Highlights
-
•
Intra-abdominal sepsis remains one the leading causes of mortality in the SICU .
-
•
Multiple surgical approaches have been introduced as part of the management of the open abdomen with varying results.
-
•
Negative pressure wound therapy with instillation in our study population appears as a safe and promising therapeutic option in the context of intraabdominal sepsis.
-
•
NPWT-I is a therapeutic alternative that showed positive results in our patient group.
Keywords: Intra-abdominal sepsis, Negative pressure wound therapy, Negative pressure wound therapy with instillation, Open abdomen
Abstract
Introduction
Despite the numerous advances in recent years, severe abdominal sepsis (with associated organ failure associated with infection) remains a serious, life-threatening condition with a high mortality rate. OA is a viable alternative to the previously used scheduled repeat laparotomy or continuous peritoneal lavage. The use of Negative Pressure Wound Therapy (NPWT) has been described as a successful method of management of the open abdomen. Adding instillation of saline solution to NPWT in a programmed and controlled manner, could offer the clinician an additional tool for the management of complex septic abdomen.
Objectives
To explore if the concept of active two-way therapy (Negative pressure wound therapy with instillation or NPWT-I) yields superior control of underlying, life-threatening abdominal infections and its effects on survival and morbidity in patients with severe abdominal sepsis when management with an open abdomen is required.
Methods
A retrospective review of 48 patients with severe abdominal sepsis, who were managed with and open abdomen and NPWT-I was performed. NPWT-I was initiated utilizing the same parameters on all patients, this consisted of cycles of instillation of saline solution, which was removed through negative pressure after a short dwell period. We observed the effects on primary fascia closure rate, mortality, hospital and SICU length of stay and associated complications.
Results
Our patient group consisted of 20 (42%) males and 28 (58%) females. Average age was 48 years. Mortality in these patients was attributed to pulmonary embolism (n = 1), acute renal failure (n = 2) and cardiopulmonary arrest (n = 1). Average total hospital stay was 24 days, and stay in the SICU (n = 26) averaged 7.5 days.
No acute complications related to the NPWT-I. All patients presenting with abdominal compartment syndrome resolved after initiation of the NPWT-I. A total of 46 patients (96%) patients achieved fascia closure after NPWT-I therapy after an average of 6 days. Four patients (8%) died during the course of treatment of causes unrelated to NPWT-I.
Conclusion
This therapy showed added benefits when compared to traditional methods such as ¨Bogota bag̈, Wittmann patch, or NPWT traditional in the management of the open abdomen pertaining to severe abdominal sepsis.
NPWT-I in patients with severe abdominal sepsis had promising results, since we obtained higher fascia closure rates, lower mortality and reduced hospital and ICU length of stay with no complications due to this therapeutic approach.
1. Introduction
Despite numerous advances in recent years, severe abdominal sepsis (organ failure associated with infection) remains a serious, life-threatening condition with a high mortality rate. Because of breakthroughs in trauma care that require damage control surgery, the use open abdomen (OA) management has increased in scope and complexity.
In non-coronary ICU’s, severe sepsis (SS) is considered the main cause of death. In the USA alone, 900,000 cases of SS are diagnosed every year and it causes 210,000 deaths every year. In Europe, the number of deaths per year is 150,000 1., [2].
Peritonitis remains the main cause of developing SS, and up to 11% of patients admitted to the surgical ICU progress to this state due to a previous intra-abdominal infection [3]. Currently, mortality rates for intra-abdominal sepsis ranges between 30% and 40% [4], [5].
During the past two decades, there has been a paradigm shift toward management of patients with severe abdominal sepsis by OA as a viable alternative to the previously used scheduled repeat laparotomy or continuous peritoneal lavage [6], as to deal with or prevent recurrent infection. In addition, it is well established that the visceral or retroperitoneal edema secondary to shock and reperfusion, may increase intra-abdominal pressure to dangerous levels, leading intra-abdominal hypertension and organ dysfunction [7]. Patients with this constellation of symptoms must have their abdomens left temporarily open to allow for visceral and renal perfusion as well as adequate pulmonary function.
