Abstract
Objective: We conducted a systematic review to evaluate whether caregiver-involved interventions improve patient outcomes among adults with dementia or Alzheimer’s disease. Method: We identified and summarized data from randomized controlled trials enrolling adults with dementia or Alzheimer’s disease by searching MEDLINE, PsycINFO, and other sources. Patient outcomes included global quality of life, physical and cognitive functioning, depression/anxiety, symptom control and management, and health care utilization. Results: We identified 31 trials; 20 compared a caregiver intervention with usual care or usual care with promise of intervention at completion of study period. Fifteen compared one caregiver intervention with another individual or caregiver intervention (active control). Compared with usual care or active controls, caregiver-involved interventions had low to insufficient strength of evidence and did not consistently improve patient outcomes. Discussion: Evidence is insufficient to endorse use of most caregiver interventions to improve outcomes for patients with dementia or Alzheimer’s disease.
Keywords: dementia, Alzheimer’s disease, family, caregivers, systematic review, patient-reported outcomes
Background
Based on 2010 census data, 4.7 million people aged 65 and older in the United States are estimated to have dementia or Alzheimer’s disease. With an aging U.S. population, 13.8 million people are expected to have dementia or Alzheimer’s disease by 2050 (Hebert, Weuve, Scherr, & Evans, 2013). By 2020, costs for dementia care purchased in the marketplace are expected to exceed US$129 billion annually (Hurd, Martorell, Delavande, Mullen, & Langa, 2013).
Responding to this impending upsurge, the United States passed the National Alzheimer’s Project Act in 2012 to create a national plan to prevent and treat Alzheimer’s disease and other dementias. Key goals in the plan are to prevent and effectively treat Alzheimer’s disease and enhance care quality and efficiency (U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, 2013). To date, however, medications to reduce or treat memory loss and the behavioral and psychological disturbances that often characterize dementia and Alzheimer’s disease show small and inconsistent benefits of uncertain clinical importance and some associated harms (Lee et al., 2004; Lin, O’Connor, Rossom, Perdue, & Eckstrom, 2013; Sink, Holden, & Yaffe, 2005). Non-pharmacological interventions, such as those that engage families or caregivers to improve patients’ quality of life and to minimize common physical and cognitive symptoms, such as physical and verbal aggression, confusion, wandering, and depression, are often considered to have an impact while being less risky.
Although the caregiver’s role in patient care quality is critical, to date the majority of evidence on interventions targeting caregivers of dementia patients have concentrated on effects on caregiver health and well-being (Goy, Kansagara, & Freeman, 2010; Sorensen, Pinquart, & Duberstein, 2002; Thompson et al., 2007; Visser-Meily, van Heugten, Post, Schepers, & Lindeman, 2005), not on patient outcomes. A summary of the evidence, therefore, is needed to determine viable strategies for improving patient outcomes. Our goal was to address the following questions: (a) What are the benefits and harms of caregiver psychosocial interventions on outcomes for adults with dementia or Alzheimer’s disease compared with usual care or wait list (i.e., efficacy of interventions)? and (b) What are the benefits and harms of a caregiver-involved psychosocial intervention compared with either a patient intervention or another alternative caregiver-oriented intervention (active controls) in improving outcomes for adults with dementia or Alzheimer’s disease (i.e., comparative effectiveness of interventions)? The key questions and scope were refined with input from a technical expert panel (TEP) comprised of clinicians, researchers, and policy makers and an earlier, version of our findings is available online (Griffin, 2013).
Method
Our systematic review protocol was considered exempt by the Minneapolis VA Institutional Review Board. Because both caregivers and patients could be included as study participants in the studies we reviewed, in our article we refer to the person with dementia or Alzheimer’s disease as a “patient” and the person who provides unpaid, direct care and support to patients with dementia or Alzheimer’s disease, regardless of relationship, as “caregiver.”
Search Strategy
We searched MEDLINE (Ovid) and PsycINFO for randomized controlled trials (RCTs) published from 1996 through December 2014 using the following search terms: family, couples, caregivers, home nursing, legal guardians, couple therapy, family therapy, or marital therapy (see Figure 1). We identified additional citations from reference lists of retrieved articles and from TEP members. We included studies written in the English language and conducted in the United States. We excluded any study with a patient population below age 18 years and any that did not include patients with dementia or Alzheimer’s disease.
Figure 1.
Electronic database search strategies.
Data Extraction and Quality Assessment
Titles, abstracts, and articles were reviewed by study authors. Study and patient characteristics and outcomes data from the included trials were extracted and then verified independently, under the supervision of the principal investigator.
We evaluated study risk of bias using criteria established by the Cochrane Collaboration (Higgins & Green, 2011). Domains included adequate allocation concealment; blinding of interventionists and/or health care providers to study assessments; blinding of assessors to participant intervention arm; whether intent-to-treat (ITT) analyses were used; and whether withdrawals and dropouts by group assignment were adequately described. We also evaluated whether the delivery of interventions was monitored for quality and consistency (i.e., treatment integrity). Trials were rated as good, fair, or poor quality. A good quality trial (low risk of bias) reported adequate allocation concealment, a minimum of single blinding (participants or investigators or assessors are blinded), and either ITT analysis was conducted or clear reasons for dropouts/attrition by group were provided.
We evaluated strength of evidence for each outcome using methods established by the Agency for Healthcare Research and Quality (Owens et al., 2010). Strength of evidence was rated as (a) high—further research is very unlikely to change the confidence in the estimate of effect, meaning that the evidence reflects the true effect; (b) moderate—further research may change our confidence in the estimate of effect and may change the estimate; (c) low—further research is very likely to have an important impact on the confidence in the estimate of effect and is likely to change the estimate, meaning that there is low confidence that the evidence reflects the true effect; or (d) insufficient—the evidence was unavailable or did not permit a conclusion. A rating of high strength of evidence indicated that the included studies were low risk of bias RCTs with consistency (i.e., the effect sizes from the included studies were similar and had the same direction, either positive or negative), directness (interventions are directly related to health outcomes of interest), and precision (the degree of certainty surrounding an estimate of effect for each outcome of interest, with uncertainty of the estimate not allowing for a clinically useful conclusion).
