Abstract
Background
NF1 is a tumor suppressor that negatively regulates Ras signaling. NF1 mutations occur in lung cancer, but their clinical significance is unknown. We evaluated clinical and molecular characteristics of NF1 mutant lung cancers with comparison to tumors with KRAS mutations.
Methods
Between July 2013 and October 2014, 591 NSCLC tumors underwent targeted next generation sequencing in a 275 gene panel that evaluates gene mutations and genomic rearrangements. NF1 and KRAS cohorts were identified, with subsequent clinical and genomic analysis.
Results
Among 591 pts, 60 had NF1 mutations (10%) and 141 (24%) had KRAS mutations. 15 NF1 mutations (25%) occurred with other oncogenic mutations (BRAF (2); ERBB2 (2); KRAS (9); HRAS (1); NRAS (1)). There were 72 unique NF1 variants. NF1 tumor pathology was diverse, including both adenocarcinoma (36, 60%) and squamous cell carcinoma (10, 17%). In contrast, KRAS mutations occurred predominantly in adenocarcinoma (136, 96%). Both mutations were common in former/current smokers. Among NF1 tumors without concurrent oncogenic alterations, TP53 mutations/2-copy deletions occurred more often (33, 65%) than with KRAS mutation (46, 35%) (p<.001). No difference between cohorts was seen with other tumor suppressors.
Conclusions
NF1 mutations define a unique population of NSCLC. NF1 and KRAS mutations present in similar patient populations, but NF1 mutations occur more often with other oncogenic alterations and TP53 mutations. Therapeutic strategies targeting KRAS activation, including inhibitors of MAP kinase signaling, may warrant investigation in NF1 mutant tumors. Tumor suppressor inactivation patterns may help further define novel treatment strategies.
Keywords: NF1, KRAS, non-small cell lung cancer, next-generation sequencing, targeted therapies
Introduction
NF1 is a tumor suppressor gene located on chromosome 17 encoding a protein known as neurofibromin. Neurofibromin negatively regulates Ras activity and has been well characterized in the clinical context of neurofibromatosis type 1, an autosomal dominant genetic disorder with an estimated incidence of 1 in 3,000 individuals.(1-3) Classically, neurofibromatosis patients may develop neurofibromas, café-au-lait macules, optic gliomas, and iris hamartomas, although the disorder can also be associated with other cardiovascular, musculoskeletal, and nervous system abnormalities.(4) NF1 was first cloned in 1990 and is one of the largest genes in the human genome, with 60 exons representing a total of 350 kb of genomic DNA.(5, 6) Functionally, several Nf1 mouse models have demonstrated that a loss of normal neurofibromin function causes a predisposition to tumor development in mice that is very similar to what is seen in human patients with neurofibromatosis.(7, 8)
A critical region in the neurofibromin protein is encoded by exons 20-27a and represents a ras-guanosine-triphosphate (GTP)-ase activation protein (GAP)-related domain (GRD). This GRD region of neurofibromin can bind to Ras proteins and downregulate their activity through the conversion of the active Ras-GTP complex to inactive Ras-GDP. Negative regulation of Ras subsequently prevents the downstream activation of Ras effector pathways, specifically the mitogen-activated protein kinase (MAPK) and PI3K/Akt/mTOR pathways that drive the pro-proliferation, survival and differentiation effects associated with Ras activation.(3, 8, 9) Indeed, the tumor suppressor function of neurofibromin has historically been attributed primarily to its ability to negatively regulate Ras function, although recent reports have also begun to expand our understanding of the cellular role of neurofibromin. Neurofibromin has been shown to have pro-apoptotic effects that are both Ras-dependent and Ras-independent.(10) Neurofibromin helps regulate cell adhesion, migration, and survival,(11) while analysis of neurofibromas from patients with neurofibromatosis suggests that loss of neurofibromin may contribute to the epithelial-mesenchymal transition (EMT) and activation of the heat shock response.(12) Together, this data suggests a complex cellular role for neurofibromin as a tumor suppressor that can function through modulation of Ras activity and influence many other intracellular signaling events.
Notably, patients with neurofibromatosis are known to have up to a four-fold increased risk of malignancy throughout their lifetime when compared to the general population.(4) These malignancies do not typically include lung cancer, but a number of relatively rare tumors are prevalent in this cohort, including malignant peripheral nerve sheath tumor (MPNST), optic pathway glioma (OPG), and juvenile myelomonocytic leukemia (JMML). However, it was not until the publication of The Cancer Genome Atlas (TCGA) data that the frequency of NF1 mutations in human tumors, including lung cancers, became more apparent. The clinical and molecular features of NSCLC tumors containing an NF1 mutation remain unknown, but TCGA data from lung cancers demonstrates that NF1 mutations are frequently seen in both adenocarcinoma (8.3%) and squamous cell carcinoma (12%).(13, 14)
The link between neurofibromin and Ras signaling suggests that NF1 mutations may be of great clinical significance in lung cancer. KRAS is the single most frequently mutated oncogene found in NSCLC and can be seen in tumors from both smokers and never-smokers. Although identification of activating mutations or rearrangements in targetable oncogenes such as the epidermal growth factor receptor (EGFR)(15, 16) or the anaplastic lymphoma kinase (ALK)(17) have led to targeted therapies with dramatic clinical implications, similar efforts to target KRAS mutations remain a challenge. As a pleiotropic GTPase, KRAS is upstream of several critical intracellular signaling pathways,(18) thus one strategy to target Ras signaling in lung cancer has been selective inhibition of downstream pathways. Recent efforts have demonstrated that inhibition of MAP kinase signaling may be effective in KRAS-mutant NSCLC.(19-21) A recent randomized phase II trial demonstrated a clinically meaningful improvement in progression-free survival in patients with KRAS mutations who received docetaxel plus the MEK inhibitor selumetinib, compared to docetaxel alone.(22) A randomized phase III clinical trial further evaluating this approach is ongoing (ClinicalTrials.gov: NCT01933932). Importantly, tumors harboring a mutation in NF1 may also be sensitive to this strategy of downstream inhibition because of the negative regulation of Ras activity associated with functional neurofibromin.