Negative pressure wound therapy (NPWT) is one of a variety of techniques used to manage the OA. NPWT was initially introduced in 1996 [8], with the intention of treating chronic soft tissue wounds. Later, it found its way into the treatment options of open abdomen. NPWT with Instillation (NPWT-I) has become a variant of this method. Although this technique was not intended for treatment of open abdomen, its potential as a new therapeutic tool, incited us to use it in patient with a septic open abdomen, and we have continued utilizing this technique thereafter.
In this report, we describe our use of negative pressure wound therapy as delivered by V.A.C.® Therapy (KCI USA, Inc., San Antonio, TX) in addition to timed, automated instillation of saline in the management of patients with SS.
2. Overview
Historically, the OA was treated with simpler approaches such as “Bogota bag” [9], Wittmann Patch [10] and Barker’s vaccum Pack [11], which yielded a variety of complications [12] such as marked adhesion formation, development of enteric fistula, non-quantifiable loss of fluids, evisceration, hemorrhage, contamination of the abdominal cavity surgical wound (especially when in proximity to stomas), spread of bacteria into the ICU and ward environment, and a high rate of subsequent ventral hernias. Different methods of temporary abdominal closure (TAC) have been developed to protect the temporary open abdomen and decrease complications [12], [13].
NPWT resulted in greater rates of fascia closure [14], [15] obviating the need for subsequent hernia repair in many patients [16]. The utility of this technique is not limited to the early postoperative period, but can be successful in for up to 3–4 weeks after the initial operation [17]. Recent large scale studies have reinforced the benefits of NPWT as compared to other TAC methods [18], and it’s early application has been showed to be beneficial [17].
Peritoneal negative pressure therapy (PNPT) decreases systemic inflammation and organ damage [19]. PNPT served to evacuate a significantly greater volume of ascites than passive drainage. Systemic inflammation as measured by serum levels of TNF-I, IL-1 and IL-6, was significantly reduced in the PNPT group and was associated with significant improvement in histology of the intestine, lung, kidney, and liver [20]. Efficacy was attributed in part to an attenuation of peritoneal inflammation by the removal of cytokines and other biochemical mediators in the ascitic fluid [19]. Interruption of peritoneal hemolymphodynamics via controlled vacuum aspiration appears to decrease entry of inflammatory mediators into the blood stream, thereby possibly limiting systemic inflammation [21]. The clinical implication of these studies was that because sepsis/trauma results in an inflammatory ascites that may perpetuate organ injury; removal of the ascites can break the cycle and decrease organ damage via source control [19].
The primary goal of TAC is to create a tension-free closure of the abdomen without increasing intra-abdominal pressure. Attempting primary fascia closure under tension in patients with extensive abdominal wall and intra-abdominal organ edema, is associated with an increased incidence of multiple organ failure (MOF), necrotizing abdominal wall infections, and mortality [7]. The optimal method of TAC should contain and protect the contents of the peritoneal cavity from external contamination and injury, preserve fascia; minimize desiccation and damage to viscera, remove and quantify third space fluid; prevent loss of domain, lower bacterial count, inflammatory response, keep patient’s abdominal wall skin dry and intact; preserve the integrity of the abdominal wall, be simple to perform and maintain, provide ease of reentry and have minimal adverse physiologic effects [13], [22], [23]. Various TAC methods have been subject to multiple studies, and their advantages and disadvantages are known [24], but are too numerous to cite. Although no prospective randomized studies are available to compare effectiveness of various TAC techniques as compared to NPWT-I, some evidence exists that shows a beneficial effect of this technique in the management of complex abdominal pathology [25].
