Abstract
Background
The aim of this study was to establish a minimally invasive defibrillation testing (DT) protocol for patients with implantable cardioverter defibrillators (ICDs).
Methods
Two different energy DTs were performed, immediately after (15 J-DT) and 7 days after (≤10 J-DT) device implantation, in 20 consecutive ICD implantation patients. Cardiac-troponin T (c-TNT) and heart-type fatty acid binding protein (H-FABP) levels were measured before implantation, 2 h after implantation, and 1 day after each DT. For an additional 122 patients with ICD, we retrospectively analyzed 203 DTs immediately and 7 days after device implantation.
Results
Serum c-TNT levels were significantly elevated 2 h after 15 J-DT [0.008 (0.004–0.019) vs. 0.053 (0.037–0.068) ng/mL, p<0.001], but not ≤10 J-DT [0.007 (0.004–0.018) ng/mL]. Similarly, serum H-FABP levels were significantly elevated 2 h after 15 J-DT (2.9±1.5 vs. 6.4±3.4 ng/mL, p<0.001), but not ≤10 J-DT (2.7±1.5 ng/mL). The changes in c-TNT and H-FABP levels between baseline and 2 h after DT were significantly greater for 15 J-DT compared with ≤10 J-DT [c-TnT: 0.039 (0.029–0.060) vs. 0 (0–0.003) ng/mL, p<0.001; H-FABP: 3.6±2.8 vs. −0.16±1.1 ng/mL, p<0.001]. The success rates of the initial shocks delivered for ventricular fibrillation were no different between ≤10 J-DT (85% [78/92]) and ≥15 J-DT (92% [103/111]).
Conclusions
Elevated levels of myocardial damage markers such as c-TNT and H-FABP were not found after ≤10 J-DT. In addition, an acceptable success rate was confirmed in ≤10 J-DT.
Keywords: Implantable cardioverter defibrillator, Defibrillation test, Myocardial injury, Heart-type fatty acid binding protein, Troponin T
1. Introduction
Implantable cardioverter defibrillator (ICD) has become a widely accepted therapy for patients with life-threatening ventricular tachyarrhythmias [1], [2], [3]. However, it has been reported that patients who received both appropriate and inappropriate shocks had a substantially higher risk of death than those who did not receive any [4]. This link between shock and poor clinical prognosis has two possible explanations. The first explanation is that arrhythmia occurred more frequently during disease progression, resulting in increased mortality, [5] and the second explanation is that shock therapy itself may worsen the clinical outcome. Although it is difficult to determine which of these explanations is correct, earlier animal and clinical studies revealed that shock caused myocardial injury [6], [7] and unstable cardiac hemodynamics [8].
Defibrillation testing (DT), conducted by inducing and terminating ventricular fibrillation (VF), has been widely considered as a part of the standard protocol for ICD implantation. DT ensures the device׳s ability to terminate VF, adequate sensing, and appropriate high-voltage electrode connections. Although the clinical importance of DT is still controversial [9], [10], [11], [12], electrophysiologists should reduce myocardial damage caused by DT as much as possible.
The aim of this study was to investigate the extent of myocardial injury after ≤10 J-DT and 15 J-DT. The levels of sensitive and highly specific circulating biomarkers, cardiac troponin T (c-TNT), and heart-type fatty acid binding protein (H-FABP), were evaluated as indicators of myocardial injury [13], [14]. In addition, we retrospectively compared the success rates of ≤10 J-DT and ≥15 J-DT.
2. Materials and methods
2.1. Study population
Between March 2010 and February 2011, 20 consecutive patients underwent pectoral ICD implantation at Yamagata University Hospital. Patients diagnosed with acute coronary syndromes within 3 months preceding admission, and those with renal failure, characterized by a serum creatinine concentration >2.0 mg/dL, were excluded from the study. Ethical approval was obtained from the institutional review committee (approval date, February 15, 2010; approval number, 147), and all patients provided informed, written consent before participating in the study.
To evaluate the success rate of ≤10 J-DT immediately after device implantation, we identified 167 patients who underwent pectoral ICD implantation between January 2004 and February 2010. Among these patients, we excluded 45 in whom DT was not performed or who received several DTs immediately after device implantation. The remaining 122 patients who underwent 203 DTs were included in a retrospective analysis.
