Abstract
The cancer microenvironment allows tumor cells to evade immune surveillance through a variety of mechanisms. While interferon-γ (IFNγ) is central to effective antitumor immunity, its effects on the microenvironment are not as clear and have in some cancers been shown to induce immune checkpoint ligands. The heterogeneity of these responses to IFNγ remains poorly characterized in desmoplastic malignancies with minimal inflammatory cell infiltration, such as pancreatic cancer (PC). Thus, the IFNγ response within and on key cells of the PC microenvironment was evaluated. IFNγ induced expression of human leukocyte antigen (HLA) class I and II on PC cell lines, primary pancreatic cancer epithelial cells (PPCE) and patient-derived tumor-associated stroma, concomitant with an upregulation of PDL1 in the absence of CD80 and CD86 expression. As expected, IFNγ also induced high levels of CXCL10 from all cell types. In addition, significantly higher levels of CXCL10 were observed in PC specimens compared to those from chronic pancreatitis, whereby intratumoral CXCL10 concentration was an independent predictor of poor survival. Immunohistochemical analysis revealed a subset of CXCR3-positive cancer cells in over 90 % of PC specimens, as well as on a subset of cultured PC cell lines and PPCE, whereby exposure to CXCL10 induced resistance to the chemotherapeutic gemcitabine. These findings suggest that IFNγ has multiple effects on many cell types within the PC microenvironment that may lead to immune evasion, chemoresistance and shortened survival.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1760-y) contains supplementary material, which is available to authorized users.
Keywords: Interferon-γ, CXCL10, Pancreatic cancer, Epithelial cell, Tumor-associated stroma, Immuno-oncology
Introduction
With mortality rates largely unchanged over the past 50 years, pancreatic cancer (PC) is projected to be the second leading cause of cancer deaths by 2030 [1]. Current cytotoxic regimens offer minimal gains in long-term survival [2]. As a promising alternative, therapies designed to induce antitumor immune responses have achieved durable cures in preclinical models of PC [3, 4]. However, as observed with systemic cytotoxic therapies, immunotherapeutic resistance dominates the landscape of the human disease [5, 6]. While mechanisms of resistance remain unclear, it is becoming increasingly evident that the pattern of local inflammation present within pancreatic tumors is one of the tolerances [7, 8]. This local tolerance serves as a major barrier to immunotherapeutic success. Efforts to further understand tolerogenic signaling within the human PC microenvironment may therefore lead to more effective immunotherapeutic approaches.
PC is an epithelial cancer with stromal elements accounting for up to 90 % of the pancreatic adenocarcinomas by mass [9]. Early infiltration of regulatory myeloid subsets into this microenvironment has been proposed as the chief mechanism behind the induction of tolerance in murine models of PC, although the mechanism of their recruitment/induction is unclear [10–12]. The physiologic function of both epithelial and stromal cells suggests a potential role in mediation of the recruitment/induction. The natural environment of the epithelial cell is one of the controlled inflammations to maintain a homeostatic balance at the interface between sterile tissue and external insult. On the other hand, the tumor-associated stroma (TAS)-induced desmoplasia (the largest component being of fibroblastic origin) accompanying PC demonstrates a striking phenotypic resemblance to the remodeling phase of a chronic wound, in which the destructive potential of adaptive immune activation is limited to promote healing [7, 13]. Thus, both PC cells and TAS may therefore significantly contribute to the tolerogenic landscape of the PC microenvironment.
While a critical role for IFNγ in cancer control has been defined [14], IFNγ within the cancer microenvironment is also capable of inducing the upregulation of immune checkpoint ligands on tumor cells promoting immune evasion [15]. The degree and characteristics of the IFNγ response remain heterogeneous across types of cancers, cell types of the microenvironment and patient populations of a given cancer [16]. As a result, efforts must be made to understand this heterogeneity in order to properly harness the immune system and develop rational approaches to overcome current therapeutic limitations. Thus, here we examine IFNγ responses of human PC cell lines, primary PC epithelial (PPCE) cells and TAS outgrowths composed of cells of fibroblastic origin. Data presented here demonstrate roles for both PC cells and TAS in local tolerogenic signaling as well as uncover a novel, immune-mediated chemoresistant phenotype of PC cells.
Methods
Pancreatic cancer (PC) cell lines
Human cell lines PANC-1 and HPDE were obtained from American Type Culture Collection (ATCC, Rockville, MD) or as a generous gift from Mokenge Malafa (Moffitt Cancer Center, Tampa, FL), respectively. L3.6pl metastatic variant was derived as previously described [17, 18]. The selection of L3.6plGemRes gemcitabine-resistant pancreatic cancer cells was conducted as previously described [19]. All human PC cell lines were authenticated within 6 months by short tandem repeat (STR) analysis. All cells were maintained and stimulated in high-nutrition media [Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) Advanced (Life Technologies, Carlsbad, CA), 10 % fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA), 4 mM GlutaMAX™ (Life Technologies), 20 ng/mL human epidermal growth factor (Life Technologies), 40 ng/mL dexamethasone (Sigma-Aldrich, St. Louis, MO) and antibiotic antimycotic solution (Sigma)] in 5 % CO2/95 % air at 37 °C. The creation of L3.6plCXCL10 CXCL10-constitutively expressing cells were created by transfection with pCMV6.puromycin vector (Origene, Rockville, MD) containing human CXCL10 cDNA and grown in selective medium containing puromycin (10 μg/mL). Isolated clones were expanded, and CXCL10 secretion was confirmed by ELISA (BD Biosciences, Franklin Lakes, NJ).
