Abstract
Purpose
Metastatic castration-resistant prostate cancer primarily affects elderly men. In this post hoc analysis we investigated the safety and efficacy of abiraterone acetate in elderly (≥75 years) and younger (<75 years) patient subgroups at the prespecified interim analysis (55% of total overall survival [OS] events) for the COU-AA-302 trial.
Materials and Methods
Patients were stratified and randomized 1:1 to abiraterone acetate 1,000 mg plus prednisone/prednisolone 5 mg bid (abiraterone-prednisone) vs placebo plus prednisone/prednisolone 5 mg bid (prednisone alone). Co-primary end points were radiographic progression-free survival (rPFS) and OS. Median time to event and hazard ratio (HR) were estimated using Kaplan-Meier method and Cox model, respectively.
Results
Elderly patients (n=350) treated with abiraterone-prednisone had significant improvements in OS and rPFS vs prednisone alone (HR=0.71 [95% CI 0.53–0.96] and HR=0.63 [95% CI 0.48–0.83], respectively), similar to younger patients (n=738, HR=0.81 [95% CI 0.63–1.03] and HR=0.49 [95% CI 0.40–0.59], respectively). All secondary end points favored the abiraterone-prednisone arm for both age subgroups. Specific adverse events with abiraterone-prednisone were similar between age subgroups. Elderly patients in both treatment arms had higher rates of fluid retention and cardiac disorders than younger patients, although rates of dose reduction or treatment interruptions due to adverse events were low in both age subgroups.
Conclusions
Abiraterone acetate demonstrated clinical benefit and was well tolerated in both elderly and younger men with chemotherapy-naïve metastatic castration-resistant prostate cancer, thus supporting it as a treatment option for elderly patients who may not tolerate other therapies with greater toxicity.
Keywords: prostatic neoplasms, aged, abiraterone acetate, safety, treatment outcome
Introduction
Prostate cancer is a leading cause of cancer death in older men.1 Compared with younger patients (aged <75 years), elderly men are more likely to present with advanced disease.2 Analysis of the Surveillance, Epidemiology and End Results database showed that almost half (48%) of all metastatic prostate cancer cases and more than half of all prostate cancer deaths were in patients aged ≥75 years.3 Age alone should not prevent patients from deriving benefit from novel therapies,2 and treatment decisions should be based on the patient's health status, including consideration of the severity of comorbid conditions.4 Optimizing therapy for elderly patients who are more likely to suffer from other medical comorbidities, physical frailty and serious toxicities from certain kinds of treatment (eg, chemotherapeutics) remains a considerable challenge.2,4–6
Abiraterone acetate is converted in vivo to abiraterone, an androgen biosynthesis inhibitor, which targets 17 α-hydroxylase/C17,20-lyase. In patients with metastatic castration-resistant prostate cancer (mCRPC) who had received prior docetaxel chemotherapy, treatment with abiraterone acetate (hereafter, abiraterone) plus low-dose prednisone improved overall survival (OS) by 4.6 months (p <0.0001, hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.64–0.86) vs prednisone alone.7,8 In a recent post hoc analysis, Mulders et al.9 showed that in elderly (≥75 years) mCRPC patients who progressed after docetaxel chemotherapy, treatment with abiraterone-prednisone, vs prednisone alone, was well tolerated and led to improved OS (p = 0.0022, HR 0.64, 95% CI 0.478–0.853), time to prostate-specific antigen (PSA) progression (TTPP) (p = 0.1995, HR 0.76, 95% CI 0.503–1.155) and radiographic progression-free survival (rPFS) (p = 0.0019, HR 0.66, 95% CI 0.506–0.859).
Study COU-AA-302 compared the efficacy and safety of abiraterone plus low-dose prednisone vs prednisone alone in asymptomatic or mildly symptomatic men with chemotherapy-naïve mCRPC.10 Abiraterone-prednisone doubled time to rPFS vs prednisone alone (median 16.5 vs 8.3 months). All secondary end points significantly favored abiraterone-prednisone vs prednisone alone.10 Here we present results from a post hoc analysis to assess the efficacy and safety of abiraterone-prednisone vs prednisone alone in elderly (≥75 years) and younger (<75 years) patient subgroups at the prespecified interim analysis for study COU-AA-302.
Materials and Methods
Patients and Study Design
Study COU-AA-302 (ClinicalTrials.gov, NCT00887198) is a phase III, multinational, randomized, double-blind, placebo-controlled study conducted at 151 sites in 12 countries.10 Patients were enrolled from April 2009 to June 2010. Screening procedures to evaluate patient eligibility for the study were conducted within 14 days prior to cycle 1 day 1. Eligible patients were randomized and returned to the site for the cycle 1 day 1 visit and dosing. Randomization took place at all study sites using a centralized Interactive Web/Voice Response System. All study personnel were blinded to the patient treatment assignments. At the time of disease progression, patient treatment assignments remained blinded.
