Abstract
The purpose of this single-subject case study was to quantify the effect of gait-like vibration training on gait abilities after an incomplete spinal cord injury (SCI). A 62-year-old male with a chronic American Spinal Injury Association Impairment Scale D SCI at T11 completed nine sessions of gait-like vibration training in a standing position. Self-selected gait speed and distance covered within 6 min were determined before and after training to evaluate the impact of training on gait performance. Associated changes in gait kinematics were assessed with a three-dimensional motion analysis system. Results showed an improvement of gait speed (0.26 vs 0.35 m s−1) and distance (23 vs 37 m) after nine gait-like vibration training sessions (+34.6%; +60.9%). In addition, more bilateral hip extension and larger left hip range of motion improved hip–knee cyclograms. Gait-like vibration training improved gait abilities in a person with chronic incomplete SCI.
Subject terms: Central pattern generators
Introduction
After a non-degenerative neurological deficit such as a spinal cord injury (SCI), improvements in gait depend largely on sensory afferents generated by repeated lower-limb gait movements during gait training. 1,2 These afferents are essential for motor learning and adaptive plasticity during locomotor training. 3 The need to provide task-specific sensory afferents explains in part the development of task-oriented approaches such as treadmill training with or without body weight support, and robotic exoskeletons. 4
Another alternative to generate muscle afferents in the absence of movements relies on localized muscle vibration. Muscle vibration is a powerful sensory stimulation that mainly activates Ia muscle afferents. 5,6 This stimulation is associated with the perception of joint movement without actual movement, 7 muscle activity of the vibrated muscle or its antagonist, 8,9 and brain activity similar to the one producing voluntary contraction of the vibrated muscle. 10,11 Application of muscle vibration may improve motor performance after SCI, and particularly during gait. After motor incomplete SCI (American Spinal Injury Association (ASIA) Impairment Scale (AIS) C and D), activity of the thigh muscles increased and had better timing during robotic-assisted walking when 80 Hz vibration was applied on the quadriceps muscle during specific phases of the gait cycle. 12 This indicates that muscle activity intensity and timing can be altered with bouts of muscle vibration after incomplete SCI. Continuous 60 Hz vibration of one lower-limb muscle can also elicit involuntary, step-like lower-limb movements, in participants with SCI (AIS A–D), lying sideways, with their lower limbs supported and free to move in the horizontal plane without friction. 13 Finally, reduction of spasticity has been observed after continuous vibration (frequency of 50 to 100 Hz) of different lower-limb muscles in individuals with AIS A–D spinal cord lesions. 14
When properly patterned, multiple localized vibrations can induce a perception of complex movements, such as writing, in healthy individuals. 15 Multiple vibrations can also be patterned on the sequence of lower-limb muscle lengthening to mimic sensory activity of normal gait. 16 Applied in individuals with SCI 16 or hemiparesis due to stroke (personal communication) in quiet standing, these gait-patterned vibrations induced small amplitude stepping-in-place movements of the lower limbs. Gait-like vibrations may therefore be an appropriate sensory stimulation for gait training after SCI. The objective of this case study was thus to test whether gait-like vibration training can improve gait abilities in an individual with incomplete SCI.
Materials and methods
Participant
A 62-year-old male with a chronic AIS D SCI at the T11 level, due to a cycling accident 10 years before, participated in the study. He was able to ambulate 10 m with a walker, no braces, and close supervision of one person as he relies on a manual wheelchair as his primary mode of locomotion.
On the AIS, his lower extremity motor score was 30/50, light touch score was 92/112 and pin/prick score was 77/112. Mild spasticity was measured (modified Ashworth scale) at the right hip adductors (1) and plantarflexors (1), left knee extensors (1) and bilateral knee flexors (1).
Vibration training program
Prior to the vibration training program, the participant completed a home-based 12-week standing program to prepare for the vibration training program. The standing time was progressed to reach 1 h of continuous standing within a standing frame daily before initiating the vibration training program.
