Abstract
Introduction
Current international guidelines for cardiac rehabilitation (CR) advocate moderate-intensity exercise training (MISS, moderate-intensity steady state). This recommendation predates significant advances in medical therapy for coronary heart disease (CHD) and may not be the most appropriate strategy for the ‘modern’ patient with CHD. High-intensity interval training (HIIT) appears to be a safe and effective alternative, resulting in greater improvements in peak oxygen uptake (VO2 peak). To date, HIIT trials have predominantly been proof-of-concept studies in the laboratory setting and conducted outside the UK. The purpose of this multicentre randomised controlled trial is to compare the effects of HIIT and MISS training in patients with CHD attending UK CR programmes.
Methods and analysis
This pragmatic study will randomly allocate 510 patients with CHD to 8 weeks of twice weekly HIIT or MISS training at 3 centres in the UK. HIIT will consist of 10 high-intensity (85–90% peak power output (PPO)) and 10 low-intensity (20–25% PPO) intervals, each lasting 1 min. MISS training will follow usual care recommendations, adhering to currently accepted UK guidelines (ie, >20 min continuous exercise at 40–70% heart rate reserve). Outcome measures will be assessed at baseline, 8 weeks and 12 months. The primary outcome for the trial will be change in VO2 peak as determined by maximal cardiopulmonary exercise testing. Secondary measures will assess physiological, psychosocial and economic outcomes.
Ethics and dissemination
The study protocol V.1.0, dated 1 February 2016, was approved by the NHS Health Research Authority, East Midlands—Leicester South Research Ethics Committee (16/EM/0079). Recruitment will start in August 2016 and will be completed in June 2018. Results will be published in peer-reviewed journals, presented at national and international scientific meetings and are expected to inform future national guidelines for exercise training in UK CR.
Trial registration number
NCT02784873; pre-results.
Keywords: HEALTH ECONOMICS
Strengths and limitations of this study.
To ensure the findings are applicable to the ‘real world’, this study will adopt a pragmatic, multicentre approach to assessing the efficacy of high-intensity interval training (HIIT) in UK cardiac rehabilitation (CR) programmes.
This study will conduct an holistic, multidisciplinary investigation into the physiological, psychosocial and economic value of HIIT in patients with CHD.
As a limitation, participants will only attend supervised exercise twice weekly for 8 weeks. This is suboptimal in relation to published data recommending three times per week for 12 weeks.
Introduction
Coronary heart disease (CHD) accounts for one-third of all deaths globally, totalling 7.4 million in 2013.1 In the UK alone, ∼175 000 myocardial infarctions (MI) are recorded annually.2 While this is a significant number, advances in preventative therapy and medical treatment have contributed to an overall reduction in CHD mortality in the UK.3 An estimated 2.3 million people are now living with the disease,2 and with a growing population of CHD survivors, the need for comprehensive and cost-effective chronic disease management is ever more apparent.
Integral to the long-term management of CHD is the provision of cardiovascular rehabilitation (CR) programmes.4 5 Exercise training is considered a key component alongside risk factor management and facilitation of long-term behavioural change.4 Compelling evidence exists for CR programmes, with meta-analyses historically highlighting a favourable effect on functional capacity, health-related quality of life (HR-QoL), hospital admissions and mortality.6–8 The most recent data, however, do not confirm a survival benefit from participation in CR.9 This may relate to the ability of contemporary medical care, interventional cardiology and secondary prevention pharmacotherapy, to achieve much of what was previously attributed to CR. However, CR does improve HR-QoL and, as such, strategies to maximise long-term physical functioning (ie, optimised, personalised exercise training programmes) should be pursued in patients with CHD. Tangible benefits are realistic for the individual and an overburdened healthcare system, and CR programmes have a vital role to play in this regard.