Isolation of primary pancreatic cancer epithelial cells (PPCE) and tumor-associated stroma (TAS)
Patient-derived PPCE were isolated from patient-derived xenografts [20]. Epithelial cell purity was confirmed by expression of HLA-ABC and cytokeratins 18 and 19 (Biolegend, San Diego, CA) by flow cytometry and immunofluorescent microscopy. Patient-derived TAS outgrowths were generated from direct culture of gross human pancreatic adenocarcinoma surgical specimens as previously validated [21]. Cell purity was confirmed by high expression levels of α-smooth muscle actin (R&D Systems, Minneapolis, MN) by flow cytometry and immunocytochemistry.
Surgical patient cohort
A review of an institutional review board-approved, prospectively maintained pancreatic cancer database at the University of Florida (UF) was performed. Informed consent was obtained from all patients. Fresh tissue lysates from resected tissue were isolated from patients who underwent surgery for chronic pancreatitis (n = 5) and PC (n = 28). Immediately adjacent tissues were preserved in formalin for histologic verification of pathology. Additionally, serum was isolated from the peripheral blood of either healthy controls or patients with PC.
Immunohistochemistry and immunofluorescence
Immunocytochemistry was performed after a brief 4 % paraformaldehyde fixation period on PC cells in culture. Anti-HLA-DR/DP/DQ (Biolegend, San Diego, CA) conjugated to Alexa Fluor® (AF) 647 and 4′,6-diamidino-2-phenylindole (DAPI) were used for staining. Sample processing and immunohistochemical (IHC) staining were performed by the University of Florida’s Molecular Pathology Core Facility. Briefly, patient tumors were formalin-fixed and paraffin-embedded. Five-micrometer sections were stained with anti-CXCR3 (R&D Systems) following antigen retrieval with citrate buffer pH 6.0 for tissue IHC analysis. Histologic slides of pancreatic specimens were reviewed by a pathologist specializing in pancreatic cancer (DHG). Immunofluorescence was performed on cells fixed for 10 min using paraformaldehyde followed by a 1-h wash with 0.1 % Triton X-100 in PBS with 3 % bovine serum albumin. Cells were then incubated overnight at 4 °C with anti-CXCR3. A goat antimouse secondary antibody conjugated to AF 647 was then applied with DAPI stains (Life Technologies, Carlsbad, CA). Fluorescent images were captured using an EVOS® FL imaging system (Life Technologies).
IFNγ cell stimulations
1 × 105 of HPDE, PANC1, L3.6pl, PPCE and TAS were plated in 24-well culture dishes, allowed to adhere overnight and stimulated with 20 ng/ml of IFNγ (R&D Systems) for 48 h. IFNγ dosing was selected based on previous investigations evaluating T cell secretory responses following anti-CD3 stimulation [22–24] as well as our own data demonstrating approximately 20 ng/mL of IFNγ secreted by peripheral blood mononuclear cells in response to anti-CD3/CD28 stimulation at 24 h (Supplemental Fig. S1e). Supernatants were harvested and stored at −80 °C until soluble mediator analysis could be performed, and the cells were subjected to flow cytometric analysis.
Enzyme-linked Immunoassay (ELISA)
Resected pancreatic tissue, cell culture supernatants or serum was probed for CXCL10 expression using an ELISA and a standard curve according to the manufacturer’s protocol (BD Biosciences, Franklin Lakes, NJ). Resected pancreatic tissue was placed in cell lysis buffer (Cell Signaling Technologies, Danvers, MA) with a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Tissues were mechanically dissociated and homogenized using the FastPrep-24 system according to the manufacturer’s protocol (MP Biomedicals, Santa Ana, CA). For tissue homogenates, all cytokine concentrations were normalized to total protein concentrations using detergent-compatible protein quantification (Bio-Rad, Hercules, CA). Soluble mediator concentrations were then converted to pg/mg of tissue as follows: pg/ml divided by mg/ml of total protein.
Flow cytometry
Cells were dissociated from 24-well culture dishes using Accutase® cell detachment solution (Sigma), washed in dPBS containing 5 % FBS, 5 mM Ethylenediaminetetraacetic acid (EDTA) and 0.1 % sodium azide (Sigma) and probed for HLA-ABC, HLA-DR/DP/DQ, CD80, CD86, PDL1 and/or CXCR3 using the following antibodies: Pacific Blue™-conjugated HLA-ABC (Biolegend) Brilliant™ Blue 515-conjugated HLA-DR/DP/DQ, phycoerythrin (PE)-conjugated CD86, allophycocyanin-conjugated PDL1, PE/Cy7-conjugated CD80 or PE/Cy7-conjugated CXCR3 (Biolegend). All antibodies were purchased from BD Biosciences unless otherwise indicated and used at manufacturer’s recommended dilutions. Manufacturer’s recommended isotype controls were used as negative controls for all antibodies used. A total of 10,000 events per sample were acquired using a BD LSR II (BD Biosciences), and data were analyzed using FlowJo data analysis software (FLOWJO LLC, Ashland, OR).