Thereafter, he attended nine vibration training sessions within 2 weeks (five sessions during week 1 and four sessions during week 2). It included only gait-like patterned muscle vibrations in various combinations of 1, 2 or 5 min vibration bouts for a total vibration duration of 20–25 min for the first six sessions and progressed to 35–40 min for the last three sessions. The vibrations were applied when the participant was in a quiet standing position, supported and helped for balance by a body weight support system. His weight was relieved by about 40% of his body weight, as measured by the support system. He was also holding himself upright using minimal upper-limb support. As previously described, gait movements of small joints amplitudes were induced during the vibration sessions. For the first and second sessions, the participant received the vibration without performing any voluntary activity. At the third session, he was encouraged to add voluntary movements to increase the amplitude of the stepping-in-place movements triggered by the patterned vibrations. Amplitudes of this stepping-in-place movements were not quantified. The first bout of vibration was applied with the eyes closed for the participant to focus on the stimulation. Then for some bouts of vibration, the subjects was asked to open his eyes and look straight ahead. Rest periods were allowed upon participant’s request.
Vibration stimulation
Twelve electromechanical vibrators (VB115, Technoconcept, Mane, France) were fixed bilaterally and transversally on the flexor and extensor muscle groups at the hips (that is, on the proximal tendon of the rectus femoris below the anterior inferior iliac spine and on the proximal tendon of the hamstring below the ischial tuberosity), knees (that is, on the patellar tendon below the patella and on the medial hamstrings above the medial femoral condyle) and ankles (that is, on the distal tendon of the dorsiflexors and of the triceps surae both at the level of the malleoli) using elastic bands (Figure 1). The vibrators were activated and deactivated cyclically according to a pattern mimicking the timing of muscle lengthening during a typical gait pattern. The duration of each cycle lasted 1 or 2 s to vary gait speed, and cycles were repeated consecutively for 1, 2 or 5 min periods. The targeted frequency during the activation of the vibrators is 80 Hz, as this frequency strongly activates muscle sensory afferents. 17,18 The feasibility of producing this stimulation has been documented previously. 16
Figure 1.
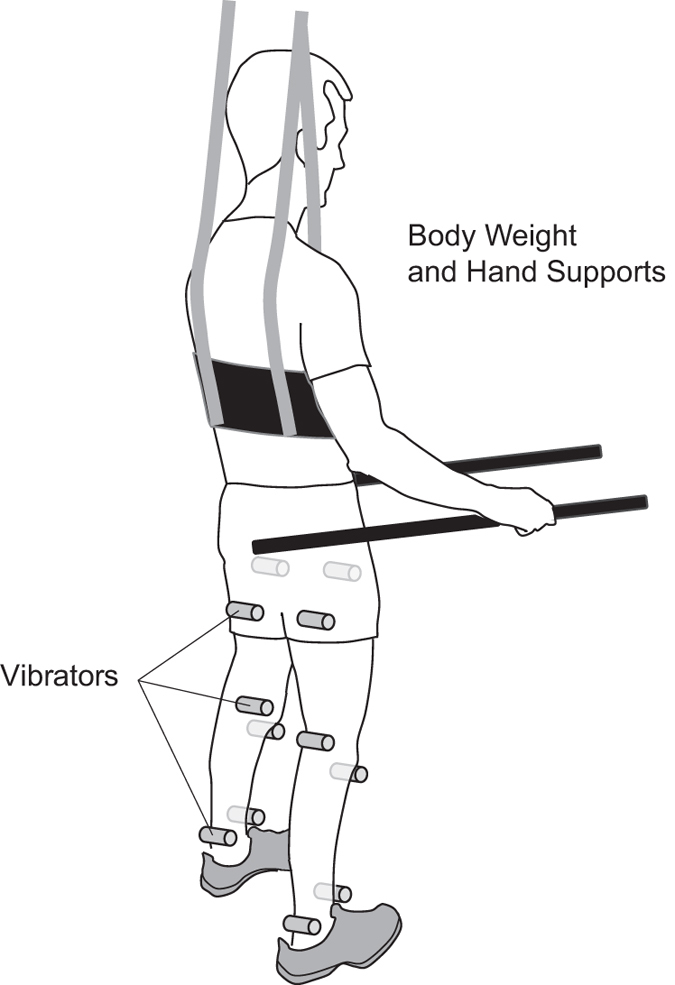
Vibration stimulation device and installation for vibration training. Twelve vibrators (gray cylinders) were placed bilaterally on the flexor and extensor muscle groups at the hips, knees and ankles. They were held in place using elastic bands. The participant was in a quiet standing position, supported and helped for balance by hand supports and a body weight support system that relieved about 40% of his body weight.
