Abstract
Charcot spinal arthropathy (CSA) is most likely increasing in patients suffering from consequences of spinal cord injury. We want to highlight initial symptoms, certain risk factors and perioperative complications of this condition. A single center retrospective case series in a specialized Center for Spinal Cord Injuries, BG Trauma Center Murnau, Germany highlighting the potential obstacles in the management of Charcot spine. We describe four female paraplegic patients (mean age: 50.75 years; range: 42–67), who developed Charcot spinal arthropathies. The mean age at the time of the accident was 21.5 years (3–35), the time lag after the accident before CSA was developed and finally diagnosed was on average 29.5 years (17–39) and the mean follow-up period was 39.5 months (6–73). Patient histories, initial symptoms, risk factors as well as the management and postoperative complications are provided. Charcot spine is an important potential sequel of spinal cord injury, which can lead to significant disability and spinal emergencies in affected individuals. More studies are needed to provide better recommendations for spine surgeons. Conservative treatment is an option. Posterior fixation alone does not seem to be sufficient.
Subject terms: Spinal cord diseases, Central nervous system
Introduction
Jean-Martin Charcot first described neuroarthropathy in 1868 in a syphilitic patient.1 Since then this condition has commonly been described as ‘Charcot spine’. In 1884, the first patient with spinal arthropathy has been reported.2 Theoretically every insult to the central or peripheral nervous system leading to an impairment of proprioceptive mechanisms can lead ultimately to the destruction of the vertebral column. Thus, a plethora of diseases such as diabetic neuropathy, tertiary syphilis, congenital absence of pain syndrome, syringomyelia as well as traumatic and non-traumatic spinal cord injury (SCI) have been described to cause Charcot spinal arthropathy (CSA).3–6
After being described first in 1978 as a sequel of traumatic SCI,7 the majority of cases with CSA was attributed to be a long-term complication of SCI.4,8 As life expectancy of this patient cohort increases and with more surgical spine procedures being performed, Charcot spine is likely to be seen more frequently by spine specialists in the future. A better understanding of the pathophysiology, altered biomechanics and bone metabolism is needed to provide the best individual care for these patients. Surgical treatment has been proposed to deliver good long-term results,5 whereas the urge of surgery as a first-line treatment has been questioned most recently.9 Here we describe four patients, where the initial treatment did not provide satisfactory results or caused a high complication rate respectively.
Methods
This analysis was performed at the Center for Spinal Cord Injuries (BG Trauma Center Murnau, Germany), a specialized surgical department in a level-I trauma center. The following study presents a retrospective chart review. To be included patient had to fulfill certain diagnostic and radiologic criteria for Charcot spine as previously described.4 Due to the retrospective character of the study, the complete absence regarding treatment interference and the clinical data sets being collected as part of institutional, national and international quality programs, a vote from the ethic committee was not indicated.
The main objective of this study was to highlight several surgical pitfalls in treating patients who developed CSA after SCI. Therefore, the complete medical records of all four selected patients were retrospectively reviewed to specify clinical and radiological findings.
Results
In the following, we present four cases of CSA in patients living with the consequences of traumatic SCI. All of the mentioned patients were female with a mean age of 50.75 years (range: 42–67) at the time when CSA was diagnosed. The mean age at the time of the accident was 21.5 years (3–35), the time lag after the accident before CSA was developed and finally diagnosed was on average 29.5 years (17–39) and the mean follow-up period was 39.5 months (6–73) (Table 1). The main goal is to highlight initial symptoms, risk factors, management and postoperative complications if applicable (Table 2).
Table 1. Demographic characteristics at the time when CSA was diagnosed.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Gender | Female | Female | Female | Female |
| Age (years) | 67 | 42 | 50 | 44 |
| AIS grade/level | A sub T3 | A sub T4 | A sub T12 | B sub T6 |
| Age at accident (years) | 35 | 3 | 20 | 28 |
| Surgical treatment of SCI | No | No | Dorsal instrumentation+laminectomy | Dorsal instrumentation+laminectomy |
| Charcot lesions (n) | 3 | 2 | 1 | 1 |
| Level of CSA | T12/L1; L2/3, L4/5 | T8/9; L5/S1 | L4/5 | L3/4 |
| Time lag betw. SCI–CSA diagnosis (years) | 33 | 39 | 29 | 17 |
| Follow-up (months) | 17 | 62 | 73 | 6 |
Abbreviations: AIS, American Spinal Injury Association Impairment Scale; CSA, Charcot spinal arthropathy; SCI, spinal cord injury.
