Abstract
Aluminum is a neurotoxic metal with known health effects in animals and humans. Glutathione-S-transferase (GST) genes and enzymes play a major role in detoxification of several heavy metals. Besides a direct relationship with oxidative stress; aluminum decreases GST enzyme activities. Using data from 116 Jamaican children; age 2–8 years; with Autism Spectrum Disorder (ASD) and 116 sex- and age-matched typically developing (TD) children; we investigated the association of polymorphisms in three GST genes (GSTP1; GSTM1; and GSTT1) with mean blood aluminum concentrations in children with and without ASD. Using log-transformed blood aluminum concentration as the dependent variable in a linear regression model; we assessed the additive and interactive effects of ASD status and polymorphisms in the three aforementioned GST genes in relation to blood aluminum concentrations. Although none of the additive effects were statistically significant (all p > 0.16); we observed a marginally significant interaction between GSTP1 Ile105Val (rs1695) and ASD status (p = 0.07); even after controlling for parental education level and consumption of avocado; root vegetables; and tuna (canned fish). Our findings indicate a significantly lower (p < 0.03) adjusted geometric mean blood aluminum concentration for TD children who had the Val/Val genotype (14.57 µg/L); compared with those with Ile/Ile or Ile/Val genotypes who had an adjusted geometric mean of 23.75 µg/L. However; this difference was not statistically significant among the ASD cases (p = 0.76). Our findings indicate that ASD status may be a potential effect modifier when assessing the association between GSTP1 rs1695 and blood aluminum concentrations among Jamaican children. These findings require replication in other populations.
Keywords: aluminum, Autism Spectrum Disorder (ASD), glutathione S-transferase (GST) genes, detoxification, interactions
1. Introduction
Aluminum (Al) is found naturally in silicates, cryolite, and bauxite rocks and is the third most abundant element in the earth’s crust [1,2,3]. Aluminum has no known biological role [4], but has an important biogeochemical cycle [1] and at high concentrations can have widespread environmental effects [1,5] and cause toxicity in a variety of living organisms, including microbes [4,6], plants [7], fish [5], and mammals [8]. High levels of aluminum in acidic soil and water can limit growth of plants and aquatic organisms [9]. At the same time, accumulation of aluminum in plants and freshwater invertebrates is a potential route for aluminum exposure in mammals [5] as well as for accumulation with repeated exposure [10]. Routes of human exposure to aluminum include food [3] or water through the digestive tract, skin, and occupational inhalation [2,3,11]. Some studies suggest that aluminum exposure in humans is associated with utilization of cookware and food packaging materials made from aluminum [12,13]. In addition, aluminum is found in some consumer products such as antacids (aluminum hydroxide), astringents, food additives (aluminum oxides), antiperspirants, fuel additives, explosives, propellants, and cosmetics [3,14]. Aluminum is excreted from the human body through the feces and urine [2,14].
As a neurotoxic agent and destabilizer of cell membranes, aluminum is associated with several neurodegenerative diseases [15,16,17]. Moreover, recent findings have implicated astrocytes, which are responsible for the physical blood-brain barrier [18] and regulate glutamate and gamma aminobutyric acid (GABA) neurotransmission [19] as the principal target of aluminum toxicity [18,20]. Other studies speculated that aluminum diminishes the ability of astrocytes to protect neurons from glutamate toxicity [18]. On the other hand, GABA level, glutamate/GABA and glutamine/glutamate ratios are reported to be significantly lower in children with Autism Spectrum Disorder (ASD) compared to typically developing (TD) controls [21,22]. Another study reported associations between reduced GABA level, neuroinflammation, and glutamate excitotoxicity [23]. A study that involved 20 children with ASD (aged 3–15 years) and 20 age- and sex-matched TD controls from Saudi Arabia reported a significant association between ASD status and levels of nine biomarkers including levels of glutathione (GSH), glutamate excitotoxicity, and impaired detoxification [24].
Due to its high reactivity, Al3+ is able to interfere with enzymatic activities in key metabolic pathways. For example, two of the enzymes that constitute the Krebs cycle are activated by aluminum [15]. The toxic effects of aluminum may also include interference with secondary messenger systems [25]; competition with Mg2+ for phosphate sites in critical biological enzymes, such as ATPase [8,26]; and enhancement of iron-induced reactive oxygen species production and oxidative stress [27]. Additionally, a well-known cause of aluminum toxicity in living organisms is by induction of oxidative stress [8,25,28,29].
Besides a direct relationship with oxidative stress accumulation, aluminum inhibits biological oxidative stress management systems by interfering with the glutathione S-transferase (GST) detoxification system [17,30]. GSTs are multi-gene isoenzymes that are encoded by three separate families of genes, including cytosolic, microsomal, and mitochondrial transferases [31,32,33,34,35], which are involved in the cellular detoxification of both xenobiotic and endobiotic compounds [31,32,36]. The human GST gene superfamily comprises eight classes: alpha, kappa, mu, omega, pi, theta, sigma, and zeta [34]. Glutathione S-transferase pi (GSTP1), glutathione S-transferase mu (GSTM1), and glutathione S-transferase theta (GSTT1) play important roles in detoxification of xenobiotics [37] and polymorphisms in these genes may affect biologic responses to heavy metals. For example, people with a double deletion of GSTM1 and GSTT1 have higher mercury concentrations in hair than other individuals, suggesting that the epistatic effect of this double deletion is a risk factor for increased susceptibility to mercury toxicity [38,39].
GST genes encode enzymes that catalyze the conjugation of the reduced form of GSH to xenobiotics, such as heavy metals, thereby reducing the toxic effects and promoting excretion of the conjugated form of the xenobiotic [34,35]. In three studies, rodents treated with aluminum have reduced amounts of GSH [17,30,40]. The aluminum-associated GST/GSH effects observed in animal models have been reproduced in human studies. For example, a study of industrial workers noted that people with the highest levels of aluminum in urine also have low GST enzymatic activity in erythrocytes [41].
Recent findings from our Jamaican study of children with ASD and TD children revealed that the relationship between GSTP1 and blood arsenic concentrations varied by ASD status (TD or ASD) in an interactive model [42] In addition, we identified a significant gene-gene interaction between GSTP1 and GSTT1 in relation to ASD, where there was a significantly higher odds of ASD for children who were heterozygous for the GSTP1 Ile105Val polymorphism and had the GSTT1 null genotype (Matched Odds Ratio (MOR) = 2.97, 95% CI (1.09, 8.01), p = 0.03) [43]. Furthermore, we found a significant gene-environment interaction between GSTP1 and blood manganese concentrations (BMC) indicating that among children who had the Ile/Ile genotype for GSTP1, those with BMC ≥ 12 µg/L had about four times higher odds of ASD than those with BMC < 12 µg/L (p = 0.03) [44].
Although a number of studies have examined the developmental effects of toxicity of aluminum in rats and mice [2], limited data are available on the toxicity of aluminum in children [2]. Higher levels of aluminum were observed in the hair [45,46,47] and urine [48] of children with ASD, compared to children without ASD. One study from Egypt additionally found that the level of aluminum in children’s hair was positively correlated with the use of aluminum pans in the home [45]. However, some studies found no association between aluminum level in hair [49,50] and aluminum level in blood and urine of children with ASD [51]. Jamaica’s soil has higher levels of aluminum than most countries around the world, with the highest aluminum concentrations near the main bauxite-mining belt and in small areas on the east and the west coasts [52]. The main objective of this study was to investigate the role of polymorphisms in three GST genes (GSTP1, GSTM1, and GSTT1) in relation to blood aluminum concentrations using data from a sex- and age-matched case-control study of ASD and TD Jamaican children, ages 2–8. Furthermore, we investigated whether ASD status is an effect modifier when exploring the association between GST gene polymorphisms and blood aluminum concentrations.
2. Materials and Methods
2.1. General Description
The Jamaican Autism Study is an age- and sex-matched case-control study of Jamaican children between 2 and 8 years of age that were enrolled during December 2009–May 2012. The overall goal of the Jamaican Autism Study is to investigate if environmental exposures to aluminum, arsenic, mercury, lead, cadmium, and manganese play a role in ASD, and to assess the role of polymorphisms in three GST genes (GSTT1, GSTM1, and GSTP1) and their interactions with these heavy metals in relation to ASD status. Detailed information regarding the recruitment and assessment of ASD cases and TD controls was reported earlier [53,54]. In brief, children included in the University of the West Indies (UWI) Jamaica Autism Database, who were previously identified as being at risk for an ASD based on Diagnostic Statistical Manual of Mental Disorders (DSM-IV-TR) criteria [55] and the Childhood Autism Rating Scale (CARS) [56], were invited to participate for reassessment of their ASD status for this study. We administered the Autism Diagnostic Observation Schedule (ADOS) [57] and the Autism Diagnostic Interview-Revised (ADI-R) [58] to confirm the diagnosis of ASD. For ascertainment of ASD, we used standard algorithms developed for scoring ADOS and ADI-R and established cut-off points. Each ASD case was confirmed based on both ADI-R and all three domains in ADOS. For each confirmed ASD case, an age- and sex-matched TD control was identified from schools and well-child clinics. The criteria for matching required that the age of the control child be within six months of the matched ASD case. For ascertainment of TD, we administered the Lifetime Form of the Social Communication Questionnaire (SCQ) [59] to the parents/guardians of potential TD control children to rule out symptoms of ASD. We set the criteria for including children in the TD control group as having a SCQ score of 0–6. This cut-off point of 6 is one standard deviation above the mean SCQ score of TD school children [60]. A SCQ score more than six indicated a possible developmental delay and the child was referred for further evaluation.