Outcomes
The effect of caregiver-involved interventions was evaluated for five patient outcome categories: (a) functional status, (b) quality of life, (c) symptom control, (d) depression/anxiety, and (e) health care utilization. Functional status was defined by multiple indicators, including the patient’s physical functioning (e.g., activities of daily living and instrumental activities of daily living) and cognitive functioning (e.g., memory capacity, problem-solving abilities). Patient depression/anxiety included reports of depressive symptoms using standardized assessments. Symptom control or management included reports of behavioral disturbances or problem behaviors associated with dementia (e.g., agitation, wandering, irritability, withdrawn behavior, incontinence, nighttime waking). Health care utilization included hospitalization, institutionalization, or emergency room visits.
Only outcomes that were assessed using previously published scales or measures or had clear end-points (e.g., hospitalization) were included. To determine both immediate benefits and long-term sustainability of the intervention, we captured, whenever possible, data at two time points: post-intervention (±1 month) and greater than 6 months post-intervention. For studies with multiple assessments after 6 months post-intervention, we extracted the last available assessment.
Categorization of Interventions
We created three categories of interventions based on common characteristics across trials. The first was caregiver training interventions, trials that provided training for families to change or manage patient behavior. These interventions typically included developing caregivers’ problem-solving skills, strategies to reduce patients’ problem behaviors, and skills training to reduce risks or hazards in a patient’s environment, but did not focus on supporting caregiver psychosocial needs or support. The second category was caregiver training and support interventions. In addition to training caregivers, these interventions often involved cognitive-behavioral techniques to assist caregivers with managing their stress and burden. Interventions included skill building and problem solving for patient safety and behavior as well as coping skills for caregivers. The third category was unique interventions with unique intervention targets. These interventions were each highly unique from one another and could not be described within the above categories.
Data Synthesis
Study findings were summarized for each outcome and within each intervention category. We summarized most findings narratively because the heterogeneity of populations, interventions, and outcomes across studies precluded us from conducting meta-analyses.
We extracted intervention effect sizes from trials when reported. We used Cohen’s (1988) guide for interpreting magnitude of effect sizes (i.e., d of 0.2-0.49 = small effect, 0.5-0.79 = medium effect, 0.8 or greater = large effect). If a trial’s effect sizes were not reported, we calculated, whenever possible, intervention effect sizes and corresponding confidence intervals (CIs) using Review Manager version 5.2 software (Review Manager [RevMan], 2014) by entering means, standard deviations, and sample sizes. If an effect size was not significant (the CI included 0), but authors reported significant findings from other statistical tests, we considered the significant test, noting the discrepancy between the effect size and the other test. Given the criticism of systematic reviews for oversimplifying findings and ignoring important differences that do not fit a review’s rubric, in cases where other statistical tests were used and effect sizes could not be calculated, the significance of the alternate parameter was noted and used.
Results
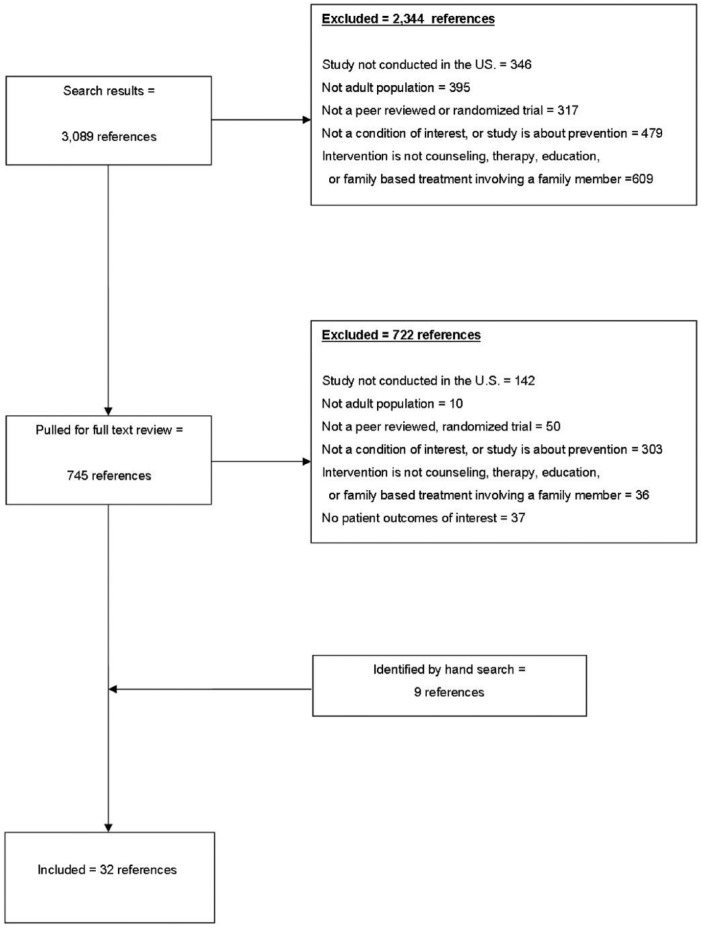
From 3,089 citations, we identified 32 references, representing 31 unique RCTs, which met our inclusion criteria (see Figure 2). Details on study characteristics, interventions, comparators, and outcomes assessed are found in Supplemental Table 1 or accessed in an earlier and expanded evidence report (Griffin, 2013).
Figure 2.
Literature flow diagram.
Description of Trials
Baseline characteristics for patients and caregivers are found in Table 1. Overall, trials varied by who provided care, the type of intervention, measures and instruments used to assess outcomes and comparators, and statistical tests for assessing post-intervention differences (e.g., effect sizes, difference in proportions, interaction terms in regression modeling). All but 4 of the 31 trials targeted caregivers of community-dwelling patients and the majority (87%) required patients to meet clinical diagnosis criteria for dementia, although the criteria used varied across trials. Use of a standardized intervention protocol was reported in 58% of trials.
Table 1.
Summary of Baseline Characteristics (31 Trials).
| Study characteristics | Total/M (range) |
|---|---|
| No. of patient/caregiver dyads randomized | 4,793/154 (36-642) |
| No. of patients/caregiver dyads analyzed | 4,261/137 (29-518) |
| Age of patients, years | 78 (73-86) |
| Age of caregivers, years | 65 (48-74) |
| Participant marital status, % married | 78 (51-100) |
| Patient gender, (%) male | 44 (11-65) |
| Manualized intervention reported | 58% (18/31) |
| Caregiver intervention with | |
| Husband/wife or male/female intimate partner only | 16% (5/31) |
| Any identified caregiver | 84% (26/31) |
| Caregiver intervention compared witha | |
| Wait list | 19% (6/31) |
| Usual care | 45% (14/31) |
| Individual treatment | 3% (1/31) |
| Other caregiver treatment(s) | 39% (12/31) |
Note. Four trials included multiple intervention conditions; therefore, total number of comparison conditions exceeds the number of trials.