Table 2. Initial symptoms, risk factors, management and complications.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Symptoms | General discomfort, low back pain | Lumbar back pain, increased spasticity, sitting imbalance | Sitting imbalance | General discomfort, low back pain, sitting imbalance |
| Additional risk factors | Posttraumatic Syringomyelia | Secondary scoliosis | Ankylosing spondylitis, type II diabetes | Idiopathic scoliosis |
| Treatment | Dorsal stabilization (L1–L4) | Shortening of inserted Harrington rod | Conservative | Dorsal stabilization (L2/3–L5) |
| Complications | Wound dehiscence, implant loosening, adjacent segment Charcot lesions | Adjacent segment Charcot lesions | Further disease progression | Implant loosening, wound healing disturbances |
Case 1
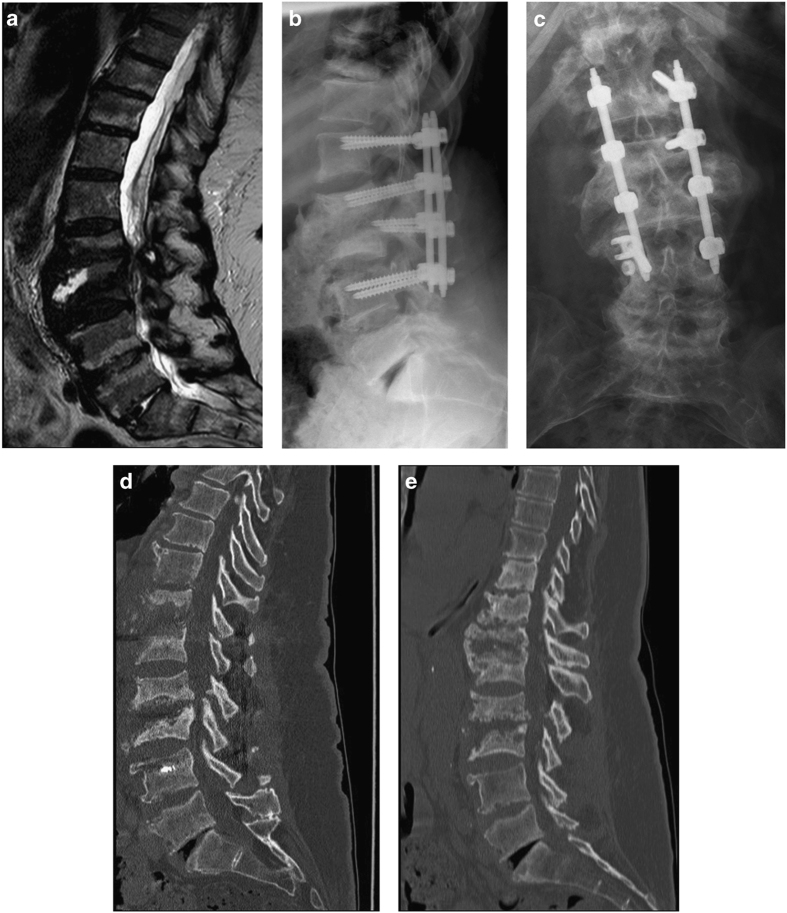
The first patient was a 67-year-old paraplegic woman. She was admitted to our institution with the suspected diagnosis of a massive spondylodiscitis at the level L2/3 from an outside hospital (Figures 1b and c). Her medical records showed a history of traumatic SCI 33 years before presentation (with conservative initial treatment), posttraumatic syringomyelia and spondylodiscitis L2/3 4 years before (Figure 1a). We performed dorsal instrumentation L1–L4 (Figure 1d) without bone grafting and provided antibiotic long-term treatment (there was no evidence for bacterial infection, but antibiosis was already started empirically). One month later she required revision surgery due to wound dehiscence. After 1 year she developed general discomfort, low back pain and sitting imbalance again. Radiologic work-up revealed CSA lesion in adjacent segments and implant loosening (Figure 1e). The dorsal instrumentation was further removed and conservative treatment via a back brace was initiated with no progression upon clinical and radiological examination over time.
