Abstract
Trichilemmal carcinoma (TC) is described as a very rare cancer of the skin adnexa.1 2 Ninety per cent of the lesions present on the scalp. Prognostic factors in TC are limited to lymph node status and surgical margins, with no statistical significance observed for age or gender of the patient, size of tumour or locoregional recurrence. We present a 46-year-old black patient who developed TC during treatment for breast cancer. Postoperative histology of the scalp lesion excision confirmed no involved margins. At the three monthly appointment, the patient was reviewed and multiple, new scalp lesions were noted. A CT scan of the head, neck found multiple lesions on the scalp, limited to the soft tissue, not involving the outer table of the skull. There was bilateral invasion of the parotid glands. To the best of our knowledge, no syndromes or associations between breast cancer and adnexal skin tumours exist.
Background
Trichilemmal carcinoma (TC) is described as a very rare cancer of the skin adnexa. It has an indolent nature and typically occurs in elderly patients on areas of the body exposed to the sun.1 2 Ninety per cent of the lesions present on the scalp.3 There is no gender predilection.2
Case presentation
A 46-year-old, black woman, a domestic worker was referred to the multidisciplinary breast unit at our institution by a general practitioner with a fungating breast mass that was first noticed 11 months prior. She had no comorbidities, reported an active lifestyle and was not overweight. On physical examination, pallor was noted. A 5×6 cm, ulcerating mass was identified in the upper outer quadrant of the breast. The patient had matted axillary lymph nodes. Incisional biopsy revealed an invasive breast carcinoma that was oestrogen receptor (ER)-positive, progesterone receptor (PR)-negative and human epidermal growth factor receptor-2 (HER 2) equivocal.
Investigations
Renal function tests, liver function tests, a chest X-ray and an abdominal ultrasonography revealed no abnormalities.
Differential diagnosis
Squamous cell carcinoma; eccrine carcinoma; trichilemmoma.
Treatment
A management plan was formulated, after review by our oncology service. The approach would be neoadjuvant chemotherapy, followed by mastectomy and radiation therapy. Tamoxifen and goserelin acetate would be initiated after the completion of chemotherapy.
A course of cytotoxic chemotherapy, which included docetaxel, was completed over an 8-week period. Tamoxifen and goserelin acetate were then initiated and the patient was referred back for a mastectomy. During the preoperative workup, a scalp lesion was identified. Clinically, this was a 5×5 cm lesion on the vertex of the scalp. A mastectomy with axillary lymphadenectomy was performed, coupled with an incisional biopsy of the scalp lesion. Pathological analysis of the breast specimen revealed an invasive breast cancer of no specific type with an in situ, high-grade, ductal component. Of the 11 lymph nodes examined, 8 were positive, with extensive perinodal spread and soft tissue deposits. The biopsy of the scalp lesion showed a trichilemmal carcinoma. Wide local excision of the scalp lesion was performed, with 1 cm margins down to the subgaleal plane and a split thickness skin graft used to cover the defect. Postoperative histology of the scalp lesion excision confirmed no involved margins.
Outcome and follow-up
The patient was subsequently reviewed as an outpatient for 8 weeks; the scalp had healed, with no subsequent lesions noted. At the 3-month follow-up appointment, the patient was reviewed and multiple scalp lesions were noted (figure 1). The patient appeared unwell and pale, and had marked facial oedema with unilateral right-sided parotid enlargement, a prominent postauricular lymph node on the left and multiple cervical and supraclavicular lymph nodes bilaterally.
Figure 1.
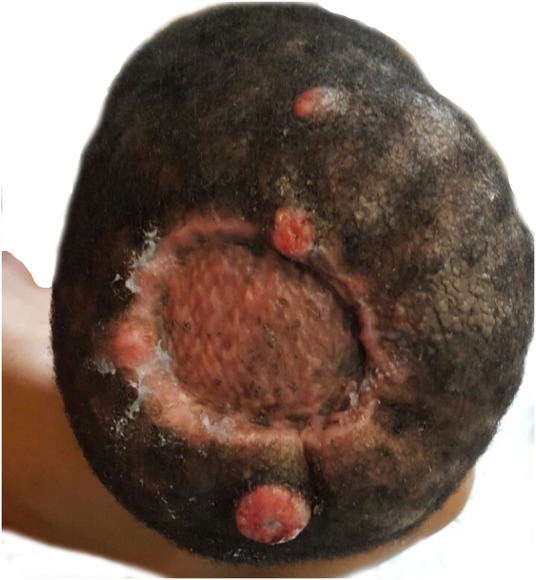
Recurrence of lesion postexcision and skin graft.