In addition to improved medical care, the prescribed intensity of the exercise training interventions included in the recent meta-analysis by Anderson et al9 may help explain the lack of improvement in mortality rates with CR. Exercise intensity ranged from 50% to 95% of peak oxygen uptake (VO2 peak), with the vast majority of protocols at the lower end of this range, that is, equivalent to moderate-intensity exercise (∼46–64% VO2 peak).10 This is in line with current international exercise guidelines for CHD which advocate moderate-intensity training (<80% VO2 peak) prescribed as either interval or steady state (MISS, moderate-intensity steady state).10 It is well known that greater improvements in VO2 peak can be expected with exercise training of a higher intensity and that a higher VO2 peak is associated with an improvement in mortality risk.11 12 Given that current guidelines predate significant advances in interventional cardiology and medical therapy, moderate-intensity exercise may be considered conservative and suboptimal for the ‘modern’ patient with CHD.13 Greater benefit may be attained by participating in high-intensity interval training (HIIT) involving repeated bursts of harder exercise interspersed with periods of recovery.14 15 High intensity, in this context, describes exercise performed above moderate intensity (ie, >64% VO2 peak) as opposed to the maximal or supramaximal exercise specified in some protocols in healthy individuals.16
Meta-analyses have indicated the superiority (∼1.7 mL/kg/min) of HIIT over MISS for improvements in VO2 peak in patients with CHD.14 15 17 These analyses, however, are limited by small sample sizes and the significant heterogeneity of study populations and HIIT protocols. HIIT protocols can be modified in numerous ways (eg, modality, intensity, interval duration) to suit the population or intended outcome,18 but there is no consensus as to the optimal configuration for the CHD population.17 In a landmark European study, high-intensity intervals lasting 4 min were deemed unfeasible in patients with CHD and offered no additional benefit over continuous training.19 As an alternative, low-volume HIIT uses 1 min intervals to provide intermittent metabolic stimulus with non-sustained cardiovascular stress. This appears to be safe and well tolerated in addition to being effective at improving VO2 peak in patients with CHD.20 21 The benefit of this ‘low-volume HIIT’ approach in ‘real world’ CR programmes in the UK, however, cannot be confirmed. Previous studies have generally been proof-of-concept studies conducted under ‘laboratory’ conditions. Carefully selected populations, tightly controlled exercise protocols and researcher-led interventions may limit the ecological validity of such studies. Likewise, substantial international variation in the provision and implementation of exercise-based CR may reduce the extent to which non-UK data can be applied to CR programmes in the UK.
The high-intensity interval training versus moderate-intensity steady-state training in UK Cardiac Rehabilitation trial (HIIT or MISS UK) is a pragmatic multicentre randomised controlled trial and economic evaluation comparing two CR exercise interventions. The primary objectives of the trial are:
To assess the effect of HIIT on VO2 peak and cardiovascular health.
To assess the acceptability of HIIT and the psychological and motivational factors associated with compliance and adherence.
To assess the effect of HIIT on lifestyle physical activity and a HR-QoL.
To conduct an economic evaluation of HIIT compared with MISS in CR programmes in the UK.
To assess the safety of HIIT.
In patients attending CR programmes in the UK, we hypothesise that HIIT will improve VO2 peak to a greater extent than MISS training. In this population, data relating to the effects of HIIT (particularly low-volume HIIT) on clinical, physiological, psychosocial and economic outcomes are limited but appear to indicate at least an equivalent effect.22–24 As such, we also hypothesise that HIIT will (1) be more acceptable than MISS and demonstrate greater patient compliance and adherence; (2) improve cardiovascular health to a greater extent than MISS; (3) improve HR-QoL to a greater extent than MISS; (4) lead to more positive motivation and attitudes to exercise than MISS; (5) increase short-term and medium-term participation in lifestyle physical activity to a greater extent than MISS; (6) be a cost-effective alternative to MISS and (7) be as safe as MISS.
Methods and analysis
The HIIT or MISS UK study is a pragmatic, single-blind, multicentre, longitudinal, randomised controlled trial and economic evaluation. In line with the median UK CR programme duration of 8.5 weeks,25 participants will be randomly allocated to 8 weeks of HIIT or MISS training (usual care). Outcomes will be measured at baseline, 8 weeks and 12 months by assessors blinded to group allocation. Study interventions will be delivered by clinical (not research) staff. The study is pragmatic in nature in that it will be conducted in existing CR programmes. It is, therefore, accepted that some variation in the delivery of usual care will be evident between study sites. This will ensure generalisability of the findings to UK CR programmes. The trial protocol adheres to the Standard Protocol Items: Recommendations for Clinical Trials (SPIRIT) guidelines.26
Setting
The HIIT or MISS study will be conducted at three community CR centres; (1) Atrium Health, Centre for Exercise & Health, Coventry, (2) Department of Sport, Health & Exercise Science, University of Hull and Hull Royal Infirmary, Kingston-upon-Hull and (3) Ystrad Fawr Hospital, Ystrad Mynach, South Wales. Programmes are commissioned by University Hospitals Coventry & Warwickshire NHS Trust, City Healthcare Partnership CIC (Hull) and Aneurin Bevan University Health Board (South Wales), respectively. Starting August 2016, 510 CR patients will be recruited over a 2-year period.
Participants
The study will recruit patients with established coronary artery disease (CAD) referred for CR exercise training. Patients with MI, coronary artery bypass graft (CABG) surgery, angiographically documented CAD and elective percutaneous coronary intervention (PCI) will be eligible.
General inclusion criteria
Successfully revascularised following PCI or CABG.