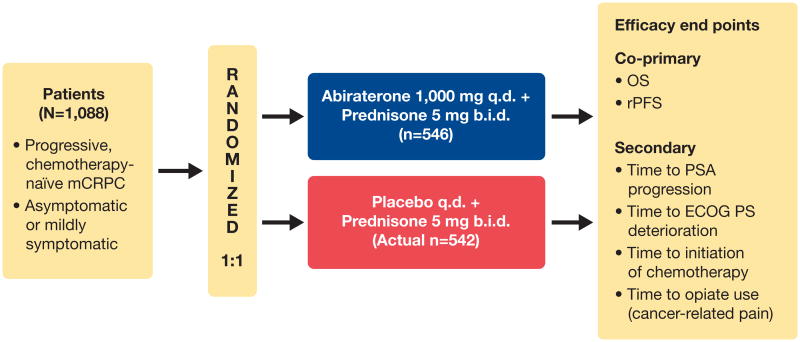
Patients were stratified by Eastern Cooperative Oncology Group performance status (ECOG PS) score (0 vs 1) and randomized 1:1 to abiraterone acetate 1 g daily plus prednisone or prednisolone 5 mg twice daily (hereafter, abiraterone-prednisone) vs placebo plus prednisone/prednisolone 5 mg twice daily (hereafter, prednisone alone) in continuous 28-day cycles (fig. 1 and supplementary fig. 1).10 Patients commenced treatment within 72 hours of randomization.
Figure 1.
Study COU-AA-302 design. OS was defined as time from randomization to death from any cause. rPFS was determined by independent radiologist unaware of study group assignments, and dates of death were confirmed. rPFS was defined as freedom from death from any cause; freedom from progression in soft tissue lesions as measured with computerized tomography or magnetic resonance imaging, defined as “progressive disease” according to modified Response Evaluation Criteria in Solid Tumors criteria; or progression on bone scan according to criteria adapted from Prostate Cancer Working Group 2.11 Changes in PSA level were not included in definition of rPFS. q.d., daily. b.i.d., twice daily.
The co-primary end points were rPFS and OS. The prespecified secondary efficacy end points were time to opiate use for cancer-related pain, time to initiation of cytotoxic chemotherapy, time to deterioration in ECOG PS and TTPP based on Prostate Cancer Working Group 2 criteria.11 Clinical assessments were conducted at prespecified visits and included medical history, vital sign measurements, body weight, physical examination, review of concomitant therapy and procedures, and review of adverse events (AEs) and serious AEs. AEs and serious AEs were graded and summarized according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
The primary and secondary end point results obtained at the time of this interim analysis have been described in detail previously.12 rPFS was determined by an independent radiologist who was unaware of study group assignments. The review boards at all participating institutions approved the study, and all patients gave written informed consent.
Key Eligibility Criteria
Study COU-AA-302 enrolled men with mCRPC, aged ≥18 years, who were medically or surgically castrated, had tumor progression and were asymptomatic or mildly symptomatic. Patients with visceral metastases or patients who had received previous therapy with ketoconazole for >7 days were excluded.
Statistical Analyses
All data for the present analyses were obtained from the prespecified interim analysis at 55% of the total death events. Patients were dichotomized by age at 75 years. This age cutoff was used in a post hoc analysis of study COU-AA-3019 in other CRPC drug trials13 and is the cutoff used in the FDA guideline to define a geriatric population in clinical trials.14 Distribution of the time-to-event end points was estimated using the Kaplan-Meier method. The Cox model was used to obtain the HR and its associated 95% CI. Stratified log-rank test was used in the treatment comparison, stratified by baseline ECOG score. Results were considered significant if p ≤0.05; no multiplicity adjustments were made for this post hoc analysis. The actual interim analysis was conducted at 56% OS events. All statistical analyses were performed using SAS® Version 9.2 (SAS Institute, Cary, North Carolina). Statistical test assumptions were verified with commonly used methods, mostly graphically. Differences between treatment arms within each age subgroup were assessed by the Wilcoxon rank test (continuous variables) and the Chi-square test (categorical variables). Missing values were uncommon and not imputed or used in the analysis. Median follow-up time for censored patients was 21.9 months for the study population based on descriptive statistics. Lost to follow-up expressed as the proportion of censored patients not evaluated during a specified time was low (about 1%).
Results
Patients
Elderly and younger patients were well balanced in each treatment arm by baseline disease characteristics (table 1). Most patients were aged <75 years (738 of 1088); there was a total of 350 elderly patients (≥75 years). A higher proportion of elderly patients (128/350, 37%) than younger patients (130/738, 18%) had an ECOG PS score of 1. Among patients with recorded Gleason scores at initial diagnosis, 44% (135/307) of elderly patients and 55% (382/689) of younger patients had scores ≥8, indicating that more younger patients than elderly patients had high-grade disease.