3. Methods
A retrospective review was performed of 48 patients with clear indication for open abdomen management due to severe abdominal sepsis that were treated with NPWT-I between November, 2007 and November, 2008 by the SICU team of the Hospital Mexico, in San Jose, Costa Rica. The following criteria were utilized in the selection of patients who would receive this treatment: Björck open abdomen class 2b or greater [24], patients with an APACHE score of 12 or greater at admission with a diagnosis of abdominal sepsis, with contamination or with secondary generalized peritonitis. All other patients that required management with an open abdomen who did not meet the previously expressed criteria were excluded from our study.
The scope of our study involves only the use of NPWT-I in regards to the management of intra-abdominal sepsis, other considerations such as abdominal closure, management of abdominal compartment syndrome and other associated conditions are not fully address and are not the main consideration of this paper.
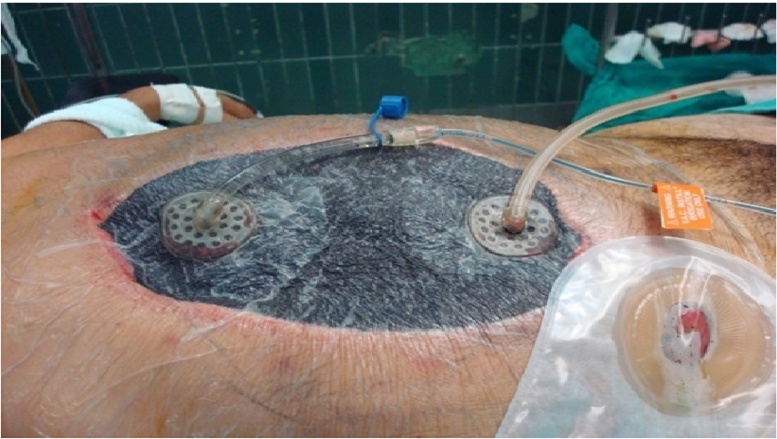
Our study group consisted of 20 males and 28 females, with ages between 22 and 76 years, in whom the infectious source was found but not controlled during the first intervention and management via OA and abdominal cavity washout for residual contamination was scheduled. All patients selected received NPWT-I. The procedure was performed by applying a sterile abdominal dressing, which consists of a fenestrated soft plastic non-adherent layer with enclosed central foam, which is placed on the surface of the viscera. Then, two layers of V.A.C. GranuFoam® dressings are applied over the plastic layer, the first layer in direct contact with the non-adherent plastic and located inside the abdominal cavity under the edges of the incision and the second, is placed directly in the abdominal incision filing the exact space of the wound opening (Fig. 1). Finally, a transparent adhesive is placed over the GranuFoam® and the wound to seal the abdominal cavity. A 1-inch diameter orifice is cut off the plastic seal on the lower or caudal area of the wound to connect a Sensa T.R.A.C.® adhesive pad, which will serve as the outflow tubing, that will be later connected from the dressing to the canister in the negative pressure device. Before the final connection is made, test of the integrity (seal) of the vacuum system must be performed by connecting a sterile suction tube to the wound system, and thus guaranteeing that no leaks are present. Finally, a second 1-inch dressing is placed over the wound to adhere the instillation tubing, which is later connected to a saline solution bag. A final irrigation plus suction test is performed just before the final settings are made by connecting all the tubing to the VAC® Instill machine, where the treatment parameters need to be programmed.
Fig. 1.
Photograph of VAC plus instillation assembly. Note the instillation line on the left and retrieval line on the right, both lying over the abdominal foam dressing.
The parameters are divided in a three step cycle, the first part involves instillation, followed by a dwell period, and finalized by vacuum aspiration.
The instillation cycle is programmed for 120 s, using 0.9% sodium chloride solution, for an estimate of approximately 40–50 mL every cycle. The volume of instillation was obtained from previous experiences in our Hospital, in which larger volumes of saline solution were used, without yielding better results. Temperature of the fluids should be maintained at a range of 21–36 °C. During instillation, the negative pressure switches off automatically. Instillation was followed by a 5-min dwell time, and then NPWT is resumed for 30 min. During the final step, a continuous vacuum set at a range of 100–125 mm Hg is used to retrieve the instilled solution along with any abdominal fluid. Approximately 37 cycles are delivered per day, depending on the time required to service the machine between replacement of canisters or saline bags, with a total volume of instillation of approximately 1.8 L per day. Dressing changes were performed every 48–72 h in all cases with the exception of faulty or clogged sponge systems when dressing changes where performed immediately upon detection.