We also administered a pre-tested questionnaire to the parents/guardians of both ASD cases and TD controls to collect information about their demographic and socioeconomic status (SES), such as ownership of a car by the family, parental education levels, and potential exposure to heavy metals through drinking water sources and food, especially the types and frequency of fruits, vegetables, and seafood consumed by the children on a weekly basis. The types of seafood, fruits, and vegetables were classified into different categories, based on their characteristics and species. For example, the types of fruits and vegetables were classified into the following categories: (1) two classes of root vegetables ((yam, sweet potato, or dasheen) and (carrot, or pumpkin)); (2) three classes of leafy vegetables ((lettuce), (callaloo, broccoli, or pak choi), and (cabbage)); (3) legumes (string beans); and (4) three different fruits (tomatoes, ackee, or avocado). For analysis, we categorized the frequency of food consumption into two levels (consumed and never consumed). Details regarding the categories of fruits and vegetables were reported earlier [53].
About 5 mL of venous whole blood was drawn from each child. Samples for trace metals analysis (2 mL) were stored at −20 °C while the remaining samples for genetic analysis (3 mL) were stored at −80 °C at UWI in Kingston, Jamaica. In addition, 2 mL of saliva was also collected from each child. Whole blood samples for trace metal analysis were shipped to the Michigan Department of Human Health Services (MDHHS), and the whole blood and saliva samples for genetic analysis were shipped to the Human Genetics Center at the University of Texas Health Science Center at Houston School of Public Health. All parents/guardians provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Boards (IRBs) of the University of Texas Health Science Center at Houston (UTHealth) and UWI, Mona Campus, in Kingston, Jamaica (Project identification code: HSC-SPH-09-0059). The data presented herein represent analysis of 116 1:1 matched case-control pairs for whom we had complete data.
2.2. Assessment of Aluminum Exposures
Aluminum exposure can be assessed using various biological specimens including blood, hair, and urine depending on duration of exposure [2]. Greater than 95% of aluminum is excreted from the human body through the kidneys. Steady state blood aluminum concentrations in serum and in whole blood are similar [3]. Occupational aluminum exposure could result in a greater increase in urinary than in plasma aluminum concentrations [3]. Depending on the type and route of exposure, clearance of aluminum from the body is estimated to vary from hours to years [3]. Although blood is considered a more reliable method for measuring recent aluminum exposures, blood aluminum concentrations may not accurately reflect the total body burden of aluminum and most literature recommends assessing aluminum in urine [2,3]. In fact brain, lung, and bone measurements may reveal much higher levels of aluminum than are found in the blood [61]. On the other hand, in extreme exposures, aluminum in hair is not associated with concentrations of aluminum in serum or bone [62,63] and is unrelated to the dietary intake [64].
Although sources of exposure to aluminum include drinking water and food [3], the extent of aluminum absorption depends on a number of factors, including pH (for aluminum speciation and solubility), bioavailability, and diet [65]. Foods that are naturally high in aluminum include potatoes, spinach, and tea. Processed dairy products, flour, and infant formula may be high in aluminum if they contain aluminum-based food additives [65]. Most studies do not consider the speciation of aluminum, as the assessment of exposure from both drinking water and food is not well characterized [65]. However, it is important to note that aluminum is not bioaccumulated to a significant extent in aquatic organisms [2]. In addition, currently there is a lack of data to support a correlation between aluminum levels measured in different biological samples [66].
Since the Jamaican community has continuous exposure to aluminum through various sources including soil, water, and the food chain, possibly through fruits and vegetables, we considered aluminum in whole blood a reliable biomarker for aluminum exposure. In this study, blood aluminum concentrations were assessed by the Trace Metals Lab at MDHHS, which is recognized by the Centers for Disease Control and Prevention (CDC). The Trace Metals Lab is certified for testing toxic metals in blood and urine by the College of American Pathology (CAP). MDHHS followed a fully validated protocol for analyzing aluminum in blood samples with a detection limit of 5.0 µg/L. All samples were diluted and analyzed on a PerkinElmer Nexion 300D inductively-coupled plasma mass spectrometer (PerkinElmer, Waltham, MA, USA). Six out of 232 children in this study had blood aluminum concentrations below the limit of detection (LoD). In data analyses, these study participants were assigned a blood aluminum concentration of 2.5 µg/L (midpoint between 0.0 and 5.0 µg/L).
2.3. Genetic Analysis
All procedures used for genetic analysis were carried out as previously described [42]. In brief, plasma, buffy coat, and red blood cells were isolated from whole blood samples collected for each participant at UWI, and stored at −80 °C for future use. Saliva was collected with either the Oragene Discover DNA Collection Kits for Research (DNA Genotek Inc., Kanata, ON, Canada) or the Oragene Discover for Assisted Collection (DNA Genotek). Frozen blood specimens or saliva samples at ambient temperature were shipped from UWI to the UTHealth School of Public Health Human Genetics Center. Genomic DNA was isolated from buffy coat using the Gentra PUREGENE Blood Kit (Qiagen, N.V., Venlo, The Netherlands), or if a buffy coat was not available, from saliva with the Gentra PUREGENE DNA Purification Kit (Qiagen protocol 400244 Rev A., Kanata, ON, Canada) [67]. GSTT1 and GSTM1 genotypes were measured using a multiplex polymerase chain reaction (PCR) [68]. The GSTP1 Ile105Val polymorphism (rs1695) was genotyped using the TaqMan Drug Metabolism SNP Genotyping Assay C_3217198_20 using the thermal cycling parameters recommended by the manufacturer (Thermo Fisher Scientific, Waltham, MA, USA). Allele detection was performed using the ABI Prism 7700 Sequence Detection System (Thermo Fisher Scientific).
2.4. Statistical Analysis
Descriptive analyses were conducted to compare demographic and SES characteristics of ASD cases and TD controls. We also assessed mean blood aluminum concentrations for children with and without ASD. Since the distribution of blood aluminum concentrations was skewed, the data were transformed using the natural logarithm (ln) to produce an approximately normal distribution. The means of the log transformed blood aluminum concentrations were transformed back to their original scale (i.e., µg/L) by applying the natural exponential function, herein called geometric means.
The PCR assay does not distinguish between a normal homozygote (I/I) and a heterozygote (I/D) for the GSTM1 and GSTT1 genes, so we used a recessive model with the genotype represented as a binary variable: I* and DD. Three genotypes, Ile/Ile, Ile/Val and Val/Val, were available for the GSTP1 gene. We analyzed the GSTP1 genotypes using different genetic models, including the recessive (Ile/* vs. Val/Val) and full models (Ile/Ile, Ile/Val and Val/Val). For the GSTP1 genotypes, we tested accordance with Hardy–Weinberg equilibrium expectations using the chi-square test in the TD control group.
In this study, we used General Linear Models (GLMs) with the log-transformed blood aluminum concentrations as the dependent variable to investigate the role of genetic variants of three GST genes in blood aluminum concentrations, while controlling for potential confounding variables. In order to minimize any potential effects of multicollinearity due to a high correlation between maternal and paternal education levels, we created a binary variable indicating whether both parents had education up to high school or at least one of the parents obtained education beyond high school. In all GLMs, we also controlled for the clustering effect of matching by including an appropriate number of dummy variables that represented the matched pairs (e.g., 105 dummy variables for 106 matched pairs used for genetic analysis and 115 dummy variables for 116 matched pairs used for analyses that did not utilize genetic data). In multivariable GLMs, we assessed interactions between each of the three GST genes and ASD status while controlling for potential confounding variables that included parental education levels, and consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish). Because we found a significant interaction between ASD status and GSTP1 genotypes in relation to blood aluminum concentrations, we used the CONTRAST statement in PROC GLM in SAS [69] to test whether there was a significant difference in geometric mean blood aluminum concentrations between individuals with the three GSTP1 genotypes, separately for ASD cases and TD controls. Similarly, we tested whether there was a significant difference in geometric mean blood aluminum concentrations between ASD cases and TD controls after stratifying by GSTP1 genotype. We calculated unadjusted and adjusted geometric mean blood aluminum concentrations for both groups of children (ASD and TD) with different GSTP1 genotypes. All analyses were performed using the SAS software [70].