Table 1. Baseline demographic and clinical characteristics of elderly and younger patients (intent-to-treat population).
| Elderly (≥75 years) | Younger (<75 years) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Abiraterone-prednisone (n=185) | Prednisone alone (n=165) | P Value | Abiraterone-prednisone (n=361) | Prednisone alone (n=377) | P Value | |
|
| ||||||
| Age, years | 0.4299 | 0.5741 | ||||
| n | 185 | 165 | 361 | 377 | ||
| Median | 79 | 79 | 67 | 66 | ||
| Range | 75–95 | 75–90 | 44–74 | 44–74 | ||
|
| ||||||
| Race, n (%) | ||||||
|
| ||||||
| White | 180 (97.3) | 161 (97.6) | 1.0000 | 340 (94.2) | 349 (92.6) | 0.4653 |
|
| ||||||
| Black | 2 (1.1) | 0 | 13 (3.6) | 13 (3.5) | ||
|
| ||||||
| Asian | 1 (0.5) | 3 (1.8) | 3 (0.8) | 6 (1.6) | ||
|
| ||||||
| Other | 2 (1.1) | 0 | 4 (1.1) | 6 (1.6) | ||
|
| ||||||
| Missing | 0 | 1 (0.6) | 1 (0.3) | 1 (0.3) | ||
|
| ||||||
| Native | 0 | 0 | 0 | 2 (0.5) | ||
|
| ||||||
| Gleason score at initial diagnosis, n (%) | ||||||
|
| ||||||
| n | 158 | 149 | 330 | 359 | ||
|
| ||||||
| ≤7 | 85 (53.8) | 87 (58.4) | 0.4870 | 140 (42.4) | 167 (46.5) | 0.3157 |
|
| ||||||
| ≥8 | 73 (46.2) | 62 (41.6) | 190 (57.6) | 192 (53.5) | ||
|
| ||||||
| PSA at initial diagnosis, ng/ml | 0.2412 | 0.5737 | ||||
|
| ||||||
| n | 152 | 127 | 318 | 327 | ||
|
| ||||||
| Median | 19.1 | 17.4 | 23.8 | 22.3 | ||
|
| ||||||
| Range | 1.4–3273.0 | 0.7–6062.0 | 0.4–5036.0 | 0.3–9726.3 | ||
|
| ||||||
| Extent of disease, n (%) | ||||||
|
| ||||||
| n | 183 | 165 | 361 | 377 | ||
|
| ||||||
| Bone | 153 (83.6) | 129 (78.2) | 299 (82.8) | 303 (80.4) | ||
|
| ||||||
| Soft tissue or node | 85 (46.4) | 79 (47.9) | 182 (50.4) | 192 (50.9) | ||
|
| ||||||
| Bone, soft tissue or node | 183 (100.0) | 165 (100.0) | 361 (100.0) | 377 (100.0) | ||
|
| ||||||
| ECOG PS, n (%) | ||||||
|
| ||||||
| n | 185 | 165 | 361 | 377 | ||
|
| ||||||
| 0 | 115 (62.2) | 107 (64.8) | 0.6820 | 301 (83.4) | 307 (81.4) | 0.5502 |
|
| ||||||
| 1 | 70 (37.8) | 58 (35.2) | 60 (16.6) | 70 (18.6) | ||
|
| ||||||
| Baseline PSA (ng/mL) | 0.0995 | 0.8564 | ||||
|
| ||||||
| n | 185 | 163 | 361 | 376 | ||
|
| ||||||
| Median | 48.4 | 37.8 | 37.6 | 37.5 | ||
|
| ||||||
| Range | 1.8–3927.4 | 0.8–2730.4 | 0.0–1715.7 | 0.7–6606.4 | ||
|
| ||||||
| Baseline hemoglobin, g/dL | 0.3617 | 0.5669 | ||||
|
| ||||||
| n | 184 | 162 | 361 | 376 | ||
|
| ||||||
| Median | 12.7 | 12.9 | 13.3 | 13.2 | ||
|
| ||||||
| Range | 9.3–15.4 | 10.1–15.4 | 7.2–16.6 | 7.0–15.7 | ||
|
| ||||||
| Lactate dehydrogenase (IU/L) | 0.7345 | 0.5810 | ||||
|
| ||||||
| n | 183 | 162 | 360 | 374 | ||
|
| ||||||
| Median | 189.0 | 186.0 | 184.5 | 183.0 | ||
|
| ||||||
| Range | 97–413 | 87–554 | 60–871 | 108–781 | ||
Primary End Points
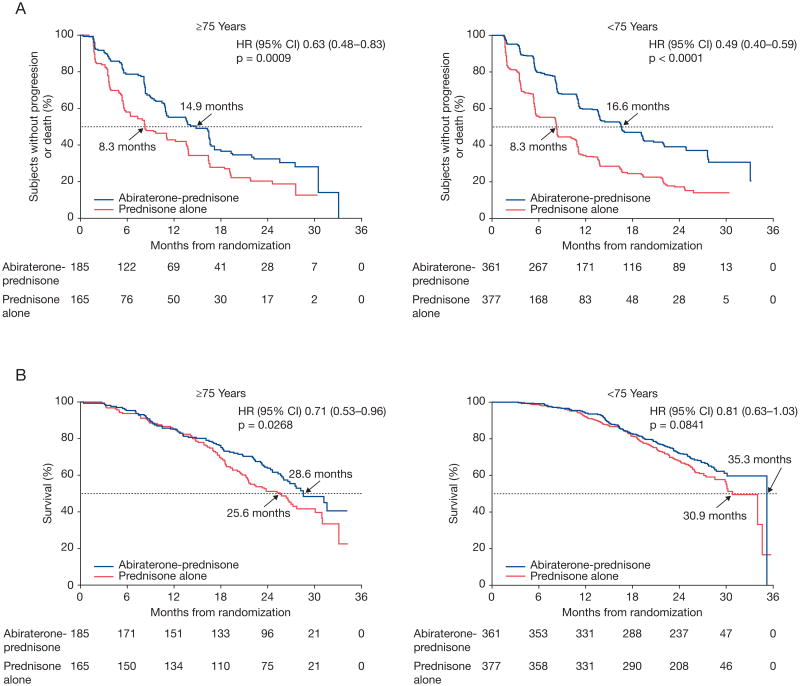