Some trials included multiple comparison conditions; therefore, groups are not mutually exclusive and, together, exceed 100%.
Key Question 1 (KQ1): What are the benefits/harms of caregiver psychosocial interventions on outcomes for adults with dementia/Alzheimer’s disease compared with usual care or wait list?
Twenty trials met our criteria. Fifteen papers from fourteen RCTs compared a caregiver intervention with usual care (Bass, Clark, Looman, McCarthy, & Eckert, 2003; Brodaty, Mittelman, Gibson, Seeher, & Burns, 2009; Burgener, Bakas, Murray, Dunahee, & Tossey, 1998; Camberg et al., 1999; Gitlin, Corcoran, Winter, Boyce, & Hauck, 2001; Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010b; McCallion, Toseland, & Freeman, 1999; Mittelman, Haley, Clay, & Roth, 2006; Mittelman, Roth, Haley, & Zarit, 2004; Robison et al., 2007; Schmitter-Edgecombe & Dyck, 2014; Teri et al., 2003; Teri, McCurry, Logsdon, & Gibbons, 2005; Wray et al., 2010; Wright, Litaker, Laraia, & DeAndrade, 2001) and 5 with a wait list control group (i.e., usual care with promise of intervention at completion of study period; Gitlin et al., 2008; Logsdon et al., 2010; Martin-Cook, Davis, Hynan, & Weiner, 2005; Ostwald, Hepburn, Caron, Burns, & Mantell, 1999; Quayhagen et al., 2000). One had both a usual care and a wait list control group (Teri, Logsdon, Uomoto, & McCurry, 1997). Four trials were rated as good, 8 as fair, and 8 as poor quality. Studies ranged in size from 47 to 406 dyads, with a median of 103 per trial. Four trials required the caregiver to be a spouse (Brodaty et al., 2009; Mittelman et al., 2004; Quayhagen et al., 2000; Wray et al., 2010); others included any caregiver involved in care. Interventions ranged from 1 to 23 sessions, typically lasting 12 to 16 weeks long. Six trials included long-term (at least 6 months) follow-up assessments (Brodaty et al., 2009; Burgener et al., 1998; Mittelman et al., 2006; Teri et al., 2003; Teri et al., 2005; Wray et al., 2010). However, 1 study, initiated 18 years prior to the paper’s publication, reported ongoing follow-up with patients to assess timing to institutionalization (Mittelman et al., 2006).
All studies reported on three or fewer outcomes; none reported on all of our outcomes. The most frequently assessed outcomes were symptom control/management (55%, 11/20 trials; Burgener et al., 1998; Camberg et al., 1999; Gitlin et al., 2001; Gitlin et al., 2003; Gitlin et al., 2010b; McCallion et al., 1999; Mittelman et al., 2004; Ostwald et al., 1999; Quayhagen et al., 2000; Robison et al., 2007; Wright et al., 2001) and physical functioning (40%, 8/20 trials; Brodaty et al., 2009; Burgener et al., 1998; Gitlin et al., 2001; Logsdon, Gibbons, McCurry, & Teri, 2002; Martin-Cook et al., 2005; Mittelman et al., 2004; Teri et al., 2003; Wright et al., 2001). Other outcomes, including cognitive functioning (30%, 6/20 trials; Martin-Cook et al., 2005; Ostwald et al., 1999; Quayhagen et al., 2000; Schmitter-Edgecombe & Dyck, 2014; Teri et al., 1997; Teri et al., 2005), global quality of life (25%, 5/20 trials; Gitlin et al., 2008; Logsdon et al., 2010; Schmitter-Edgecombe & Dyck, 2014; Teri et al., 1997; Wright et al., 2001), depression/anxiety (30%, 6/20 trials; Gitlin et al., 2008; Logsdon et al., 2010; McCallion et al., 1999; Schmitter-Edgecombe & Dyck, 2014; Teri et al., 2003; Teri et al., 1997), and health care utilization (25%, 5/20 trials; Bass et al., 2003; Brodaty et al., 2009; Mittelman et al., 2006; Wray et al., 2010; Wright et al., 2001), were assessed in less than one third of trials.
Summary of Trials
More than half of the 20 trials (55%) reported significant differences in outcomes between usual care and caregiver-involved interventions (Table 2). The cumulative strength of evidence for intervention effectiveness, however, was low for all outcomes (Table 3), due to moderate to high risk of bias and inconsistency and imprecision of the effect sizes calculated. Although single trials had significant effects on outcomes, reported differences were rarely consistent across outcomes or even within different assessments of the same outcome. For example, 11 trials assessed symptom management and 5 found significant differences post-intervention. Of these, 1 trial had a large, significant effect size (0.72; Gitlin et al., 2008; Logsdon et al., 2010; Teri et al., 1997; Wright et al., 2001). However, others demonstrated a small effect size (1 trial), reported mean differences only (point estimates could not be calculated), or reported significant differences without data being shown (2 trials) to determine the magnitude of effect. Non-significant effect sizes were mostly small in magnitude but had wide CIs (3 trials). In other studies, statistical significance was either not reported or could not be determined (4 trials).
Table 2.
Outcomes Reported in Trials Comparing Interventions With Caregiver Component Versus Usual Care or Wait List Control.