Angiographically documented non-obstructive CAD.
Left ventricular ejection fraction >40%.
Clinically stable (symptoms and medication) for >2 weeks.
18–75-year of age.
General exclusion criteria
Symptoms of ischaemia.
Significant left main stem stenosis.
NYHA class III–IV symptoms.
Compromising ventricular arrhythmia.
Significant valvular heart disease.
Inability to comply with guidelines for participation in exercise testing and training.27–29
Significant limiting comorbidities that would prevent full participation.
Additional exclusion criteria
Further to the analysis of cardiopulmonary exercise test (CPET) and resting echocardiography by the research team at baseline, and prior to randomisation, patients will be prevented from continuing their involvement in the study if there is indication of:
Study procedures
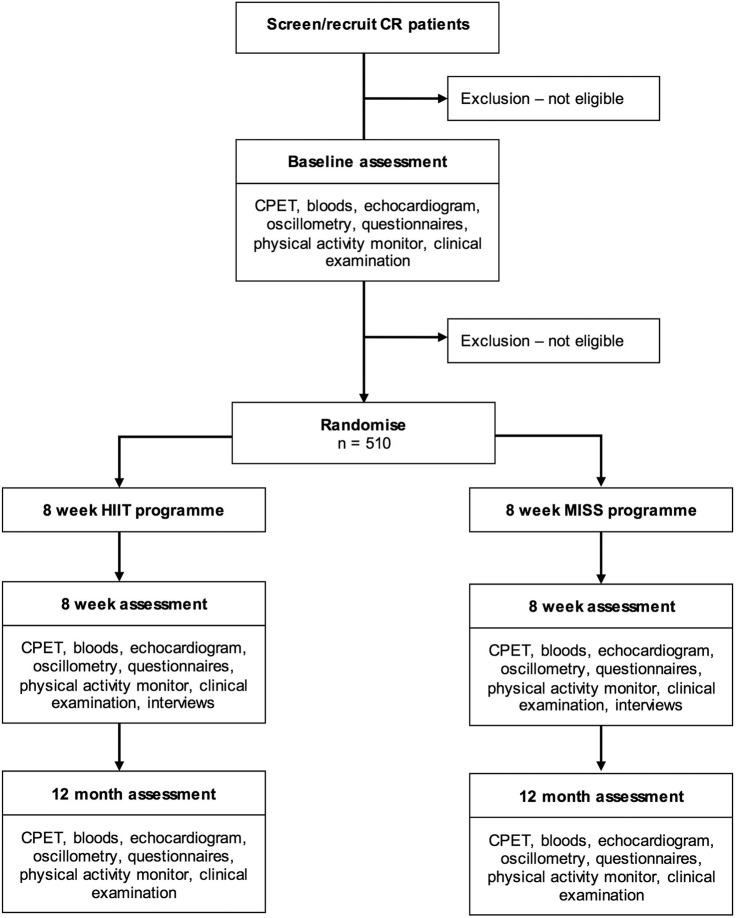
An outline of the participant pathway for the study is presented in figure 1. Eligibility will be assessed by the research team at each site under the supervision of the local principal investigator (PI). Potential participants will be approached at their first outpatient CR appointment by a member of the study team: verbal and written information will be provided. A subsequent phone call (at least 48 hours later) will confirm those who wish to participate. Informed consent will be attained at the baseline assessment visit, which will coincide with an outpatient CR appointment. Baseline procedures will include CPET, echocardiogram, venipuncture, arterial oscillometry and clinical examination. Instruments to assess HR-QoL, health and social care use and the psychological and motivational factors associated with compliance and adherence will be administered, and a lifestyle physical activity monitor will be fitted (removed 1 week later). Further to the analysis of CPET and echocardiography at baseline, the local research team will rescreen potential participants for eligibility. Those who are ineligible will take no further part in the study but will continue with usual care CR. Eligible participants will subsequently be randomised to 8 weeks of twice weekly HIIT or MISS training. All measures completed at baseline will be repeated at 8 weeks and 12 months.
Figure 1.
Study flow chart.
Interventions
The study will compare HIIT with current usual care in the UK—that is, moderate-intensity interval training progressing towards moderate-intensity steady-state (MISS) training.29 Table 1 provides a summary of both interventions, and table 2 details the framework within which the HIIT intervention will be progressed. Participants will attend twice weekly CR exercise sessions for 8 weeks, performing either HIIT or MISS training for the cardiovascular component of their programme. In accordance with current UK standards,29 a muscular strength and endurance training programme will also be completed by both study groups, and participation in additional home-based exercise recommended as standard. Participants who are unable/unwilling to comply with the HIIT protocol will be permitted to cease involvement in the HIIT intervention and continue with usual care CR (not as part of the trial). Where two or more consecutive training sessions are missed, the intervention period can be extended to 10 weeks. As is commonplace for CR programmes in the UK, there will be some variation in the structure and delivery of the MISS intervention at each of the study sites. This is in keeping with the pragmatic nature of the trial. Each centre will, however, adhere to current UK standards.29
Table 1.