Clinical evaluations
Self-selected gait speed was evaluated using the 10-m walk test and the walking distance was measured using the 6-min walk test. 19,20 During these evaluations, the participant used a rolling walker and no other physical assistance was provided. The tests were performed twice before the beginning of the training session to establish initial locomotor capacity and measure natural variability of these measures, and once the day after the last training session. A familiarization test session was also completed before the first evaluation session. The pre-training locomotor capacity (that is, speed and distance) represents the mean of the values obtained during the two pre-training assessment sessions.
Gait assessment
Lower-limb kinematics was recorded using an NDI Certus motion capture system (Waterloo, ON, Canada), with at least three non-collinear markers on each major lower-limb segments (feet, shanks and thighs) and on the pelvis. The participant walked continuously (30 consecutive steps) on a Bertec dual-belt instrumented treadmill (Columbus, OH, USA), using handrails to support part of his body weight. A security harness was used only to prevent fall and provided no body weight support. To test the effects of training on kinematics, the same self-selected speed was used in both pre- and post-training assessment (that is, 0.185 m s−1). No vibration was applied during gait evaluation. Flexion peak, extension peak and range of motion at the ankle, knee and hip were calculated on both sides from 10 gait cycles for each gait trial.
Data analysis
Descriptive statistics were used for pre- and post-training clinical evaluations (gait speed and walking distance over 6 min). The effect of the vibration training program on kinematic data was assessed by comparing each variable pre- and post-training. Kinematics was also analyzed using hip–knee cyclograms. Their shape was compared with normative data 21 before and after vibration training.
Results
Clinical evaluations
Gait speed increased from 0.26 m s−1 (0.26 m s−1 at each pre-training trial) to 0.35 m s−1 in post-training (increase of 34.6%). Walking distance over 6 min increased from 23 m (25 and 20 m for each pre-training trial) to 37 m in post-training (increase of 60.9%).
Kinematic data
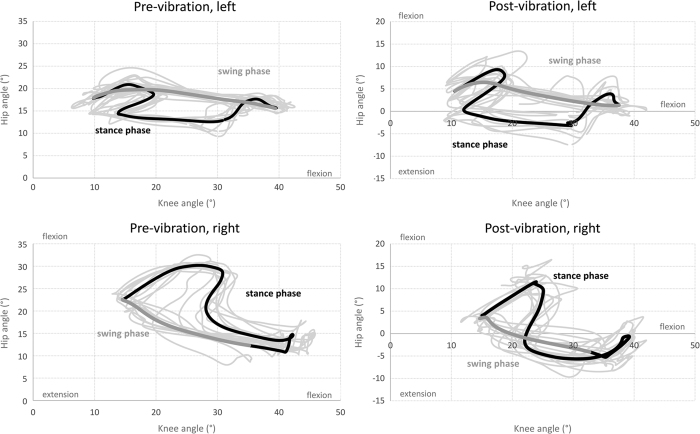
Joint amplitudes were overall smaller than expected based on normative data. Pre-training cyclograms (Figure 2) were mainly affected due to alterations of both hip and knee joint motions. During stance phase, hip and knee flexions were exaggerated, leading to an ‘upward–rightward’ cyclogram after heel contact. This showed the difficulty to maintain the lower-limb position during weight acceptance, particularly on the right lower-limb. Hip and knee joints were also more in flexion throughout the cycle, leading to a shift of the whole cyclograms toward flexion values. At the end of stance phase, hip flexion was initiated but stopped and reversed toward extension, to a larger extent at the right hip, before knee flexion and a limited flexion only on the right hip generated swing. The swing phase was largely produced by knee extension and limited hip flexion, particularly small at the left hip.
Figure 2.
Joint angle analysis, hip–knee cyclograms. Top of the panel: left lower-limb, bottom of the panel: right lower-limb, left part of the panel: cyclograms before vibration training, right part of the panel: cyclograms after vibration training, bold black (stance phase) and dark gray (swing phase) lines are for average gait trial and thin light gray lines represent each gait trial. Note that the vertical scale was adapted due to the shift of the cyclograms towards hip extension in post-training.
After training, there were little changes in range of motion (ROM) at the knees (Table 1). At the left hip, the ROM increased (ROM pre=9.8°, ROM post=14.1°) due to an increase in the maximal hip extension angle. At the right hip, ROM did not change. However, for both hips, the whole ROM shifted toward extension by more than 20°, resulting in hip motions reaching below-zero values, that is, in the actual extension range (Table 1). In addition, the relative motion of the hip and knee improved at the right lower-limb during swing phase, leading to a cyclogram more similar to the one of the left lower-limb particularly before swing phase (Figure 2).