| Author, year | na | Study qualityb | Physical functioning | Cognitive function | Quality of life/overall functioning | Symptom management/control | Depression/anxiety | Utilization |
|---|---|---|---|---|---|---|---|---|
| Caregiver training interventions (n = 7) | ||||||||
| Burgener 1998 (Burgener, Bakas, Murray, Dunahee, & Tossey, 1998)c | 54 | Poor | ± | ± | ||||
| Gitlin 2001 (Gitlin, Corcoran, Winter, Boyce, & Hauck, 2001) | 202 | Poor | ↔/↑ | ↔/↑ | ||||
| Gitlin 2008 (Gitlin et al., 2008) | 60 | Good | ↔ | ↔/↑ | ↔ | |||
| Martin-Cook 2005 (Martin-Cook, Davis, Hynan, & Weiner, 2005) | 47 | Poor | ↔ | ↔ | ||||
| Quayhagen 2000 (Quayhagen et al., 2000)c | 103 | Poor | ↔ | ↔ | ||||
| Teri 2005 (Teri, McCurry, Logsdon, & Gibbons, 2005) | 95 | Fair | ↔/↑ | ↔/↑ | ||||
| Wright 2001 (Wright, Litaker, Laraia, & DeAndrade, 2001) | 93 | Poor | ↔ | ± | ↔ | ↔ | ||
| Caregiver training and support interventions (n = 7) | ||||||||
| Brodaty 2009 (Brodaty, Mittelman, Gibson, Seeher, & Burns, 2009) | 52 | Poor | ↔ | ↔ | ||||
| Gitlin 2010 (Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010b) | 272 | Fair | ±/↑ | |||||
| Mittelman 2004 (Mittelman, Roth, Haley, & Zarit, 2004) 2006 (Mittelman, Haley, Clay, & Roth, 2006) | 406 | Good | ± | ↔ | ↔/↑ | |||
| Ostwald 1999 (Ostwald, Hepburn, Caron, Burns, & Mantell, 1999) | 117 | Good | ↔ | ↔ | ||||
| Schmitter-Edgecombe 2014 (Schmitter-Edgecombe & Dyck, 2014) | 55 | Good | ↔/↑ | ↔/↑ | ↔ | |||
| Teri 1997 (Teri, Logsdon, Uomoto, & McCurry, 1997)c | 72 | Fair | ↔/↑ | ↔/↑ | ||||
| Wray 2010 (Wray et al., 2010) | 158 | Fair | ↔ | |||||
| Unique interventions with unique intervention targets (n = 6) | ||||||||
| Bass 2003 (Bass, Clark, Looman, McCarthy, & Eckert, 2003) | 182 | Fair | ↔ | |||||
| Camberg 1999 (Camberg et al., 1999)c | 54 | Fair | ↔ | |||||
| Logsdon 2010 (Logsdon et al., 2010) | 142 | Poor | ↔ | ↑ | ↑ | |||
| McCallion 1999 (McCallion, Toseland, & Freeman, 1999) | 66 | Fair | ↔/↑ | ↔/↑ | ||||
| Robison 2007 (Robison et al., 2007) | 388 | Poor | ↑ | |||||
| Teri 2003 (Teri et al., 2003) | 153 | Fair | ↔/↑ | ↑ | ↔ | |||
Source. Adapted from evidence report (Griffin et al, 2013).
Note. Ratings: ↑ = treatment significantly better than comparator; ↔ = no significant difference between intervention and comparator; ↓ = treatment significantly worse than comparator; ± = significance not reported or could not be determined; two ratings separated by “/” indicates multiple assessments were used, and the significance of outcomes varied across assessments.
Number randomized.
Good (low risk of bias): The trial reported adequate allocation concealment, a minimum of single blinding (participants or investigators or assessors are blinded), and that either intent-to-treat analysis was conducted or clear reasons for dropouts/attrition by group were provided. Fair (moderate risk of bias): The trial met or was unclear for allocation concealment and blinding with no more than one of the remaining domains (ITT, withdrawals) unmet. A trial with adequate allocation concealment that did not meet other domains was rated fair. Poor (high risk of bias): The trial had inadequate allocation concealment or blinding and/or clearly met only one of the established risks of bias domains.
Multi-arm trials that are also evaluated in KQ2.
Table 3.
Strength of Evidence for Trials Comparing Intervention With Caregiver Component to Usual Care or Wait List Control.
| Outcome | No. of studies (na) | Risk of biasb | Directnessc | Precisiond | Consistencye | Evidence rating |
|---|---|---|---|---|---|---|
| Physical functioning | 8 (1,149) | High | Direct | Imprecise | Inconsistent | Low |
| Cognitive functioning | 6 (489) | Moderate | Direct | Imprecise | Inconsistent | Low |
| Quality of life | 5 (445) | Moderate | Direct | Imprecise | Inconsistent | Low |
| Symptom control/management | 11 (1,815) | Moderate | Direct | Imprecise | Inconsistent | Low |
| Depression/anxiety | 6 (548) | Moderate | Direct | Imprecise | Inconsistent | Low |
| Utilization | 6 (1,044) | Moderate | Direct | Imprecise | Consistent | Low |
Source. Adapted from evidence report (Griffin et al, 2013).
Number randomized.
Internal validity. Study design and the quality of individual studies included in the review. Study design limitations may bias the estimates of treatment effect (such as lack of allocation concealment or lack of blinding).
Interventions are directly related to health outcomes of interest.
The degree of certainty surrounding an estimate of effect for each outcome of interest. Uncertainty of effect does not allow for a clinically useful conclusion, and is unable to rule out an important benefit or harm.
The effect sizes from the included studies are similar and have the same direction of effect (positive or negative).
Benefits by Intervention Category
Seven studies (1 of good quality) assessed caregiver training interventions to improve patient outcomes. Seven (3 of good quality) evaluated caregiver training and support interventions and six (none rated good quality) involved a unique intervention. Details on effect sizes for trials in each intervention category are found in Supplemental Tables 3 to 5.
No one category of intervention appeared to be more effective than another category. Likewise, no one category consistently had significant findings for a specific outcome. In trials that included training to improve caregiving skills, three of seven reported significant improvements in at least one outcome versus comparators (Table 2). One trial of good quality (Gitlin et al., 2008) included training families to tailor activities to the capabilities of dementia patients and reported a large reduction in problem behaviors (d = 0.72). Of the trials that utilized both caregiver skill building and caregiver coping and problem solving, four of seven trials reported significant outcomes versus comparators, with two of three being of good quality. One of those trials (Mittelman et al., 2006) found that counseling and support groups for caregivers had persistent and long-term effects on delaying time to nursing home placement compared with usual care controls (unadjusted hazard ratio = 0.71, CI = [0.54, 0.94]). The other, a trial that compared a combination of cognitive rehabilitation and multi-family group treatment for patients with mild cognitive impairment and a family member to usual care, reported significant improvements post-intervention in mean scores of everyday functioning and cognitive functioning, but calculated that effect sizes were not significant (Schmitter-Edgecombe & Dyck, 2014). None of the unique interventions were rated good quality, but four of six trials reported significant improvements in outcomes over comparators. Effect sizes with CIs were not uniformly reported in these trials.