Comparison of HIIT and MISS training interventions
| HIIT | MISS |
|---|---|
|
|
CPET, cardiopulmonary exercise test; HIIT, high-intensity interval training; HR, heart rate; HRR, heart rate reserve; MISS, moderate-intensity steady state; PA, physical activity; PPO, peak power output; RPE, rating of perceived exertion; VO2 peak, peak oxygen uptake.
Table 2.
Breakdown of HIIT training programme by week
| Week | High-intensity intervals (number×time in min) | Low-intensity intervals (number×time in min) | Total high-intensity exercise (min) | Total low-intensity exercise (min) | Total exercise time (min) |
|---|---|---|---|---|---|
| 1 | 5×0.5 | 5×1 | 2.5 | 5 | 7.5 |
| 2 | 5×1 | 5×1 | 5 | 5 | 10 |
| 3 | 7×1 | 7×1 | 7 | 7 | 14 |
| 4–8 | 10×1 | 10×1 | 10 | 10 | 20 |
HIIT, high-intensity interval training.
The following exercise training criteria must be satisfied for participants to be regarded as having sufficiently adhered to the treatment protocol:
A minimum of 80% of sessions completed (13 of 16).
HIIT—10×1 min protocol achieved by week 4.
MISS—20 min continuous CV exercise achieved by week 4.
The number of participants who do not meet the above criteria will be recorded.
Randomisation and blinding
Trial participants will be randomised to HIIT or MISS on a 1:1 basis. The random allocation sequence will be generated by the trial statistician using a random number generator and implemented by a central telephone registration and randomisation service at Warwick Clinical Trials Unit. Randomisation will be stratified by site using random permuted blocks randomisation within each site to ensure approximately equal numbers of patients are allocated to HIIT and MISS. To ensure allocation concealment, researchers will request randomisation on completion of all baseline assessments. Outcome assessors will be blinded to group allocation, as will the trial statistician. Clinical staff delivering the interventions cannot be blinded, however, they will not be involved in data analysis or reporting.
Study outcome measures
The primary outcome measure is the change in peak oxygen uptake (VO2 peak) at 8 weeks. A number of secondary outcome measures will also be assessed, namely (1) acceptability and the motivational and attitudinal factors associated with compliance and adherence; (2) HR-QoL; (3) service and resource use; (4) lifestyle physical activity; (5) cardiovascular reserve; (6) cardiac remodelling; (7) arterial remodelling; (8) cardiovascular health and (9) safety. Table 3 provides the complete schedule for outcome assessment.
Table 3.
Outcome measures and assessment schedule
| Measure | Instrument | Assessment time point |
|---|---|---|
| Primary outcome | ||
| VO2 peak | CPET | Baseline, 8 weeks, 12 months |
| Secondary outcomes | ||
| Compliance, adherence | Compliance/adherence/drop-out rates | Continuous |
| MSES | Baseline, 8 weeks, 12 months | |
| BREQ-2 | Baseline, 8 weeks, 12 months | |
| PNSES | Baseline, 8 weeks, 12 months | |
| Bipolar adjectival rating scale | Baseline, 8 weeks, 12 months | |
| SC-IAT | Baseline, 8 weeks, 12 months | |
| Acceptability | Semistructured interviews | 8 weeks |
| HR-QOL | EQ-5D | Baseline, 8 weeks, 12 months |
| Service and resource use | CSRI | Baseline, 8 weeks, 12 months |
| Lifestyle physical activity | Physical activity monitor | Baseline, 8 weeks, 12 months |
| Cardiovascular reserve | CPET | Baseline, 8 weeks, 12 months |
| Cardiac remodelling | Echocardiography | Baseline, 8 weeks, 12 months |
| Arterial remodelling | Arterial oscillometry | Baseline, 8 weeks, 12 months |
| Cardiovascular health | Clinical examination | Baseline, 8 weeks, 12 months |
| Blood sampling | Baseline, 8 weeks, 12 months | |
| Safety | Adverse event monitoring | Continuous |
BREQ-2, Behavioural Regulation in Exercise Questionnaire-2; CPET, cardiopulmonary exercise test; CSRI, client service receipt inventory; EQ-5D, 5 item EuroQol; HR-QoL, health-related quality of life; MSES, Multidimensional Self-Efficacy for Exercise Scale; PNSES, Psychological Need Satisfaction in Exercise Scale; SC-IAT, Single-Category Implicit Association Test; VO2 peak, peak oxygen uptake.