Chemoresistance assay
1 × 105 of PANC1, L3.6pl, L3.6plGemRes or L3.6plCXCL10 cells were plated in 24-well culture dishes and allowed to adhere overnight. After which gemcitabine (Eli Lilly, Indianapolis, IN) suspended in Dulbecco’s PBS (D-PBS) was added at concentrations for the lethal dose (LD50) previously published (L3.6pl:100 nM, PANC-1; 5 μM) [25, 26], in the presence and/or absence of 100 nM CXCL10 (R&D Systems (Minneapolis, MN)), 1ug/ml of anti-CXCR3 (R&D Systems) and/or 1ug/ml of anti-CXCL10 (R&D Systems) for 72 h. Cellular viability was quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT) (Trevigen, Gaithersburg, MD) according to the manufacturer’s protocol.
T cell stimulation
1 × 106 human peripheral blood mononuclear cells from a healthy donor were stimulated with anti-CD3/CD28 beads (Life Technologies). Supernatants were collected at 24 and 48 h post-stimulation. Secreted IFNγ was measured by ELISA (BD Biosciences).
Statistical analysis
All continuous variables were assessed for normality using the Shapiro–Wilk test. Normally distributed variables were compared using independent samples t tests. All other continuous variables were compared using the Mann–Whitney U test. Survival analysis was performed using SPSS version 22.0 (IBM SPSS Statistics for Windows; IBM Corp). Patients were dichotomized at median intratumoral CXCL10 concentration, and Kaplan–Meier survival curves were employed to analyze overall survival. The log-rank test was used to evaluate significance (P < 0.05). A univariate Cox proportional hazards model further examined the effect of stage, grade, margin, tumor size and intratumoral CXCL10 on overall survival.
Results
IFNγ upregulates antigen presentation machinery in the context of negative co-stimulation
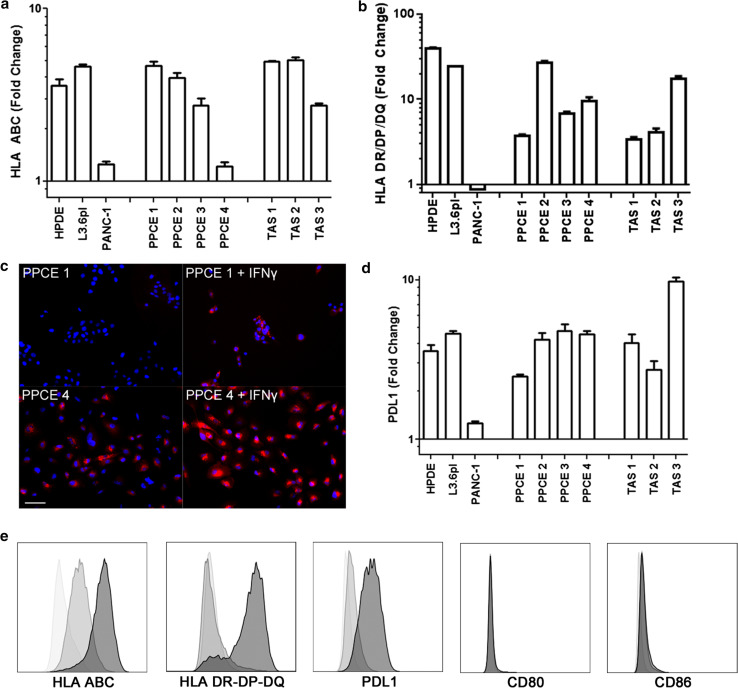
To investigate the effect of IFNγ on innate immune function of the PC microenvironment, PC cell lines, primary PC epithelial (PPCE) cells and TAS cultures were assessed for changes in expression of antigen presentation machinery and co-stimulatory molecules following IFNγ exposure. Following 48 h of exposure, each cell type demonstrated a two- to fivefold increase in class I HLA expression (Fig. 1a, e). In addition, IFNγ stimulated HLA class II expression on all cell types except for the PC cell line PANC-1 (Fig. 1b, e). Interestingly, three of the four PPCE lines contained a proportion of unstimulated cells expressing class II HLA (Fig. 1c, S1). Importantly, none of the cell types evaluated expressed the positive co-stimulatory ligands CD80 or CD86, regardless of IFNγ stimulation (Fig. 1e). Conversely, 48 h of IFNγ exposure did induce a two- to tenfold increase in PDL1 expression on PC cell lines, PPCE and TAS (Fig. 1d, e).Taken together, these data demonstrate that IFNγ within the tumor microenvironment upregulates antigen presentation machinery in the context of negative co-stimulation on both tumor and stromal cells, potentially resulting in immune evasion.
