH, Park S, Kang SM et al. . Effect of atorvastatin monotherapy and low-dose atorvastatin/ezetimibe combination on fasting and postprandial triglycerides in combined hyperlipidemia. J Cardiovasc Pharmacol Ther 2012;17:65–71. 10.1177/1074248411399762 [DOI] [PubMed] [Google Scholar]
- 52.Skulas-Ray AC, Flock MR, Richter CK et al. . Red blood cell docosapentaenoic acid (DPA n-3) is inversely associated with triglycerides and C-reactive protein (CRP) in healthy adults and dose-dependently increases following n-3 fatty acid supplementation. Nutrients 2015;7:6390–404. 10.3390/nu7085291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tapola N, Karvonen H, Niskanen L et al. . Glycemic responses of oat bran products in type 2 diabetic patients. Nutr Metab Cardiovasc Dis 2005;15:255–61. 10.1016/j.numecd.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 54.Sonia S, Witjaksono F, Ridwan R. Effect of cooling of cooked white rice on resistant starch content and glycemic response. Asia Pac J Clin Nutr 2015;24:620–5. [DOI] [PubMed] [Google Scholar]
- 55.Tack J, Stanghellini V, Dubois D et al. . Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil 2014;26:21–7. 10.1111/nmo.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pocock SJ. Clinical trials: a practical approach. Chichester, UK: John Wiley & Sons Ltd, 1983. [Google Scholar]
- 57.Cust AE, Skilton MR, van Bakel MM et al. . Total dietary carbohydrate, sugar, starch and fibre intakes in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr 2009;63(Suppl 4):S37–60. 10.1038/ejcn.2009.74 [DOI] [PubMed] [Google Scholar]
- 58.Whitton C, Nicholson SK, Roberts C et al. . National Diet and Nutrition Survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br J Nutr 2011;106:1899–914. 10.1017/S0007114511002340 [DOI] [PMC free article] [PubMed] [Google Scholar]