Cardiopulmonary exercise testing will be performed to measure VO2 peak and other parameters representative of cardiovascular reserve. Tests will be conducted using a standard bicycle ramp protocol in accordance with American Thoracic Society guideline.30 Participants will be encouraged to maintain a cadence of 70 rpm until symptom-limited volitional fatigue prevents continuation. Criteria for the assessment of a good participant effort will include peak respiratory exchange ratio (RER) >1.10, peak HR≥85% predicted and RPE ≥18.31
Compliance and adherence will be determined by recording the number of training sessions attended and successfully completed in accordance with the exercise protocol. Drop-out from the programme will also be documented for both study groups in addition to reason for drop-out, where provided voluntarily by participants. To assess the psychological and motivational factors associated with compliance and adherence to the exercise training interventions, the predictive effects of self-efficacy, motivation, need satisfaction and implicit and explicit attitudes and, reciprocally, the effects of training on self-efficacy, motivation, need satisfaction and implicit and explicit attitudes, will be quantitatively measured using validated tools: (1) the Multidimensional Self-Efficacy for Exercise Scale (MSES);32 (2) the Behavioural Regulation in Exercise Questionnaire-2 (BREQ-2);33 (3) the Psychological Need Satisfaction in Exercise Scale (PNSES);32 (4) Courneya and Bobick's 7-point Bipolar Adjectival Rating Scale34 and (5) a Single-Category Implicit Association Test (SC-IAT).35 Semistructured interviews will qualitatively evaluate acceptability in a subgroup of 40 patients, representative of completers and drop-outs in both intervention groups. Verbatim transcripts will be thematically analysed.36
Health-related quality of life will be assessed with the five-item EuroQoL (EQ-5D-5L),37 as recommended by the National Institute for Health and Care Excellence in the UK for economic evaluation in clinical trials.38 General population preference-based tariffs for the UK allow for the comparison of EQ-5D index scores with population norms and other health conditions.39 An adapted client service receipt inventory (CSRI), based on examples in the DIRUM database,40 will be administered at each time point to capture participant health and social care service use since the last time point (plus a retrospective 2-month period at baseline).
Lifestyle physical activity will be recorded over a 7-day period with an ActiGraph GT9X Link (Actigraph, Pensacola, Florida, USA) worn on the wrist. Comprehensive evaluation of participants' daily physical activity patterns will be derived from the unit's 3-axis accelerometer, magnetometer and gyroscope. The Actigraph GT9X Link is considered the gold standard in non-invasive research grade physical activity monitoring and has been extensively validated.
To quantify cardiac remodelling, echocardiographic images will be obtained and analysed as recommended in current guidelines.41 42 To assess cardiac structure and function (systolic and diastolic), standard techniques will be used including 2D, M-mode, pulse wave Doppler and tissue Doppler echocardiography. To investigate arterial remodelling, pulse wave velocity will be determined through the non-invasive method of brachial oscillometry (Mobil-O-Graph PWA Monitor, IEM GmbH, Stolberg, Germany). A blood pressure cuff will be placed on the participant's upper left arm and will inflate and deflate automatically. Mobil-O-Graph PWA has been validated against internationally recognised invasive and non-invasive gold standards.43
Standard clinical examination will include medical history, stature, body mass and cardiovascular risk factor assessment, that is, resting blood pressure, diabetes, family history of premature CHD and smoking status. Blood sampling will be performed to allow the measurement of biomarkers of cardiovascular and metabolic health. Routine testing will include full blood cell count, liver function, urea and electrolytes, glycaemic control and a full lipid profile. Serum and plasma will be stored for the analysis of current and emerging biochemical markers of cardiovascular and metabolic health relating to inflammation, cardiac remodelling, pro-thrombosis, endocrine function and lipids.44 45
To verify the safety of HIIT and MISS training performed in CR, adverse and serious adverse events will be carefully monitored, recorded and reported. In line with the principles of Good Clinical Practice, the nature and severity of the event, in addition to its potential association with the exercise training intervention, will be ascertained by the local PI and ratified by the trial clinician.46
Sample size
Given the pragmatic nature of the trial, a 1.5 mL/kg/min larger improvement of VO2 peak in the HIIT group compared to the MISS group is considered a clinically relevant difference. Keteyian and colleagues reported a reduction of ∼15% in all-cause mortality for each 1 mL/kg/min increase in VO2 peak in a large CR cohort with revascularised coronary disease.47 In the present study, a sample size of 191 patients in each group will be sufficient to detect this difference assuming a SD of 4.5 mL/kg/min, a power of 90% and a significance level of 5%. The assumed SD is based on observations from Conraads et al.19 This trial is similar to HIIT or MISS and reported a loss to follow-up from baseline to post-intervention of 13%. A conservative drop-out of ∼25% yields a required sample size of 510 patients (255 per group) to be randomised. Should the drop-out rate at 12 months be 50%, then the study would retain power of 76% to detect a difference of 1.5 mL/kg/min in the primary outcome at this time point using the aforementioned assumptions.