3. Results
The mean age of children with ASD was 67.5 months and the mean age for TD children was 68.4 months. As expected, about 85.3% of the ASD cases were male, and consequently, the same percentage of TD controls were male. Nearly all of the ASD cases (93.1%) and TD controls (99.1%) were Afro-Caribbean. Similarly, 96.6% of mothers and 95.7% of fathers were Afro-Caribbean. Our data indicated a frequency of 25.5% and 20.8% for the GSTM1 and GSTT1 null genotype, respectively, for TD children. There was no significant deviation from Hardy–Weinberg equilibrium for the GSTP1 genotype in the TD controls (p = 0.70). In addition, there were no significant differences between ASD cases and TD controls with respect to the genotype frequencies of GSTM1, GSTP1, and GSTT1 (all p > 0.16). The arithmetic mean blood aluminum concentration for children with ASD was 30.9 µg/L, the mean blood aluminum concentration for TD children was 36.9 µg/L. Demographic and other characteristics of children and their parents by ASD case status are presented in Table 1.
Table 1.
Characteristics of children and their parents by ASD case status (116 matched pairs).
| Variables | Categories | ASD Case (n = 116) N (%) | TD Control (n = 116) N (%) | p-Value * |
|---|---|---|---|---|
| Child’s sex | Male | 99 (85.3) | 99 (85.3) | 1.00 |
| Child’s age (months) | Age < 48 | 22 (19.0) | 19 (16.4) | 0.29 |
| 48 ≤ age < 72 | 51 (44.0) | 52 (44.8) | ||
| Age ≥ 72 | 43 (37.0) | 45 (38.8) | ||
| Child’s race | Afro-Caribbean | 108 (93.1) | 115 (99.1) | 0.19 |
| Maternal age a (at child’s birth) | <35 years | 87 (75.0) | 100 (99.1) | <0.01 |
| ≥35 years | 29 (25.0) | 11 (9.0) | ||
| Paternal age b (at child’s birth) | <35 years | 57 (50.9) | 78 (72.9) | <0.01 |
| ≥35 years | 55 (49.1) | 29 (27.1) | ||
| Maternal race | Afro-Caribbean | 109 (94.0) | 115 (99.1) | 0.25 |
| Paternal race c | Afro-Caribbean | 109 (94.0) | 113 (98.3) | 0.82 |
| Maternal education d (at child’s birth) | Up to high school † | 59 (50.9) | 87 (77.0) | <0.01 |
| Beyond high school †† | 57 (49.1) | 26 (23.0) | ||
| Paternal education e (at child’s birth) | Up to high school † | 61 (54.0) | 98 (88.3) | <0.01 |
| Beyond high school †† | 52 (46.0) | 13 (11.7) | ||
| Socioeconomic status (SES) | Car ownership | 77 (66.4) | 41 (35.3) | <0.01 |
| GSTP1 h | Ile/Ile | 30 (28.3) | 26 (24.5) | 0.71 |
| Ile/Val | 55 (51.9) | 55 (51.9) | ||
| Val/Val | 21 (19.8) | 25 (23.6) | ||
| GSTM1 h | DD f | 28 (26.4) | 27 (25.5) | 0.87 |
| I/I or I/D g | 78 (73.6) | 79 (74.5) | ||
| GSTT1 h | DD f | 31 (29.2) | 22 (20.8) | 0.16 |
| I/I or I/D g | 75 (70.8) | 84 (79.2) | ||
| Blood aluminum concentration (µg/L) Arithmetic mean (SD) |
30.9 (29.8) | 36.9 (40.0) | 0.71 ** | |
* p-values are based on Wald’s test in conditional logistic regression models that compares the distribution of independent variables between ASD case and TD control groups; ** p-value is based on Related-Samples Wilcoxon Signed Rank Test that compares the distribution of blood aluminum concentration between ASD case and TD control groups; † Up to high school education means attended Primary/Jr. Secondary, and Secondary/High/Technical schools; †† Beyond high school education means attended a Vocational, Tertiary College, or University; a Maternal age was missing for five TD controls; b Paternal age was missing for four ASD cases and nine TD controls; c Paternal race was missing for one ASD case; d Maternal education was missing for three TD controls; e Paternal education was missing for three ASD cases and five TD controls; f DD indicates the null alleles for GSTT1 and GSTM1; g I/I or I/D indicate the homozygote (I/I) or a heterozygote (I/D) for GSTT1 and GSTM1; h Results based on 106 matched pairs.
A comparison of dietary consumption between ASD cases and TD controls revealed that a significantly lower proportion of ASD cases reported eating avocado (Matched Odds Ratio (MOR) = 0.18, 95% CI (0.09, 0.35), p < 0.01) and root vegetables (yam, sweet potato, or dasheen) (MOR = 0.48, 95% CI (0.25, 0.93), p = 0.03). In addition, a significantly lower proportion of ASD cases reported consumption of other fruits and vegetables. Comparisons of other variables related to dietary consumption between children with and without ASD are displayed in Table 2.
Table 2.
Associations between dietary consumption and ASD case status using Conditional Logistic Regression (CLR) (232 children or 116 matched pairs).
| Exposure Variables | Category | ASD Case N (%) | TD Control N (%) | Matched OR (MOR) | 95% CI for MOR | p-Value d | |
|---|---|---|---|---|---|---|---|
| Source of drinking water a | Piped water | 110 (94.8) | 111 (96.5) | 0.67 | (0.19, 2.36) | 0.53 | |
| Source of water for cooking b | Piped water | 110 (94.8) | 111 (96.5) | 0.67 | (0.19, 2.36) | 0.53 | |
| Fruits and vegetables consumption c | Root vegetables | Yam, sweet potato, or dasheen | 82 (78.7) | 95 (82.6) | 0.48 | (0.25, 0.93) | 0.03 |
| Carrot or pumpkin | 101 (87.1) | 113 (98.3) | 0.14 | (0.03, 0.63) | 0.01 | ||
| Leafy vegetables | Lettuce | 53 (45.7) | 73 (63.5) | 0.53 | (0.33, 0.88) | 0.01 | |
| Callaloo, broccoli, or pakchoi | 84 (72.4) | 108 (93.9) | 0.22 | (0.10, 0.50) | <0.01 | ||
| Cabbage | 77 (66.4) | 108 (93.9) | 0.18 | (0.08, 0.40) | <0.01 | ||
| Fruits | Tomatoes | 72 (62.1) | 96 (83.5) | 0.29 | (0.14, 0.58) | <0.01 | |
| Ackee | 68 (58.6) | 107 (93.0) | 0.05 | (0.01, 0.20) | <0.01 | ||
| Avocado | 31 (26.7) | 77 (67.0) | 0.18 | (0.09, 0.35) | <0.01 | ||
| Green banana | 82 (70.7) | 103 (89.6) | 0.28 | (0.14, 0.55) | <0.01 | ||
| Fried plantains | 77 (66.4) | 104 (90.4) | 0.19 | (0.07, 0.48) | <0.01 | ||
| Seafood consumption | Ate salt water fish | 90 (77.6) | 104 (89.7) | 0.39 | (0.18, 0.85) | 0.02 | |
| Ate fresh water fish (Pond fish, Tilapia) | 50 (43.1) | 65 (56.0) | 0.56 | (0.32, 0.98) | 0.04 | ||
| Ate sardine, mackerel (Canned fish) | 87 (75.0) | 107 (92.2) | 0.26 | (0.11, 0.60) | <0.01 | ||
| Ate tuna (Canned fish) | 40 (34.5) | 50 (43.1) | 0.67 | (0.38, 1.17) | 0.16 | ||
| Ate salted fish (Pickled mackerel) | 82 (70.7) | 106 (91.4) | 0.20 | (0.08, 0.48) | <0.01 | ||
| Ate shellfish (Lobsters, Crabs) | 8 (6.9) | 16 (13.8) | 0.43 | (0.17, 1.12) | 0.08 | ||
| Ate shrimp | 24 (20.7) | 33 (28.40) | 0.64 | (0.34, 1.20) | 0.16 | ||
a Source of drinking water was missing for one TD control; b Source of water for cooking was missing for one TD control; c For all variables under fruits and vegetables consumption data were missing for one TD control; d p-values are based on Wald’s test in conditional logistic regression models that compares the distribution of dietary consumption between and ASD case and TD control groups.
We compared geometric mean blood aluminum concentrations between children who had different levels and types of exposures. In the univariable analysis, there was no significant association between blood aluminum concentrations and GSTM1 and GSTT1 (GSTM1 (p = 0.31), GSTT1 (p = 0.98), or GSTP1 polymorphisms (all pairwise comparisons p > 0.10)). Additionally, there were no significant associations between blood aluminum concentrations and the consumption of seafood or fruits and vegetables. There was also no significant association between blood aluminum concentrations and ASD status (geometric mean blood aluminum concentrations for ASD group = 21.49 µg/L vs. 20.95 µg/L for the TD control group, p = 0.78). Associations of other exposure variables with blood aluminum concentrations are reported in Table 3.
Table 3.