A CT scan of the head, neck and upper chest found multiple lesions on the scalp, limited to the soft tissue and not involving the outer table of the skull. There was also a bilateral invasion of the parotid glands with preauricular and postauricular lymphadenopathy. Significant cervical adenopathy, including supraclavicular nodes, was identified. Biopsy revealed a histological diagnosis of TC in the new scalp lesions and cervical lymph nodes. The patient unfortunately died before further management could be instituted.
Discussion
TC is a neoplasm of ‘adnexal keratinocytes’4 from the ‘outer root sheath of the hair follicle’,5 and is the malignant counterpart of trichilemmoma.6 It is often mistaken for other skin cancers, such as squamous cell carcinoma, basal cell carcinoma or nodular melanoma.7
TC can develop de novo from actinically damaged skin, from chronic burn scars, in patients with repeated ionising radiation exposure and in xeroderma pigmentosum.1 8 9 Immunosuppression and human papilloma virus infection have also been implicated.10
The tumour is typically exophytic with a keratotic crust and is usually present for less than a year.1 11 Accelerated growth prompts consultation.11
The clinical features of malignant transformation result from the combination of an overlying area of scalp alopecia, with a size >5 cm, locations other than the scalp, accelerated growth and areas of ulceration.5 Histologically, one observes increased mitotic activity, increased proliferative index and activity beyond the basal layer of the skin.12 Other features include characteristic trabeculae with peripheral palisading of cells extending to the level of the dermis.7 These findings are presented in table 1.
Table 1.
| Clinical | Overlying scalp alopecia Size >5 cm Locations other than the scalp Accelerated growth Ulceration |
| Histological | Increased mitotic activity Increased proliferative index Activity beyond the basal layer Peripheral palisading of cells extending to the dermal level |
Malignant transformation progresses histologically through three stages, namely the adenomatous, epitheliomatous and finally the carcinomatous stage.13 Malignant cells are observed to reflect a loss of CD34 expression.14 TC exhibits biology that is unpredictable with a capacity to invade local structures plus distant metastasis.5 Ye et al15 proposed three groupings of proliferating trichilemmal tumours within their 76-patient case series, as presented in table 2.
Table 2.
Classification of proliferating trichilemmal tumours15
| Grade | Behaviour | Spread | Recurrence |
|---|---|---|---|
| Benign |
|
Nil | Nil |
| Low grade |
|
Locally invasive | Local recurrence |
| High grade |
|
Lymph node involvement | High local recurrence rate Distant metastasis |
The characteristically indolent nature of TC suggests that a cure is possible with wide local excision.16 There is no current consensus on surgical margins or follow-up for patients with TC. Wilkie et al2 suggested using margins for cutaneous squamous cell carcinoma, specifically those that are 4–6 mm in size. Zhuang et al16 showed improved overall survival in their study when a surgical margin of 1 cm was taken. Mohs micrographic surgery could also be used for excision of TC, as it allows for immediate assessment of surgical margins while preserving as much normal tissue as possible; Kim et al17 reported no recurrence in 19 months of follow-up when using Mohs micrographic surgery for excision of TC.
Metastatic workup for a patient with TC could include a CT scan of the head and neck, a chest X-ray and a positron emission tomography scan.18 Experience in the management of advanced or metastatic cases of TC is limited. There is no data on the utility of radiotherapy, although one patient has been reported to be disease-free after wide local excision and adjuvant radiotherapy.19 Furthermore, no well-defined chemotherapy protocol for advanced TC exists.16 Partial remission with combination cisplatin-cyclophosphamide treatment has been achieved.20 A regimen of cisplatin, adriamycin and vindesine, as used in cases of advanced squamous cell carcinoma, showed an ability to restrict the progression of the tumour.21 Despite the lack of consensus on a chemotherapy regimen for advanced TC, one should still consider chemotherapy in cases of metastatic TC.16
Prognostic factors in TC are limited to lymph node status and surgical margins (1 cm), with no statistical significance observed regarding the age or gender of the patient, the size of the tumour or locoregional recurrence.16
Our case features a patient with breast cancer who developed the TC after chemotherapy and while undergoing tamoxifen/goserelin acetate treatment. The rapid progression of the tumour over a 4-week period resulted in metastases that were incurable. To the best of our knowledge, there are no existing syndromes or associations between breast cancer and adnexal skin tumours.