In the current study, we evaluate the clinical and molecular features of a cohort of NSCLC characterized by NF1 mutation and compare the results to a cohort of tumors characterized by KRAS mutation. Our findings begin to identify a novel cohort of NSCLC defined by NF1 mutation and suggest that ongoing therapeutic targeting strategies for KRAS tumors may also have efficacy in this population.
Methods
Next generation sequencing and identification of patient cohorts
Between July 2013 and October 2014, 4267 patients underwent targeted next generation sequencing (NGS) at the Dana-Farber Cancer Institute/Brigham and Women's Hospital under an IRB-approved research protocol. All patients provided written informed consent prior to sequencing of their tumors. Among these population, 591 patients with non-small cell lung cancer (NSCLC) were identified using the Oncology Data Retrieval System (OncDRS), an internal system developed at the Dana-Farber Cancer Institute to integrate clinical and genomic data. No germline sequencing was performed in this cohort.
Sequencing was performed on tumor DNA extracted from fresh, frozen, or formalin-fixed paraffin-embedded samples and evaluated for single nucleotide variants, copy number variations, and structural variants (including rearrangements).(23) The initial gene panel surveyed all exons of the 275 genes in the panel, along with 91 introns across 30 genes for rearrangement detection. The full list of genes is available as supplemental data (Supplemental Figure 1). DNA was isolated from tissue containing at least 20% tumor nuclei and analyzed by massively parallel sequencing using a solution-phase Agilent SureSelect hybrid capture kit and an Illumina HiSeq 2500 sequencer. Data was analyzed by an internally-developed bioinformatics pipeline composed of reconfigured publically-available tools and internally-developed algorithms. Single nucleotide variants (SNVs) were called using MuTect(24) and indels using Indelocator (http://www.broadinstitute.org/cancer/cga/indelocator). Annotation was performed using Oncotator.(25) Tumor tissues were tested without a paired normal from individual patients, thus additional informatics steps were taken to identify common single nucleotide polymorphisms (SNPS): any SNP present at >0.1% in Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), Seattle, WA (URL: http://evs.gs.washington.edu/EVS/ accessed May 30, 2013) or present in dbSNP was filtered. Variants also present in COSMIC were rescued for manual review. VisCap Cancer calls copy number changes based on log2 ratios that are calculated using a normalized depth of coverage against a median from a panel of normal (non-cancer) samples. Samples with a mean target coverage of <50× were failed and excluded from further analysis. Individual variants present at <10% allele fraction or in regions with <50× coverage were flagged for manual review and interpreted by the reviewing laboratory scientists and molecular pathologists based on overall tumor percentage, read depth, complexity of alteration, and evidence for associated copy number alterations.
Genetic mutation analysis
Identified mutations in NF1 were analyzed using the Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) and Human Splicing Finder (http://www.umd.be/HSF/) programs to evaluate whether a given mutation was predicted to have a benign or damaging effect on the NF1 protein. The introduction of a stop codon or predicted loss of function was also determined. The NF1 mutations identified in our cohort were cross-referenced with reported NF1 variants in dbSNP (http://www.ncbi.nlm.nih.gov/SNP/), COSMIC (http://cancer.sanger.ac.uk/cosmic), ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/), ExAC (http://exac.broadinstitute.org), Exome Variant Server (http://evs.gs.washington.edu), and Pubmed (http://www.ncbi.nlm.nih.gov/pubmed). An institutional instance of cBioPortal was used to visualize the data across specimens.(26)
Clinical characteristics
Following identification of patients with NF1 and KRAS mutations, clinical and molecular characteristics were determined. Patient age, gender, date of diagnosis, stage at diagnosis, location of metastatic lesions at diagnosis, smoking status at diagnosis, and date of death were determined from a review of the medical record and the social security death index. NGS data from each patient cohort was then reviewed to identify concurrent alterations in other known oncogenic drivers with relevance in lung cancer (EGFR, ALK, ROS1, NRAS, HRAS, BRAF, ERBB2). Sequencing data was also reviewed to identify mutation or two-copy deletion in commonly altered tumor suppressors including TP53, LKB1, PTEN, RB1, CDKN2A, and CDKN2B.
Statistical analysis
Overall survival is defined as the time in months from the date of diagnosis of metastatic disease to the date of death from any cause; patients alive at the time of analysis have been censored at their last known follow-up date. The Kaplan-Meier method was used to estimate event-time distributions. Fisher's exact test was used to compare frequency of gene mutations in tumor suppressors and selected clinical variables between the NF1 and the KRAS cohorts. The Mann-Whitney test was used to compare the age distribution between the NF1 and the KRAS cohorts.