2.2. Defibrillator implantation and DT
All 20 patients received a transvenous lead system via the subclavian vein, and a pulse generator was placed in the left pectoral region (Medtronic Inc., Minneapolis, MN, USA, n=5; St. Jude Medical Inc., St. Paul, MN, USA, n=10; Boston Scientific Inc., Marlborough, MA, USA, n=5). A single-chamber transvenous ICD system was used for 10 (50%) patients. All patients were implanted with a dual coil ICD lead at the right ventricular apex.
DTs examining two different energies were performed in each patient. The first DT (15 J-DT) was immediately after ICD implantation, and the second DT [≤10 J-DT (9 J or 10 J)] was performed 7 days after device implantation (Fig. 1). DTs were performed under short-duration, deep sedation using thiopental. VF was induced via the device׳s test program with a 50-Hz burst. In cases of initial shock failure, the energy was increased in 10-J steps in subsequent tests until defibrillation was successful. In the retrospective analysis, which included 122 patients with ICDs, the initial shock energy was selected by a physician who implanted the device.
Fig. 1.
Blood sampling and defibrillation testing protocols. Two different energy defibrillation testing were performed, immediately after and 7 days after device implantation. Cardiac damage markers were measured before implantation, 2 h after implantation, and 1 day after each defibrillation testing. d, Day; DT, defibrillation testing; h, hour.
2.3. Measurement of c-TNT and H-FABP
Baseline c-TNT and H-FABP levels were measured before ICD implantation when the patient was stable and free from clinical ventricular arrhythmia for at least 2 weeks. Blood sampling was performed at least 1 week after electrophysiological studies and VF induction. Postoperative blood samples were obtained 2 h and 1 day after the first and second DTs.
Blood samples used to measure serum c-TNT and H-FABP levels were centrifuged at 2500g for 15 min at 4 °C, within 30 min of collection, and the serum was stored at −70 °C until analysis. The concentrations of c-TNT were measured using a fourth-generation electrochemiluminescence immunoassay on an Elecsys 2010 automatic analyzer (Elecsystroponin-T, Roche Diagnostics, Basel, Switzerland) [13], [14]. H-FABP levels were measured using a two-step sandwich enzyme-linked immunosorbent assay (MARKIT-M H-FABP, Dainippon Pharmaceutical Co. Ltd., Tokyo, Japan) [13], [14].
2.4. Statistical analysis
Continuous variables were expressed as the mean±the standard deviation. Skewed variables were presented as medians with interquartile ranges (IQRs). We employed t-tests and chi-square tests to compare continuous and categorical variables, respectively. Mann–Whitney U tests were conducted for non-normally distributed data, and Wilcoxon signed rank sum tests were used for comparing pre- and postoperative values. Univariate analysis and logistic regression were used to identify significant predictors of ≤10 J-DT failure. Values of p<0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
All 20 patients underwent successful ICD implantation and testing. The initial shocks were successful for both the first (15 J-DT) and second (≤10 J-DT) sessions. No patients required additional shocks, and no serious complications were noted. The clinical characteristics of the 20 patients for whom DTs and blood sampling were conducted are shown in Table 1. The patients had a mean age of 56.4±12.2 years, and the majority (i.e., 80%, n=16) were male. Overall, 5 (25%), 2 (10%), and 7 (35%) patients had hypertension, diabetes mellitus, and dyslipidemia, respectively. Five patients had a history of ischemic heart disease. ICDs were implanted for primary prevention in 5 patients and secondary prevention in 15 patients. Three (15%) patients received a resynchronization ICD device. The median plasma B-type natriuretic polypeptide level was 51.9 pg/mL. The estimated glomerular filtration rate was preserved in all patients. Baseline c-TNT and H-FABP levels were 0.008 (0.004–0.019) ng/mL and 2.9±1.5 ng/mL, respectively. Echocardiography showed a preserved left ventricular end-diastolic dimension and left ventricular ejection fraction. Seven (35%) patients received amiodarone.
Table 1.