A case report of a kidney transplant recipient on immunosuppression, described by Hiramatsu22, brought the relationship between immunosuppression and a higher risk of cancer to the fore; of note is that this patient specifically developed an aggressive form of TC with subsequent metastatic spread. In the aforementioned study, resultant clear margins were obtained; however, the patient presented a year later with nodal spread, which was further complicated by liver and lung metastases 5 months after that. Radiotherapy to the affected lymph nodes, wedge excision of the liver metastases and pneumonectomy followed by further radiotherapy did not prevent the eventual demise of the patient.
Interestingly, our patient was dark skinned. The first reported case of TC in a black patient (skin phototype VI) was described in 2002.9 Actinic damage has been described as an important factor in the pathogenesis of TC,1 and cutaneous malignancies are rare in dark-skinned individuals.9 The pathogenesis of the TC in our patient could have, therefore, been related to something other than actinic damage, although none of the other proposed pathogenic mechanisms seemed to apply. Docetaxel was part of the chemotherapy regimen administered to our patient for her breast cancer. Docetaxel is known to cause a variety of skin toxicities23 and has been linked to cases of skin fibrosis.24 However, it has never been linked to skin cancer per se. Other factors in the pathogenesis of TC are therefore important and require further investigation.
In summary, we present a case of a black patient who developed TC during her treatment for breast cancer. Despite wide local excision and careful follow-up, the patient died. The aggressive nature of the tumour prevented chemotherapy or radiotherapy. The pathogenesis of TC needs further study, and the development of standard treatment approaches in metastatic disease is also required.
Learning points.
The characteristically indolent nature of trichilemmal carcinoma suggests a cure is possible with wide local excision.
There is no current consensus for excision margins for trichilemmal carcinoma.
Prognostic factors in trichilemmal carcinoma are limited to lymph node status and surgical margins (1 cm), with no statistical significance observed regarding the age or gender of the patient, the size of the tumour, or locoregional recurrence.
Footnotes
Twitter: Follow Chrysis Sofianos at @sofianosc
Contributors: CS was involved with the conception of the work; literature review; interpretation of the case report; drafting of the article; critical revision of the article. NYC was involved with the conception of the work; data collection; interpretation of the case report; critical revision of the article. AG was involved with the conception of the work; critical revision of the article. All authors of this article have made substantial contributions to the article. All authors gave final approval of the submitted manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Reis JP, Tellechea O, Cunha MF et al. Trichilemmal carcinoma: review of 8 cases. J Cutan Pathol 1993;20:44–9. 10.1111/j.1600-0560.1993.tb01248.x [DOI] [PubMed] [Google Scholar]
- 2.Wilkie MD, Munir N, Roland NJ et al. Trichilemmal carcinoma: an unusual presentation of a rare cutaneous lesion. BMJ Case Rep 2013;2013:doi:10.1136/bcr-2012-008369 10.1136/bcr-2012-008369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshmukh BD, Kulkarni MP, Momin YA et al. Malignant proliferating trichilemmal tumor: a case report and review of literature. J Cancer Res Ther 2014;10:767–9. 10.4103/0973-1482.136036 [DOI] [PubMed] [Google Scholar]
- 4.Headington JT. Tumors of the hair follicle. A review. Am J Pathol 1976;85:479–514. [PMC free article] [PubMed] [Google Scholar]
- 5.Kini JR, Kini H. Fine-needle aspiration cytology in the diagnosis of malignant proliferating trichilemmal tumor: Report of a case and review of the literature. Diagn Cytopathol 2009;37:744–7. 10.1002/dc.21100 [DOI] [PubMed] [Google Scholar]