Fig. 1.
IFNγ induces upregulation of antigen presentation machinery in the context of negative co-stimulation in PC cell lines, PPCE and TAS. Surface expression of HLA a class I, b, c class II and d PDL1 was evaluated on normal ductal epithelial cells (HPDE), the pancreatic cancer cell lines L3.6pl and PANC-1, primary pancreatic cancer epithelial cells (PPCE) from four individual donors as well as primary tumor-associated stroma (TAS) from three individual donors in the presence and absence of 48 h of IFNγ treatment (20 ng/mL). a, b Data are presented as fold change calculated from peak intensities of treated cells divided by untreated cells. c Class II HLA was examined by microscopy. Scale bar indicates 50 μm. d Data are presented as fold change calculated from peak intensities of treated cells divided by untreated cells. e Representative histograms from flow cytometric analysis for isotype controls (white), unstimulated (gray) and IFNγ-stimulated (black) cells
Local CXCL10 secretion is associated with poor long-term outcomes in PC
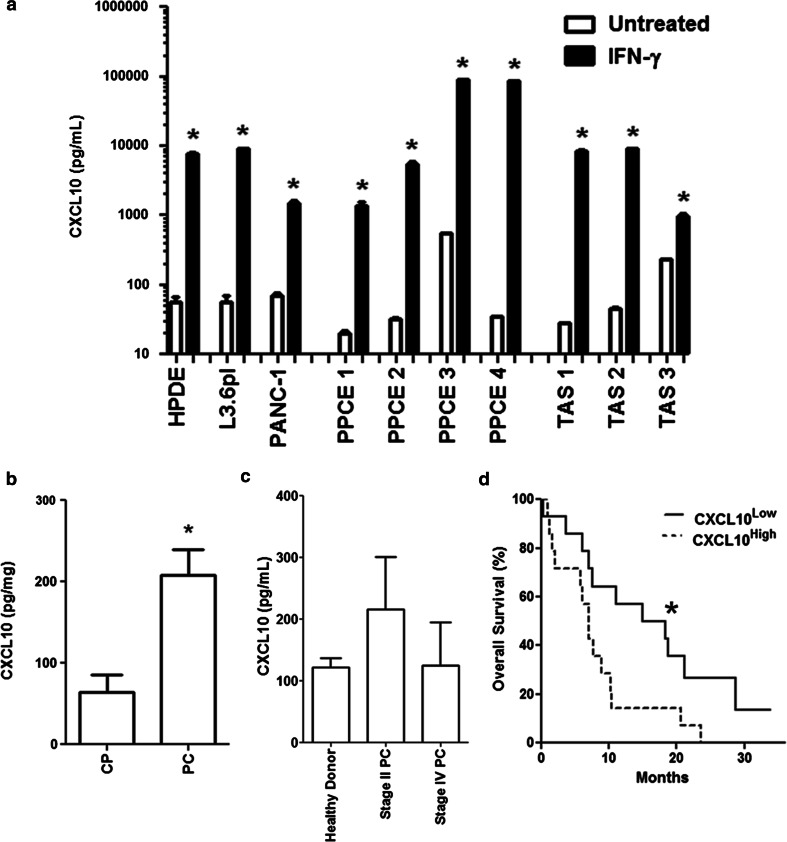
CXCL10 is an IFNγ inducible protein which was expressed at high levels by PC cell lines, PPCE and TAS upon IFNγ exposure (Fig. 2a). In addition, local pancreatic CXCL10 expression was significantly elevated in malignancy when compared to chronic pancreatitis (CP) (208 vs. 64 pg/mg; P = .013) (Fig. 2b). This diagnostic relationship between CXCL10 and PC appeared to be a local phenomenon, as serum CXCL10 levels were not significantly different between healthy controls and patients with either resectable or metastatic PC (Fig. 2c). In the cohort of patients with surgically resected PC, patients with high intratumoral CXCL10 levels demonstrated significantly reduced overall survival (OS) on Kaplan–Meier analysis (median OS 7.1 vs. 14.9 months; P = .022) (Fig. 2d). This trend was further validated using a proportional hazards model, which revealed a significant correlation between intratumoral CXCL10 concentration and reduced overall survival (hazard ratio 82.9; P = .003) (Table 1). Therefore, intratumoral CXCL10 expression may harbor prognostic value in PC.
Fig. 2.