Results
NF1 mutations are heterogeneous and widely distributed throughout the NF1 gene
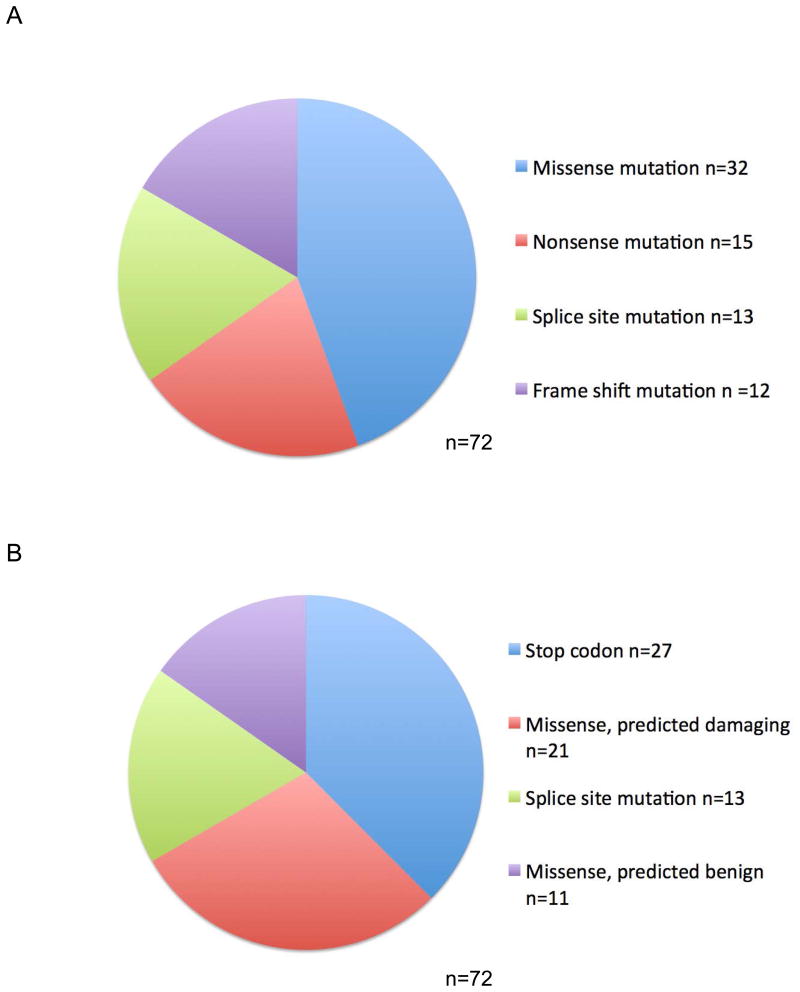
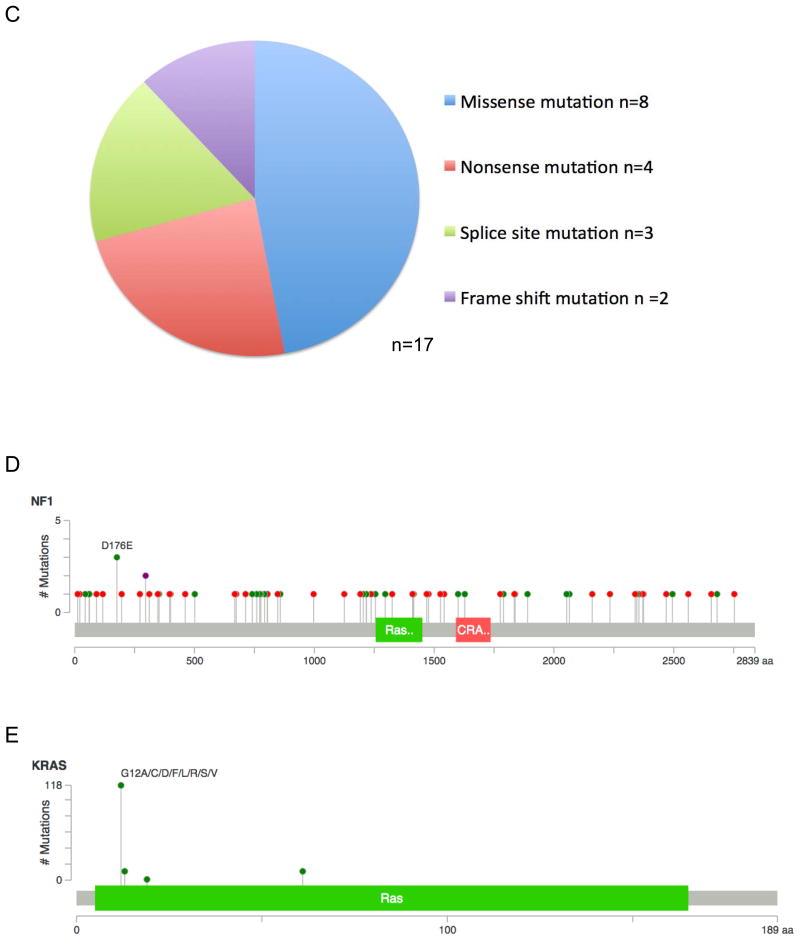
72 unique NF1 nucleotide variants were identified in our cohort of 60 distinct tumors. The majority of these variants have not been previously reported or functionally characterized (Supplemental Table 1). Variants in NF1 included missense mutations (n=32), nonsense mutations (n=15), splice site mutations (n=13), and frameshift mutations (n=12) (Figure 1A). Polyphen-2 or Human Splicing Finder were used to predict the functional effect of identified NF1 variants. All splice site mutations result in a broken acceptor site with predicted loss of function of the NF1 protein. Of the 59 missense, nonsense, and frameshift variants identified by NGS in our cohort, 11 variants were predicted to be benign and 48 were predicted to have a damaging effect. Notably, 27 of the 59 variants result in the generation of a stop codon with predicted loss of function of the NF1 protein (Figure 1B). Nine tumors had more than one NF1 variant, with 7 tumors having 2 variants and 2 tumors having 3 variants (Supplemental Table 2). The NF1 mutations found concurrently with other driver mutations (n=15 tumors, 17 NF1 variants) were noted to be representative of the spectrum of NF1 variants found in the larger cohort, with a similar proportion of missense mutations, nonsense mutations, splice site mutations, and frameshift mutations (Figure 1C). The distribution of all identified NF1 mutations in our cohort is represented in Figure 1D. Mutations are spread throughout all exons of the NF1 gene. Similarly, 13 KRAS mutations were identified in our cohort of 141 distinct tumors (Supplemental Table 3). No tumors had more than one KRAS mutation, and the majority of mutations identified represent known activating mutations in exons 1 or 2 (Figure 1E).
Figure 1. Characterization of identified NF1 mutations reveals a heterogeneous population with distribution throughout the gene.
A. Pie chart demonstrating mutation classification of all 72 identified NF1 variants in our cohort. B. Pie chart demonstrating functional classification of all 72 identified NF1 variants with functional prediction by Polyphen-2 analysis. C. Pie chart demonstrating mutation classification of 17 NF1 variants indentified in 15 tumors with concurrent NF1 variants and additional oncogenic alterations (n=17 variants). D. Lollipop plot demonstrating distribution of all identified NF1 variants in our cohort. Green circles represent missense mutations; red circles represent known truncating mutations (nonsense, frameshift, splice site); purple circles indicated residues affected by multiple mutation types. Indicated in green is the RasGAP domain and indicated in red is the CRAL-TRIO domain which functions as a lipid binding pocket. E. Lollipop plot demonstrating distribution of all identified KRAS mutations in our cohort. Green circles represent missense mutations; red circles represent known truncating mutations (nonsense, frameshift, splice site); purple circles indicated residues affected by multiple mutation types.