Elderly patients treated with abiraterone-prednisone had significant improvements in rPFS and OS vs those treated with prednisone alone, similar for younger patients (fig. 2). In elderly patients, rPFS was significantly longer in the abiraterone-prednisone arm vs the prednisone-alone arm (p = 0.0009, HR 0.63, 95% CI 0.48–0.83, median 14.9 vs 8.3 months). Younger patients taking abiraterone-prednisone also experienced significantly longer rPFS (p <0.0001, HR 0.49, 95% CI 0.40–0.59, median 16.6 vs 8.3 months) vs those who received prednisone alone. OS in elderly patients receiving abiraterone-prednisone was significantly longer than for those receiving prednisone alone (p = 0.0268, HR 0.71; 95% CI 0.53–0.96, median 28.6 vs 25.6 months). Among younger patients, abiraterone-prednisone treatment had a favorable effect on OS vs patients taking prednisone alone (p = 0.0841, HR 0.81, 95% CI 0.63–1.03, median 35.3 vs 30.9 months). Subsequent therapy with docetaxel was more common among patients taking prednisone alone vs abiraterone-prednisone and among younger vs elderly patients (supplementary table 1).
Figure 2.
Primary end points. A and C, rPFS by age group. B and D, OS by age group. A and B, 75 years old or older. C and D, younger than 75 years.
Secondary End Points
Secondary end point outcomes favored abiraterone-prednisone for both age subgroups (table 2). The TTPP in elderly patients receiving abiraterone-prednisone was significantly better than in those receiving prednisone alone (p = 0.0002, HR 0.60, 95% CI 0.46–0.79, median 8.6 vs 5.5 months). Younger patients taking abiraterone-prednisone also had significantly longer TTPP (p <0.0001, HR 0.46, 95% CI 0.38–0.55, median 11.1 vs 5.6 months) vs those who received prednisone alone. Both elderly and younger patients treated with abiraterone-prednisone had significant improvements in time to initiation of chemotherapy and time to opiate use for cancer-related pain vs those treated with prednisone alone (p value range <0.0001–0.0278, HRs <1.0). Time to ECOG deterioration was improved with abiraterone-prednisone vs prednisone alone in both elderly (p = 0.1078, HR 0.83, 95% CI 0.66–1.04, median 10.3 vs 8.6 months) and younger patients (p = 0.0111, HR 0.81, 95% CI 0.68–0.95, median 14.3 vs 11.2 months).
Table 2. Effect of treatment among elderly and younger subgroups (intent-to-treat population).
| Elderly (≥75 years) | Younger (<75 years) | |||
|---|---|---|---|---|
|
| ||||
| Abiraterone-prednisone(n=185) | Prednisone alone (n=165) | Abiraterone-prednisone (n=361) | Prednisone alone (n=377) | |
| TTPP,* months | 8.6 | 5.5 | 11.1 | 5.6 |
| HR (95% CI) | 0.602 (0.460–0.789) | 0.457 (0.382–0.546) | ||
| p value | 0.0002 | <0.0001 | ||
| Deterioration of ECOG PS score by ≥1 point,* months | 10.3 | 8.6 | 14.3 | 11.2 |
| HR (95% CI) | 0.828 (0.657–1.043) | 0.806 (0.682–0.952) | ||
| p value | 0.1078 | 0.0111 | ||
| Time to initiation of cytotoxic therapy,* months | NE | 25.4 | 23.8 | 15.0 |
| HR (95% CI) | 0.622 (0.43–0.872) | 0.601 (0.496–0.728) | ||
| p value | 0 | .0055 | <0.00 | 01 |
| Time to opiate use,* months | NE | 30.3 | NE | 22.3 |
| HR (95% CI) | 0.678 (0.478–0.961) | 0.720 (0.581–0.892) | ||
| p value | 0.0278 | 0.0026 | ||
Median time to event.