All dressing changes were performed in a sterile fashion in the operating room. All patients were monitored with daily blood work, which consisted of a complete metabolic panel and complete blood count. Bladder pressure was monitored periodically in search of intra-abdominal hypertension.
Discontinuation of NPWT-I was performed when the abdominal cavity was ready for fascia closure.
Fascia closure was performed when the following criteria where met:
-
•
The source of contamination was controlled and the abdominal cavity was macroscopically clean.
-
•
Absence of intra-abdominal hypertension.
-
•
No clinical evidence of septic syndrome or hemodynamic instability attributable to intra-abdominal disease.
4. Results
In this study, 48 patients with open abdomen were treated with NPWT-I. 20 (42%) were male and 28 (58%) were female. Average age was 48 years. Causes of management via OA included: damage control surgery with contaminated abdominal cavity (n = 9), non-traumatic intra-abdominal sepsis (n = 29) and abdominal compartment syndrome with peritonitis (n = 10). Mortality in these patients was attributed to pulmonary embolism (n = 1), acute renal failure (n = 2) and cardiopulmonary arrest (n = 1). A total of 26 patients were admitted to our surgical ICU after initial evaluation, the other 22 patients were not taken to the SICU because of lack of beds, and were managed in the surgical recovery ward. Average total hospital stay was 24 days, and stay in the ICU for patients requiring aggressive monitoring (n = 26) averaged 7.5 days.
During treatment there were no acute complications related to the NPWT-I. No enteric fistulae were detected post NPWT-I. All patients presenting with abdominal compartment syndrome resolved after initiation of the NPWT-I, no patients developed intra-abdominal hypertension associated with the NPWT-I. A total of 46 patients (96%) patients achieved fascia closure after NPWT-I therapy after an average of 6 days. Four patients (8%) died during the course of treatment of causes unrelated to NPWT-I. A six month follow up of all the patients revealed no further complications related to their initial episode except for 2 patients (4%) who presented with a ventral hernia which were treated in the recommended time periods [24]. Table 1 summarizes the key characteristics and outcomes of our study.
Table 1.
Characteristics and outcomes of our study population.
| Average Age | Gender Male/Female | Average SICU LOS | Fascia closure rate | Enteric fistula rate | Ventral Hernia rate | Overall Mortality |
|---|---|---|---|---|---|---|
| 48 years | 20/28 | 7.48 days | 96% | 0% | 4% | 8.3% |
SICU: Surgical Intensive Care Unit. LOS: Length of Stay.
5. Discussion
Surgical source control in patients with severe abdominal sepsis is critical to patient survival [26], and is especially important with the current increase in antibiotic resistance in surgical ICU patients [27], as well as suboptimal practices related to the use of antibiotics [28]. Novel therapeutic approaches, such as NPWT-I, that allow for rapid source control without the use of drugs, appear to be valuable addition to traditional therapeutic strategies for management of contaminated abdominal cavities. The instillation and fluid retrieval, using NPWT-I device appeared to be a key element for obtaining safe and satisfactory results in the management of abdominal sepsis in our patient group. The use of normal saline appears to be sufficent in the management of complex wounds [29], while other substances seem to be ineffective [30], [31] and exposes patients to added risks, therefore there is no clear benefit for their use in abdominal instillation [32].
Our experience with this technique initiated in 2004, and has been in use continuously, but unfortunately, the executive decree that allowed medical research to be performed in Costa Rica was repealed by constitutional resolution number 2010-001668, impeding the continuation of our study, therefore no patient data from 2009 onward was used.