Baseline patient characteristics.
| All patients (n=20) | |
|---|---|
| Age, yrs | 56.4±12.2 |
| Female | 4 (20) |
| Hypertension | 5 (25) |
| Diabetes mellitus, n (%) | 2 (10) |
| Dyslipidemia, n (%) | 7 (35) |
| Diagnosis, n (%) | |
| Ischemic heart disease | 5 (25) |
| Non-ischemic heart disease | 15 (75) |
| Device | |
| Primary prevention, n (%) | 5 (25) |
| Resynchronization ICD implanted, n (%) | 3 (15) |
| Dual chamber ICD implanted, n (%) | 10 (50) |
| Dual coil ICD lead, n (%) | 20 (100) |
| Right-sided device implant, n (%) | 0 |
| Laboratory data | |
| BNP, pg/mL (IQR) | 51.9 (20.4–172.4) |
| eGFR, mL/min/1.72 m2 (IQR) | 87.3 (75.3–93.0) |
| Troponin T, ng/mL (IQR) | 0.008 (0.004–0.019) |
| H-FABP, ng/mL | 2.9±1.5 |
| Echocardiography | |
| LV end-diastolic dimension (mm) | 54.3±10.1 |
| LV ejection fraction (%) | 55.3±16.6 |
| Drugs, n (%) | |
| ACE inhibitor/ARB | 10 (50) |
| β Blocker | 7 (35) |
| Amiodarone | 7 (35) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, B-type natriuretic polypeptide; eGFR, estimated glomerular filtration rate; H-FABP, heart-type fatty acid binding protein; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LV, left ventricular.
3.2. Myocardial injury
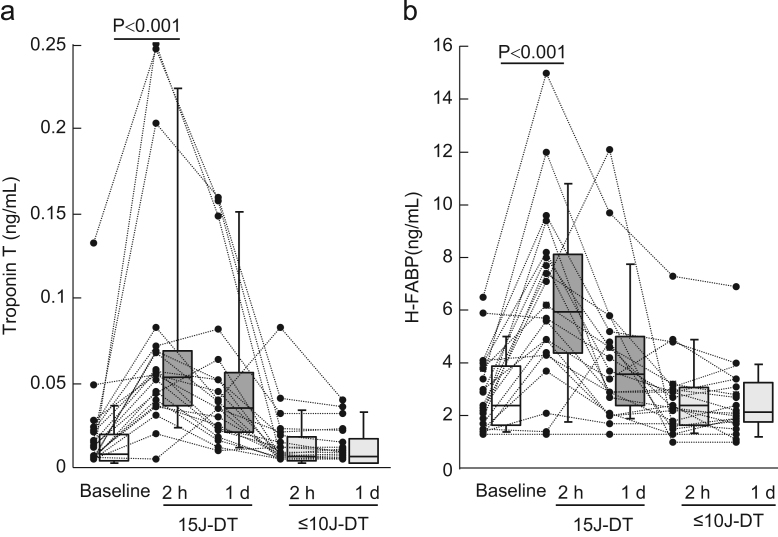
Serum c-TNT levels were significantly elevated 2 h after 15 J-DT [0.053 (0.037–0.068) ng/mL]; however, these value decreased 1 day after 15 J-DT [0.035 (0.021–0.053) ng/mL]. On the other hand, serum c-TNT levels were not significantly elevated 2 h after ≤10 J-DT [0.007 (0.004–0.018) ng/mL] or 1 day after ≤10 J-DT [0.007 (0.003–0.015) ng/mL] (Fig. 2A). The change in c-TNT level from baseline to 2 h after DT was significantly greater in 15 J-DT compared with ≤10 J-DT [0.039 (0.029–0.060) ng/mL vs. 0 (0–0.003) ng/mL, p<0.001]. Normal values of c-TNT (≤0.014 ng/mL) were found in 14 of 20 (70%) patients at baseline. Nineteen (95%) patients had an increase in c-TNT level (>0.014 ng/mL) 2 h after 15 J-DT; however, ≤10 J-DT did not affect the prevalence of the normal c-TNT level (70% [14/20]). Similarly, serum H-FABP levels were significantly elevated 2 h after 15 J-DT (6.4±3.4 ng/mL), but these value decreased 1 day after 15 J-DT (4.2±2.7 ng/mL). Serum levels of H-FABP were not significantly elevated 2 h or 1 day after ≤10 J-DT, 2.7±1.5 and 2.6±1.4 ng/mL, respectively (Fig. 2B). The change in H-FABP level from baseline to 2 h after DT was significantly greater for 15 J-DT compared with ≤10 J-DT (3.6±2.8 vs. −0.2±1.1 ng/mL, p<0.001). A majority of patients [95% (19/20)] showed normal H-FABP levels (≤6.2 ng/mL) at baseline. Ten (50%) patients showed an increase in H-FABP level (>6.2 ng/mL) 2 h after 15 J-DT; however, ≤10 J-DT did not affect the prevalence of the normal H-FABP level (95% [19/20]).