Figure 1.
Patient 1. (a) Sagittal T2-weighted MR image of the lumbar spine 4 years later to a; (b) lateral X-ray showing dorsal spondylodesis; (c) Anterior-posterior X-ray showing implant loosening 1 year after (d); (d) Sagittal CT indicating Charcot spine lesions in adjacent segments; (e) Sagittal CT 7 months after (d).
Case 2
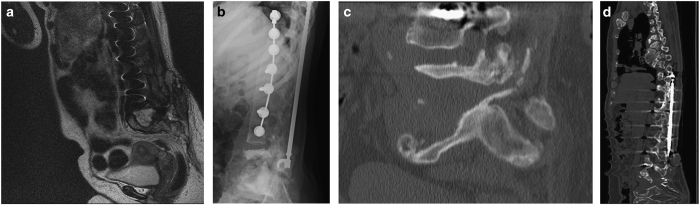
This female patient was admitted to our service because of lumbar back pain, increased spasticity and sitting imbalance. Radiologic examination showed a massive fluid-filled substance defect in the lumbosacral region (Figure 2a). She was dealing with the consequences of paraplegia due to traumatic thoracic SCI since 39 years and spinal surgery with Harrington rods due to secondary neuropathic scoliosis 26 years ago (Figure 2b). Her medical condition refused major spine surgery. The inserted Harrington rod was shortened at the caudal end (Figure 2c) and conservative treatment was initiated. Over the years no disease progression was observed in the lumbar region, but adjacent CSA lesion was observed rostral to the instrumentation material (Figure 2d).
Figure 2.
Patient 2. (a) Sagittal T2-weighted MR image showing a lumbosacral substance defect; (b) Lateral X-ray 3 years before (a); (c) According sagittal CT image to a; (d) Sagittal CT 4 years after (a) showing shortening of Harrington rod and adjacent CSA above the instrumentation.
Case 3
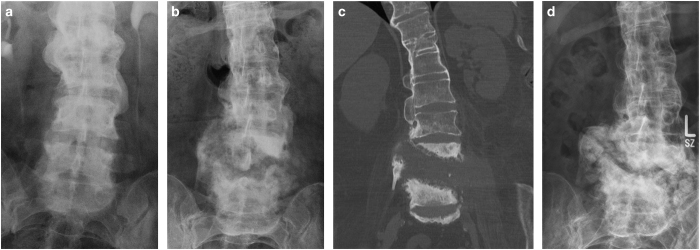
During a radiologic control examination due to discrete sitting imbalance and mild low back pain for a couple of months, a CSA lesion was found at the level of L4/5 (Figures 3b and c) in a paraplegic 50-year-old woman. An examination 4 years earlier showed radiologic signs of ankylosing spondylitis (Figure 3a). Her medical history was also indicative for type II diabetes and morbid obesity. Due to the relatively low burden of disease, the patient underwent conservative management with a back brace (Figure 3d).
Figure 3.
Patient 3. (a) X-ray taken during an urodynamic control examination showing signs of ankylosing spondylitis 4 years before (b). (b) X-ray with a CSA lesion at the level of L4/5. (c) According coronar CT to b. (d) X-ray after a 6-year follow-up period showing radiologic progression with a clear pseudotumoral appearance.