NF1 mutations do exist with concurrent oncogenic alterations but the majority of NF1 mutations in NSCLC occur independently
45 of 60 tumors (75%) with an NF1 mutation did not have any concurrent oncogenic alterations. Among the 15 NF1 tumors with a concurrent oncogenic alteration, 9 tumors had a coexisting KRAS mutation; 1 tumor had an NRAS or HRAS mutation; and 2 tumors had a BRAF or an ERBB2 mutation (Table 1). Concurrent KRAS mutations were all found in the G12 or G13 position, with three G12D and G12V mutations; two G13C mutations; and one G13V mutation. The HRAS mutation was noted to be a Q61H mutation, and the NRAS mutation was identified as a G13R. BRAF mutations included the previously identified G469V mutation and a C685S variant. C685S is predicted to be a benign variant with a Polyphen-2 prediction score of 0.271. ERBB2 mutations included the previously identified S310Y mutation and a V308L variant, both found in the furin-like, cysteine-rich region of exon 8. V308L is predicted to be a possibly damaging mutation with a Polyphen-2 score of 0.691.
Table 1. NF1 variants concurrent with other known NSCLC oncogenic alterations.
Shown are the oncogenic alterations found concurrently in the NF1 tumor cohort (n=15). Polyphen-2 prediction scores for the concurrent oncogenic alteration are also shown for the BRAF and ERBB2 variants that have not been previously reported.
| Mutation | Incidence (n=60) | Concurrent mutation predictiona (Polyphen-2 score) | |
|---|---|---|---|
|
| |||
| NF1 alone | 45 | ||
|
| |||
| KRAS | 9 | ||
| G12D | 3 | ||
| G12V | 3 | ||
| G13C | 2 | ||
| G13D | 1 | ||
|
| |||
| HRAS | 1 | ||
| Q61H | 1 | ||
|
| |||
| NRAS | 1 | ||
| G13R | 1 | ||
|
| |||
| BRAF | 2 | ||
| C685S | 1 | Benign | (0.271) |
| G469V | 1 | ||
|
| |||
| ERBB2 | 2 | ||
| S310Y | 1 | ||
| V308L | 1 | Damaging | (0.691) |
For previously unreported variants
NF1 and KRAS cohorts share similar clinical characteristics with some differences in tumor histology
Median age, gender, smoking status, stage at diagnosis, locations of metastases at diagnosis, and tumor histology were determined for both the NF1 and the KRAS cohort (Table 2). In total, tumors with NF1 mutations represented 10% of our NSCLC cohort and tumors with KRAS mutations represented 24% of our NSCLC cohort. Overall, the NF1 and KRAS cohorts shared very similar clinical profiles. Median age was similar, at 62 years (range 44-83) for the NF1 cohort and 65 years (range 42-86) for the KRAS cohort (p=.14, Mann-Whitney test). Gender distribution included 52% women in the NF1 cohort (31/60) compared to 59% women in the KRAS cohort (83/141) (p=.36, Fisher's exact test). Current or former smokers represented the largest subgroup within both cohorts (53/60, 88% for NF1 and 135/141, 96% for KRAS), but there were more never-smokers in the NF1 cohort (7/60, 12%) compared to the KRAS cohort (6/141, 4%), with a trend towards significance (p=.06, Fisher's exact test).
Table 2. Clinical characteristics of patients included in both NF1 and KRAS cohorts.
Shown is a comparison of age, gender, smoking status, tumor histology, stage at diagnosis, and location of metastatic lesions at diagnosis for all patients in both NF1 and KRAS cohorts.
| Variable | NF1 (n=60) | KRAS (n=141) | p value |
|---|---|---|---|
|
| |||
| Median age (range) | 62 (44-83) | 65 (42-86) | 0.14 |
|
| |||
| Sex | |||
| Female | 31 (52%) | 83 (58%) | 0.36 |
| Male | 29 (48%) | 58 (41%) | |
|
| |||
| Smoking status | |||
| Never-smoker | 7 (12%) | 6 (4%) | 0.06 |
| Former/current | 53 (88%) | 135 (96%) | |
|
| |||
| Stage at diagnosis | |||
| I | 17 (28%) | 55 (39%) | 0.34* |
| II | 5 (8%) | 14 (10%) | |
| III | 10 (17%) | 19 (13%) | |
| IV | 27 (47%) | 53 (38%) | |
|
| |||
| Tumor Histology | |||
| Squamous cell carcinoma | 10 (17%) | 2 (1%) | |
| Adenocarcinoma | 36 (60%) | 136 (96%) | p<0.001 |
| Large cell neuroendocrine | 2 (3%) | 1 (1%) | |
| Giant cell carcinoma | 1 (2%) | 2 (1%) | |
| Poorly differentiated carcinoma | 11 (18%) | 0 (0%) | |
|
| |||
| Distant Metastases | |||
| Bone | 13 (22%) | 28 (20%) | 0.81 |
| Brain | 5 (8%) | 14 (10%) | 0.58 |
| Visceral | 25 (42%) | 48 (34%) | 1.0 |
|
| |||
| % of total NSCLC cohort | 10% | 24% | |
p-value for comparison between patients with Stage IV disease vs Stage I-III disease
A majority of patients in both groups presented with advanced Stage III/IV disease (NF1=38/60, 63%; KRAS=72/141, 51%). A greater percentage of patients with an NF1 mutation presented with Stage IV disease compared to patients whose tumors harbored a KRAS mutation (NF1=27/60, 47%; KRAS=53/141, 38%) but this difference was not found to be statistically significant (p=.34, Fisher's exact test). Patients with metastatic disease at diagnosis presented with a similar distribution of metastatic sites, including bone (NF1=13/60, 22%; KRAS=28/141, 20%); brain (NF1=5/60, 8%; KRAS=14/141, 10%); and visceral disease (NF1=25/60, 42%; KRAS=48/141, 34%). No statistically significant differences between the NF1 and KRAS cohorts were observed when comparing the location of metastatic sites for patients with Stage IV disease.