IFNγ induces expression of CXCL10 in PC cell lines, PPCE and TAS, whereby intratumoral CXCL10 concentration is prognostic in PC. a CXCL10 secretion was evaluated by ELISA in normal ductal epithelial cells (HPDE), the pancreatic cancer cell lines L3.6pl and PANC-1, primary pancreatic cancer epithelial cells (PPCE) from four individual donors as well as primary tumor-associated stroma (TAS) from three individual donors in the presence and absence of 48 h of IFNγ treatment (20 ng/mL). White bars = untreated, Black bars = IFNγ-treated. All data are presented as mean ± SEM *P < .05 versus unstimulated by Mann–Whitney U test. b Pancreatic CXCL10 concentration was compared between patients with chronic pancreatitis (CP; n = 5) and PC (n = 28). CXCL10 concentrations within fresh tissue lysates (ng) were normalized to total protein concentration (mg). Data are presented as mean ± SEM *P < .05 using the Mann–Whitney U test. c Serum CXCL10 concentration was compared between healthy controls (n = 8), patients with stage II PC (n = 10) and patients with stage IV PC (n = 12). Data are presented as mean ± SEM. d Patients with PC were dichotomized by median intratumoral CXCL10 concentration into CXCL10Low and CXCL10High groups. Kaplan–Meier survival curves were generated and overall survival compared using the log-rank test. *P < .05
Table 1.
Univariate analysis of overall survival in surgically resected pancreatic adenocarcinoma
| Parameter | Hazard ratio | P value |
|---|---|---|
| Positive lymph node ratio | 63.9 | .002* |
| Intratumoral CXCL10 (ng/mg) | 82.9 | .003* |
| Positive resection margin (R1) | 2.2 | .071 |
| Major vascular resection | 2.5 | .115 |
| Poor tumor differentiation | 1.6 | .241 |
| Tumor size (cm) | 1.03 | .740 |
* P < .05
A subset of cells, within in the PC microenvironment, express the CXCL10 receptor, CXCR3
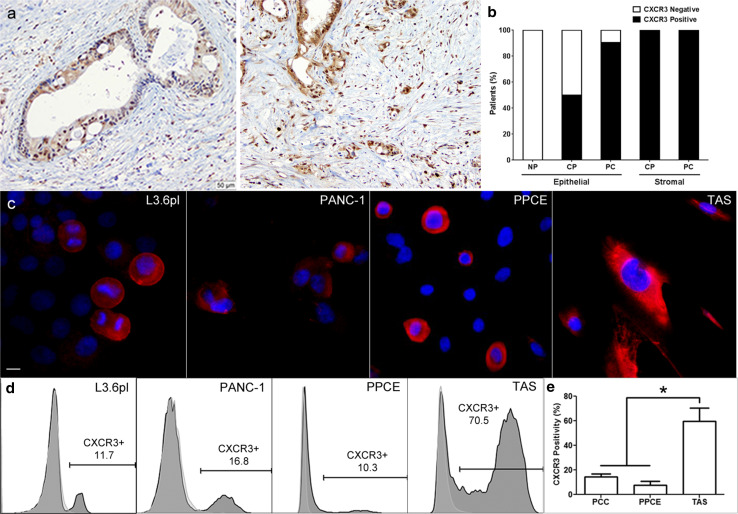
To interrogate the functional consequences of this prognostic association, surgical specimens were probed for the CXCL10 receptor, CXCR3. Here, a subset of malignant epithelial cells and the surrounding stroma consistently demonstrated CXCR3 expression (Fig. 3a). Upon pathological quantification, >90 % of PC specimens exhibited a subset of epithelial cells expressing CXCR3, which was not observed in normal pancreatic specimens (Fig. 3b). Further, all specimens from patients with either chronic pancreatitis (CP) or PC exhibited a subpopulation of stromal cells expressing CXCR3 (Fig. 3b). CXCR3 expression was then examined on PC cell lines, PPCE and TAS in culture. Again, CXCR3 expression was observed in 1–20 % of PC cell lines or PPCE and in up to 80 % of the cells of the TAS (Fig. 3c–e). Together, these data suggest that both epithelial and stromal components contribute to IFNγ-induced CXCL10-dependent mechanisms of poor outcomes in PC.
Fig. 3.
A subset of cells, within in the PC microenvironment, expresses the CXCL10 receptor, CXCR3. a Representative images of immunohistochemistry probing for CXCR3 were performed on formalin-fixed, paraffin-embedded PC specimens. b Quantification of CXCR3-positive ductal epithelial cells in surgically resected normal pancreas (NP; n = 2), chronic pancreatitis (CP; n = 4) and PC (n = 21) specimens. Specimens were considered CXCR3-positive if at least 5 % of ductal epithelial cells expressed CXCR3. White bars = CXCR3 negative, Black bars = CXCR3 positive. c Immunofluorescent staining for CXCR3 (red) and DAPI (blue) was performed on cultured PC cell lines (n = 2), PPCE (n = 4) and TAS (n = 2). Representative images are displayed. Scale bar indicates 10 μm. d Representative histograms from flow cytometric analysis of PC cell lines, PPCE and TAS for surface CXCR3 staining. e The average percentage of CXCR3-positive cells was quantified for each cell population. PC cell lines (PCC) consisted of L3.6pl and PANC-1. PPCE represents the average from four individual donors. Primary TAS represents the average from two individual donors. Data are presented as mean ± SEM *P < .05 using the Mann–Whitney U test
CXCL10 induces gemcitabine resistance in PC cells in a CXCR3-dependent manner
Gemcitabine is an antimetabolite commonly used in the systemic treatment of PC, whereby chemoresistance leads to poor clinical outcomes. Previous reports have demonstrated that CXCL10 halts progression of the endothelial cell cycle [27]. As the toxicity induced by antimetabolites is dependent on a rapid mitotic progression that bypasses DNA repair mechanisms [28], we sought to investigate whether CXCL10 exposure induces antimetabolite chemoresistance. Thus, viability was assessed on gemcitabine-treated PC cell lines in culture with and without CXCL10 treatment. Simultaneous treatment with CXCL10 and gemcitabine imparted a survival advantage to PC cell lines, which was abrogated by both anti-CXCR3 and anti-CXCL10 agents (Fig. 4a). In order to determine the effect of chronic exposure of PC cells to CXCL10 on gemcitabine resistance, we incorporated a constitutive CXCL10-expressing vector into the L3.6pl PC cell line (L3.p6lCXCL10) and treated with multiple doses of gemcitabine for 72 h (Fig. 4b). Here, L3.p6lCXCL10 had similar viability to that of L3.6plGemRes, a gemcitabine-resistant clone previously isolated by our group [26], even at high doses of gemcitabine (Fig. 4c, d). These data demonstrate one potential mechanism leading to IFNγ-induced CXCL10-dependent poor clinical outcomes in PC.