Table 1. Mean bilateral amplitudes at the ankle, knee and hip joints during gait evaluation on the treadmill (mean of 10 gait cycles).
|
Right ankle
|
Left ankle
|
|||||
|---|---|---|---|---|---|---|
| Dorsi flexion (°) | Plantar flexion (°) | ROM (°) | Dorsi flexion (°) | Plantar flexion (°) | ROM (°) | |
| Pre | 7.7 | −17.1 | 24.8 | 8.6 | −13.2 | 21.8 |
| Post | 4.6 | −17.5 | 22.1 | 8.8 | −13.9 | 22.6 |
|
Right knee
|
Left knee
|
|||||
|---|---|---|---|---|---|---|
| Flexion (°) | Extension (°) | ROM (°) | Flexion (°) | Extension (°) | ROM (°) | |
| Pre | 42.9 | 14.2 | 28.8 | 40.0 | 8.8 | 31.2 |
| Post | 39.6 | 13.9 | 25.6 | 38.1 | 9.5 | 28.6 |
|
Right hip
|
Left hip
|
|||||
|---|---|---|---|---|---|---|
| Flexion (°) | Extension (°) | ROM (°) | Flexion (°) | Extension (°) | ROM (°) | |
| Pre | 30.4 | 10.4 | 20.0 | 21.6 | 11.7 | 9.8 |
| Post | 11.7 | −7.0 | 18.7 | 9.6 | −4.5 | 14.1 |
Abbreviation: ROM, range of motion.
Variability of the lower-limb movements also increased between cycles post-training with less superimposition between cycles on the cyclograms compared with pre-training (Figure 2).
Discussion
The results showed that patterned gait-like vibrations, used repeatedly and exclusively in a gait training program, can improve self-selected gait speed and total distance traveled over 6 min in a male with a chronic incomplete SCI. These improvements in performance are similar to those obtained in training programs requiring actual gait practice. 1,4 Interestingly, these training programs lasted often more than 20 sessions and up to 144 session in some cases. 1,4 Further study will have to test whether longer vibration training would be more efficient, and to what extent it relates to the initial level of performance or time since injury.
The vibration training also altered the participant’s lower-limb kinematics toward an improved gait pattern that likely contributed to improve gait performances. Among the positive kinematic changes, a shift toward extension was obtained at both hips post training. The more extended hip position is not likely due only to the sessions in standing position, as the participant prepared for this training by standing daily during the month before the vibration training. It is more likely that the motor facilitation induced by the vibration improved the voluntary control of lower-limb muscle activity 12 at the hips and particularly at the right lower-limb that also showed improved coordination. This might also explain the larger variability of the cyclograms observed post-training. The increased variability suggests that the participant has more ability to alter his gait pattern than before training and represents a favorable outcome. 22
Overall, this study strengthens existing evidence that sensory activity patterned according to the task can improve this task as already proposed before. 1,3 However, the importance of the pattern of the sensory stimulation remains to be thoroughly evaluated since whole-body vibration has also been found to improve gait speed to a similar extent (0.06 m s−1) among 17 persons with a chronic SCI participants with similar clinical characteristics. 23 One can suspect that the mechanisms of action differ slightly, between a global, non-specific stimulation during whole-body vibration compared with a task-oriented specific stimulation during patterned vibration.
This case study aimed to test whether gait-like vibration training could improve gait abilities in one person with chronic AIS D SCI. The results of this study provide preliminary evidence of a positive effect of patterned vibration training associated with voluntary movements on gait abilities after SCI. Vibration training uses low-tech and low-cost technology that can easily be integrated into clinical practice in rehabilitation facilities or at home. Further studies are now warranted to further test the generalizability of the results, the perceived satisfaction of the users, and the efficiency of this novel rehabilitation intervention.
Acknowledgments
DHG and CD are members of the SensoriMotor Rehabilitation Research Team (SMRRT). DHG co-chairs the Initiative for the Development of New Technologies and Practices in Rehabilitation (INSPIRE) funded by the LRH Foundation via the Lindsay Rehabilitation Prize. We thank the participant for his commitment to the training program and Ann-Julie Côté and Andréanne Bergeron for their technical assistance.
The authors declare no conflict of interest.