NE = not estimable.
Safety
The median duration of exposure was 11.8 and 8.5 months among elderly patients and 14.4 and 8.2 months among younger patients in the abiraterone-prednisone and prednisone-alone groups, respectively. Most patients in both age subgroups tolerated study treatment well, with ≤6% of patients having dose reductions across treatment arms. Two elderly patients and 3 younger patients taking abiraterone-prednisone had dose reductions due to AEs, and 1 patient (younger) in the prednisone-alone arm had a dose reduction due to AEs. More elderly patients experienced an abiraterone-prednisone dose interruption than younger patients, although treatment interruptions were uncommon in both age groups. More than 1 dose interruption was reported by 12% (22/184) of patients in the abiraterone-prednisone arm vs 4% (7/164) in the prednisone-alone arm for elderly patients and 4% (14/360) vs 3% (10/376), respectively, for younger patients.
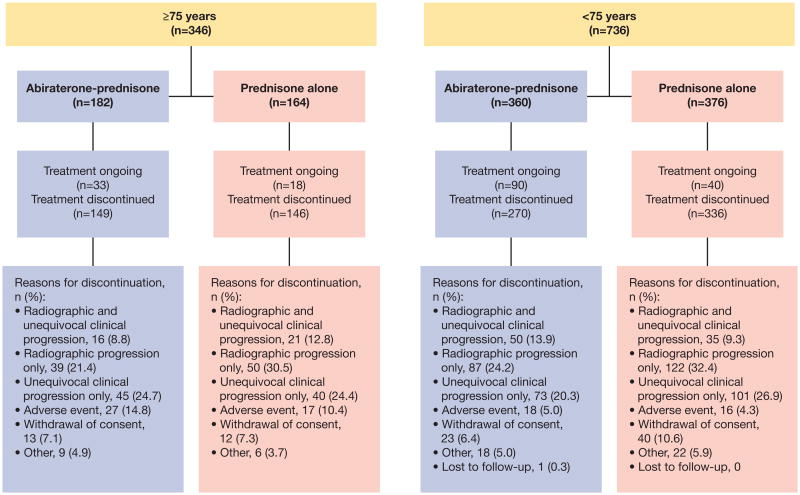
In both elderly and younger subgroups, fewer patients receiving abiraterone-prednisone discontinued treatment due to disease progression vs patients receiving prednisone alone (fig. 3). Discontinuations due to AEs in patients receiving abiraterone-prednisone vs prednisone alone were 15% (27/182) vs 10% (17/164) among elderly patients and 5% (18/360) vs 4% (16/376) among younger patients, respectively. Among elderly patients, the incidence of withdrawal from the study was low (7% [25/346]) in both treatment arms.
Figure 3. Patient disposition by age group.
The most common AE for the abiraterone-prednisone arm vs the prednisone-alone arm was fatigue in both elderly (42% [76/182] vs 38% [62/164], respectively) and younger (39% [139/360] vs 33% [125/376], respectively) patients (table 3). The incidence of grade 3/4 fatigue was higher in elderly (6% [11/182] abiraterone-prednisone vs 4% [7/164] prednisone alone) vs younger patients (<1% [2/360] abiraterone-prednisone vs <1% [3/376] prednisone alone). AEs of special interest related to the known effect of abiraterone-prednisone on mineralocorticoid excess (fluid retention/edema, hypokalemia and hypertension) were reported more frequently with abiraterone-prednisone vs prednisone alone. The incidence of AEs of special interest was higher with abiraterone-prednisone treatment in both elderly and younger patients (74% [134/182] abiraterone-prednisone vs 59% [97/164] prednisone alone, and 66% [237/360] abiraterone-prednisone vs 48% [180/376] prednisone alone, respectively). Elderly patients had a higher incidence of peripheral edema (35% [63/182] abiraterone-prednisone vs 32% [52/164] prednisone alone), although the difference between treatment arms was greater in younger patients (22% [78/360] vs 16% [61/376], respectively). The incidence of hypokalemia was higher with abiraterone-prednisone treatment in both elderly and younger patients (17% [30/182] abiraterone-prednisone vs 11% [17/164] prednisone alone, and 18% [63/360] abiraterone-prednisone vs 14% [52/376] prednisone alone, respectively). Hepatotoxicity and cardiac disorders were the most frequent grade 3/4 AEs of special interest. The incidence of grade 3/4 hepatotoxicity was higher with abiraterone-prednisone treatment in both elderly and younger patients (8% [15/182] abiraterone-prednisone vs 4% [6/164] prednisone alone, and 8% [28/360] abiraterone-prednisone vs 2% [9/376] prednisone alone, respectively). In addition, the incidence of grade 3/4 cardiac disorders was higher with abiraterone-prednisone treatment in both elderly and younger patients (9% [16/182] abiraterone-prednisone vs 5% [8/164] prednisone alone, and 6% [20/360] abiraterone-prednisone vs 3% [11/376] prednisone alone, respectively). Overall, hepatotoxicity and cardiac disorders were infrequent and rarely led to treatment discontinuation. Grade 3/4 hypertension and hypokalemia were infrequent and were medically managed. The incidence of treatment-emergent AEs leading to death in the abiraterone-prednisone and prednisone alone treatment groups was 8% (14/182) and 6% (9/164), respectively, in elderly subgroups and 2% each (7/360 and 7/376, respectively) in the younger subgroups. Grade 3/4 AEs were reported in 40% (54/135), 48% (109/225) and 57% (104/182) of patients aged <65, 65–74, and ≥75 years in the abiraterone-prednisone arm, respectively, and in 36% (55/154), 40% (88/222) and 56% (92/164) of patients in the prednisone-alone arm, respectively (supplementary table 2).