Associations of various independent variables with blood aluminum concentrations based on univariable General Linear Models (116 matched pairs).
| Variables | Category | Yes | No | p-Value ** | |||
|---|---|---|---|---|---|---|---|
| Mean Al * (μg/L) | N | Mean Al * (μg/L) | N | ||||
| Genes a | GSTT1 (I *) b | 22.91 | 157 | 23.02 | 55 | 0.98 | |
| GSTM1 (I *) b | 25.99 | 159 | 22.03 | 53 | 0.31 | ||
| GSTP1 (Ile/Ile) c | 22.39 | 56 | 24.76 | 156 | 0.54 | ||
| GSTP1 (Val/Val) c | 18.43 | 46 | 24.45 | 166 | 0.10 | ||
| GSTP1 (Ile/Val) c | 24.25 | 110 | 21.71 | 102 | 0.43 | ||
| ASD status | Autism Spectrum Disorder | 21.49 | 116 | 20.95 | 116 | 0.78 | |
| Child’s age (months) | Age > 48 | 22.12 | 191 | 17.49 | 41 | 0.62 | |
| Child’s sex | Male | 21.19 | 198 | 21.39 | 34 | 0.96 | |
| Socioeconomic status | Own a car | 22.35 | 118 | 20.11 | 114 | 0.44 | |
| Maternal age d (at child’s birth) | ≥35 years | 22.76 | 40 | 20.93 | 187 | 0.65 | |
| Parental education levels e (at child’s birth) | At least one of the parents had education beyond high school | 23.39 | 108 | 18.36 | 113 | 0.06 | |
| Source of drinking water f | Piped water | 20.93 | 221 | 26.93 | 10 | 0.43 | |
| Fruits and vegetables consumption g | Root vegetables | Yam, sweet potato, or dasheen | 22.37 | 177 | 17.83 | 54 | 0.16 |
| Carrot or pumpkin | 21.14 | 214 | 22.23 | 17 | 0.85 | ||
| Leafy vegetables | Lettuce | 21.43 | 126 | 20.98 | 105 | 0.86 | |
| Callaloo, broccoli, or pak choi | 21.24 | 192 | 21.15 | 39 | 0.98 | ||
| Cabbage | 20.92 | 185 | 22.53 | 46 | 0.65 | ||
| Fruits | Tomatoes | 22.06 | 168 | 19.14 | 63 | 0.38 | |
| Ackee | 21.92 | 175 | 19.23 | 56 | 0.48 | ||
| Avocado | 24.12 | 108 | 18.97 | 123 | 0.10 | ||
| Green banana | 22.10 | 181 | 18.30 | 50 | 0.22 | ||
| Fried plantains | 21.71 | 185 | 19.36 | 46 | 0.55 | ||
| Seafood consumption | High seafood consumption (more than 6 meals per week) | 23.20 | 82 | 20.21 | 150 | 0.35 | |
| Ate salt water fish | 22.04 | 194 | 17.46 | 38 | 0.20 | ||
| Ate fresh water fish (pond fish, tilapia) | 20.40 | 115 | 22.05 | 117 | 0.58 | ||
| Ate sardine, mackerel (canned fish) | 21.83 | 194 | 18.37 | 38 | 0.34 | ||
| Ate tuna (canned fish) | 24.63 | 90 | 19.31 | 142 | 0.09 | ||
| Ate salted fish (pickled mackerel) | 21.43 | 188 | 20.34 | 44 | 0.77 | ||
| Ate shellfish (lobsters, crabs) | 16.97 | 24 | 21.77 | 208 | 0.27 | ||
| Ate shrimp | 20.69 | 57 | 21.39 | 175 | 0.83 | ||
* Mean Al indicates the geometric mean = Exp. [Mean (ln Al)]; ** p-values are based on GLMs that compare geometric mean blood aluminum concentrations between children who had the characteristic described (in the “Yes” column) and those who did not (in the “No” column); The “Yes” column includes participants who had the characteristic described for the categories in each variable; The “No” column includes participants who did not have the characteristic described for the categories in each variable; a Results based on 106 matched pairs; b I* indicates the homozygote (I/I) or a heterozygote (I/D) for GSTT1 and GSTM1; c GSTP1 has three categories (Ile/Ile, Ile/Val, and Val/Val); d Maternal age was missing for five participants; e Parental education level was missing for 11 participants; f Source of drinking water was missing for one participant; g Fruits and vegetables consumption was missing for one participant.
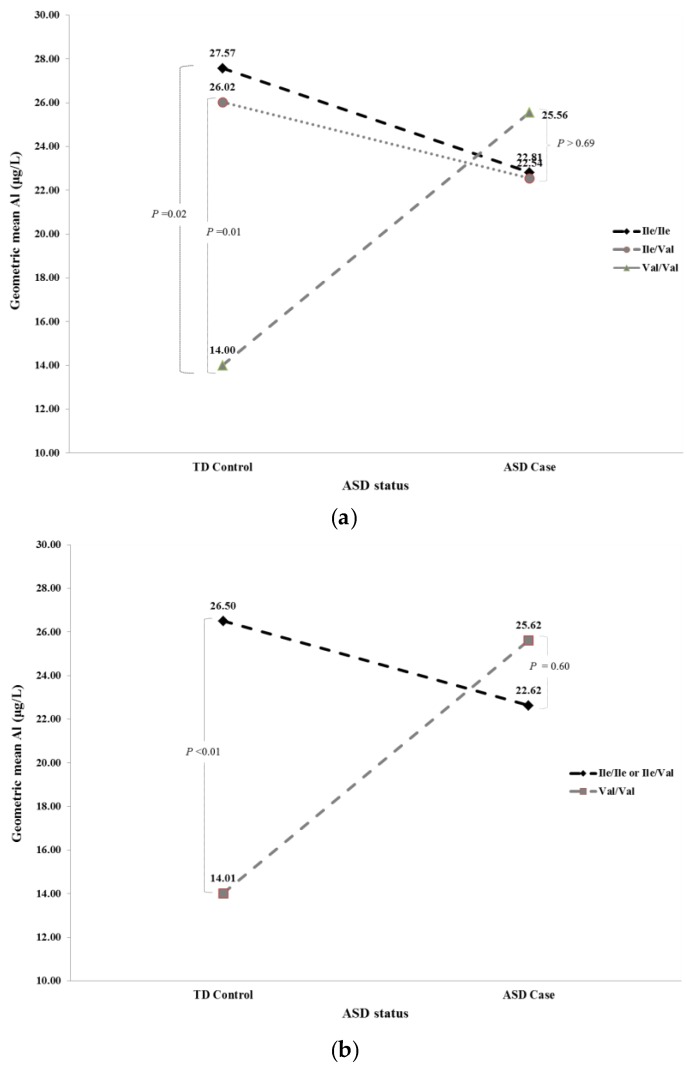
In an unadjusted recessive multivariable model where we assessed the relationship between GSTP1 genotype and blood aluminum concentrations within the ASD case and TD control groups, we identified a significant interaction between ASD status and GSTP1 genotype (p < 0.02) in relation to blood aluminum concentrations. Further analysis of this interaction revealed that while there was no significant association between GSTP1 rs1695 and blood aluminum concentrations within the ASD case group, there was a significant association between GSTP1 rs1695 and blood aluminum concentrations within the TD control group. For example, in the recessive model, ASD cases with either an Ile/Ile or Ile/Val genotype had a geometric mean blood aluminum concentration of 22.62 µg/L, not significantly different (p = 0.60) from 25.62 µg/L for ASD cases with the Val/Val genotype. However, in the TD control group, we observed a geometric mean blood aluminum concentration of 14.01 µg/L for children with the Val/Val genotype significantly lower (p < 0.01) than the geometric mean of 26.50 µg/L among those with Ile/Ile or Ile/Val genotypes. Similar findings were observed for the TD control group in the adjusted recessive model. Specifically, we observed a significantly lower (p < 0.03) geometric mean blood aluminum concentration of 14.57 µg/L for children who had the Val/Val genotype, compared with a geometric mean of 23.75 µg/L among those with either Ile/Ile or Ile/Val genotypes. Details regarding the comparison of the unadjusted and adjusted geometric blood aluminum concentrations between individuals with different GSTP1 genotypes by ASD status are shown in Table 4. Graphical illustrations of unadjusted geometric blood aluminum concentrations between those with different GSTP1 genotypes by ASD status based on the full and recessive models are provided in Figure 1a,b.
Table 4.