References
- Harkema SJ , Hillyer J , Schmidt-Read M , Ardolino E , Sisto SA , Behrman AL . Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil 2012; 93: 1588–1597. [DOI] [PubMed] [Google Scholar]
- D'Amico JM , Condliffe EG , Martins KJ , Bennett DJ , Gorassini MA . Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Neurosci 2014; 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubli M , Dietz V . The physiological basis of neurorehabilitation--locomotor training after spinal cord injury. J Neuroeng Rehabil 2013; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawietz C , Moffat F . Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 2013; 94: 2297–2308. [DOI] [PubMed] [Google Scholar]
- Roll JP , Vedel JP . Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 1982; 47: 177–190. [DOI] [PubMed] [Google Scholar]
- Fallon JB , Macefield VG . Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve 2007; 36: 21–29. [DOI] [PubMed] [Google Scholar]
- Gilhodes JC , Roll JP , Tardy-Gervet MF . Perceptual and motor effects of agonist-antagonist muscle vibration in man. Exp Brain Res 1986; 61: 395–402. [DOI] [PubMed] [Google Scholar]
- Calvin-Figuiere S , Romaiguere P , Gilhodes JC , Roll JP . Antagonist motor responses correlate with kinesthetic illusions induced by tendon vibration. Exp Brain Res 1999; 124: 342–350. [DOI] [PubMed] [Google Scholar]
- Forner-Cordero A , Steyvers M , Levin O , Alaerts K , Swinnen SP . Changes in corticomotor excitability following prolonged muscle tendon vibration. Behav Brain Res 2008; 190: 41–49. [DOI] [PubMed] [Google Scholar]
- Romaiguere P , Anton JL , Roth M , Casini L , Roll JP . Motor and parietal cortical areas both underlie kinaesthesia. Brain Res Cogn Brain Res 2003; 16: 74–82. [DOI] [PubMed] [Google Scholar]
- Duclos C , Roll R , Kavounoudias A , Roll JP . Cerebral correlates of the "Kohnstamm phenomenon": An fMRI study. NeuroImage 2007; 34: 774–783. [DOI] [PubMed] [Google Scholar]
- Cotey D , Hornby TG , Gordon KE , Schmit BD . Increases in muscle activity produced by vibration of the thigh muscles during locomotion in chronic human spinal cord injury. Exp Brain Res 2009; 196: 361–374. [DOI] [PubMed] [Google Scholar]
- Field-Fote E , Ness LL , Ionno M . Vibration elicits involuntary, step-like behavior in individuals with spinal cord injury. Neurorehabil Neural Repair 2012; 26: 861–869. [DOI] [PubMed] [Google Scholar]
- Murillo N , Valls-Sole J , Vidal J , Opisso E , Medina J , Kumru H . Focal vibration in neurorehabilitation. Eur J Phys Rehabil Med 2014; 50: 231–242. [PubMed] [Google Scholar]
- Thyrion C , Roll JP . Predicting any arm movement feedback to induce three-dimensional illusory movements in humans. J Neurophysiol 2010; 104: 949–959. [DOI] [PubMed] [Google Scholar]
- Duclos C , Kemlin C , Lazert D , Gagnon D , Dyer JO , Forget R . Complex muscle vibration patterns to induce gait-like lower-limb movements: proof of concept. J Rehabil Res Dev 2014; 51: 245–251. [DOI] [PubMed] [Google Scholar]
- Roll JP , Vedel JP . Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 1982; 47: 177–190. [DOI] [PubMed] [Google Scholar]
- Fallon JB , Macefield VG . Vibration sensitivity of human muscle spindles and golgi tendon organs. Muscle Nerve 2007; 36: 21–29. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ , Wirz M , Dietz V . Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil 2005; 86: 190–196. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ , Wirz M , Curt A . Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord 2006; 44: 352–356. [DOI] [PubMed] [Google Scholar]
- Awai L , Curt A . Intralimb coordination as a sensitive indicator of motor-control impairment after spinal cord injury. Front Hum Neurosci 2014; 8: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K , Newell KM . The structure of variability in human walking and running is speed-dependent. Exerc Sport Sci Rev 2008; 36: 200–204. [DOI] [PubMed] [Google Scholar]
- Ness LL , Field-Fote EC . Whole-body vibration improves walking function in individuals with spinal cord injury: a pilot study. Gait Posture 2009; 30: 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]