Fig. 2.
Myocardial damage marker levels after defibrillation testing (DT). (A) Serum levels of cardiac troponin-T (c-TNT) were significantly elevated 2 h after 15 J-DT [0.053 (0.037–0.068) ng/mL] but decreased 1 day after 15 J-DT [0.035 (0.021–0.053) ng/mL]. Serum c-TNT levels were not significantly elevated 2 h [0.007 (0.004–0.018) ng/mL] or 1 day after ≤10 J-DT [0.007 (0.003–0.015) ng/mL]. (B) Serum levels of heart-type fatty acid binding protein (H-FABP) were significantly elevated 2 h after 15 J-DT (6.4±3.4 ng/mL) but decreased 1 day after 15 J-DT (4.2±2.7 ng/mL). Serum H-FABP levels were not significantly elevated 2 h (2.7±1.5 ng/mL) or 1 day after ≤10 J-DT (2.6±1.4 ng/mL).
3.3. DT success rate
In our retrospective cohort of 122 patients with ICD implantation, a total of 203 DTs were performed. Forty-one patients underwent a single DT immediately after device implantation. The remaining 81 patients underwent two DTs, one immediately after device implantation and another 7 days after ICD implantation. Among 122 patients, ≤10 J-DT and ≥15 J-DT were performed in 76 (62%) and 46 (38%) patients, respectively. Among 203 DTs, ≤10 J-DT and ≥15 J-DT accounted for 92 (45%) and 111 patients (55%), respectively. The success rate of ≤10 J-DT was 85% (78/92), while that of ≥15 J-DT was 92% (103/111). Although an acceptable success rate was found for ≤10 J-DT, the success rate was relatively low compared to that of ≥15 J-DT (p=0.07).
3.4. Predicting ≤10 J-DT failure
The baseline characteristics of the 76 patients who underwent ≤10 J-DT are summarized in Table 2. Hypertension, dyslipidemia, and ischemic heart disease tended to be frequently observed in the ≤10 J-DT success group (Table 2). No significant difference was found between the two groups in terms of age, prevalence of resynchronization ICD implantation, dual chamber ICD, right-sided device, blood examination, echocardiographic findings, or amiodarone usage.
Table 2.
Comparison of baseline characteristics between ≤10 J-DT successes and failures.
| All patients (n=76) | Success (n=62) | Failure (n=14) | p Value | |
|---|---|---|---|---|
| Age, yrs | 60.5±13.5 | 61.4±12.9 | 56.6±16.0 | 0.31 |
| Female | 21 (27.6) | 19 (31.6) | 2 (14.3) | 0.22 |
| Hypertension | 12 (15.8) | 12 (19.4) | 0 | 0.07 |
| Diabetes mellitus, n (%) | 11 (14.5) | 8 (12.9) | 3 (21.4) | 0.41 |
| Dyslipidemia, n (%) | 25 (32.9) | 23 (37.1) | 2 (14.3) | 0.10 |
| Diagnosis, n (%) | ||||
| Ischemic heart disease | 11 (14.5) | 11 (17.7) | 0 | 0.09 |
| Non-ischemic heart disease | 65 (85.5) | 51 (82.3) | 14 (100) | 0.86 |
| Device | ||||
| Primary prevention, n (%) | 28 (36.8) | 25 (40.3) | 3 (21.4) | 0.19 |
| Resynchronization ICD implanted, n (%) | 17 (22.4) | 14 (22.6) | 3 (21.4) | 0.93 |
| Dual chamber ICD implanted, n (%) | 50 (65.8) | 43 (69.4) | 7 (50.0) | 0.17 |
| Right sided device implant, n (%) | 3 (3.9) | 2 (3.2) | 1 (7.1) | 0.50 |
| Laboratory data | ||||
| BNP, pg/mL (IQR) | 127.4 (49.0–345.1) | 121.8 (46.0–333.4) | 135.3 (79.9–426.9) | 0.66 |
| eGFR, mL/min/1.72 m2, (IQR) | 72.5 (50.4–88.2) | 72.5 (51.2–87.9) | 72.8 (50.9–98.4) | 0.77 |
| Transthoracic echocardiography | ||||
| LV end-diastolic dimension (mm) | 57.5±10.3 | 57.7±9.9 | 56.7±12.3 | 0.78 |
| LV ejection fraction (%) | 45.7±18.6 | 45.4±18.4 | 47.1±20.1 | 0.78 |
| Drugs, n (%) | ||||
| ACE inhibitor/ARB | 46 (60.5) | 38 (61.3) | 8 (57.1) | 0.77 |
| β blocker | 45 (59.2) | 38 (61.3) | 7 (50.0) | 0.44 |
| Amiodarone | 35 (46.1) | 28 (45.2) | 7 (50.0) | 0.74 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, B-type natriuretic polypeptide; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LV, Left ventricular.