Data collection and management
Study data will be collected on a case report form by the research team at baseline, 8 weeks and 12 months. Each participant will be allocated a unique study ID number; a list of participants will be stored electronically by UHCW NHS Trust. Data will be anonymously entered into REDCap (Research Electronic Data Capture),48 a secure, web-based application designed to support data capture for research studies. This will be hosted by Cardiff Metropolitan University.
Statistical analysis
The primary end point for the statistical analysis is the mean change in VO2 peak (mL/kg/min) from baseline to 8 weeks of follow-up. The primary end point will be compared between intervention arms using a general linear model with the treatment group and baseline VO2 peak fitted as covariates, and 8-week VO2 peak as the dependant variable. The linear model will be adjusted for the continuous covariate age, and the categorical covariates sex and study site. Further adjustment variables may be investigated as part of the exploratory analysis. The robustness of the primary outcome analysis will be investigated using three standard multiple imputation methods (monotone regression, fully conditional specification regression and Markov chain Monte Carlo (MCMC)).
The secondary outcome measures will be analysed using the same covariates as the primary outcome analysis. Likewise, the differences between groups in terms of continuous secondary outcome measures will be assessed with the same statistical model as the primary outcome analysis. Differences between treatment arms for binary, unordered categorical and ordinal secondary outcome variables will be analysed using logistic regression, multinomial logistic regression and proportional odds models, respectively.
The primary and secondary outcome analyses will be conducted at the conventional (two-sided) 5% level. To reduce the risk of false-positive claims, all secondary analyses will be considered to be exploratory if a non-significant result is obtained from the primary analysis and, whenever reported, the failure to achieve a significant result in the primary analysis will be declared. It is not proposed to formally adjust for multiple testing among the secondary end points as these are likely to be correlated so that standard adjustment techniques such as the Bonferroni method would be conservative. All analyses will be performed on an intention-to-treat basis. A per protocol analysis will be conducted as an exploratory analysis, as will subgroup analyses (for subgroups prespecified in the protocol) and repeated measures mixed models.
All data will be summarised and reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guideline.49 No formal interim analyses are anticipated.
Economic evaluation
In line with the National Institute for Health and Clinical Excellence (NICE) guidance on the economic evaluation of public health interventions,50 from a societal perspective, a cost-consequence analysis of HIIT (embedded within CR) compared with MISS training (representing usual care) will be undertaken. Within the cost-consequence analysis, there will be an embedded cost-utility analysis, using Quality Adjusted Life Years (QALYs) gained with HR-QoL weights drawn from EQ-5D-5L. This approach has been chosen because QALYs allow comparison with the value for money of other medical and public health interventions but do not capture the full range of relevant outcomes in public health prevention.50 We will use STATA V.14 to bootstrap (5000 replications) the differences in cost and outcomes, to produce a 95% CI, cost-effectiveness planes and cost-effectiveness acceptability curves, to present to healthcare policymakers and local commissioners the probability that the intervention is cost-effective at different payer thresholds.
The health economics component of the study will be written up in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement for the reporting of published economic evaluations.51
Patient and public involvement
Patient and public involvement has been integral to protocol development. A formal consultation event was attended by a representative sample of current CR participants in August 2015. Participants were introduced to various different approaches to exercise training and asked to comment on their suitability. A range of opinions and views were recorded with the overriding sentiment being that participants would be prepared to engage in HIIT with minimal concern. Close supervision by experienced CR exercise professionals was considered essential. The only significant negative comment related to the fact that HIIT would be performed solely on an exercise bike as opposed to a range of cardiovascular exercise equipment. However, participants confirmed that they would be prepared to tolerate this in the short term. Two CR participants will sit on the trial steering group for the duration of the trial.
Dissemination and impact
Throughout the trial, media outlets (including social media) will be informed of progress, and the experiences gained will be presented at national conferences and non-academic outlets such as national governing body publications. On completion, the study results will be published in peer-reviewed journals and presented at scientific meetings. The results will also be disseminated in newsletter form throughout the UK via national governing bodies and at local research and patient conference events. It is anticipated that the results of the study will inform future national guidelines for exercise training in UK CR.