Unadjusted and adjusted geometric mean blood aluminum concentrations by GSTP1 genotypes based on General Linear Models (GLM) that include interaction between GSTP1 and ASD case status (ASD and TD control) (106 matched pairs).
| Models | Gene | (Column A) Genotypes Compared | Referent Genotypes | Group | Unadjusted (μg/L) ** | Adjusted (μg/L) c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean Al of Children with Genotypes in Column A * | Geometric Mean Al of Children with Referent Genotypes * | p d | Geometric Mean Al of Children with Genotypes in Column A * | Geometric Mean Al of Children with Referent Genotypes * | p d | |||||
| Full a | GSTP1 | Ile/Ile | Ile/Val | TD Control | 27.57 | 26.02 | 0.81 | 24.19 | 23.39 | 0.89 |
| GSTP1 | Ile/Ile | Ile/Val | ASD Case | 22.81 | 22.54 | 0.96 | 21.71 | 23.16 | 0.77 | |
| GSTP1 | Ile/Ile | Val/Val | TD Control | 27.57 | 14.00 | 0.02 | 24.19 | 14.60 | 0.07 | |
| GSTP1 | Ile/Ile | Val/Val | ASD Case | 22.81 | 25.56 | 0.69 | 21.71 | 24.39 | 0.68 | |
| GSTP1 | Ile/Val | Val/Val | TD Control | 26.02 | 14.00 | 0.01 | 23.39 | 14.60 | 0.05 | |
| GSTP1 | Ile/Val | Val/Val | ASD Case | 22.54 | 25.56 | 0.62 | 23.16 | 24.39 | 0.84 | |
| Recessive b | GSTP1REC | Ile/Ile or Ile/Val | Val/Val | TD Control | 26.50 | 14.01 | <0.01 | 23.75 | 14.57 | 0.03 |
| GSTP1REC | Ile/Ile or Ile/Val | Val/Val | ASD Case | 22.62 | 25.62 | 0.60 | 22.62 | 24.38 | 0.76 | |
* Mean AL indicates the geometric mean = Exp. [Mean (ln AL)]; ** In the univariable GLMs, the independent variables include pairs, ASD status, GSTP1, and GSTP1 interaction with ASD; a GSTP1 in the full model has three categories (Ile/Ile, Ile/Val, and Val/Val); b GSTP1 (REC) = GSTP1 in the recessive model has two categories (Val/Val, Ile/Ile or Ile/Val); c In multivariable GLMs in addition to the variables in the univariable model we adjusted for parental education levels, consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish); d p-values are for the comparison of mean blood aluminum concentrations of children with genotypes in “Column A” compared to those with “referent genotypes”, stratified by ASD case status (ASD and TD control), based on CONTRAST option in the SAS program for GLMs as described in the Methods Section.
Figure 1.
(a) Unadjusted geometric blood aluminum concentrations based on result from Full Model in Table 4; and (b) unadjusted geometric blood aluminum concentrations based on result from Recessive Model in Table 4.
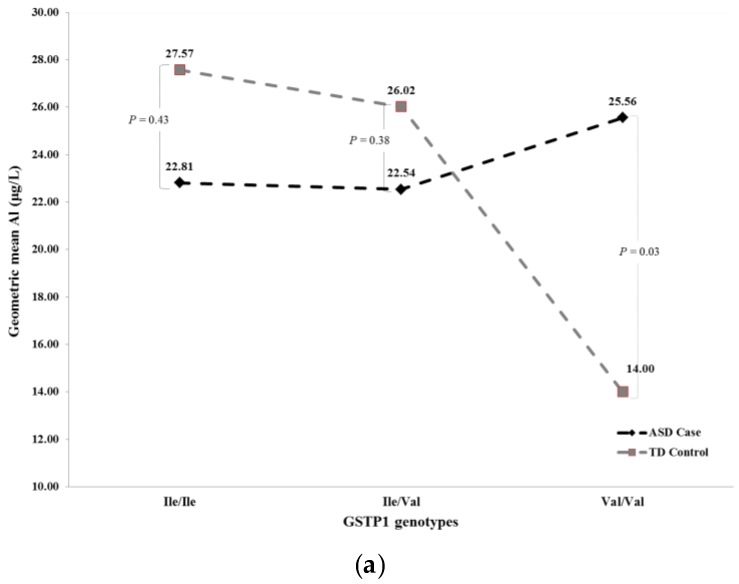
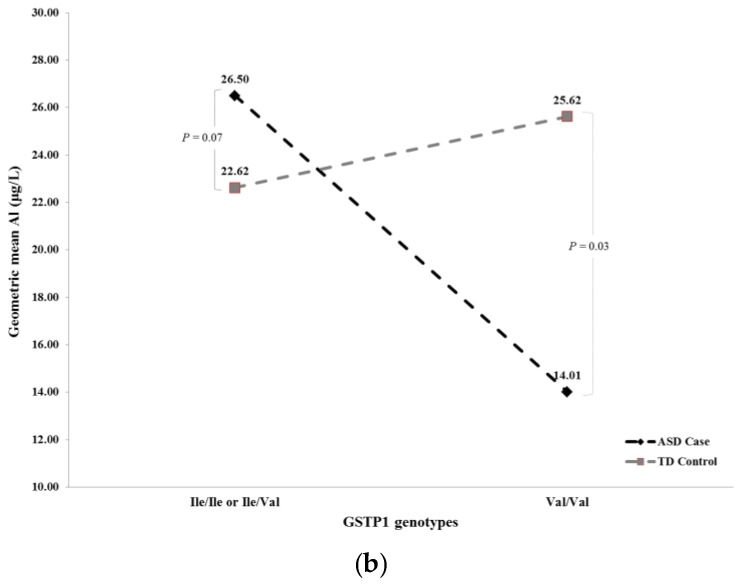
Further analysis of the significant interaction between ASD status and GSTP1 genotype in relation to blood aluminum concentrations revealed that while there was no significant association between ASD status and blood aluminum concentrations in children with the Ile/Ile and Ile/Val genotype (p = 0.43 and p = 0.38, respectively), there was a significant (p = 0.03) association between ASD status and blood aluminum concentrations in children with the Val/Val genotype. Similarly, in the recessive model, ASD children with the Val/Val genotype had a geometric mean blood aluminum concentrations of 25.62 µg/L, significantly higher (p = 0.03) than that observed for TD control children with the same Val/Val genotype (14.01 µg/L). However, when we adjusted for potential confounders, including parental education levels and consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish), these associations became marginally significant (p = 0.07) in the analysis using both the full and recessive models. Details regarding the comparison of the unadjusted and adjusted geometric blood aluminum concentrations between ASD cases and TD controls by GSTP1 genotype are shown in Table 5. Graphical illustrations of unadjusted geometric blood aluminum concentrations between ASD cases and TD control groups by GSTP1 genotype based on the full and recessive models are provided in Figure 2a,b.
Table 5.
Unadjusted and adjusted geometric mean blood aluminum concentrations by ASD status (ASD and TD control) based on General Linear Models (GLM) that includes interaction between GSTP1 genotypes and ASD case status (ASD and TD control) (106 matched pairs).
| Models | (Column A) Group Compared | Referent Group | GSTP1 Genotypes | Unadjusted Interactive Model (μg/L) ** | Adjusted Interactive Model (μg/L) c | ||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean Al of Children with Group Compared in Column A * | Geometric Mean Al of Children with Referent Group * | p d | Geometric Mean Al of Children with Group Compared in Column A * | Geometric Mean Al of Children with Referent Group * | p d | ||||
| Full a | ASD Case | TD Control | Ile/Ile | 22.81 | 27.57 | 0.43 | 21.71 | 24.19 | 0.66 |
| ASD Case | TD Control | Ile/Val | 22.54 | 26.02 | 0.38 | 23.16 | 23.39 | 0.96 | |
| ASD Case | TD Control | Val/Val | 25.56 | 14.00 | 0.03 | 24.39 | 14.60 | 0.07 | |
| Recessive b | ASD Case | TD Control | Val/Val | 25.62 | 14.01 | 0.03 | 24.38 | 14.57 | 0.07 |
| ASD Case | TD Control | Ile/Ile or Ile/Val | 22.62 | 26.50 | 0.18 | 22.62 | 23.75 | 0.73 | |
* Mean AL indicates the geometric mean = Exp. [Mean (ln AL)]; ** In the univariable GLMs, the independent variables include pairs, ASD status, GSTP1, and GSTP1 interaction with ASD; a GSTP1 in the full model has three categories (Ile/Ile, Ile/Val, and Val/Val); b GSTP1 (REC) = GSTP1 in the recessive model has two categories (Val/Val, Ile/Ile or Ile/Val); c In multivariable GLMs in addition to the variables in the univariable model we adjusted for parental education levels, consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish); d p-values are for the comparison of mean blood aluminum concentrations of children with the ASD case status in “Column A” compared to those with the TD control status in “referent group”, stratified by GSTP1 genotypes , based on CONTRAST option in the SAS program for GLMs as described in the Methods Section.
Figure 2.
(a) Unadjusted geometric blood aluminum concentrations based on result from Full Model in Table 5; and (b) unadjusted geometric blood aluminum concentrations based on result from Recessive Model in Table 5.
4. Discussion
4.1. Blood Aluminum Concentrations in Jamaican Children
In this research, to our knowledge, we are the first to assess and report distributions of blood aluminum concentrations for Jamaican children with and without ASD (2–8 years). The arithmetic mean blood aluminum concentration for children with ASD was 30.9 µg/L and the mean blood aluminum concentration for TD children was 36.9 µg/L. Data regarding blood aluminum concentrations in children are limited [2], and, to our knowledge, published reference ranges are not established for children. However, for healthy individuals the Agency for Toxic Substances and Disease Registry (ATSDR) considers a serum aluminum concentration of 1.00–3.00 µg/L as “normal” [2,71,72]. Therefore, it will not be possible to reliably estimate the proportion of Jamaican children with abnormal blood aluminum concentrations. On the other hand, aluminum toxicity is defined as a plasma aluminum concentration of >500 μg/L [2,71,72]. In our sample, the highest blood aluminum concentration was 221 μg/L. Therefore, none of the children in our sample had blood aluminum concentrations at the defined toxicity level.