Tumor histology was also evaluated for all tumors in both NF1 and KRAS cohorts. The majority of tumors in both cohorts represented were adenocarcinomas (NF1=36/60 (60%, KRAS=136/141, 96%), but there was substantially more diversity in histopathology in the NF1 cohort. Most notably, compared to KRAS tumors (2/141, 1%), NF1 mutations were also found in squamous cell carcinoma (10/60, 17%, p<0.001, Fisher's exact test). Both NF1 tumors and KRAS tumors included large cell neuroendocrine tumors and giant cell carcinoma at low frequency. The NF1 cohort also included several tumors with poorly differentiated/undifferentiated histology (11/60, 18%) that was not seen in any of the KRAS tumors.
Concurrent tumor suppressor mutations show varying frequencies in the NF1 and KRAS NSCLC cohorts
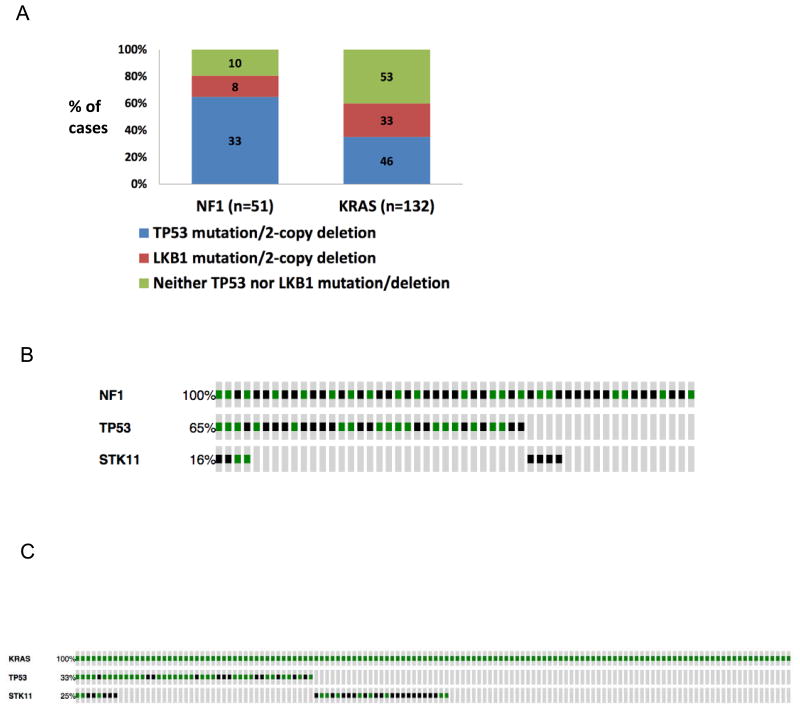
The frequency of mutations or 2-copy deletions in key tumor suppressors was identified in non-overlapping NF1 and KRAS cohorts. Nine tumors were found to have both an NF1 and a KRAS mutation, thus the NF1 cohort for this analysis included 51 tumors while the KRAS cohort included 132 tumors. TP53 and LKB1 were the most frequently mutated tumor suppressors found in tumors harboring either NF1 or KRAS mutations. Furthermore, among the non-overlapping NF1 and KRAS cohort, TP53 mutations or 2-copy deletions were found to occur more frequently in the NF1 cohort (33/51, 65%) than in the KRAS cohort (46/132, 35%), with p<.001 (Figure 2A). In contrast, no significant difference was noted between the frequency of LKB1 mutations or 2-copy deletions in the NF1 cohort (8/51, 16%) compared to the KRAS cohort (33/132, 25%), p=.24 (Figure 2A). All mutations in TP53 and STK11 are shown collectively for the NF1 cohort (Figure 2B) and the KRAS cohort (Figure 2C).
Figure 2. Analysis of tumor suppressor co-mutations in independent NF1 and KRAS cohorts demonstrates differences in the frequency of TP53 mutations.
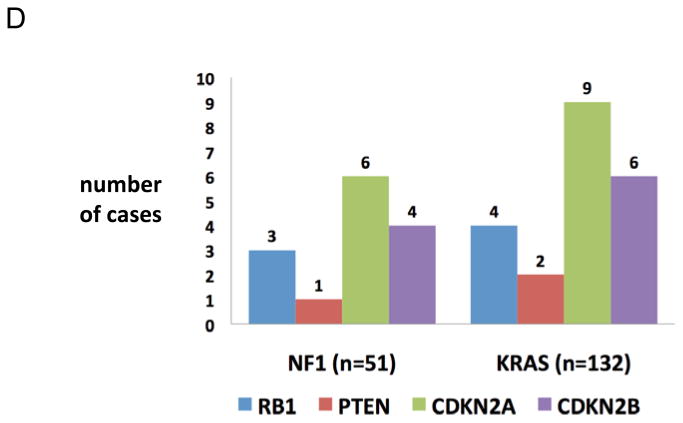
A. Frequency of concurrent TP53 mutations/2-copy deletion and STK11 mutations/2-copy deletion in non-overlapping NF1 and KRAS cohorts with statistical analysis using Fisher's exact test. B. Representation of concurrent TP53 and STK11 mutations in non-overlapping NF1 cohort (n=51) using OncoPrint analysis from cBiopPortal. Green lines represent missense mutations and black lines represent known truncating mutations. C. Representation of concurrent TP53 and STK11 mutations in non-overlapping KRAS cohort (n=132) using OncoPrint analysis from cBioPortal. Green lines represent missense mutations and black lines represent known truncating mutations. D. Frequency of concurrent RB1, CDKN2A, CDKN2B, and PTEN mutations/2-copy deletion in non-overlapping NF1 and KRAS cohorts.
When compared to TP53 or LKB1, mutation/deletions in RB1, PTEN, CDKN2A, or CDKN2B were rare in both cohorts (Figure 2D). RB1 alterations were found in 3/51 NF1 tumors and 4/132 KRAS tumors; PTEN alterations were found in 1/51 NF1 tumors and 2/132 KRAS tumors; CDKN2A alterations were found in 6/51 NF1 tumors and 9/132 KRAS tumors; and CDKN2B alterations were found in 4/51 NF1 tumors and 5/132 KRAS tumors (all non significant).