Case 4
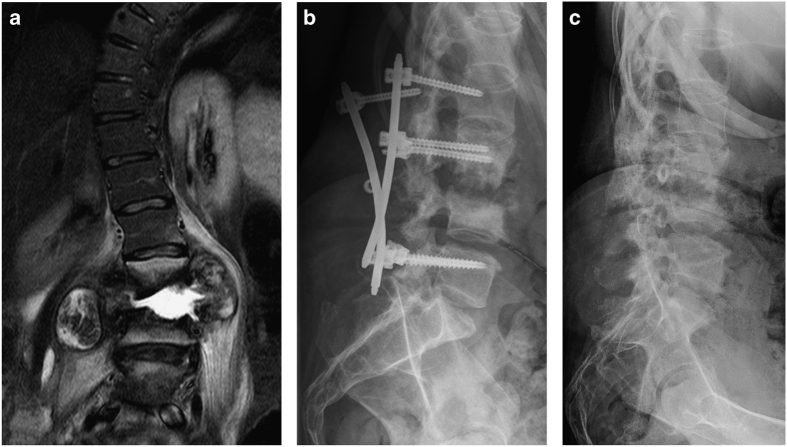
This 44-year-old woman was sent to our department with the suspected diagnosis of spondylodiscitis at the level L3/L4 with coexisting abscesses in the psoas muscle bilaterally (Figure 4a). She presented with low back pain, an audible crepitus upon transfer and general discomfort for 10 days. Her past medical history was significant for motor complete, sensory incomplete paraplegia below the level of T6 since 17 years and preexisting idiopathic scoliosis, which required several spine surgeries even her traumatic SCI. Needle biopsies did not show any evidence regarding bacterial infection. A posterior percutaneous fixation was carried out from L2/L3 to L5 without any bone grafting (Figure 4b).
Figure 4.
Patient 4. (a) Coronar T2-weighted MR image showing a fluid-filled disc space at the level of L3/4 with significant bone destruction and paravertebral abscess formation; (b) lateral X-ray highlighting implant loosening; (c) lateral X-ray showing similar erosion to prior examinations.
Ten days after surgery the patient realized a different cracking noise and the feeling of sitting imbalance immediately after mobilization into her wheelchair. The following X-ray investigation showed an implant loosening (Figure 4c). Because the patient refused revision surgery with a circumferential approach, the dorsal spondylodesis was completely removed. Postoperatively she developed wound-healing disturbances. After approximately 6 months no further disease progression was observed after conservative treatment (Figure 4d).
Discussion
Neurogenic arthropathy involves extensive disc degeneration as well as massive vertebral degeneration with coexisting osteosclerosis and additional bone formation at the same time. This eventually results in dislocation and instability of the vertebral column4 as seen in all of our presented patients. The most accepted pathophysiological hypothesis highlights the initial loss of proprioception and pain/temperature sensation. This leads to altered joint defense mechanisms and abnormal stress in these joints. Moreover, the loss of sympathetic innervation probably has an essential role in the multi-faceted occurrence of Charcot spine (for further reading please see Barrey et al.4).
Previously suggested diagnostic criteria are:4,5
Presence of an underlying disease, which impairs adequate proprioception and pain sensation.
Bone destruction and resorption as well as new bone formation on radiological examinations.
Evidence of non-specific chronic inflammation on histologic examination.
These processes lead to several radiologic features:4,5,10–12
Profound disc generation (seen in all presented cases).
Vertebral destruction/erosion accompanied with osteolysis and/or osteosclerosis (seen in all presented individuals).
Hypertrophic hyperostosis within surrounding soft tissue, with a ‘pseudotumoral’ appearance.
Early destructive changes are seen at the facet joints.