Other studies have reported blood aluminum concentrations of children living in other countries. For example, a study from Riyadh, Saudi Arabia that involved 533 girls (6–8 years) reported a mean serum aluminum concentration of 23.21 μg/L (range 5.98–206.93 μg/L) [73]. Another study conducted in Romania during 2006–2007 involved two samples of children (8–12 years), one sample from Bucharest (N = 37) and the other sample from Pantelimon (N = 46), a city near a metal-processing plant. The Romania study reported geometric mean blood aluminum concentrations of 36 μg/L and 49 μg/L for Bucharest and Pantelimon, respectively [74]. While the mean blood aluminum concentration of Jamaican children is similar to that of children from Bucharest, it is higher than the mean serum aluminum concentration reported for children from Riyadh, Saudi Arabia. We acknowledge that the aluminum concentrations reported by the study from Saudi Arabia were measured in serum, which are shown to be similar to that of whole blood in the steady state [3].
4.2. Blood Aluminum Concentrations and ASD
Although we did not find a significant difference between the geometric mean blood aluminum concentrations of the ASD and TD control groups, in a recessive and in a full model that included GSTP1, ASD status, and their interaction term (i.e., GSTP1 × ASD) status we found significant interactions between ASD status and the GSTP1 Ile105Val polymorphism in relation to blood aluminum concentrations in both recessive and full models. Further analysis of the significant interaction between ASD status and the GSTP1 variant in relation to blood aluminum concentrations revealed that while there was no significant difference between ASD and TD children with respect to blood aluminum concentrations in children with the Ile/Ile and Ile/Val genotypes, there was a significant association between ASD status and blood aluminum concentrations in children with the Val/Val genotype. Similarly, in the recessive model, ASD children with the Val/Val genotype had a geometric mean blood aluminum concentration of 25.62 µg/L, significantly higher than 14.01 µg/L for TD control children with the same Val/Val genotype. However, in multivariable analysis when we also added potential confounders, including parental education levels and consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish), these associations became marginally significant in both full and recessive models. To our knowledge, we are the first to report an interactive association of ASD status and GSTP1 rs1695 in relation to blood aluminum concentrations of Jamaican children.
Other studies have reported higher levels of aluminum in the hair [45,46,47] and urine [48] of children with ASD compared to children without ASD. Although it appears that our results contrast with results from other studies, the difference could be due to the differences in methods of assessment of aluminum concentrations; we determined the concentration of aluminum in blood while these other studies assessed aluminum concentrations in hair and urine. Some studies investigated whether there is a correlation between hair and serum aluminum concentrations, but none found any significant associations [62,63,75]. Another major difference between our study and these previous studies is that when we compared blood aluminum concentrations of children with and without ASD, we accounted for a significant interaction between the GSTP1 variant and ASD status and also controlled for potential confounders. Specifically, in the multivariable GLM, we controlled for potential confounding by adjusting for parental education levels and the consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish). However, none of these other studies adjusted their results by such factors.
4.3. Role of GST Genes in Blood Aluminum Concentrations of Jamaican Children with and without ASD
Another unique aspect of our study is the availability of genetic data related to GSTT1, GSTM1, and GSTP1 genotypes. These data allowed assessment of associations between genetic variation in GSTT1, GSTM1, and GSTP1 and aluminum concentrations in blood. In an additive model, we did not find a significant association between the three GST polymorphisms and aluminum concentrations in blood. However, as mentioned earlier, we found a significant interaction between GSTP1 genotype and ASD status (p < 0.02) in relation to aluminum concentration in blood using an unadjusted recessive multivariable model. This finding indicates that the association between GSTP1 rs1695 and blood aluminum concentrations varies by ASD status. Specifically, we did not find a significant difference between geometric mean blood aluminum concentrations when comparing children with the Val/Val genotype and the Ile/Ile or Ile/Val genotypes for ASD cases. However, in the TD control group we observed a significantly lower (p < 0.01) geometric mean blood aluminum concentration for children who had the Val/Val genotype (14.01 µg/L), compared with the geometric mean blood aluminum concentration among children with Ile/Ile or Ile/Val genotypes (26.50 µg/L). Similar findings were observed for the TD control group in the adjusted recessive models that controlled for covariates. Specifically, after controlling for parental education levels and the consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish), we observed a significantly lower (p < 0.03) geometric mean blood aluminum concentration for children who had the Val/Val genotype (14.57 µg/L), compared with the geometric mean blood aluminum concentration among children with the Ile/Ile or Ile/Val genotypes (23.75 µg/L). A possible biological explanation for our finding that geometric mean blood aluminum concentrations in TD control children with the Val/Val genotype is lower than that of TD children with Val/Ile or Ile/Ile genotypes is that Val/Val homozygotes may detoxify aluminum more efficiently than those with other genotypes. This mechanism for detoxifying aluminum may be less efficient in ASD children with the same Val/Val genotype, leading to higher geometric blood aluminum concentrations compared to TD children. However, we did not find any previous literature supporting these hypotheses. To our knowledge, we are the first to report ASD status as an effect modifier when assessing the association between GSTP1 rs1695 and blood aluminum concentrations. Confirmation of this finding in other populations is warranted.
5. Limitations
We acknowledge several limitations in this study. First, since the control children for this study were selected to match the ASD cases by sex and age from the Kingston area, they may not represent a random sample from the population of all children in Jamaica. Additionally, our controls belonged to a lower SES group than our ASD cases. Therefore, the findings reported in this study may not be generalizable to populations other than that in which the samples were selected. Although our analyses for food consumption (fruits, vegetables, and seafood) were conducted under the assumption that most products were grown and caught locally, we acknowledge that some participants may have consumed foods imported from other locations; however, we did not assess this possibility in the food frequency questionnaire. In addition, it is possible that our analysis did not account for unmeasured confounding variables that may have a strong correlation with blood aluminum concentrations and GSTP1 rs1695 in the ASD group. We also acknowledge that it is possible that the observed association between GSTP1 rs1695 and blood aluminum concentrations may not necessarily represent an effect of the sequence variant if it is in linkage disequilibrium with a true causal polymorphism that was not measured in this study. Finally, we acknowledge that the p-values reported in this study were not adjusted to account for multiple comparisons.
6. Conclusions
In this study, we reported mean blood aluminum concentrations for Jamaican children that could serve as baseline data for exposure to aluminum in Jamaican children, age 2–8 years. Specifically, the mean blood aluminum concentration was 36.87 µg/L for the TD Jamaican children. In addition, based on multivariable analysis that controlled for potential confounding by adjusting for parental education levels and consumption of root vegetables (yam, sweet potato, or dasheen), avocado, and tuna (canned fish), we reported that ASD status may serve as an effect modifier of the association between GSTP1 rs1695 and blood aluminum concentrations of Jamaican children. However, it is difficult to provide a clear biological explanation for this finding. Future research should focus on better understanding this finding from our study.
Acknowledgments
This research is co-funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health Fogarty International Center (NIH-FIC) by a grant (R21HD057808) as well as National Institute of Environmental Health Sciences (NIEHS) by a grant (R01ES022165) awarded to University of Texas Health Science Center at Houston. We also acknowledge the support provided by the Biostatistics/Epidemiology/Research Design (BERD) component of the Center for Clinical and Translational Sciences (CCTS) for this project. CCTS is mainly funded by the NIH Centers for Translational Science Award (NIH CTSA) grant (UL1 RR024148), awarded to University of Texas Health Science Center at Houston in 2006 by the National Center for Research Resources (NCRR) and its renewal (UL1 TR000371) by the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH-FIC or NIEHS or the NCRR or the NCATS.