Benefits and Harms for Outcomes of Interest
Symptom control
Eleven studies (2 rated good quality; Gitlin et al., 2008) assessed symptom management or control. Effect sizes were reported in 5 of the 11 trials and ranged from −0.19 to 0.72 (Gitlin et al., 2001; Gitlin et al., 2008; McCallion et al., 1999; Ostwald et al., 1999; Quayhagen et al., 2000). Five trials, each using a different assessment for controlling problem behaviors, reported significant improvements compared with usual care or wait list control group (Gitlin et al., 2001; Gitlin et al., 2008; Gitlin et al., 2010b; McCallion et al., 1999; Robison et al., 2007; Table 2). Two of the 5 reported significant reductions in either the number or frequency of problem behaviors, with effect sizes ranging from 0.32 to 0.72, respectively (Gitlin et al., 2001; Gitlin et al., 2008). These 2 trials included patients who needed a great deal of assistance with daily tasks and included multi-component interventions with targeted plans for reducing problem behaviors. The other studies that reported significant improvements in symptom management either reported significant interaction between time and comparator group, but not significant effect sizes (McCallion et al., 1999); reported significant differences across comparator groups, but did not show data (Robison et al., 2007); or, reported only mean differences by comparator group (Gitlin et al., 2010b).
Depression
Six trials assessed how interventions affected patient depressive symptoms. Two were good quality (Gitlin et al., 2008; Schmitter-Edgecombe & Dyck, 2014), but found no significant difference by comparator group. The remaining four trials, all rated poor or fair quality, used multiple depression assessments and found significant differences in at least one of the depression scales used (Logsdon et al., 2010; McCallion et al., 1999; Teri et al., 2003; Teri et al., 1997). The clinical significance of these differences is uncertain. Three trials used unique interventions: a patient exercise promotion intervention (Teri et al., 2003); training for effective caregiver visits with institutionalized patients (McCallion et al., 1999); and, an early-stage memory loss support group for patients and caregivers (Logsdon et al., 2010). The fourth intervention included either behavior therapy and problem solving for caregivers or behavior therapy and training for caregivers to provide pleasant activities for the patient (Teri et al., 1997). Because effect sizes and associated CIs were not consistently reported, the magnitude of effects was not always transparent. For example, one trial (Teri et al., 1997), which compared the intervention group with both a usual care and wait list arm, reported significant reductions across two different measures of depression, with effect sizes ranging from d = −0.86 to −1.4. In another trial (Teri et al., 2003), however, no effect size was reported, although the mean difference in depression scale scores was significantly lower for the treatment group after adjustment for baseline depression. In the two other trials (Logsdon et al., 2010; McCallion et al., 1999), the effect size was reported as significant, but the actual effect size estimate was not provided and CIs were not reported (Logsdon et al., 2010) or the main effect size was not significant, but the intervention group by time interaction was (McCallion et al., 1999).
Quality of life
Five trials assessed quality of life. Three trials, varying in methodological quality from good to poor, reported significant improvements in quality of life versus controls (Logsdon et al., 2010; Schmitter-Edgecombe & Dyck, 2014; Teri et al., 2005). One good quality trial, by Schmitter-Edgecombe and Dyck (2014), found improvements in mean scores of everyday functioning, but effect sizes were not significant. A trial by Logsdon et al. (2010) compared an early-stage memory loss support group for families with controls consisting of dyads on a support group wait list. Another compared usual care with an intervention with home visits to caregivers to teach them problem-solving strategies to change patient behavior (Teri et al., 2005). Teri et al. (2005) reported effect sizes that were not significant post-intervention, but were at 6 months after treatment, adjusting for baseline and 2-month follow-up assessments. Logsdon et al. (2010) reported that effect sizes were statistically significant, but did not report CIs. Another trial that assessed quality of life was rated good quality, and although a large reduction in problem behaviors was found, there was no significant difference in patients’ quality of life (Gitlin et al., 2008).
Physical and cognitive functioning
Two of nine trials, one of poor and the other of fair quality, assessed physical functioning and showed significant improvements compared with controls (Gitlin et al., 2001; Teri et al., 2003). The trial by Teri et al. (Gitlin et al., 2001; Teri et al., 2003) specifically targeted promotion of exercise to improve symptoms. Gitlin et al. (2001) used a multi-component intervention that included modifications of the social and physical environment and a targeted plan to reduce symptoms.
Three of six trials assessing cognitive functioning, one rated good and two rated fair, reported significant improvement versus comparators (Schmitter-Edgecombe & Dyck, 2014; Teri et al., 1997; Teri et al., 2005), but effect sizes were not significant.
Utilization
Described in more detail below, only one of the six trials assessing health care utilization reported significant differences. In that trial, compared with usual care, patients of caregivers who received counseling and support groups were able to avoid nursing home placement for longer periods of time (Mittelman et al., 2006).
No harm to caregivers or patients was reported in any studies.
Long-Term Outcomes
Of the six trials that assessed long-term outcomes (>6 months), three reported persistent intervention effects. Mittelman et al. conducted a large trial that included tailored counseling and ad hoc telephone support for caregivers and followed patients over 18 years. Although the physical functioning and symptom control of patients in the comparator groups did not differ, they found that patients were able to remain at home longer. The intervention group showed a 28.3% less nursing home placement for patients, an equivalent to a delay of 557 days compared with usual care. Of the remaining two trials showing significant long-term outcomes, one noted improvements in cognitive functioning and quality of life after 6 months (Teri et al., 2005) and the other found an improvement in physical functioning and depressive symptoms after 2 years (Teri et al., 2003).
Key Question 2 (KQ2): What are the benefits of one caregiver-involved psychosocial intervention compared with either a patient intervention or another alternative caregiver-oriented intervention (active controls) in improving outcomes for adults with dementia or Alzheimer’s disease?
Summary of Trials
We identified 15 trials that met inclusion criteria. Study characteristics and abbreviations are provided in Supplemental Tables 1 and 2, respectively. Although 6 of 15 trials (40%) reported significant differences between interventions for any outcome (Table 4), the cumulative strength of evidence for intervention effectiveness was low for all outcomes (Table 5) due to moderate to high risk of bias, imprecision of the effect size, and poor methodological quality. Non-significant effect sizes were mostly small, though some outcomes had wide CIs and we could not rule out potentially clinically important effects. Data from two trials were not reported or could not be determined from the data reported.
Table 4.