Fig. 4.
CXCL10 induces gemcitabine resistance in PC cells. a, b PC cell lines were treated with gemcitabine corresponding to median lethal doses (LD50) (a L3.6pl: 100 nM, b PANC-1: 5 μM), CXCL10 (100 nM), anti-CXCR3 (1 μg/mL) or anti-CXCL10 (1 μg/mL) as indicated for 72 h. Viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and normalized to untreated cells. Data are presented as mean ± SEM and compared using the Mann–Whitney U test. c L3.6pl cells transfected with a CXCL10-expressing vector (L3.6plCXCL10) were assayed for CXCL10 secretion by ELISA and compared to empty vector control L3.6pl cells (L3.6plVC). d L3.6pl, L3.6plVC, L3.6plCXCL10 and the previously established gemcitabine-resistant L3.6pl (L3.6plGemRes) cells were treated with the indicated dose of gemcitabine for 72 h. Viability was calculated using the MTT assay and normalized to untreated cells. Data are presented as mean ± SEM and compared using the Mann–Whitney U test. *P < .05 compared to either L3.6pl or L3.6plVC
Discussion
It is becoming increasing clear that the tumor microenvironment of many cancers plays a critical role in disease progression, although the totality of mechanisms at play remains elusive. In addition to epithelial cell dysplasia, the microenvironment of PC has an abundant stromal/desmoplastic reaction which is composed of not only fibroblast-like stellate cells which produce a collagenous extracellular matrix, but immune cells, endothelial cells and neural cells [29, 30]. Here, evidence is accumulating to suggest interactions between these stromal elements and the cancer cells themselves. In addition, the close proximity of immune cell infiltration to the fibroblast compartment of the microenvironment implies a certain interaction between these compartments as well [30, 31]. Regardless, all of these interactions may be highly dependent on the tumor stage, the specific tissue context and other components of the microenvironment. Therefore, a detailed understanding of the different components involved is crucial for the effective development of future therapies [31]. Thus, here we evaluated the immunological response of two key cellular components of the PC microenvironment: the PC epithelial cell and the PC-associated fibroblast (TAS).
Like many other immunological mediators involved in cancer biology, IFNγ has been demonstrated to have pleiotropic effects on cancer progression [32, 33]. On the one hand, IFNγ is indispensable for the antitumor effect of both CD4+ and CD8 + T cells [34]. On the other hand, IFNγ is also responsible for the upregulation of checkpoint ligands within the tumor microenvironment, leading to immunological tolerance [35]. Importantly, the source of IFNγ within the tumor is likely multifactorial, as a variety of innate immune cells are capable of secreting IFNγ in response to damage or pathogen-associated molecular patterns. Further, comparable levels of IFNγ secretion as those used here have been demonstrated following lymphocyte activation [22–24]. Regardless of the source of IFNγ, the secretion of high levels of CXCL10 represents a consistent type II interferon response present in both PC cells and TAS, which likely contributes to the high levels of CXCL10 observed in PC specimens compared to nonmalignant controls.
Here, we demonstrate that IFNγ has direct and indirect consequences on the microenvironment of PC. Directly, IFNγ treatment stimulated the upregulation of antigen presentation machinery, but did do so in the context of negative co-stimulation through the induction of PDL1. Most importantly, IFNγ had this direct effect not only on the PC epithelial cell, but also on the fibroblastic component of the stromal environment. Indeed others have demonstrated a role for the fibroblastic component in regulating the immune microenvironment in PC. For instance, fibroblast activation protein-α (FAP) has been shown to govern the immune suppression of CXCL12 by excluding T cells from the microenvironment, whereby the depletion of FAP-expressing cells facilitates CD8 + T cell-mediated tumor killing [36]. Importantly, in this model, the therapeutic efficacy of the T cell checkpoint antagonist PDL1 was also improved [36]. In the current report, IFNγ-induced class II HLA expression on primary TAS was associated with a three- to fivefold increase in PDL1 and negligible expression of the positive co-stimulatory markers, CD80 and CD86. As TAS dominates the microenvironment by mass in PC, it is feasible that infiltrating lymphocytes may frequently contact stromal cells presenting tumor-associated antigens with tolerogenic co-stimulation, and this interaction contributes to the observed histopathologic phenomenon of stromal “trapping,” in which infiltrating lymphocytes fail to migrate through dense layers of activated stroma in PC [37]. Together, these data provide strong evidence that the fibroblastic component can not only shape the soluble and solid stromal environment in PC, but may also control and mediate the local immune suppression.