Author Contributions
Mohammad H. Rahbar, Maureen Samms-Vaughan, Katherine A. Loveland, Jan Bressler, and Eric Boerwinkle have made substantial contributions to conception and study design; Maureen Samms-Vaughan, Sydonnie Shakespeare-Pellington, Compton Beecher, and Wayne McLaughlin contributed to acquisition of data; Manouchehr Hessabi, Sydonnie Shakespeare-Pellington, Maureen Samms-Vaughan, Megan L. Grove, Compton Beecher, Wayne McLaughlin, and Mohammad H. Rahbar have made contributions to data quality assurance procedures; Mohammad H. Rahbar and Manouchehr Hessabi conducted data analysis; Mohammad H. Rahbar, Jan Bressler, Meagan R. Pitcher, and Manouchehr Hessabi have contributed to interpretation of data; Mohammad H. Rahbar, Meagan R. Pitcher, MacKinsey A. Christian, and Manouchehr Hessabi significantly contributed to drafting of the manuscript; and Mohammad H. Rahbar, Jan Bressler, Meagan R. Pitcher, Maureen Samms-Vaughan, and Eric Boerwinkle provided critical revision of the manuscript. All authors have read and approved the final version submitted for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Exley C. A biogeochemical cycle for aluminium? J. Inorg. Biochem. 2003;97:1–7. doi: 10.1016/S0162-0134(03)00274-5. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Aluminum. ATSDR; Atlanta, GA, USA: 2008. [PubMed] [Google Scholar]
- 3.Krewski D., Yokel R.A., Nieboer E., Borchelt D., Cohen J., Harry J., Kacew S., Lindsay J., Mahfouz A.M., Rondeau V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B Crit. Rev. 2007;10(Suppl. 1):1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruins M.R., Kapil S., Oehme F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 2000;45:198–207. doi: 10.1006/eesa.1999.1860. [DOI] [PubMed] [Google Scholar]
- 5.Rosseland B.O., Eldhuset T.D., Staurnes M. Environmental effects of aluminium. Environ. Geochem. Health. 1990;12:17–27. doi: 10.1007/BF01734045. [DOI] [PubMed] [Google Scholar]
- 6.Lemire J., Mailloux R., Auger C., Whalen D., Appanna V.D. Pseudomonas fluorescens orchestrates a fine metabolic-balancing act to counter aluminium toxicity. Environ. Microbiol. 2010;12:1384–1390. doi: 10.1111/j.1462-2920.2010.02200.x. [DOI] [PubMed] [Google Scholar]
- 7.Delhaize E., Ma J.F., Ryan P.R. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012;17:341–348. doi: 10.1016/j.tplants.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Katyal R., Desigan B., Sodhi C.P., Ojha S. Oral aluminum administration and oxidative injury. Biol. Trace Elem. Res. 1997;57:125–130. doi: 10.1007/BF02778195. [DOI] [PubMed] [Google Scholar]
- 9.Sparling D.W., Lowe T.P. Environmental hazards of aluminum to plants, invertebrates, fish, and wildlife. Rev. Environ. Contam. Toxicol. 1996;145:1–127. doi: 10.1007/978-1-4612-2354-2_1. [DOI] [PubMed] [Google Scholar]
- 10.Yokel R.A. The toxicology of aluminum in the brain: A review. Neurotoxicology. 2000;21:813–828. [PubMed] [Google Scholar]
- 11.Shaw C.A., Seneff S., Kette S.D., Tomljenovic L., Oller J.W., Jr., Davidson R.M. Aluminum-induced entropy in biological systems: Implications for neurological disease. J. Toxicol. 2014;2014:491316. doi: 10.1155/2014/491316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai-Ashmawy M.A. Prevalence and public health significance of aluminum residues in milk and some dairy products. J. Food Sci. 2011;76:T73–T76. doi: 10.1111/j.1750-3841.2011.02064.x. [DOI] [PubMed] [Google Scholar]
- 13.Weidenhamer J.D., Kobunski P.A., Kuepouo G., Corbin R.W., Gottesfeld P. Lead exposure from aluminum cookware in Cameroon. Sci. Total Environ. 2014;496:339–347. doi: 10.1016/j.scitotenv.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Agency for Toxic Substances and Disease Registry (ATSDR) Public Health Statement for Aluminum. ATSDR; Atlanta, GA, USA: 2008. [Google Scholar]
- 15.Zatta P., Lain E., Cagnolini C. Effects of aluminum on activity of krebs cycle enzymes and glutamate dehydrogenase in rat brain homogenate. Eur. J. Biochem. 2000;267:3049–3055. doi: 10.1046/j.1432-1033.2000.01328.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z., Du Y., Xue H., Wu Y., Zhou B. Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiol. Aging. 2012;33:199.e1–199.e12. doi: 10.1016/j.neurobiolaging.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Prakash D., Gopinath K., Sudhandiran G. Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. Neuromol. Med. 2013;15:192–208. doi: 10.1007/s12017-012-8210-1. [DOI] [PubMed] [Google Scholar]
- 18.Aremu D.A., Meshitsuka S. Some aspects of astroglial functions and aluminum implications for neurodegeneration. Brain Res. Rev. 2006;52:193–200. doi: 10.1016/j.brainresrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Schousboe A. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem. Res. 2003;28:347–352. doi: 10.1023/A:1022397704922. [DOI] [PubMed] [Google Scholar]
- 20.Campbell A., Hamai D., Bondy S.C. Differential toxicity of aluminum salts in human cell lines of neural origin: Implications for neurodegeneration. Neurotoxicology. 2001;22:63–71. doi: 10.1016/S0161-813X(00)00007-3. [DOI] [PubMed] [Google Scholar]
- 21.Abu Shmais G.A., Al-Ayadhi L.Y., Al-Dbass A.M., El-Ansary A.K. Mechanism of nitrogen metabolism-related parameters and enzyme activities in the pathophysiology of autism. J. Neurodev. Disord. 2012;4:4. doi: 10.1186/1866-1955-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada M., Taki M.M., Nose A., Kubo H., Mori K., Nishitani H., Matsuda T. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J. Autism Dev. Disord. 2011;41:447–454. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- 23.El-Ansary A., Al-Ayadhi L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2014;11:189. doi: 10.1186/s12974-014-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Ansary A. Data of multiple regressions analysis between selected biomarkers related to glutamate excitotoxicity and oxidative stress in Saudi autistic patients. Data Brief. 2016;7:111–116. doi: 10.1016/j.dib.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rengel Z. Aluminium cycling in the soil-plant-animal-human continuum. Biometals. 2004;17:669–689. doi: 10.1007/s10534-004-1201-4. [DOI] [PubMed] [Google Scholar]
- 26.Martin R.B. The chemistry of aluminum as related to biology and medicine. Clin. Chem. 1986;32:1797–1806. [PubMed] [Google Scholar]
- 27.Crichton R.R., Wilmet S., Legssyer R., Ward R.J. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 2002;91:9–18. doi: 10.1016/S0162-0134(02)00461-0. [DOI] [PubMed] [Google Scholar]
- 28.Oguz E.O., Enli Y., Sahin B., Gonen C., Turgut G. Aluminium sulphate exposure increases oxidative stress and suppresses brain development in Ross broiler chicks. Med. Sci. Monit. 2012;18:BR103–BR108. doi: 10.12659/MSM.882515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan C.Y., Lee Y.J., Hsu G.S. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J. Biomed. Sci. 2012;19:51. doi: 10.1186/1423-0127-19-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumathi T., Shobana C., Kumari B.R., Nandhini D.N. Protective role of Cynodon dactylon in ameliorating the aluminium-induced neurotoxicity in rat brain regions. Biol. Trace Elem. Res. 2011;144:843–853. doi: 10.1007/s12011-011-9029-6. [DOI] [PubMed] [Google Scholar]
- 31.Hayes J.D., Pulford D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 32.Salinas A.E., Wong M.G. Glutathione S-transferases—A review. Curr. Med. Chem. 1999;6:279–309. [PubMed] [Google Scholar]
- 33.Higgins L.G., Hayes J.D. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- 34.Josephy P.D. Genetic variations in human glutathione transferase enzymes: Significance for pharmacology and toxicology. Hum. Genom. Proteom. 2010;2010:876940. doi: 10.4061/2010/876940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidegard J., Ekstrom G. The role of human glutathione transferases and epoxide hydrolases in the metabolism of xenobiotics. Environ. Health Perspect. 1997;105(Suppl. 4):791–799. doi: 10.1289/ehp.97105s4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebert D.W., Vasiliou V. Analysis of the glutathione S-transferase (GST) gene family. Hum. Genom. 2004;1:460–464. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossignol D.A., Genuis S.J., Frye R.E. Environmental toxicants and autism spectrum disorders: A systematic review. Transl. Psychiatry. 2014;4:e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gundacker C., Komarnicki G., Jagiello P., Gencikova A., Dahmen N., Wittmann K.J., Gencik M. Glutathione-S-transferase polymorphism, metallothionein expression, and mercury levels among students in Austria. Sci. Total Environ. 2007;385:37–47. doi: 10.1016/j.scitotenv.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 39.Klautau-Guimarães M.D.N., DAscenção R., Caldart F.A., Grisolia C.K., de Souza J.R., Barbosa A.C., Cordeiro C.M.T., Ferrari I. Analysis of genetic susceptibility to mercury contamination evaluated through molecular biomarkers in at-risk Amazon Amerindian populations. Genet. Mol. Biol. 2005;28:827–832. doi: 10.1590/S1415-47572005000500027. [DOI] [Google Scholar]