Table 3. Adverse events (grades 1 to 4 or grades 3 and 4 [>15% in any subgroup]) reported during treatment (safety population).
| Elderly (≥75 years) | Younger (<75 years) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Abiraterone-prednisone (n=182) | Prednisone alone (n=164) | Abiraterone-prednisone (n=360) | Prednisone alone (n=376) | |||||
|
| ||||||||
| Grades 1–4 | Grades 3/4 | Grades 1–4 | Grades 3/4 | Grades 1–4 | Grades 3/4 | Grades 1–4 | Grades 3/4 | |
| Subjects with TEAE, n (%) | ||||||||
| General AEs | 182 (100.0) | 104 (57.1) | 163 (99.4) | 92 (56.1) | 356 (98.9) | 163 (45.3) | 361 (96.0) | 143 (38.0) |
| Fatigue | 76 (41.8) | 11 (6.0) | 62 (37.8) | 7 (4.3) | 139 (38.6) | 2 (<1) | 125 (33.2) | 3 (<1) |
| Peripheral edema | 63 (34.6) | 2 (1.1) | 52 (31.7) | 3 (1.8) | 78 (21.7) | 0 | 61 (16.2) | 2 (<1) |
| Back pain | 52 (28.6) | 7 (3.8) | 56 (34.1) | 5 (3.0) | 128 (35.6) | 8 (2.2) | 123 (32.7) | 16 (4.3) |
| Arthralgia | 49 (26.9) | 4 (2.2) | 42 (25.6) | 4 (1.8) | 110 (30.6) | 6 (1.7) | 90 (23.9) | 6 (1.6) |
| Nausea | 54 (29.7) | 2 (1.1) | 36 (22.0) | 0 | 76 (21.1) | 3 (<1) | 88 (23.4) | 1 (<1) |
| Constipation | 51 (28.0) | 1 (<1) | 42 (25.6) | 1 (<1) | 77 (21.4) | 1 (<1) | 68 (18.1) | 2 (<1) |
| Hot flush | 33 (18.1) | 0 | 25 (15.2) | 0 | 90 (25.0) | 1 (<1) | 74 (19.7) | 0 |
| Diarrhea | 42 (23.1) | 2 (1.1) | 33 (20.1) | 0 | 85 (23.6) | 4 (1.1) | 65 (17.3) | 5 (1.3) |
| Bone pain | 35 (19.2) | 2 (1.1) | 33 (20.1) | 4 (1.8) | 78 (21.7) | 5 (1.4) | 70 (18.6) | 7 (1.9) |
| Pain in extremity | 28 (15.4) | 0 | 35 (21.3) | 2 (1.2) | 65 (18.1) | 4 (1.1) | 52 (13.8) | 3 (<1) |
| Muscle spasms | 24 (13.2) | 0 | 32 (19.5) | 0 | 53 (14.7) | 0 | 79 (21.0) | 1 (<1) |
| Contusion | 35 (19.2) | 0 | 23 (14.0) | 0 | 39 (10.8) | 0 | 27 (7.2) | 0 |
| Dizziness | 35 (19.2) | 3 (1.6) | 29 (17.7) | 1 (<1) | 37 (10.3) | 2 (<1) | 45 (12.0) | 0 |
| Cough | 33 (18.1) | 0 | 21 (12.8) | 0 | 65 (18.1) | 0 | 53 (14.1) | 1 (<1) |
| Vomiting | 32 (18.1) | 1 (<1) | 17 (10.4) | 0 | 45 (12.5) | 3 (<1) | 44 (11.7) | 0 |
| Dyspnea | 33 (18.1) | 8 (4.4) | 19 (11.6) | 2 (1) | 35 (9.7) | 6 (1.7) | 36 (9.6) | 3 (<1) |
| Asthenia | 24 (13.2) | 1 (<1) | 27 (16.5) | 5 (3) | 23 (6.4) | 0 | 20 (5.3) | 2 (<1) |
| Musculoskeletal pain | 28 (15.4) | 2 (1.1) | 23 (14.0) | 2 (1) | 60 (16.7) | 5 (1.4) | 58 (15.4) | 4 (1.1) |
| Headache | 16 (8.8) | 1 (<1) | 16 (9.8) | 0 | 58 (16.1) | 1 (<1) | 50 (13.3) | 1 (<1) |
| Insomnia | 23 (12.6) | 1 (<1) | 18 (11.0) | 0 | 56 (15.6) | 0 | 44 (11.7) | 0 |
| Total TEAEs of special interest, n (%) | 134 (73.6) | 44 (24.2) | 97 (59.1) | 28 (17) | 237 (65.8) | 64 (17.8) | 180 (47.9) | 35 (9.3) |
| Fluid retention/edema | 69 (37.9) | 2 (1.1) | 57 (34.8) | 4 (2) | 89 (24.7) | 3 (<1) | 73 (19.4) | 5 (1.3) |
| Hypokalemia | 30 (16.5) | 8 (4.4) | 17 (10.4) | 5 (3) | 63 (17.5) | 6 (1.7) | 52 (13.8) | 5 (1.3) |
| Hypertension | 40 (22.0) | 8 (4.4) | 28 (17.1) | 9 (6) | 78 (21.7) | 15 (4.2) | 45 (12.0) | 8 (2.1) |
| Hepatotoxicity | 38 (20.9) | 15 (8.2) | 24 (14.6) | 6 (4) | 64 (17.8) | 28 (7.8) | 37 (9.8) | 9 (2.4) |
| Cardiac disorders | 49 (27.0) | 16 (8.8) | 43 (26.2) | 8 (5) | 64 (17.8) | 20 (5.6) | 52 (13.8) | 11 (2.9) |
TEAE = treatment-emergent adverse event.
Discussion