IFNγ exposure to the PC epithelial cells and fibroblast components also led to high levels of CXCL10 expression in the absence of traditional immune cells. Like IFNγ, CXCL10 also has pleiotropic effects on cancer progression. CXCL10 has been most robustly studied in the context of activation and/or recruitment of immune cells including T lymphocytes, NK cells, dendritic cells, macrophages and B cells through its cognate receptor CXCR3. As ligation of CXCR3 on these immune cells leads to the induction of cytokines such as IFNγ, interleukin-2 (IL-2) or tumor necrosis factor-α (TNF-α), which would promote cellular immune responses in the tumors, one would imagine this chemokine to have antitumor effects [38]. But once again, the expression of this immune mediator appears to be a double-edged sword within the microenvironment of PC. Here, we demonstrate that high levels of intratumoral CXCL10 are associated with poor clinical outcomes. These data support recent findings from Lunardi et al. [39] demonstrating an association between intratumoral CXCL10 mRNA expression and poor outcomes in PC. Lunardi et al. further implicate the chemotaxis of CXCR3-expressing regulatory T cells as a result of intratumoral CXCL10 secretion. Importantly, the literature also supports a direct effect of CXCL10 on PC cancer cells, promoting invasion and metastasis [40–43]. Here, we uncovered a novel direct effect of CXCL10 on PC, whereby chemoresistance is induced.
While chemotherapy is a commonly used strategy in the treatment of PC, the clinical response mediated by anticancer cytotoxic agents is also limited, whereby a large majority of cancers develop chemoresistance [44]. An ever-increasing number of factors contribute to chemoresistance including the inability of the treatment to eliminate the entire population of malignant cells. Whereas the bulk of the tumor mass can be reduced successfully via cytotoxic treatment, a small population of cells can survive and give rise to a new generation of cancer cells that are resistant to chemotherapy [44]. Here, we identified a subset of cells within PC cell lines, the PC lesion and isolated primary pancreatic cancer epithelial cells (PPCE) that expressed the receptor for CXCL10 and CXCR3. In addition, we describe a novel direct effect of CXCL10 on PC epithelial cells, whereby gemcitabine resistance is induced which is CXCR3 dependent. These data support previous work implicating cell cycle-dependent CXCR3 expression in endothelial cells, whereby exposure to CXCL10 induced cell cycle arrest, ultimately reducing the rate of proliferation [27]. While speculative, in this manner CXCL10 stimulation may bypass antimetabolite-dependent toxicity in PC cells, which is reliant on a rapid mitotic progression that precludes DNA repair mechanisms.
Other groups have characterized additional PC cell subsets that are also prone to chemoresistance. For instance, cancer stem cells and cells that have undergone the process of epithelial mesenchymal transition (EMT) have been demonstrated to be resistant to chemotherapeutics [44, 45]. It is unclear as of yet whether the CXCR3+ cell populations described here are one in the same as tumor stem cells and/or those which have under gone EMT, but together these data illustrate that the tumor microenvironment and/or the state of the targeted cell population play a role in the development of chemoresistance [44]. Further functional and in vivo analyses are necessary to further define the role of IFNγ-induced tolerogenic PC epithelial cell and the PC-associated fibroblast (TAS) in disease progression, as well as the role of IFNγ-induced expression of CXCL10 in chemoresistance. But in its totality, this present work provides data supporting IFNγ-induced tolerogenic phenotypes in the human PC microenvironment, combined with the novel description of IFNγ-induced CXCL10-mediated PC epithelial cell chemoresistance. These data significantly add to the body of the literature of mechanisms within the PC tumor microenvironment associated with poor clinical outcomes. In addition, the association between high intratumoral CXCL10 expression and poor prognosis coupled with the CXCL10-CXCR3-dependent chemoresistance lends credence to intratumoral CXCL10 as a prognostic marker.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank the National Cancer Institute (NCI 5T32 CA106493-09), the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK F31 DK104492-01A), the National Institute of Dental and Craniofacial Research (NIDCR T90 DE021990-02), the Cracchiolo Foundation and the Frederick A. Coller Surgical Society for their support in these investigations.