In patients who required management with OA therapy under our care, the use NPWT-I showed encouraging results. Our patients had low morbidity and mortality, as well a shorter duration of stay both for the surgical ICU unit as well as the hospital. When comparing our group, to similar case studies, both in our country and abroad, we see that NPWT-I had an almost four-fold reduction in mortality [33], a 52% increase in facial closure rates [34] a reduction of 69% in the ICU stay, and a hospital stay reduction of 57% in comparison to the use of silo closure methods [33], [34]. Even when compared to traditional NPWT, our study population showed reduced mortality (8.3% vs 23%), no intra-abdominal bleeding and lower incidence of enteric fistula [35]. We were unable to document any complications associated with this therapy in our patient group.
Due to the limited scope of our study, and scarce amount of literature available relating to the use of NPWT-I, we recommend that larger, multicenter studies be performed to validate and further explore the results associated with this approach. We recommend a control group using other methods of management of the open abdomen that may permit a more accurate comparison of the outcomes associated with each technique that due to the nature of our cohort outcome review, was not performed. Ideally the two study groups would encompass one way versus two way therapy.
Conflicts of interest
No conflict of interest to disclose.
Funding
No funding provided.
Ethical approval
No ethical approval was solicited.
Author contribution
Dr. Pablo Sibaja is the main author, took part in study design, data collection, data analysis and writing.
Dr. Alfredo Sanchez took part in the writing process, literature review and text revision.
Dr. Guillermo Villegas took part in data analysis and text revision.
Dr. Alvaro Apestegui wrote part of the text and took part in the literature review.
Dr. Esteban more took part in data collections.
Guarantor
Dr. Pablo J. Sibaja.
References
- 1.Vincent J.-L., Gerlach H. Fluid resuscitation in severe sepsis and septic shock: an evidence-based review. Crit. Care Med. 2004;32(November Suppl. (11)):S451–S454. doi: 10.1097/01.ccm.0000142984.44321.a4. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15542955. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger R.P. Cardiovascular management of septic shock. Crit. Care Med. 2003;31(March (3)):946–955. doi: 10.1097/01.CCM.0000057403.73299.A6. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/12627010. [DOI] [PubMed] [Google Scholar]
- 3.Anaya D.A., Nathens A.B. Risk factors for severe sepsis in secondary peritonitis. Surg. Infect. (Larchmt) 2003;4(4):355–362. doi: 10.1089/109629603322761418. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15012862. [DOI] [PubMed] [Google Scholar]
- 4.Holzheimer R.G., Dralle H. Paradigm change in 30 years peritonitis treatment – a review on source control. Eur. J. Med. Res. 2001;6(4):161–168. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11309228. [PubMed] [Google Scholar]
- 5.Lamme B., Boermeester M.A., Belt E.J.T., van Till J.W.O., Gouma D.J., Obertop H. Mortality and morbidity of planned relaparotomy versus relaparotomy on demand for secondary peritonitis. Br. J. Surg. 2004;91(8):1046–1054. doi: 10.1002/bjs.4517. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15286969. [DOI] [PubMed] [Google Scholar]
- 6.Waibel B.H., Rotondo M.F. Damage control for intra-abdominal sepsis. Surg. Clin. North Am. 2012;92(April (2)):243–257. doi: 10.1016/j.suc.2012.01.006. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22414411. [DOI] [PubMed] [Google Scholar]
- 7.Ivatury R.R., Porter J.M., Simon R.J., Islam S., John R., Stahl W.M. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: prophylaxis, incidence, and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J. Trauma. 1998;44(June (6)):1016–1021. doi: 10.1097/00005373-199806000-00014. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9637157. [DOI] [PubMed] [Google Scholar]