In SCI patients—especially in active paraplegic patients (like patients 1, 2 and 4)—the thoracolumbar and lumbosacral spine is especially susceptible to develop Charcot lesions because excessive loads are operating repetitively on this area.5 In paraplegic patients lumbar lordosis is flattened and kyphosis might develop, which may intensify further degenerative changes.13,14 This explains the predominance of the mentioned spine areas (as also seen in cases 1–4), where the L3/4 segment seems to be affected the most.4
Other risk factors may include spinal fusion and laminectomy for the treatment of SCI as well as very active paraplegic patients.5,8,15,16 We want to highlight the relative frequency of scoliosis, either idiopathic (patient 4) or secondary to SCI (patient 2) in this series. Since diabetic neuropathy and syringomyelia are risk factors per se,3,6 it seems logical that their concomitant presence (patients 1 and 3) might boost the development of CSA. Ankylosing spinal hyperostosis (patient 3) might be another risk factor.3
Patients may present with a variety of different symptoms. Sitting imbalance, an audible cracking noise upon transfer, pain in an otherwise insensitive area and a change in the neurological status seem to be the most frequent ones.3,4,8,13 Noteworthy changes in the spasticity, usually toward a flaccid paralysis might occur at the time of CSA diagnosis.3 Interestingly patient 2 presented with increased spasticity, but this might have also been present due to a urinary tract infection at that time. Increased episodes of autonomic dysreflexia might also be indicative for CSA.4,17
The main differential diagnoses are tumorous or infectious processes.4,18,19 Unfortunately, there are no pathognomonic features that can help to differentiate among these. Two of the aforementioned patients were sent to our institution with the suspected diagnosis of acute spondylodiscitis. These patients also showed general discomfort and elevated leukocytosis. A paravertebral mass or abscess can be evident in Charcot spine (as seen in patient 4) as well as in spondylodiscitis. It is challenging to discriminate between spondylodiscitis and a superinfected Charcot spine if the detailed patient history is not present.20,21 Furthermore, patients suffering from recurrent infections (for example, urinary tract infections) are more likely to develop spinal infections.4 A needle biopsy—ideally before the initiation of empiric antibiotic treatment—should be centered in the disc space to help in the distinction. Tissue pathology of Charcot spine involves fibrosis without any indications for acute inflammation, normal granulation tissue and osteosclerosis without any signs of malignancy.4 Sometimes a tumoral process, like a paravertebral mass, is present in spinal arthropathy.22 It is crucial to obtain a biopsy or remove the mass during surgery.
In the published literature, CSA develops around 17.3 years after the initial trauma (in this study after 29.5 years). In about two-thirds of the patients Charcot spine affects only one segment, whereas two joints are impacted in more than 25% and three joints in the remaining.4 These numbers are also reflected in this case series. The fact, that only female patients are described here, although a clear male predominance has been reported, might just be a coincidence.3,4
Surgical treatment is able to deliver excellent long-term results and the outcome might be slightly better in the spine than for Charcot arthropathies in the limbs.5 However, conservative treatment should be considered9 and is sometimes necessary due to the medical condition of these patients (our own experience). Postoperative complications include implant loosening (especially if only dorsal instrumentation has been performed as seen in patients 1 and 4), wound healing delays and the development of additional Charcot lesion either within or adjacent to the operated area8 (patients 1, 2 and 4).8 If surgery is performed, then a stable reconstruction of all three spine columns, especially the anterior one is necessary. In our opinion, a circumferential arthrodesis with a single staged posterolateral approach23 seems to be an attractive option in complete paraplegic patients.
In general the vague clinical presentation with the potential severe clinical impact of CSA, emphasizes the value of regular, long-term clinical and radiological follow-up of spinal cord-injured patients in specialized centers.
Data archiving
There were no data to deposit.