This post hoc analysis evaluated the efficacy and safety of abiraterone-prednisone among elderly and younger patents with mCRPC and an ECOG PS score of 0 or 1. Abiraterone-prednisone appears to be safe and well tolerated among elderly patients; consistent results were shown with analysis of safety data based on additional age subgroups. The clinical benefit of abiraterone-prednisone vs prednisone alone in elderly patients was similar to that in younger patients. Moreover, patients treated with abiraterone-prednisone had improved OS despite a higher proportion of patients in the prednisone arm having received docetaxel. Despite a median treatment exposure of more than 11 months in the abiraterone-prednisone arm and 8 or more months in the prednisone-alone arm, patients were adherent to treatment. In the abiraterone-prednisone treatment arm, the incidence of withdrawal of consent was 6.4% (23/360) and 7.1% (13/182) among younger and elderly patients, respectively. The incidence of withdrawal of consent in the prednisone alone treatment arm was 10.6% (40/376) and 7.3% (12/164) among younger and elderly patients, respectively.
There is a need to better understand age-related changes that can affect the risk of toxicities and overall benefit of cancer treatment in elderly patients.5,6,15 More recently, elderly patients have been well represented in clinical trials of novel agents for advanced prostate cancer. In a retrospective study of patients aged ≥75 years with CRPC, PSA response rates were not significantly different between the standard and adapted docetaxel treatment regimen groups.16 In 2 placebo-controlled phase III clinical trials, enzalutamide prolonged survival of men aged ≥65 years with mCRPC after chemotherapy17 and abiraterone improved OS, TTPP, rPFS and PSA response rate among patients aged ≥75 years with mCRPC after chemotherapy vs patients treated with prednisone alone.9
The improved survival outcome and secondary end points shown in our analysis were consistent with results of the overall population in study COU-AA-302,10 including improved outcomes in the abiraterone-prednisone treatment arm vs prednisone alone in all secondary end points measured for either age subgroup.9 These findings corroborate the outcomes with abiraterone treatment among elderly patients from study COU-AA-301.9 Our findings are similar to those of COU-AA-301, although patients in study COU-AA-302 were chemotherapy-naïve and had longer treatment exposure.
A limitation of our analysis is that the treatment effect was evaluated retrospectively in subgroups. Despite this limitation, we confirmed that in elderly and younger patients with mCRPC, abiraterone significantly improved rPFS, significantly delayed clinical initiation of chemotherapy and use of opiates for cancer-related pain and improved all other measures of efficacy end points, consistent with the overall patient population.10 Thus, abiraterone represents a treatment option for elderly patients who may not tolerate other therapies with greater toxicity.
Conclusions
This post hoc analysis of study COU-AA-302 demonstrates that the clinical benefit of abiraterone was preserved in the elderly. These results confirm those from a similar analysis of younger and elderly patients treated with abiraterone post-docetaxel in study COU-AA-301.9 Taken together, these observations demonstrate that the efficacy and safety of abiraterone are similar for elderly and younger patients with mCRPC and a favorable ECOG PS, and support the use of abiraterone therapy in elderly men with mCRPC regardless of prior chemotherapy.