Overall survival for patients with NF1 mutant tumors is similar to overall survival for patients with KRAS mutant tumors
Kaplan-Meier analysis was used to determine the overall survival (OS) of patients with Stage IV NSCLC at diagnosis and with either an NF1 or KRAS mutation (Figure 3). OS in both Stage IV patient cohorts was similar, with patients whose tumors harbored an NF1 mutation having an OS of 12.4 months, while Stage IV patients whose tumors harbored a KRAS mutation had an OS of 11.6 months.
Figure 3. Overall survival in patients from NF1 and KRAS cohorts.
A. Kaplan-Meier curve representing overall survival of patients with Stage IV disease at diagnosis and tumors containing NF1 mutations (n=28). B. Kaplan-Meier curve representing overall survival of patients with Stage IV disease at diagnosis and tumors containing KRAS mutations (n=53).
Discussion
Advances in our understanding of the genomic drivers of NSCLC have profoundly altered the diagnostic workup, treatment options, and prognosis for this disease. Genetic testing at time of diagnosis is now standard-of-care, and targeted therapy options for activating mutations in EGFR, ALK, and ROS1 have significantly improved outcomes for these patients. Ongoing trials continue to develop strategies for targeting other oncogenic alterations.(27-30) In the last decade, NSCLC has gone from a disease in which genetic characterization was limited to identification of KRAS as a frequently occurring oncogene without targeted treatment options, to a disease in which an ever-growing number of low frequency yet targetable mutations have been identified.(31)
However, despite the success of this approach, there are still many patients for whom treatment options for NSCLC remain limited. First, the advances of the targeted therapy era have yet to make a substantial difference for patients with KRAS mutations. As a GTPase, activated KRAS is not amenable to the direct targeting approach that works well for many kinases. Second, despite the growing number of small cohorts of NSCLC defined by targetable activating mutations, some 30-40% of NSCLC is still characterized by the lack of a clearly identifiable oncogenic alteration. For these patients, cytotoxic chemotherapy remains the mainstay of oncology care. Finally, the histology of NSCLC helps determine the likelihood that a patient will have targeted therapy options, as targetable oncogenic alterations are found almost exclusively in adenocarcinoma. TCGA data demonstrates that the squamous subset of NSCLC is enriched for mutations in several tumor suppressors (TP53, CDKN2A, PTEN, RB1), along with PIK3CA and NOTCH1, yet none of these are easily targeted.(13) Against this landscape, the identification of NF1 as an oft-mutated tumor suppressor in both the adenocarcinoma and squamous subsets of NSCLC is particularly intriguing.(13, 14) NF1 is a widely studied tumor suppressor, but research in the field to date has been primarily in the context of Type I neurofibromatosis. However, if downstream signaling caused by NF1 mutations can be targeted in NSCLC, this treatment approach could have relevance for patients with both adenocarcinoma and squamous tumors.
In our cohort, the frequency of KRAS mutations is generally consistent with published reports,(31) while our observed frequency of NF1 mutations is congruent with TCGA data.(13, 14) Importantly, we demonstrated that although there is some overlap between tumors harboring both mutations, the NF1 cohort is at least partially independent, with 75% of mutations (45/60) occurring independently from other oncogenic alterations. Notably, the institutional database used to identify the NF1 and KRAS cohorts described here is biased against including EGFR mutant tumors because many of these patients would have undergone rapid PCR-based testing for clinical decision-making, thus it may be important to reconsider NF1 variants specifically in the setting of concurrent EGFR mutations.(32) However, in light of the well-characterized role of NF1 in regulating Ras signaling through the MAPK/ERK pathway, it is not surprising that all of the concurrent driver mutations identified with NF1 mutations in this cohort also represent genes involved with MAPK/ERK signaling (KRAS, HRAS, NRAS, BRAF, ERBB2).
The specific mutations affecting NF1 were found to be highly variable and distributed throughout the genome, as expected for mutations in a tumor suppressor. Genomic prediction programs were used to evaluate each individual mutation, with the majority of identified variants predicted to have damaging effects. Some of the variants identified in our cohort do correspond to previously reported SNPs at the same NF1 codon, but this represents a minority of the 73 variants in our cohort. A similar pattern of NF1 mutations was also seen in the tumors containing a concurrent oncogenic alteration. However, in the absence of paired germline assessment, it is impossible to completely exclude variants that represent germline inheritance instead of somatic changes in the tumor. Furthermore, the analysis reported here does not include the functional validation of NF1 mutations that will be an important next step in preclinical evaluation. However, it is notable that the majority of the variants identified in this NSCLC cohort have not been previously reported as germline variants or functionally characterized.
In comparison to patients with KRAS mutant tumors, several clinical similarities were noted in the NF1 cohort, a finding that is not surprising given the functional activation of downstream Ras signaling that would be anticipated in both populations. However, there was a clear difference seen in the histopathology observed in each cohort, with NF1 mutant tumors displaying greater histologic diversity. Further distinctions between the two cohorts were identified in an evaluation of concurrent tumor suppressor mutations. Mutations in TP53 and LKB1 have previously been identified in KRAS mutant tumors,(33) with some suggestion that prognostic outcome in these tumors, particularly the success of MEK inhibitors, can be influenced by whether or not a tumor has intact LKB1 signaling.(33, 34) Notably, in the NF1 cohort, both TP53 and LKB1 mutations were identified. However, when compared to the KRAS tumors, a statistically significant increase in TP53 mutations was noted without a significant difference in the frequency of LKB1 mutations. In light of prior studies evaluating LKB1 in KRAS mutant tumors, this finding provides some suggestion that NF1 tumors may be enriched in a population that is more likely to respond to targeted therapies acting downstream of Ras activation. Interestingly, the malignant peripheral nerve sheath tumors (MPNSTs) that develop in neurofibromatosis patients are also frequently found to have concurrent mutations in TP53, while mutations in LKB1 have not been widely reported.(35) Overall, these findings continue to emphasize that NSCLC tumors defined by NF1 mutation share many overlapping features and characteristics with KRAS mutant tumors but are also a tumor cohort with some unique distinctions that may be exploitable for therapeutic benefit.