4. Discussion
Our results demonstrated that patients who underwent ICD implantation and received 15 J-DT exhibited evidence of myocardial damage as indicated by increased serum c-TNT and H-FABP levels. On the other hand, ≤10 J-DT (9 J or 10 J) was associated with an acceptable successful DT rate and no significant elevation in either marker.
We used the specific myocardial injury markers c-TNT and H-FABP. Some reports clearly show the clinical usefulness of c-TNT among patients with myocardial infarctions [15], [16] and cardiac contusions [17]. In addition, H-FABP elevation is associated with minimal damage to cardiomyocytes and reflects superficial myocardial injury. Basic and clinical research in rats, as well as human autopsy analyses have revealed that H-FABP leakage occurs despite the absence of myocyte necrosis [18]. H-FABP is a low-molecular-weight protein that is normally confined to the cytoplasm and released into the circulation through the porous membranes of damaged myocardial cells [19], [20].
There are some reports regarding the relationship between internal shocks and myocardial injury. Hurst et al. reported that mean defibrillation energy during DT was significantly higher in patients with cardiac troponin I (c-TNI) elevation (20.0±3.8 J) than in those without marker elevation (14.6±3.4 J). Multivariate analysis revealed that a mean defibrillation energy ≥18 J was a strong risk factor for a rise in c-TNI [6]. Boriani et al. confirmed asymptomatic, minor myocardial injuries in patients with persistent atrial fibrillation who underwent atrial cardioversion. In these subjects, two catheters were placed in the right atrium and the coronary sinus, respectively, to administer internal shocks. The level of c-TNI was elevated in 15 of 35 (43%) patients, and the total delivered energy ranged from 28.7±10.4 to 35.3±32.6 J [21]. In accordance with earlier reports, we found that both c-TNT and H-FABP levels were elevated in patients undergoing 15 J-DT. If myocardial damage only affected the limited focal myocardium, it might not be a serious problem. However, endocardial shock affects the entire heart. Schirmer et al. showed that in 13 fox hounds, the use of endocardial lead systems with low-energy countershocks caused severe myocardial alterations such as swollen mitochondria, disruption of mitochondrial crests, and the loss of integrity of the inner and outer mitochondrial membranes [22]. Takano et al. assessed 17 patients with ICD implantation and found a significant correlation between shock strength and the change in cardiac index; lower energy shocks did not affect cardiac hemodynamics [8]. Although the precise relationship between increased myocardial damage marker levels and altered hemodynamic status is unclear, high-energy DT may induce focal myocardial damage and affect cardiac hemodynamics.
Several studies concluded that neither ICD shock frequency nor mortality was different between patients who underwent DT and those who did not [10], [12]. Conversely, patients who did not undergo intraoperative DT had significantly higher overall mortality rates than those who did [9], [11]. In addition to this controversy, we need to consider the possibility of device malfunction. A single successful DT for VF was just as useful as repeat DTs [23], [24]. Recently, sub-analysis of the Shockless IMPLant Evaluation (SIMPLE) trial demonstrated that elevated troponin levels after ICD implantation were associated with a high mortality rate (adjusted hazard ratio 1.43, p=0.001) and a high risk of arrhythmic death (adjusted hazard ratio 1.80, p=0.002). Abnormal troponin level elevation was frequently observed in patients who underwent DT (>17 J) compared with those without DT (42.1% vs 37.5%, p=0.04) [25]. From this viewpoint, a single minimally invasive DT may be acceptable.