Supplementary Material
Supplementary Table 1. Subsequent therapy with docetaxel among elderly and younger subgroups (intent-to-treat population)
Supplementary Table 2. Adverse events reported during treatment, by 3 age categories (safety population)
Acknowledgments
Writing assistance was provided by Ann P. Tighe, PhD, of PAREXEL, and was funded by Janssen Global Services, LLC.
Funding: This work was supported by Janssen Research & Development (formerly Ortho Biotech Oncology Research & Development, unit of Cougar Biotechnology [no grant number]).
Abbreviations
- mCRPC
metastatic castration-resistant prostate cancer
- OS
overall survival
- HR
hazard ratio
- CI
confidence interval
- PSA
prostate-specific antigen
- TTPP
time to prostate-specific antigen progression
- rPFS
radiographic progression-free survival
- ECOG PS
Eastern Cooperative Oncology Group performance status
- AE
adverse event
- NE
not estimable
- TEAE
treatment-emergent adverse event
Footnotes
ClinicalTrials.gov: NCT00887198
Disclosure: Matthew R. Smith, Charles J. Ryan and Joan Carles have served as consultants/advisors to and received honoraria from Janssen.
Dana E. Rathkopf has served as a consultant/advisor to and received research funding from Janssen.
Jinhui Li, Thian Kheoh, Thomas W. Griffin and Arturo Molina are employees of Janssen Research & Development and hold stock in Johnson & Johnson.
Peter F. A. Mulders has served as an advisor to and speaker for Janssen. Hendrik Van Poppel has no relevant disclosures.
Presented in part at The European Multidisciplinary Cancer Congress. 27 September – 1 October 2013, Amsterdam, The Netherlands. Abstract 2932.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
Contributor Information
Matthew R. Smith, Department of Genitourinary Oncology, Massachusetts General Hospital Cancer Center, Boston, Massachusetts.
Dana E. Rathkopf, Department of Oncology and Internal Medicine, Memorial Sloan Kettering Cancer Center, New York, New York
Peter. F.A. Mulders, Department of Urology, Radboud University Medical Centre, Nijmegen, The Netherlands
Joan Carles, Department of Oncology, Vall d'Hebron University Hospital, Barcelona, Spain.
Hendrik Van Poppel, Department of Urology, University Hospitals of KU Leuven, Leuven, Belgium.
Jinhui Li, Department of Biostatistics Oncology, Janssen Research & Development, Raritan, New Jersey.
Thian Kheoh, Department of Biostatistics & Programming, Janssen Research & Development, San Diego.
Thomas W. Griffin, Department of WC Clinical Oncology
Arturo Molina, Department of Oncology, Janssen Research & Development, Los Angeles.
Charles J. Ryan, Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, California
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Mukherji D, Pezaro CJ, Shamseddine A, et al. New treatment developments applied to elderly patients with advanced prostate cancer. Cancer Treat Rev. 2013;39:578. doi: 10.1016/j.ctrv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Scosyrev E, Messing EM, Mohile S, et al. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118:3062. doi: 10.1002/cncr.26392. [DOI] [PubMed] [Google Scholar]
- 4.Droz JP, Aapro M, Balducci L, et al. Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol. 2014;15:e404–e414. doi: 10.1016/S1470-2045(14)70018-X. [DOI] [PubMed] [Google Scholar]
- 5.Fung C, Dale W, Mohile SG. Prostate cancer in the elderly patient. J Clin Oncol. 2014;32:2523. doi: 10.1200/JCO.2014.55.1531. [DOI] [PubMed] [Google Scholar]
- 6.Shelke AR, Mohile SG. Treating prostate cancer in elderly men: how does aging affect the outcome? Curr Treat Options Oncol. 2011;12:263. doi: 10.1007/s11864-011-0160-6. [DOI] [PubMed] [Google Scholar]
- 7.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 9.Mulders PF, Molina A, Marberger M, et al. Efficacy and safety of abiraterone acetate in an elderly patient subgroup (aged 75 and older) with metastatic castration-resistant Prostate cancer after docetaxel-based chemotherapy. Eur Urol. 2014;65:875. doi: 10.1016/j.eururo.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry: E7 Studies in Support of Special Populations: Geriatrics Questions and Answers. U.S. Food and Drug Administration Web site; [Accessed April 2, 2014]. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM189544.pdf. [Google Scholar]
- 15.Halabi S, Vogelzang NJ, Ou SS, et al. Clinical outcomes by age in men with hormone refractory prostate cancer: a pooled analysis of 8 Cancer and Leukemia Group B (CALGB) studies. J Urol. 2006;176:81. doi: 10.1016/S0022-5347(06)00566-0. [DOI] [PubMed] [Google Scholar]
- 16.Italiano A, Ortholan C, Oudard S, et al. Docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-resistant prostate cancer. Eur Urol. 2009;55:1368. doi: 10.1016/j.eururo.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Subsequent therapy with docetaxel among elderly and younger subgroups (intent-to-treat population)
Supplementary Table 2. Adverse events reported during treatment, by 3 age categories (safety population)