Footnotes
Twitter: Follow Gordon McGregor at @HIITorMISSUK
Contributors: GM is the chief investigator for the trial, leading on protocol writing, ethics application and manuscript preparation. GM, KB, RS, SN, LI, SE, RP, SB, MJH, BB, TH and PB all contributed fully to study design. TH (statistics), RT-E, LB (health economics), DM (quantitative psychology) and JM (qualitative psychology) provided expertise in their respective discipline and authored the relevant section of the protocol and manuscript. KB, RS and SN edited the manuscript. All authors read and approved the final version of the manuscript.
Competing interests: None declared.
Ethics approval: The study protocol V.1.0, dated 1 February 2016, was approved by the NHS Health Research Authority, East Midlands—Leicester South Research Ethics Committee on 4 March 2016 (16/EM/0079).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.WHO. The top 10 causes of death. Fact sheet No. 310. World Health Organization, 2014. http://www.who.int/mediacentre/factsheets/fs310/en/ (updated May 2014). [Google Scholar]
- 2.BHF. Coronary Heart Disease Statistics. A compendium of health statistics. 2012 edition, 2012.
- 3.Allender S, Scarborough P, O'Flaherty M et al. Patterns of coronary heart disease mortality over the 20th century in England and Wales: possible plateaus in the rate of decline. BMC Public Health 2008;8:148 10.1186/1471-2458-8-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley JP, Furze G, Doherty P et al. BACPR scientific statement: British standards and core components for cardiovascular disease prevention and rehabilitation. Heart 2013;99:1069–71. 10.1136/heartjnl-2012-303460 [DOI] [PubMed] [Google Scholar]
- 5.NICE. Secondary prevention after a myocardial infarction. NICE quality standard (QS99), 2015. https://www.nice.org.uk/guidance/qs99 [Google Scholar]
- 6.Valkeinen H, Aaltonen S, Kujala UM. Effects of exercise training on oxygen uptake in coronary heart disease: a systematic review and meta-analysis. Scand J Med Sci Sports 2010;20:545–55. 10.1111/j.1600-0838.2010.01133.x [DOI] [PubMed] [Google Scholar]
- 7.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J 2011;162:571–84.e2. 10.1016/j.ahj.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 8.Heran BS, Chen JM, Ebrahim S et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011;(7):CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson L, Oldridge N, Thompson DR et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016;67:1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 10.ACSM. Guidelines for exercise testing and prescription. 9th edn Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 11.Kodama S, Saito K, Tanaka S et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024–35. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 12.Garber CE, Blissmer B, Deschenes MR et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. [DOI] [PubMed] [Google Scholar]
- 13.West RR, Jones DA, Henderson AH. Rehabilitation After Myocardial Infarction Trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012;98:637–44. 10.1136/heartjnl-2011-300302 [DOI] [PubMed] [Google Scholar]
- 14.Liou K, Ho S, Fildes J et al. High intensity interval versus moderate intensity continuous training in patients with coronary artery disease: a meta-analysis of physiological and clinical parameters. Heart Lung Circ 2016;25:166–74. [DOI] [PubMed] [Google Scholar]
- 15.Elliott AD, Rajopadhyaya K, Bentley DJ et al. Interval training versus continuous exercise in patients with coronary artery disease: a meta-analysis. Heart Lung Circ 2015;24:149–57. 10.1016/j.hlc.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 16.Gillen JB, Martin BJ, MacInnis MJ et al. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS One 2016;11:e0154075 10.1371/journal.pone.0154075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattyn N, Coeckelberghs E, Buys R et al. Aerobic interval training vs. moderate continuous training in coronary artery disease patients: a systematic review and meta-analysis. Sports Med 2014;44:687–700. [DOI] [PubMed] [Google Scholar]
- 18.Gibala MJ, Little JP, Macdonald MJ et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 2012;590(Pt 5):1077–84. 10.1113/jphysiol.2011.224725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conraads VM, Pattyn N, De Maeyer C et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol 2015;179:203–10. 10.1016/j.ijcard.2014.10.155 [DOI] [PubMed] [Google Scholar]
- 20.Currie KD, Dubberley JB, McKelvie RS et al. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc 2013;45:1436–42. 10.1249/MSS.0b013e31828bbbd4 [DOI] [PubMed] [Google Scholar]
- 21.Currie KD, Bailey KJ, Jung ME et al. Effects of resistance training combined with moderate-intensity endurance or low-volume high-intensity interval exercise on cardiovascular risk factors in patients with coronary artery disease. J Sci Med Sport 2015;18:637–42. [DOI] [PubMed] [Google Scholar]