Although the success rate for DT was not significantly different between ≤10 J-DT and ≥15 J-DT, the former tended to have a lower success rate. Minimally invasive DT is a promising strategy; however, unsuccessful attempts are followed by the administration of additional higher energy DTs. Therefore, ≤10 J-DT should be avoided in patients who may have a high risk for an unsuccessful DT. Unfortunately, we could not identify specific risk factors associated with an unsuccessful DT in the present investigation. Earlier studies reported that atrial fibrillation, left ventricular systolic dysfunction, left ventricular hypertrophy, and amiodarone usage were predictive of a high defibrillation threshold [26], [27].
4.1. Study limitations
First, DTs were performed twice in each patient. Myocardial damage associated with the first test (15 J-DT) may influence marker levels during the second test (≤10 J-DT). Despite this, we found that c-TNT and H-FABP levels were not increased after ≤10 J-DT. Second, baseline blood sampling was performed before device implantation, and not immediately before the first DT. Although a previous report concluded that cardiac marker levels did not increase after lead implantation [5], device implantation itself might have influenced myocardial damage markers in the present study [6]. Third, all 20 patients who underwent blood sampling and DT were implanted with a dual coil ICD lead in the present study. The difference between single coil and dual coil ICDs may influence myocardial damage. Finally, the SIMPLE study revealed the efficacy of ICD implantation without DT. However, in this study, all patients were programmed for shocks of >17 J [12]. Future investigations may be necessary to compare the efficacies of ICD implantation without DT versus those performed with minimally invasive DT.
5. Conclusion
Elevated levels of the myocardial damage markers c-TNT and H-FABP were not found after ≤10 J-DT (9 J or 10 J) in patients who underwent device implantation. Our findings confirm that minimally-invasive DT has an acceptable success rate. To prevent unnecessary myocardium damage, ≤10 J-DT may be an ideal strategy; however, it should not be used in patients at high risk for a high defibrillation threshold.
Conflict of interest
None.
Acknowledgments
This work was supported, in part, by a grant-in-aid for Scientific Research (No. 25461039) from the Ministry of Education Culture, Sport, Science, and Technology of Japan. All authors declare no conflict of interest related to this study.
References
- 1.Moss A.J., Zareba W., Hall W.J. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Fauchier L., Marijon E., Defaye P. Effect of age on survival and causes of death after primary prevention implantable cardioverter-defibrillator implantation. Am J Cardiol. 2015;115:1415–1422. doi: 10.1016/j.amjcard.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Wanezaki M., Arimoto T., Takahashi H. Electroanatomical mapping of the atrialized right ventricle: placement of a transvenous implantable cardioverter-defibrillator in a patient with Ebstein׳s anomaly. J Arrhythm. 2014;30:382–384. [Google Scholar]
- 4.Poole J.E., Johnson G.W., Hellkamp A.S. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dichtl W., Wolber T., Paoli U. Appropriate therapy but not inappropriate shocks predict survival in implantable cardioverter defibrillator patients. Clin Cardiol. 2011;34:433–436. doi: 10.1002/clc.20910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurst T.M., Hinrichs M., Breidenbach C. Detection of myocardial injury during transvenous implantation of automatic cardioverter-defibrillators. J Am Coll Cardiol. 1999;34:402–408. doi: 10.1016/s0735-1097(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 7.Cevik C., Perez-Verdia A., Nugent K. Implantable cardioverter defibrillators and their role in heart failure progression. Europace. 2009;11:710–715. doi: 10.1093/europace/eup091. [DOI] [PubMed] [Google Scholar]