Outcomes Reported in Trials Comparing Interventions With Caregiver Component to Alternative Caregiver or Patient Interventions.
| Author, year | na | Study qualityb | Physical functioning | Cognitive function | Quality of life/overall functioning | Symptom management/control | Depression/anxiety | Utilization |
|---|---|---|---|---|---|---|---|---|
| Caregiver training interventions (n = 5) | ||||||||
| Bourgeois 2002 (Bourgeois, Schulz, Burgio, & Beach, 2002) | 63 | Good | ↔\↑ | |||||
| Burgener 1998 (Burgener, Bakas, Murray, Dunahee, & Tossey, 1998) | 54 | Poor | ± | ± | ||||
| Chang 1999 (Chang, 1999) | 65 | Poor | ↔ | ↔ | ||||
| Gerdner 2002 (Gerdner, Buckwalter, & Reed, 2002) | 241 | Fair | ± | ± | ||||
| Quayhagen 2000 (Quayhagen et al., 2000) | 103 | Poor | ↔ | ↔ | ||||
| Caregiver training and support interventions (n = 7) | ||||||||
| Belle 2006 (Belle et al., 2006) | 518 | Fair | ↑ | ↔ | ↔ | |||
| Burns 2003 (Burns, Nichols, Martindale-Adams, Graney, & Lummus, 2003) | 76 | Poor | ↔ | |||||
| Gaugler 2013 (Gaugler, Reese, & Mittelman, 2013) | 107 | Good | ↑ | |||||
| Gitlin 2003 (Gitlin et al., 2003) | 255 | Fair | ↔ | ↔ | ↔ | |||
| Gitlin 2010 (Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010a) | 237 | Good | ↔\↑ | ↔ | ↔ | |||
| Gonyea 2006 (Gonyea, O’Connor, & Boyle, 2006) | 91 | Poor | ↔ | |||||
| Teri 1997 (Teri, Logsdon, Uomoto, & McCurry, 1997) | 72 | Fair | ↔ | ↔ | ||||
| Unique interventions with unique intervention targets (n = 3) | ||||||||
| Camberg 1999 (Camberg et al., 1999) | 54 | Fair | ↔ | |||||
| Jirovec 2001 (Jirovec & Templin, 2001) | 118 | Poor | ↔ | ↔/↑ | ||||
| McCurry 2005 (McCurry, Gibbons, Logsdon, Vitiello, & Teri, 2005) | 36 | Good | ↔ | ↔/↑ | ↔/↑ | |||
Source. Adapted from evidence report (Griffin et al, 2013).
Note. Bourgeois (Bourgeois et al., 2002) compared a caregiver intervention with a patient intervention; all other trials compared one caregiver-involved intervention with an alternative caregiver intervention. Ratings: ↑ = treatment significantly better than comparator; ↔ = no significant difference between intervention and comparator; ↓ = treatment significantly worse than comparator; ± = significance not reported or could not be determined; two ratings separated by “/” indicates multiple assessments were used, and the significance of outcomes varied across assessments.
Number randomized.
Good (low risk of bias): The trial reported adequate allocation concealment, a minimum of single blinding (participants or investigators or assessors are blinded), and that either intent-to-treat analysis was conducted or clear reasons for dropouts/attrition by group were provided. Fair (moderate risk of bias): The trial met or was unclear for allocation concealment and blinding with no more than one of the remaining domains (ITT, withdrawals) unmet. A trial with adequate allocation concealment that did not meet other domains was rated fair. Poor (high risk of bias): The trial had inadequate allocation concealment or blinding and/or clearly met only one of the established risks of bias domains.
Table 5.
Strength of Evidence for Trials Comparing Interventions With Caregiver Component to Alternative Caregiver or Patient Interventions.
| Outcome | No. of studies (na) | Risk of biasb | Directnessc | Precisiond | Consistencye | Evidence rating |
|---|---|---|---|---|---|---|
| Physical functioning | 5 (852) | Moderate | Direct | Imprecise | Unknown | Low |
| Cognitive functioning | 6 (675) | Moderate | Direct | Imprecise | Consistent | Low |
| Quality of life | 2 (755) | Moderate | Direct | Imprecise. | Unknown | Low |
| Symptom control/management | 12 (1,820) | Moderate | Direct | Imprecise | Consistent | Low |
| Depression/anxiety | 2 (108) | Moderate | Direct | Imprecise | Consistent | Low |
| Utilization | 2 (625) | Moderate | Direct | Imprecise | Inconsistent | Low |
Source. Adapted from expanded evidence report (Authors, 2013).
Number randomized.
Internal validity. Study design and the quality of individual studies included in the review. Study design limitations may bias the estimates of treatment effects (such as lack of allocation concealment or lack of blinding).
Interventions are directly related to health outcomes of interest.
The degree of certainty surrounding an estimate of effect for each outcome of interest. Uncertainty of effect does not allow for a clinically useful conclusion, and is unable to rule out an important benefit or harm.
The effect sizes from the included studies are similar and have the same direction of effect (positive or negative).
Only one trial compared an individual intervention (i.e., targeting self-change for the caregiver) with a caregiver-involved intervention (i.e., targeting patient behavior; Bourgeois, Schulz, Burgio, & Beach, 2002). The remaining trials directly compared an active caregiver intervention with either an attention control (typically an education component with or without a supportive phone call; Belle et al., 2006; Burns, Nichols, Martindale-Adams, Graney, & Lummus, 2003; Chang, 1999; Gitlin et al., 2003; Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010a; Gonyea, O’Connor, & Boyle, 2006; McCurry, Gibbons, Logsdon, Vitiello, & Teri, 2005; Gaugler, Reese, & Mittelman, 2013), one other caregiver intervention (Gerdner, Buckwalter, & Reed, 2002), or multiple alternative caregiver interventions (Bourgeois et al., 2002; Burgener et al., 1998; Camberg et al., 1999; Quayhagen et al., 2000; Teri et al., 1997). Four trials were rated as good, five as fair, and six as poor quality. Studies ranged in size from 36 to 518 dyads with a median of 97 per trial. Interventions included 1 to 38 sessions, averaging 9. Two trials included only spousal caregivers (Bourgeois et al., 2002; Quayhagen et al., 2000); one included only children of care recipients (Gaugler et al., 2013), and all others included any caregiver or primary caregiver involved in care. Two trials (Gaugler et al., 2013; Teri et al., 1997) included long-term (at least 6 months post-intervention) follow-up assessments.