Acknowledgments
We thank the institutional radiology department for the good collaboration.
The authors declare no conflict of interest.
References
- Charcot JM . Sur quelques arthropathies qui paraissant dependre dune lesion due cerveau ou de la modelle epiniere. Arch Physiol Norm Pathol 1868; 1: 161. [Google Scholar]
- Kroning G . Spondylolisthese bei einem Tabiker. Zeit Klin Med 1884; suppl 7, 165. [Google Scholar]
- Morita M , Miyauchi A , Okuda S , Oda T , Yamamoto T , Iwasaki M . Charcot spinal disease after spinal cord injury. J Neurosurg Spine 2008; 9, 419–426. [DOI] [PubMed] [Google Scholar]
- Barrey C , Massourides H , Cotton F , Perrin G , Rode G . Charcot spine: two new case reports and a systematic review of 109 clinical cases from the literature. Ann Phys Rehabil Med 2010; 53, 200–220. [DOI] [PubMed] [Google Scholar]
- Suda Y , Shioda M , Kohno H , Machida M , Yamagishi M . Surgical treatment of Charcot spine. J Spinal Disord Tech 2007; 20, 85–88. [DOI] [PubMed] [Google Scholar]
- Silber JS , Vaccaro AR , Green B . Summary statement: chronic long-term sequelae after spinal cord injury: post-traumatic spinal deformity and post-traumatic myelopathy associated with syringomyelia. Spine (Phila Pa 1976) 2001; 26, S128. [DOI] [PubMed] [Google Scholar]
- Slabaugh PB , Smith TK . Neuropathic spine after spinal cord injury. A case report. J Bone Joint Surg Am 1978; 60, 1005–1006. [PubMed] [Google Scholar]
- Aebli N , Potzel T , Krebs J . Characteristics and surgical management of neuropathic (Charcot) spinal arthropathy after spinal cord injury. Spine J 2014; 14, 884–891. [DOI] [PubMed] [Google Scholar]
- Moreau S , Lonjon G , Jameson R , Judet T , Garreau de Loubresse C . Do all Charcot Spine require surgery? Orthop Traumatol Surg Res 2014; 100, 779–784. [DOI] [PubMed] [Google Scholar]
- Harrison MJ , Sacher M , Rosenblum BR , Rothman AS . Spinal Charcot arthropathy. Neurosurgery 1991; 28, 273–277. [DOI] [PubMed] [Google Scholar]
- Vaccaro AR , Silber JS . Post-traumatic spinal deformity. Spine (Phila Pa 1976) 2001; 26, S111–S118. [DOI] [PubMed] [Google Scholar]
- Brown CW , Jones B , Donaldson DH , Akmakjian J , Brugman JL . Neuropathic (Charcot) arthropathy of the spine after traumatic spinal paraplegia. Spine (Phila Pa 1976) 1992; 17, S103–S108. [DOI] [PubMed] [Google Scholar]
- De Iure F , Chehrassan M , Cappuccio M , Amendola L . Sitting imbalance cause and consequence of post-traumatic Charcot spine in paraplegic patients. Eur Spine J 2014; 23(Suppl 6), 604–609. [DOI] [PubMed] [Google Scholar]
- Seelen HA , Potten YJ , Huson A , Spaans F , Reulen JP . Impaired balance control in paraplegic subjects. J Electromyogr Kinesiol 1997; 7, 149–160. [DOI] [PubMed] [Google Scholar]
- Sobel JW , Bohlman HH , Freehafer AA . Charcot's arthropathy of the spine following spinal cord injury. A report of five cases. J Bone Joint Surg Am 1985; 67, 771–776. [PubMed] [Google Scholar]
- Standaert C , Cardenas DD , Anderson P . Charcot spine as a late complication of traumatic spinal cord injury. Arch Phys Med Rehabil 1997; 78, 221–225. [DOI] [PubMed] [Google Scholar]
- Selmi F , Frankel HL , Kumaraguru AP , Apostopoulos V . Charcot joint of the spine, a cause of autonomic dysreflexia in spinal cord injured patients. Spinal Cord 2002; 40, 481–483. [DOI] [PubMed] [Google Scholar]
- Jones EA , Manaster BJ , May DA , Disler DG . Neuropathic osteoarthropathy: diagnostic dilemmas and differential diagnosis. Radiographics 2000; 20 Spec No, S279–S293. [DOI] [PubMed] [Google Scholar]
- Vialle R , Mary P , Tassin JL , Parker F , Guillaumat M . Charcot's disease of the spine: diagnosis and treatment. Spine (Phila Pa 1976) 2005; 30, E315–E322. [DOI] [PubMed] [Google Scholar]
- Suda Y , Saito M , Shioda M , Kato H , Shibasaki K . Infected Charcot spine. Spinal Cord 2005; 43, 256–259. [DOI] [PubMed] [Google Scholar]
- Mikawa Y , Watanabe R , Yamano Y , Morii S . Infected Charcot spine following spinal cord injury. Spine (Phila Pa 1976) 1989; 14, 892–895. [DOI] [PubMed] [Google Scholar]
- Son SB , Lee SH , Kim ES , Eoh W . Charcot arthropathy of the lumbosacral spine mimicking a vertebral tumor after spinal cord injury. J Korean Neurosurg Soc 2013; 54, 537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW , Seo EM , Hwang JT , Kwak BC . Charcot spine treated using a single staged posterolateral costotransversectomy approach in a patient with traumatic spinal cord injury. J Korean Neurosurg Soc 2013; 54, 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]