Intriguingly, recent clinical trials in neurofibromatosis patients support the potential efficacy of a targeted therapy approach in tumors driven by NF1 mutations. Pre-clinical studies of neurofibromas in a genetically engineered murine model of neurofibromatosis have shown that tumor size can be controlled with the MEK inhibitor PD-0325901.(36) Similar findings have also been observed in xenograft models of human MPNSTs.(37) Interestingly, in animal models of Nf1 deficiency with a myeloproliferative disorder (MPD) similar to the juvenile myelomonocytic leukemia (JMML) seen in pediatric neurofibromatosis patients, treatment with PD-0325901 also demonstrated improvement in clinical measures.(38) A phase I trial evaluating the MEK inhibitor selumetinib in pediatric patients with inoperable plexiform neurofibromas resulted in all 11 patients having some response on restaging exam,(39) and a phase II trial evaluating PD-0325901 in these patients is ongoing (Clinical Trials identifier: NCT02096471). Notably, NF1 mutations have also been noted in melanoma, with suggestion of dependence upon MEK signaling,(40, 41) although the potential applications in this clinical setting remain under investigation.(42) The safe use of MEK inhibitors in NSCLC patients has been previously established in patients with BRAF or KRAS mutations, (27, 43) and it remains to be determined whether MEK inhibitors may also be effective in NSCLC harboring NF1 mutations. Pre-clinical studies in animal models of neurofibromatosis have also identified a role for the mTOR pathway in facilitating the growth of NF1 deficient tumors. TORC1 is an essential component of tumorigenesis in neurofibromatosis-associated malignancies,(44) and mouse models of Nf1 deficient tumors are sensitive to treatment with an Hsp-90 inhibitor combined with the mTOR inhibitor rapamycin.(45) Preclinical studies have revealed that combination MEK and mTORC1 inhibition is required for optimal growth inhibition in human MPNST cell lines and a genetically engineered mouse model of MPNST.(46) Moving forward, it is possible that in the right genetically-defined subset of tumors with an NF1 mutation, dual inhibition of mTOR signaling along with inhibition of a parallel pathway may lead to greater clinical efficacy.
The complex and overlapping milieu of Ras signaling pathways suggests that effective downstream targeting in lung cancer may depend upon identifying subpopulations of patients who are sensitive to inhibition of one pathway over another. Multiple lines of evidence indicate that variables as divergent as specific exon/base-pair KRAS mutation(47) or co-existing mutations in key tumor suppressor genes(21, 48, 49) may help shape the biological heterogeneity of tumors with a KRAS mutation. Although KRAS has historically been considered an “undruggable” target,(50) novel approaches to silencing Ras-driven pathways are currently ongoing.(43) The biological function of NF1 suggests that the same targeting strategies under ongoing evaluation in KRAS tumors may also have efficacy in NF1 mutant tumors. Our findings identify the clinical and molecular characteristics of a new cohort of NSCLC defined by NF1 mutation and reveal both similarities and differences with tumors characterized by KRAS mutation. These findings establish a role for further preclinical studies to validate the functional activity of NF1 mutations in NSCLC and to investigate the efficacy of targeted inhibition of downstream Ras signaling pathways in such tumors.
Supplementary Material
Translational relevance.
Mutations in the tumor suppressor NF1 occur in lung cancer, but their clinical significance and molecular characterization remain unknown. NF1 functions as a negative regulator of Ras signaling, suggesting that lung cancers with NF1 mutations may also be characterized by downstream activation of Ras signaling. Further evaluation of lung cancers defined by NF1 mutation may reveal a unique cohort of tumors, distinct from KRAS mutant lung cancers, for which therapeutic targeting of pathways downstream of activated Ras may have clinical efficacy.
Acknowledgments
Financial support: National Cancer Institute T32CA009172 (supporting AJR)
References
- 1.Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. American journal of epidemiology. 2000 Jan 1;151(1):33–40. doi: 10.1093/oxfordjournals.aje.a010118. [DOI] [PubMed] [Google Scholar]
- 2.Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990 Nov 16;63(4):843–9. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 3.Bollag G, McCormick F. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature. 1991 Jun 13;351(6327):576–9. doi: 10.1038/351576a0. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. The New England journal of medicine. 1986 Apr 17;314(16):1010–5. doi: 10.1056/NEJM198604173141603. [DOI] [PubMed] [Google Scholar]
- 5.Cawthon RM, Weiss R, Xu GF, Viskochil D, Culver M, Stevens J, et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990 Jul 13;62(1):193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- 6.Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990 Jul 13;249(4965):181–6. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 7.Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature genetics. 1994 Jul;7(3):353–61. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- 8.Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999 Dec 10;286(5447):2172–6. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 9.Xu GF, O'Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990 Aug 10;62(3):599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 10.Shapira S, Barkan B, Friedman E, Kloog Y, Stein R. The tumor suppressor neurofibromin confers sensitivity to apoptosis by Ras-dependent and Ras-independent pathways. Cell death and differentiation. 2007 May;14(5):895–906. doi: 10.1038/sj.cdd.4402057. [DOI] [PubMed] [Google Scholar]
- 11.Kweh F, Zheng M, Kurenova E, Wallace M, Golubovskaya V, Cance WG. Neurofibromin physically interacts with the N-terminal domain of focal adhesion kinase. Molecular carcinogenesis. 2009 Nov;48(11):1005–17. doi: 10.1002/mc.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai C, Santagata S, Tang Z, Shi J, Cao J, Kwon H, et al. Loss of tumor suppressor NF1 activates HSF1 to promote carcinogenesis. The Journal of clinical investigation. 2012 Oct;122(10):3742–54. doi: 10.1172/JCI62727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012 Sep 27;489(7417):519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014 Jul 31;511(7511):543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004 May 20;350(21):2129–39. doi: 10.1056/NEJMoa040938. Epub 2004/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 16.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004 Jun 4;304(5676):1497–500. doi: 10.1126/science.1099314. Epub 2004/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. Jun 20;368(25):2385–94. doi: 10.1056/NEJMoa1214886. Epub 2013/06/04. eng. [DOI] [PubMed] [Google Scholar]