No trial reported on all outcomes and most reported on only one or two. The most frequently assessed outcome was symptom control/management (80%, 12/15 trials; Belle et al., 2006; Bourgeois et al., 2002; Burgener et al., 1998; Burns et al., 2003; Camberg et al., 1999; Chang, 1999; Gerdner et al., 2002; Gitlin et al., 2003; Gitlin et al., 2010a; Jirovec & Templin, 2001; McCurry et al., 2005; Quayhagen et al., 2000), followed by cognitive functioning (40%, 6/15 trials; Gitlin et al., 2003; Gonyea et al., 2006; Jirovec & Templin, 2001; McCurry et al., 2005; Quayhagen et al., 2000; Teri et al., 1997), physical functioning (33.3%, 5/15 trials; Burgener et al., 1998; Chang, 1999; Gerdner et al., 2002; Gitlin et al., 2003; Gitlin et al., 2010a), and global quality of life (20%, 3/15 trials; Belle et al., 2006; Gitlin et al., 2010a; Teri et al., 1997). The remaining outcomes of interest, depression/anxiety and utilization, were each assessed in 13% (2/15) of trials (depression: McCurry et al., 2005; Teri et al., 1997; utilization: Belle et al., 2006; Gaugler et al., 2013).
Benefits by Intervention Category
None of the intervention categories emerged as the superior approach for improving patient outcomes. Details on effect sizes for trials in each intervention category are found in Supplemental Tables 6 to 8. Of the five trials that included caregiver training only, one reported significant improvements in outcomes versus comparators (Bourgeois et al., 2002). This good quality trial, described in detail below, showed that training caregivers to manage patient behavior was more effective at improving symptoms than providing caregivers personal strategies to cope with patient behavior. In the second category of trials that included both caregiver training and support, three of six trials, two of good (Gaugler et al., 2013; Gitlin et al., 2010a) and one of poor quality (Belle et al., 2006), reported significant differences. One good quality trial showed significant improvements in patient outcomes for caregivers who received support and training to reduce environmental stressors at home compared with those who received psychoeducation only over the telephone. The other good quality trial showed less placement in residential care among care recipients of caregivers involved in the study’s intervention (Gaugler et al., 2013). In the third category of interventions, two of three unique interventions showed significant differences in symptom control (Jirovec & Templin, 2001; McCurry et al., 2005), compared with supportive controls. Each targeted a specific symptom to change (toileting intervention to reduce incontinence and sleep education to reduce nighttime wakening, respectively).
Benefits and Harms of Caregiver Interventions Compared With Individual or Another Caregiver-Involved Intervention
Just more than half of the 15 trials (Burns et al., 2003; Camberg et al., 1999; Chang, 1999; Gaugler et al., 2013; Gitlin et al., 2003; Gonyea et al., 2006; Quayhagen et al., 2000; Teri et al., 1997) reported significant differences between comparator groups. Of these, 5 trials reported superior intervention benefits on one outcome of interest (Belle et al., 2006; Bourgeois et al., 2002; Gaugler et al., 2013; Gitlin et al., 2010a; Jirovec & Templin, 2001), and 1 trial reported benefits for two outcomes (McCurry et al., 2005; Table 4). Two trials reported significant findings, but did not report their findings with sufficient detail to evaluate differences in outcomes by comparator groups (Burgener et al., 1998; Gerdner et al., 2002).
The overall strength of evidence for the effectiveness of one intervention compared with an alternative active treatment was low for all outcomes, due to moderate risk of bias and imprecision of the effect size (Table 5). Trials comparing a caregiver-involved intervention with attention controls, such as psychoeducation, showed few improvements on outcomes. Evidence was insufficient to suggest that interventions, beyond providing education and minimal support to caregivers, are beneficial to patients.
Symptom control
Twelve trials reported symptom control outcomes. Of these, 3 reported significant differences in symptom control due to interventions (Bourgeois et al., 2002; Jirovec & Templin, 2001; McCurry et al., 2005). Although 2 of the 3 were of good quality (Bourgeois et al., 2002; McCurry et al., 2005), none had significant effect sizes and instead reported either significant mean differences by intervention or reported p values without reporting data. All were narrowly focused interventions intended to change specific symptoms. They included a sleep hygiene intervention to improve patient sleep behavior (McCurry et al., 2005) and a toileting intervention to reduce incontinence (Jirovec & Templin, 2001). The third included a comparison of one intervention to train caregivers to manage patient behavior with another intervention that taught caregivers self-care strategies to cope with caregiving burden (Bourgeois et al., 2002).
Quality of life, physical and cognitive functioning, depression/anxiety, and utilization
Of the 15 trials, 12 assessed outcomes of interest other than symptom control. Of these, 4 trials showed significant differences between intervention and controls for any one outcome. One was a cognitive-behavioral intervention that improved physical functioning of patients by reducing environmental stressors at home (Gitlin et al., 2010a). The second was an intervention designed to improve patients’ quality of life by enhancing their family members’ skills in caregiving (Belle et al., 2006). The third replicated the Mittelman study previously reported. The intervention, which included counseling and support specifically for adult children of care recipients, was effective at reducing or delaying placement in residential care for care recipients compared with attention controls. The fourth was the sleep hygiene study that reported significant differences by comparator group in patient depression, but using data provided, our calculated effect size was not significant (McCurry et al., 2005).
No harm to caregivers or patients was reported in any studies.
Long-Term Outcomes
Two trials (Gaugler et al., 2013; Teri et al., 1997) included long-term (at least 6 months post-intervention) follow-up. The first trial replicated the Mittelman trial and had similar findings to this earlier trial. Researchers found that the intervention was successful at keeping patients at home significantly longer. The time from baseline to residential placement for care recipients of caregivers in the control group was 228 days earlier than for the intervention group. In the second trial, comparisons between intervention and controls at 6 months were not reported, only changes within groups over time.
Discussion
Family roles are significantly disrupted when an individual with cognitive impairment develops worsening function or symptoms. Functional decline typically means a greater demand on family caregivers for patient assistance, care management, and support. Thus, interventions that reduce caregiver burden and enhance patient assistance, care management, and support are important. Previous work has demonstrated the effectiveness of certain interventions on caregiver outcomes. In this review, we sought to determine whether caregiver interventions were effective at improving or reducing decline in patient outcomes, including functional status, quality of life, managing problem behaviors, and health care utilization. We did not identify specific types of interventions (i.e., training, training and support or unique interventions) that demonstrated greater mitigation of declines in or greater statistical improvement in patient outcomes than comparators, although we cannot rule out that clinically meaningful differences exist. Of the larger, good quality trials that showed significant effects on at least one outcome, interventions were tailored to the needs of patients and caregivers (Gitlin et al., 2010a) and included long-term, ongoing support (Gaugler et al., 2013; Mittelman et al., 2006).