Abbreviations
- AF
Alexa Fluor®
- APC
Allophycocyanin
- CP
Chronic pancreatitis
- CXCL10
CXC chemokine ligand 10
- CXCR3
CXC chemokine receptor 3
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- EDTA
Ethylenediaminetetraacetic acid
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- HLA
Human leukocyte antigen
- IFNγ
Interferon-γ
- LD50
Median lethal dose
- NK
Natural killer
- OS
Overall survival
- PC
Pancreatic cancer
- PDL1
Programmed death ligand 1
- PE
Phycoerythrin
- PPCE
Primary pancreatic cancer epithelial cells
- R1
Positive resection margin
- SEM
Standard error of the mean
- STR
Short tandem repeat
- TAS
Tumor-associated stroma
- UF
University of Florida
Compliance with ethical standards
Conflict of interest
All authors declare no conflicts of interest.
Contributor Information
Shannon M. Wallet, Email: swallet@dental.ufl.edu
Steven J. Hughes, Email: steven.hughes@surgery.ufl.edu
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winograd R, Byrne KT, Evans RA, et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 8.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer—science driving clinical progress. Nat Rev Cancer. 2005;5:459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 9.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 10.Beatty GL, Winograd R, Evans RA, et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 13.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 16.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 17.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trevino JG, Summy JM, Gray MJ, Nilsson MB, Lesslie DP, Baker CH, Gallick GE. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005;65:7214–7222. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- 19.Trevino JG, Verma M, Singh S, et al. Selective disruption of rb-raf-1 kinase interaction inhibits pancreatic adenocarcinoma growth irrespective of gemcitabine sensitivity. Mol Cancer Ther. 2013;12:2722–2734. doi: 10.1158/1535-7163.MCT-12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delitto D, Pham K, Vlada AC, et al. Patient-derived xenograft models for pancreatic adenocarcinoma demonstrate retention of tumor morphology through incorporation of murine stromal elements. Am J Pathol. 2015;185:1297–1303. doi: 10.1016/j.ajpath.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/S0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 22.Meng H, Lee Y, Ba Z, Fleming JA, Furumoto EJ, Roberts RF, Kris-Etherton PM, Rogers CJ. In vitro production of IL-6 and IFN-gamma is influenced by dietary variables and predicts upper respiratory tract infection incidence and severity respectively in young adults. Front Immunol. 2015;6:94. doi: 10.3389/fimmu.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Rendon JL, Akhtar S, Choudhry MA. Activation of toll-like receptor 2 prevents suppression of T-cell interferon gamma production by modulating p38/extracellular signal-regulated kinase pathways following alcohol and burn injury. Mol Med. 2012;18:982–991. doi: 10.2119/molmed.2011.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhoef CM, Van Roon JA, Vianen ME, Glaudemans CA, Lafeber FP, Bijlsma JW. Lymphocyte stimulation by CD3-CD28 enables detection of low T cell interferon-gamma and interleukin-4 production in rheumatoid arthritis. Scand J Immunol. 1999;50:427–432. doi: 10.1046/j.1365-3083.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 25.Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trevino JG, Pillai S, Kunigal S, Singh S, Fulp WJ, Centeno BA, Chellappan SP. Nicotine induces inhibitor of differentiation-1 in a Src-dependent pathway promoting metastasis and chemoresistance in pancreatic adenocarcinoma. Neoplasia. 2012;14:1102–1114. doi: 10.1593/neo.121044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romagnani P, Annunziato F, Lasagni L, et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8–16. doi: 10.1016/j.ejphar.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 29.Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Lohr JM, Friess H. Pancreatic cancer microenvironment. Int J Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 30.Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, Wilson JS. Pancreatic cancer: the microenvironment needs attention too! Pancreatology. 2015;15:S32–S38. doi: 10.1016/j.pan.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Neesse A, Algul H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64:1476–1484. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 32.Lange F, Rateitschak K, Fitzner B, Pohland R, Wolkenhauer O, Jaster R. Studies on mechanisms of interferon-gamma action in pancreatic cancer using a data-driven and model-based approach. Mol Cancer. 2011;10:13. doi: 10.1186/1476-4598-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 34.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.CAN-03-3259. [DOI] [PubMed] [Google Scholar]
- 36.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20:3422–3433. doi: 10.1158/1078-0432.CCR-13-2972. [DOI] [PubMed] [Google Scholar]
- 38.Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, Ramakrishna S. Immune system: a double-edged sword in cancer. Inflamm Res. 2013;62:823–834. doi: 10.1007/s00011-013-0645-9. [DOI] [PubMed] [Google Scholar]
- 39.Lunardi S, Jamieson NB, Lim SY, et al. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget. 2014;5:11064–11080. doi: 10.18632/oncotarget.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi P, Gilkes DM, Wong CC, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, Witz IP, Ben-Baruch A. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007;67:3396–3405. doi: 10.1158/0008-5472.CAN-06-3087. [DOI] [PubMed] [Google Scholar]
- 42.Walser TC, Rifat S, Ma X, et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 43.Kawada K, Sonoshita M, Sakashita H, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 44.Schober M, Jesenofsky R, Faissner R, Weidenauer C, Hagmann W, Michl P, Heuchel RL, Haas SL, Lohr JM. Desmoplasia and chemoresistance in pancreatic cancer. Cancers (Basel) 2014;6:2137–2154. doi: 10.3390/cancers6042137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.