- 6.Garrett AB, Azmi FH, Ogburia KS. Trichilemmal carcinoma: a rare cutaneous malignancy: a report of two cases. Dermatol Surg 2004;30:113–15. [DOI] [PubMed] [Google Scholar]
- 7.Chan KO, Lim IJ, Baladas HG et al. Multiple tumour presentation of trichilemmal carcinoma. Br J Plast Surg 1999;52:665–7. 10.1054/bjps.1999.3180 [DOI] [PubMed] [Google Scholar]
- 8.Misago N, Tanaka T, Kohda H. Trichilemmal carcinoma occurring in a lesion of solar keratosis. J Dermatol 1993;20:358–64. 10.1111/j.1346-8138.1993.tb01298.x [DOI] [PubMed] [Google Scholar]
- 9.Laochumroonvorapong P, Kokta V, Quan MB. Trichilemmal carcinoma in an African-American. Dermatol Surg 2002;28:284–6. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Flores A. Study of proliferating trichilemmal tumor with PCR for HPV. Appl Immunohistochem Mol Morphol 2009;17:85–6. 10.1097/PAI.0b013e318169fad6 [DOI] [PubMed] [Google Scholar]
- 11.Swanson PE, Marrogi AJ, Williams DJ et al. Tricholemmal carcinoma: clinicopathologic study of 10 cases. J Cutan Pathol 1992;19:100–9. 10.1111/j.1600-0560.1992.tb01350.x [DOI] [PubMed] [Google Scholar]
- 12.Rutty GN, Richman PI, Laing JH. Malignant change in trichilemmal cysts: a study of cell proliferation and DNA content. Histopathology 1992;21:465–8. 10.1111/j.1365-2559.1992.tb00432.x [DOI] [PubMed] [Google Scholar]
- 13.Saida T, Oohara K, Hori Y et al. Development of a malignant proliferating trichilemmal cyst in a patient with multiple trichilemmal cysts. Dermatologica 1983;166:203–8. 10.1159/000249868 [DOI] [PubMed] [Google Scholar]
- 14.Herrero J, Monteagudo C, Ruiz A et al. Malignant proliferating trichilemmal tumours: an histopathological and immunohistochemical study of three cases with DNA ploidy and morphometric evaluation. Histopathology 1998;33:542–6. 10.1046/j.1365-2559.1998.00549.x [DOI] [PubMed] [Google Scholar]
- 15.Ye J, Nappi O, Swanson PE et al. Proliferating pilar tumors: a clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am J Clin Pathol 2004;122:566–74. 10.1309/21DK-LY2R-94H1-92NK [DOI] [PubMed] [Google Scholar]
- 16.Zhuang SM, Zhang GH, Chen WK et al. Survival study and clinicopathological evaluation of trichilemmal carcinoma. Mol Clin Oncol 2013;1:499–502. 10.3892/mco.2013.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YH, Lee YK, Choi KW et al. A case of trichilemmal carcinoma treated with Mohs micrographic surgery. Ann Dermatol 2008;20:157–61. 10.5021/ad.2008.20.3.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung J, Cho SB, Yun M et al. Metastatic malignant proliferating trichilemmal tumor detected by positron emission tomography. Dermatol Surg 2003;29:872–4. [DOI] [PubMed] [Google Scholar]
- 19.Dubhashi SP, Jadhav SK, Parasnis A et al. Recurrent malignant proliferating trichilemmal tumor with lymph node metastasis in a young woman. J Postgrad Med 2014;60:400–2. 10.4103/0022-3859.143973 [DOI] [PubMed] [Google Scholar]
- 20.Yi HS, Sym SJ, Park J et al. Recurrent and metastatic trichilemmal carcinoma of the skin over the thigh: a case report. Cancer Res Treat 2010;42:176–9. 10.4143/crt.2010.42.3.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi I, Harada T, Muraoka M et al. Malignant proliferating trichilemmal tumour and CAV (cisplatin, adriamycin, vindesine) treatment. Br J Dermatol 2004;150:156–7. 10.1111/j.1365-2133.2004.05670.x [DOI] [PubMed] [Google Scholar]
- 22.Hiramatsu K, Sasaki K, Matsuda M et al. A case of trichilemmal carcinoma with distant metastases in a kidney transplantation patient. Transplant Proc 2015;47:155–7. 10.1016/j.transproceed.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 23.Poi MJ, Berger M, Lustberg M et al. Docetaxel-induced skin toxicities in breast cancer patients subsequent to paclitaxel shortage: a case series and literature review. Support Care Cancer 2013;21:2679–86. 10.1007/s00520-013-1842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleveland MG, Ajaikumar BS, Reganti R. Cutaneous fibrosis induced by docetaxel: a case report. Cancer 2000;88:1078–81. [DOI] [PubMed] [Google Scholar]