- 22.Aamot IL, Karlsen T, Dalen H et al. Long-term exercise adherence after high-intensity interval training in cardiac rehabilitation: a randomized study. Physiother Res Int 2016;21:54–64. 10.1002/pri.1619 [DOI] [PubMed] [Google Scholar]
- 23.Pattyn N, Vanhees L, Cornelissen VA et al. The long-term effects of a randomized trial comparing aerobic interval versus continuous training in coronary artery disease patients: 1-year data from the SAINTEX-CAD study. Eur J Prev Cardiol 2016;23: 1154–64. 10.1177/2047487316631200 [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro PA, Boidin M, Juneau M et al. High-intensity interval training in patients with coronary heart disease: prescription models and perspectives. Ann Phys Rehabil Med 2016. doi:10.1016/j.rehab.2016.04.004 10.1016/j.rehab.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 25.The National Audit of Cardiac Rehabilitation Annual Statistical Report. 2015. http://www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2015.pdf
- 26.Chan AW, Tetzlaff JM, Altman DG et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AACVPR. Guidelines for cardiac rehabilitation and secondary prevention programs. 4th edn Champaign: (IL: ): Human Kinetics, 2003. [Google Scholar]
- 28.ACSM. Guidelines for exercise testing and prescription. 8th edn Riverwoods: (IL: ): Lippincott Williams & Wilkins, 2009. [Google Scholar]
- 29.ACPICR. Standards for physical activity and exercise in the cardiac population 2015. http://acpicr.com/sites/default/files/ACPICR%20Standards%202015_0.pdf
- 30.Ross RM. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:1451; author reply 51. [DOI] [PubMed] [Google Scholar]
- 31.Balady GJ, Arena R, Sietsema K et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010;122:191–225. 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 32.Rodgers WM, Wilson PM, Hall CR et al. Evidence for a multidimensional self-efficacy for exercise scale. Res Q Exerc Sport 2008;79:222–34. 10.1080/02701367.2008.10599485 [DOI] [PubMed] [Google Scholar]
- 33.Markland D, Tobin V. A modification of the Behavioral Regulation in Exercise Questionnaire to include an assessment of amotivation. J Sport Exerc Psychol 2004;26:191–6. 10.1123/jsep.26.2.191 [DOI] [Google Scholar]
- 34.Courneya KS, Bobick TM. Integrating the theory of planned behaviour with processes and stages of change in the exercise domain. Psychol Sport Exerc 2000;1:41–56. 10.1016/S1469-0292(00)00006-6 [DOI] [Google Scholar]
- 35.Karpinski A, Steinman RB. The single category implicit association test as a measure of implicit social cognition. J Pers Soc Psychol 2006;91:16–32. 10.1037/0022-3514.91.1.16 [DOI] [PubMed] [Google Scholar]
- 36.Clarke BA. Successful qualitative research. London: Sage, 2013. [Google Scholar]
- 37.The EuroQol Group. EQ-5D user guide. Rotterdam, The Netherlands: The EuroQol Group, 1996. [Google Scholar]
- 38.NICE. Guide to the methods of technology appraisal 2013. http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf
- 39.Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. Discussion Paper 172 UK: Centre for Health Economics Discussion Paper Series, University of York, 1999. [Google Scholar]
- 40.Ridyard CH, Hughes DA. Methods for the collection of resource use data within clinical trials: a systematic review of studies funded by the UK Health Technology Assessment program. Value Health 2010;13:867–72. 10.1111/j.1524-4733.2010.00788.x [DOI] [PubMed] [Google Scholar]
- 41.Nagueh SF, Smiseth OA, Appleton CP et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 42.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28: 1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 43.Luzardo L, Lujambio I, Sottolano M et al. 24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res 2012;35:980–7. 10.1038/hr.2012.78 [DOI] [PubMed] [Google Scholar]
- 44.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 2006;113:2335–62. 10.1161/CIRCULATIONAHA.104.482570 [DOI] [PubMed] [Google Scholar]
- 45.Montgomery JE, Brown JR. Metabolic biomarkers for predicting cardiovascular disease. Vasc Health Risk Manag 2013; 9:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.1996. ICH Harmonised Tripartite guideline—guideline for good clinical practice E6 (R1) http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.html.
- 47.Keteyian SJ, Brawner CA, Savage PD et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 2008;156:292–300. 10.1016/j.ahj.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 48.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moher D, Hopewell S, Schulz KF et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NICE. Methods for the development of NICE public health guidance. 3rd ed NICE article [pmg4], 2012. https://www.nice.org.uk/article/pmg4/chapter/1%20introduction [PubMed] [Google Scholar]
- 51.Husereau D, Drummond M, Petrou S et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346:f1049 10.1136/bmj.f1049 [DOI] [PubMed] [Google Scholar]