- 8.Tokano T., Bach D., Chang J. Effect of ventricular shock strength on cardiac hemodynamics. J Cardiovasc Electrophysiol. 1998;9:791–797. doi: 10.1111/j.1540-8167.1998.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 9.Pires L.A., Johnson K.M. Intraoperative testing of the implantable cardioverter-defibrillator: how much is enough? J Cardiovasc Electrophysiol. 2006;17:140–145. doi: 10.1111/j.1540-8167.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M., Occhetta E., Bongiorni M.G. Clinical evaluation of defibrillation testing in an unselected population of 2,120 consecutive patients undergoing first implantable cardioverter-defibrillator implant. J Am Coll Cardiol. 2012;60:981–987. doi: 10.1016/j.jacc.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Sadoul N., Defaye P., Mouton E. Defibrillation testing in everyday medical practice during implantable cardioverter defibrillator implantation in France: analysis from the LEADER registry. Arch Cardiovasc Dis. 2013;106:562–569. doi: 10.1016/j.acvd.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Healey J.S., Hohnloser S.H., Glikson M. Cardioverter defibrillator implantation without induction of ventricular fibrillation: a single-blind, non-inferiority, randomised controlled trial (SIMPLE) Lancet. 2015;385:785–791. doi: 10.1016/S0140-6736(14)61903-6. [DOI] [PubMed] [Google Scholar]
- 13.Daidoji H., Arimoto T., Nitobe J. Circulating heart-type fatty acid binding protein levels predict the occurrence of appropriate shocks and cardiac death in patients with implantable cardioverter-defibrillators. J Card Fail. 2012;18:556–563. doi: 10.1016/j.cardfail.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kutsuzawa D., Arimoto T., Watanabe T. Ongoing myocardial damage in patients with heart failure and preserved ejection fraction. J Cardiol. 2012;60:454–461. doi: 10.1016/j.jjcc.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Mueller M., Vafaie M., Biener M. Cardiac troponin T: from diagnosis of myocardial infarction to cardiovascular risk prediction. Circ J. 2013;77:1653–1661. [PubMed] [Google Scholar]
- 16.Kitamura M., Hata N., Takayama T. High-sensitivity cardiac troponin T for earlier diagnosis of acute myocardial infarction in patients with initially negative troponin T test—comparison between cardiac markers. J Cardiol. 2013;62:336–342. doi: 10.1016/j.jjcc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Sybrandy K.C., Cramer M.J., Burgersdijk C. Diagnosing cardiac contusion: old wisdom and new insights. Heart. 2003;89:485–489. doi: 10.1136/heart.89.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X., Ming M., Wang E. Heart fatty acid binding protein as a marker for postmortem detection of early myocardial damage. Forensic Sci Int. 2006;160:11–16. doi: 10.1016/j.forsciint.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Viswanathan K., Kilcullen N., Morrell C. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010;55:2590–2598. doi: 10.1016/j.jacc.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 20.Haltern G., Peiniger S., Bufe A. Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol. 2010;105:1–9. doi: 10.1016/j.amjcard.2009.08.645. [DOI] [PubMed] [Google Scholar]
- 21.Boriani G., Biffi M., Cervi V. Evaluation of myocardial injury following repeated internal atrial shocks by monitoring serum cardiac troponin I levels. Chest. 2000;118:342–347. doi: 10.1378/chest.118.2.342. [DOI] [PubMed] [Google Scholar]
- 22.Schirmer U., Hemmer W., Lindner K.H. Ultrastructural alterations in the right and left ventricular myocardium following multiple low energy endocardial countershocks in anesthetized dogs. Pacing Clin Electrophysiol. 1997;20:79–87. doi: 10.1111/j.1540-8159.1997.tb04815.x. [DOI] [PubMed] [Google Scholar]
- 23.Gold M.R., Breiter D., Leman R. Safety of a single successful conversion of ventricular fibrillation before the implantation of cardioverter defibrillators. Pacing Clin Electrophysiol. 2003;26:483–486. doi: 10.1046/j.1460-9592.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 24.Higgins S., Mann D., Calkins H. One conversion of ventricular fibrillation is adequate for implantable cardioverter-defibrillator implant: an analysis from the Low Energy Safety Study (LESS) Heart Rhythm. 2005;2:117–122. doi: 10.1016/j.hrthm.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Vamos M., Healey J.S., Wang J. Troponin levels after ICD implantation with and without defibrillation testing and their predictive value for outcomes: insights from the SIMPLE trial. Heart Rhythm. 2016;13:504–510. doi: 10.1016/j.hrthm.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Lin E.F., Dalal D., Cheng A. Predictors of high defibrillation threshold in the modern era. Pacing Clin Electrophysiol. 2013;36:231–237. doi: 10.1111/pace.12039. [DOI] [PubMed] [Google Scholar]
- 27.Mizukami K., Yokoshiki H., Mitsuyama H. Predictors of high defibrillation threshold in patients with implantable cardioverter-defibrillator using a transvenous dual-coil lead. Circ J. 2014;79:77–84. doi: 10.1253/circj.CJ-14-0860. [DOI] [PubMed] [Google Scholar]