- 18.Cully M, Downward J. SnapShot: Ras Signaling. Cell. 2008 Jun 27;133(7):1292–e1. doi: 10.1016/j.cell.2008.06.020. Epub 2008/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008 Dec;14(12):1351–6. doi: 10.1038/nm.1890. Epub 2008/11/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji H, Wang Z, Perera SA, Li D, Liang MC, Zaghlul S, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007 May 15;67(10):4933–9. doi: 10.1158/0008-5472.CAN-06-4592. Epub 2007/05/19. eng. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. Mar 29;483(7391):613–7. doi: 10.1038/nature10937. Epub 2012/03/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. Jan;14(1):38–47. doi: 10.1016/S1470-2045(12)70489-8. Epub 2012/12/04. eng. [DOI] [PubMed] [Google Scholar]
- 23.Wagle N, Berger MF, Davis MJ, Blumenstiel B, Defelice M, Pochanard P, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer discovery. 2012 Jan;2(1):82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature biotechnology. 2013 Mar;31(3):213–9. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos AH, Lichtenstein L, Gupta M, Lawrence MS, Pugh TJ, Saksena G, et al. Oncotator: cancer variant annotation tool. Human mutation. 2015 Apr;36(4):E2423–9. doi: 10.1002/humu.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012 May;2(5):401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planchard DGH, Kim TM, et al. Interim results of a phase II study of the BRAF inhibitor dabrafenib in combintation with the MEK inhibitor trametinib in patietns with BRAF V600E mutated metastatic non-small cell lung cancer. J Clin Oncol. 2015;22 suppl; abstr 8006. [Google Scholar]
- 28.Gandhi L, Bahleda R, Tolaney SM, Kwak EL, Cleary JM, Pandya SS, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol. 2014 Jan 10;32(2):68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 29.Paik PKDA, Yu HE, et al. Response to crizotinib and cabozantinib in stage IV lung adenocarcinoma patients with mutations that cause MET exon 14 skipping. J Clin Oncol. 2015;33 suppl abstr 8021. [Google Scholar]
- 30.Drilon AESC, Somwar R, et al. Phase II study of cabozantinib for patients with advanced RET-rearranged lung cancers. J Clin Oncol. 2015;33 suppl abstr 8007. [Google Scholar]
- 31.Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nature medicine. 2012 Mar;18(3):349–51. doi: 10.1038/nm.2697. [DOI] [PubMed] [Google Scholar]
- 32.de Bruin EC, Cowell C, Warne PH, Jiang M, Saunders RE, Melnick MA, et al. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer discovery. 2014 May;4(5):606–19. doi: 10.1158/2159-8290.CD-13-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calles A, Sholl LM, Rodig SJ, Pelton AK, Hornick JL, Butaney M, et al. Immunohistochemical Loss of LKB1 Is a Biomarker for More Aggressive Biology in KRAS-Mutant Lung Adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 Jun 15;21(12):2851–60. doi: 10.1158/1078-0432.CCR-14-3112. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012 Mar 29;483(7391):613–7. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birindelli S, Perrone F, Oggionni M, Lavarino C, Pasini B, Vergani B, et al. Rb and TP53 pathway alterations in sporadic and NF1-related malignant peripheral nerve sheath tumors. Laboratory investigation; a journal of technical methods and pathology. 2001 Jun;81(6):833–44. doi: 10.1038/labinvest.3780293. [DOI] [PubMed] [Google Scholar]
- 36.Jousma E, Rizvi TA, Wu J, Janhofer D, Dombi E, Dunn RS, et al. Preclinical assessments of the MEK inhibitor PD-0325901 in a mouse model of Neurofibromatosis type 1. Pediatric blood & cancer. 2015 Apr 22; doi: 10.1002/pbc.25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. The Journal of clinical investigation. 2013 Jan;123(1):340–7. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang T, Krisman K, Theobald EH, Xu J, Akutagawa J, Lauchle JO, et al. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. The Journal of clinical investigation. 2013 Jan;123(1):335–9. doi: 10.1172/JCI63193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widemann BLJ, Fisher MJ, et al. Phase I study of the MEK 1/2 inhibitor selumetinib (AZD6244) hydrogen sulfate in children and young adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas. J Clin Oncol. 2014;32 suppl: 10018. [Google Scholar]
- 40.Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nature genetics. 2015 Sep;47(9):996–1002. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nissan MH, Pratilas CA, Jones AM, Ramirez R, Won H, Liu C, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer research. 2014 Apr 15;74(8):2340–50. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whittaker SR, Theurillat JP, Van Allen E, Wagle N, Hsiao J, Cowley GS, et al. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer discovery. 2013 Mar;3(3):350–62. doi: 10.1158/2159-8290.CD-12-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. The Lancet Oncology. 2013 Jan;14(1):38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 44.Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC, et al. TORC1 is essential for NF1-associated malignancies. Current biology : CB. 2008 Jan 8;18(1):56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 45.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer cell. 2011 Sep 13;20(3):400–13. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malone CF, Fromm JA, Maertens O, DeRaedt T, Ingraham R, Cichowski K. Defining key signaling nodes and therapeutic biomarkers in NF1-mutant cancers. Cancer discovery. 2014 Sep;4(9):1062–73. doi: 10.1158/2159-8290.CD-14-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. Feb 8;104(3):228–39. doi: 10.1093/jnci/djr523. Epub 2012/01/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007 Aug 16;448(7155):807–10. doi: 10.1038/nature06030. Epub 2007/08/07. eng. [DOI] [PubMed] [Google Scholar]
- 49.Calles ALX, Sholl L, Butaney M, Rodig S, Freeman GJ, Lydon CA, Oxnard GR, Jackman DM, Janne PA. Differential expression of LKB1, PD-L1, and PD-L2 in KRAS-mutant non-small cell lung cancer in never-smokers. J Clin Oncol. 2014;32 [Google Scholar]
- 50.Stephen AG, Esposito D, Bagni RK, McCormick F. Dragging ras back in the ring. Cancer cell. 2014 Mar 17;25(3):272–81. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.