- 40.Khanna P., Nehru B. Antioxidant enzymatic system in neuronal and glial cells enriched fractions of rat brain after aluminum exposure. Cell. Mol. Neurobiol. 2007;27:959–969. doi: 10.1007/s10571-007-9233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halatek T., Trzcinka-Ochocka M., Matczak W., Gruchala J. Serum Clara cell protein as an indicator of pulmonary impairment in occupational exposure at aluminum foundry. Int. J. Occup. Med. Environ. Health. 2006;19:211–223. doi: 10.2478/v10001-006-0033-6. [DOI] [PubMed] [Google Scholar]
- 42.Rahbar M.H., Samms-Vaughan M., Ma J., Bressler J., Loveland K.A., Ardjomand-Hessabi M., Dickerson A.S., Grove M.L., Shakespeare-Pellington S., Beecher C., et al. Role of Metabolic Genes in Blood Arsenic Concentrations of Jamaican Children with and without Autism Spectrum Disorder. Int. J. Environ. Res. Public Health. 2014;11:7874–7895. doi: 10.3390/ijerph110807874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahbar M.H., Samms-Vaughan M., Ma J., Bressler J., Loveland K.A., Hessabi M., Dickerson A.S., Grove M.L., Shakespeare-Pellington S., Beecher C., et al. Interaction between GSTT1 and GSTP1 allele variants as a risk modulating-factor for autism spectrum disorders. Res. Autism Spectr. Disord. 2015;12:1–9. doi: 10.1016/j.rasd.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahbar M.H., Samms-Vaughan M., Ma J., Bressler J., Dickerson A.S., Hessabi M., Loveland K.A., Grove M.L., Shakespeare-Pellington S., Beecher C., et al. Synergic effect of GSTP1 and blood manganese concentrations in Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2015;18:73–82. doi: 10.1016/j.rasd.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed F.B., Zaky E.A., El-Sayed A.B., Elhossieny R.M., Zahra S.S., Salah E.W., Youssef W.Y., Khaled R.A., Youssef A.M. Assessment of Hair Aluminum, Lead, and Mercury in a Sample of Autistic Egyptian Children: Environmental Risk Factors of Heavy Metals in Autism. Behav. Neurol. 2015;2015:545674. doi: 10.1155/2015/545674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda H., Tsutsui T. Assessment of infantile mineral imbalances in autism spectrum disorders (ASDs) Int. J. Environ. Res. Public Health. 2013;10:6027–6043. doi: 10.3390/ijerph10116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blaurock-Busch E., Amin O.R., Dessoki H.H., Rabah T. Toxic Metals and Essential Elements in Hair and Severity of Symptoms among Children with Autism. Maedica (Buchar) 2012;7:38–48. [PMC free article] [PubMed] [Google Scholar]
- 48.Blaurock-Busch E., Amin O.R., Rabah T. Heavy metals and trace elements in hair and urine of a sample of arab children with autistic spectrum disorder. Maedica (Buchar) 2011;6:247–257. [PMC free article] [PubMed] [Google Scholar]
- 49.Fido A., Al-Saad S. Toxic trace elements in the hair of children with autism. Autism. 2005;9:290–298. doi: 10.1177/1362361305053255. [DOI] [PubMed] [Google Scholar]
- 50.Al-Ayadhi L.Y. Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neurosciences (Riyadh) 2005;10:213–218. [PubMed] [Google Scholar]
- 51.Albizzati A., More L., Di C.D., Saccani M., Lenti C. Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr. 2012;64:27–31. [PubMed] [Google Scholar]
- 52.Lalor G.C. Geochemical mapping in Jamaica. Environ. Geochem. Health. 1996;18:89–97. doi: 10.1007/BF01771284. [DOI] [PubMed] [Google Scholar]
- 53.Rahbar M.H., Samms-Vaughan M., Ardjomand-Hessabi M., Loveland K.A., Dickerson A.S., Chen Z., Bressler J., Shakespeare-Pellington S., Grove M.L., Bloom K., et al. The role of drinking water sources, consumption of vegetables and seafood in relation to blood arsenic concentrations of Jamaican children with and without Autism Spectrum Disorders. Sci. Total Environ. 2012;433:362–370. doi: 10.1016/j.scitotenv.2012.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahbar M.H., Samms-Vaughan M., Loveland K.A., Ardjomand-Hessabi M., Chen Z., Bressler J., Shakespeare-Pellington S., Grove M.L., Bloom K., Pearson D.A., et al. Seafood Consumption and Blood Mercury Concentrations in Jamaican Children With and Without Autism Spectrum Disorders. Neurotox. Res. 2013;23:22–38. doi: 10.1007/s12640-012-9321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Publishing, Inc.; Washington, DC, USA: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 56.Schopler E., Reichler R.J., DeVellis R.F., Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J. Autism Dev. Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 57.Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- 58.Rutter M., Le C.A., Lord C. Autism Diagnostic Interview-Revised (ADI-R) Western Psychological Services; Los Angeles, CA, USA: 2003. [Google Scholar]
- 59.Rutter M., Bailey A., Lord C. The Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA, USA: 2003. [Google Scholar]
- 60.Mulligan A., Richardson T., Anney R.J., Gill M. The Social Communication Questionnaire in a sample of the general population of school-going children. Ir. J. Med. Sci. 2009;178:193–199. doi: 10.1007/s11845-008-0184-5. [DOI] [PubMed] [Google Scholar]
- 61.Analytical Research Laboratories . Aluminum Toxicity. Analytical Research Laboratories, Inc.; Oklahoma City, OK, USA: 1989. [Google Scholar]
- 62.Wilhelm M., Passlick J., Busch T., Szydlik M., Ohnesorge F.K. Scalp hair as an indicator of aluminium exposure: Comparison to bone and plasma. Hum. Toxicol. 1989;8:5–9. doi: 10.1177/096032718900800102. [DOI] [PubMed] [Google Scholar]
- 63.Pineau A., Guillard O., Huguet F., Speich M., Gelot S., Boiteau H.L. An evaluation of the biological significance of aluminium in plasma and hair of patients on long-term hemodialysis. Eur. J. Pharmacol. 1993;228:263–268. doi: 10.1016/0926-6917(93)90059-Y. [DOI] [PubMed] [Google Scholar]
- 64.Naylor G.J., Sheperd B., Treliving L., McHarg A., Smith A., Ward N., Harper M. Tissue aluminum concentrations stability over time, relationship to age, and dietary intake. Biol. Psychiatry. 1990;27:884–890. doi: 10.1016/0006-3223(90)90469-I. [DOI] [PubMed] [Google Scholar]
- 65.Giddings M.M., Magara Y., Ohanian E. Aluminium in Drinking-Water, Background Document for Development of WHO Guidelines for Drinking-Water Quality. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 66.Zeager M., Woolf A.D., Goldman R.H. Wide variation in reference values for aluminum levels in children. Pediatrics. 2012;129:e142–e147. doi: 10.1542/peds.2010-3481. [DOI] [PubMed] [Google Scholar]
- 67.Dols M., Chartier J., Lem P. Compatibility of the PUREGENE DNA Purification Kit with the Oragene Self-Collection Kit. DNA Genotek Inc.; Kanata, ON, Canada: 2014. pp. 3–6. [Google Scholar]
- 68.Li R., Boerwinkle E., Olshan A.F., Chambless L.E., Pankow J.S., Tyroler H.A., Bray M., Pittman G.S., Bell D.A., Heiss G. Glutathione S-transferase genotype as a susceptibility factor in smoking-related coronary heart disease. Atherosclerosis. 2000;149:451–462. doi: 10.1016/S0021-9150(99)00483-9. [DOI] [PubMed] [Google Scholar]
- 69.Kleinbaum D.G., Klein M. Logistic Regression: A Self-Learning Text. 3rd ed. Springer; New York, NY, USA: 2010. [Google Scholar]
- 70.SAS Institute Inc. SAS® 9.4. SAS Institute Inc.; Cary, NC, USA: 2013. [Google Scholar]
- 71.House R.A. Factors affecting plasma aluminum concentrations in nonexposed workers. J. Occup. Med. 1992;34:1013–1017. [PubMed] [Google Scholar]
- 72.Liao Y.H., Yu H.S., Ho C.K., Wu M.T., Yang C.Y., Chen J.R., Chang C.C. Biological monitoring of exposures to aluminium, gallium, indium, arsenic, and antimony in optoelectronic industry workers. J. Occup. Environ. Med. 2004;46:931–936. doi: 10.1097/01.jom.0000137718.93558.b8. [DOI] [PubMed] [Google Scholar]
- 73.Al-Saleh I., Shinwari N. Aluminum in Saudi children. Biometals. 1996;9:385–392. doi: 10.1007/BF00140608. [DOI] [PubMed] [Google Scholar]
- 74.Nicolescu R., Petcu C., Cordeanu A., Fabritius K., Schlumpf M., Krebs R., Kramer U., Winneke G. Environmental exposure to lead, but not other neurotoxic metals, relates to core elements of ADHD in Romanian children: Performance and questionnaire data. Environ. Res. 2010;110:476–483. doi: 10.1016/j.envres.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Hewitt C.D., Day J.P. Aluminium and copper concentrations in hair and serum are unrelated in renal patients. Acta Pharmacol. Toxicol. (Copenh) 1986;59(Suppl. 7):442–445. doi: 10.1111/j.1600-0773.1986.tb02798.x. [DOI] [PubMed] [Google Scholar]