- 8.Morykwas M.J., Argenta L.C., Shelton-Brown E.I., McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann. Plast. Surg. 1997;38(June (6)):553–562. doi: 10.1097/00000637-199706000-00001. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9188970. [DOI] [PubMed] [Google Scholar]
- 9.Borráez O.A. Abdomen abierto. Infecc en Cirugía. 2001;16:230–237. [Google Scholar]
- 10.J.G. Hadeed, G.W. Staman, H.S. Sariol, S. Kumar, S.E. Ross, Delayed Primary Closure in Damage Control Laparotomy: The Value of the Wittmann Patch. Southeastern Surgical Congress. [PubMed]
- 11.Barker D.E., Kaufman H.J., Smith L.A., Ciraulo D.L., Richart C.L., Burns R.P. Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patient. J. Trauma. 2000;48(2):201–206. doi: 10.1097/00005373-200002000-00001. https://www.ncbi.nlm.nih.gov/pubmed/10697075 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan M., Banwell P., Orgill D., Ivatury R. HMP Commun; 2005. Guidelines for the Management of the Open Abdomen. [Google Scholar]
- 13.Open Abdomen Advisory Panel, Campbell A., Chang M., Fabian T., Franz M., Kaplan M. Management of the open abdomen: from initial operation to definitive closure. Am. Surg. 2009;75(November Suppl. (11)):S1–S22. ([Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19998714) [PubMed] [Google Scholar]
- 14.Miller P.R., Thompson J.T., Faler B.J., Meredith J.W., Chang M.C. Late fascial closure in lieu of ventral hernia: the next step in open abdomen management. J. Trauma. 2002;53(November (5)):843–849. doi: 10.1097/00005373-200211000-00007. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/12435933. [DOI] [PubMed] [Google Scholar]
- 15.De Waele J.J., Kaplan M., Sugrue M., Sibaja P., Björck M. How to deal with an open abdomen? Anestezjol. Intens. Ter. 2015;47(September (4)):372–378. doi: 10.5603/AIT.a2015.0023. [Internet] [cited 2016 Jun 2] Available from: http://czasopisma.viamedica.pl/ait/article/view/41693. [DOI] [PubMed] [Google Scholar]
- 16.Dubose J.J., Scalea T.M., Holcomb J.B., Shrestha B., Okoye O., Inaba K. Open abdominal management after damage-control laparotomy for trauma: a prospective observational American Association for the Surgery of Trauma multicenter study. J. Trauma Acute Care Surg. 2013;74(1):113–120. doi: 10.1097/TA.0b013e31827891ce. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23271085. [DOI] [PubMed] [Google Scholar]
- 17.Miller P.R., Meredith J.W., Johnson J.C., Chang M.C. Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann. Surg. 2004;239(May (5)):608–614. doi: 10.1097/01.sla.0000124291.09032.bf. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15082964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirocchi R., Birindelli A., Biffl W.L., Mutafchiyski V., Popivanov G., Chiara O. What is the effectiveness of the negative pressure wound therapy (NPWT) in patients treated with open abdomen technique? A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2016;81(September (3)):575–584. doi: 10.1097/TA.0000000000001126. [Internet] [cited 2016 Oct 3] Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=01586154-201609000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Kubiak B.D., Albert S.P., Gatto L.A., Snyder K.P., Maier K.G., Vieau C.J. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock. 2010;34(November (5)):525–534. doi: 10.1097/SHK.0b013e3181e14cd2. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20823698. [DOI] [PubMed] [Google Scholar]
- 20.Batacchi S., Matano S., Nella A., Zagli G., Bonizzoli M., Pasquini A. Vacuum-assisted closure device enhances recovery of critically ill patients following emergency surgical procedures. Crit. Care. 2009;13(6):R194. doi: 10.1186/cc8193. [Internet] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2811940&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korotaev G.M., Sheprinskiĭ P.E., Osmokesku K.I. The role of lymphatic collector in the spreading infections in peritonitis. Khirurgiia (Sofiia) 1991;(5):19–23. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/2072639. [PubMed] [Google Scholar]
- 22.Stonerock C.E., Bynoe R.P., Yost M.J., Nottingham J.M. Use of a vacuum-assisted device to facilitate abdominal closure. Am. Surg. 2003;69(December (12)):1030–1034. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/14700286. [PubMed] [Google Scholar]
- 23.Kreis B.E., de Mol van Otterloo A.J., Kreis R.W. Open abdomen management: a review of its history and a proposed management algorithm. Med. Sci. Monit. 2013;19:524–533. doi: 10.12659/MSM.883966. [Internet] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3706408&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiara O., Cimbanassi S., Biffl W., Leppaniemi A., Henry S., Scalea T.M. International consensus conference on open abdomen in trauma. J. Trauma Acute Care Surg. 2016;80(1):173–183. doi: 10.1097/TA.0000000000000882. [Internet] Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=01586154-201601000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald J.E.F., Gupta S., Masterson S., Sigurdsson H.H. Laparostomy management using the ABThera™ open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int. Wound J. 2013;10(2):138–144. doi: 10.1111/j.1742-481X.2012.00953.x. https://www.ncbi.nlm.nih.gov/pubmed/22487377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azuhata T., Kinoshita K., Kawano D., Komatsu T., Sakurai A., Chiba Y. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit. Care. 2014;18(3):R87. doi: 10.1186/cc13854. [Internet] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4057117&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahu M.K., Siddharth B., Choudhury A., Vishnubhatla S., Singh S.P., Menon R. Incidence, microbiological profile of nosocomial infections, and their antibiotic resistance patterns in a high volume Cardiac Surgical Intensive Care Unit. Ann. Card. Anaesth. 2016;19(2):281–287. doi: 10.4103/0971-9784.179625. [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/27052070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llor C., Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014;5(December (6)):229–241. doi: 10.1177/2042098614554919. [Internet] [cited 2016 Jun 3] Available from: http://taw.sagepub.com/lookup/doi/10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fluieraru S., Bekara F., Naud M., Herlin C., Faure C., Trial C. Sterile-water negative pressure instillation therapy for complex wounds and NPWT failures. J. Wound Care. 2013;22(6) doi: 10.12968/jowc.2013.22.6.293. [Internet] 293-294, 296, 298-299. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24049811. [DOI] [PubMed] [Google Scholar]
- 30.Hau T., Nishikawa R., Phuangsab A. Irrigation of the peritoneal cavity and local antibiotics in the treatment of peritonitis. Surg. Gynecol. Obstet. 1983;156(January (1)):25–30. [Internet] [cited 2016 Jun 22] Available from: http://www.ncbi.nlm.nih.gov/pubmed/6847944. [PubMed] [Google Scholar]
- 31.Rambo W.M. Irrigation of the peritoneal cavity with cephalothin. Am. J. Surg. 1972;123(2):192–195. doi: 10.1016/0002-9610(72)90332-7. [DOI] [PubMed] [Google Scholar]
- 32.Ozmen V., Thomas W.O., Healy J.T., Fish J.M., Chambers R., Tacchi E. Irrigation of the abdominal cavity in the treatment of experimentally induced microbial peritonitis: efficacy of ozonated saline. Am. Surg. 1993;59(May (5)):297–303. [Internet] [cited 2016 Jun 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/8489098. [PubMed] [Google Scholar]
- 33.Sánchez Arias M. Abdomen abierto. Acta Med. Costarric. 2000;42(2):76–80. nc-nd/3.0/ [Google Scholar]
- 34.Tremblay L.N., Feliciano D.V., Schmidt J., Cava R.A., Tchorz K.M., Ingram W.L. Skin only or silo closure in the critically ill patient with an open abdomen. Am. J. Surg. 2001;182(December (6)):670–675. doi: 10.1016/s0002-9610(01)00805-4. [Internet] [cited 2016 Jun 23] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11839336. [DOI] [PubMed] [Google Scholar]
- 35.Seternes A., Rekstad L.C., Mo S., Klepstad P., Halvorsen D.L., Dahl T., Björck M., Wibe A. Open abdomen treated with negative pressure wound therapy: indications, management and survival. World J. Surg. 2016 doi: 10.1007/s00268-016-3694-8. https://www.ncbi.nlm.nih.gov/pubmed/27541031 1-0. [DOI] [PubMed] [Google Scholar]