The strength of evidence is low regarding the effectiveness of caregiver-involved interventions in improving patient outcomes in adults with dementia compared with usual care or wait list. We also did not find that caregiver-involved interventions were superior to the ones that are patient focused or provide only health education, support, or psychoeducation. Few trials with statistically significant findings have been replicated. The one exception is the New York University Caregiver Intervention (NYUCI), which in two trials (three articles; Gaugler et al., 2013; Mittelman et al., 2006; Mittelman et al., 2004), with different comparator groups, showed significant effects of caregiver counseling and support in delaying institutionalization. Other trials that have not been replicated suggest that symptom control and depression/anxiety were outcomes most amenable to change. However, additional research is needed before recommending widespread adoption.
Previous research and reviews have been equivocal in their summative conclusions about the effects of caregiver interventions on outcomes for patients with dementia/Alzheimer’s disease. Our review not only updates previous reviews but also provides a different perspective for examining the evidence. First, unlike previous reviews by Brodaty and Arasaratnam (2012); Torti, Gwyther, Reed, Friedman, and Schulman (2004); and Opie, Rosewarne, and O’Connor (1999), that included observational, quasi-experimental, and case studies, we included only RCTs. By reviewing evidence only from RCTs, we minimized the potential that reported effects may be due to secular trends or imbalances or from variations in patient or caregiver baseline characteristics, issues that cannot be controlled in non-randomized studies. Second, we assessed the evidence from trials that compared two different interventions instead of limiting the review only to interventions that were compared with usual care. Third, previous reviews also included studies conducted outside the United States. We limited our review to studies conducted in the United States to reflect the unique social, cultural, and clinical norms and resources for caregiver support that can vary across countries (Corbett et al., 2012; Torti et al., 2004) and to better inform the public health needs outlined in the National Plan to Address Alzheimer’s Disease (U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, 2013). Fourth, unlike other reviews that targeted a specific set of outcomes (Brodaty & Arasaratnam, 2012; Opie et al., 1999), we included a broad set of outcomes that affect patients with dementia. We included not only problem behaviors, such as wandering, aggression, and agitation but also patient depression and anxiety, quality of life and functional status. Finally, we included a broad range of psychosocial interventions targeting caregivers and did not limit the review to only trials that included face-to-face interventions for the caregiver/care recipient dyad (Van’t Leven et al., 2013).
The evidence summarized in our review has limitations. Although the intention of each trial was not always stated, a number of studies in our review likely were designed to improve caregiver outcomes (e.g., reducing caregiver burden) and patient outcomes were secondary. For some interventions, it is likely that the intention was to reduce the burden of care for caregivers by helping them manage patient functioning and care. Consequently, their limited impact on patient outcomes is not surprising; such interventions were likely not explicitly designed to directly benefit patients. The Resources for Enhancing Alzheimer’s Caregiver Health (REACH) trials, for example, have shown significant improvements in caregiver outcomes over comparators (Belle et al., 2006; Elliott, Burgio, & Decoster, 2010; Mausbach et al., 2004), but patient outcomes examined for this review were not consistently different than comparators (Belle et al., 2006; Gitlin et al., 2003). It is possible that effective interventions targeting caregiver outcomes may subsequently benefit patients or slow the progression of poor outcomes, but the effect on families must be large enough to result in perceptible patient benefit and sufficient follow-up to detect subsequent patient effects. Our review did not include large-scale interventions or public-health-oriented programs, such as support lines, mass educational campaigns, or cash and counseling programs that families may use in ways that affect patient outcomes.
The National Plan to Address Alzheimer’s Disease provides a charge to harness efforts to improve research quality and consistency of measurement and to more clearly define specific caregiver strategies to improve care quality, especially among high-risk groups, and fill gaps in the body of evidence. With these goals in mind, we have a number of recommendations for policy makers, researchers, and providers. First, other studies have shown that caregiver interventions can reduce caregiver burden (Sorensen et al., 2002). However, it remains unclear if reducing caregiver burden enhances patient care, which, in turn, improves patient outcomes. Future research that can rigorously test this question is needed. Understanding the link between caregiver health and patient health is critical for understanding whether separate interventions should address caregiver issues and patient issues, or if investing in caregiver interventions will provide downstream improvements in patient outcomes. Second, more replication of good quality studies that have shown significant effects, such as those conducted by Mittelman et al. (2004) and Gaugler et al. (2013), which both have shown persistent effects over long periods of time, is critical to determine whether interventions can affect targeted outcomes in similar populations and subpopulations. Third, adequately powered research is needed to improve the precision of the estimated intervention effects. Likewise, improved documentation of methodology including blinding, allocation concealment, descriptions of dropouts by experimental group, and use of ITT analyses is needed to assess potential bias. In addition, consensus in the field about which criteria should be used to assess dementia severity and which instruments should be employed to assess common outcomes of interest would allow for meta-analysis of data that could help elucidate the net impact of interventions. Outcome data should be reported post-treatment for each comparator group for direct group comparison and, when feasible, longer term outcomes should be included to assess intervention sustainability and downstream effects of caregiver intervention on patient outcomes. Trials such as Teri et al.’s (2005), which did not show post-intervention effects, but did find significant differences at 6 months, may indicate a need to study if a lack of evidence is in part due to delayed interventions that are missed when long-term assessments are not included.
Conclusion
We identified a wide range of interventions directed at caregivers of adults with dementia or Alzheimer’s disease that also reported patient outcomes. Most were evaluated solely in single randomized trials. Variability in study populations, interventions, and outcomes precluded data pooling and limited generalizing findings from any single study. Harms appeared to be few but data were rarely reported. Although many did not demonstrate improved outcomes in adults with dementia or Alzheimer’s disease compared with usual care or an active treatment, an intervention that combined counseling and support groups for caregivers, resulted in persistent delays in nursing home placement. However, current evidence does not demonstrate that most caregiver interventions provide consistent or clinically meaningful improvements for adults with dementia or Alzheimer’s disease. Additional research is needed before widespread implementation of caregiver interventions can be implemented if the goal is to improve outcomes in patients with dementia or Alzheimer’s disease.
Acknowledgments
The authors thank members of the Technical Expert Panel who provided consultation on this review.
Footnotes
Authors’ Note: The findings and conclusions are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or QUERI. The sponsor was not involved in any aspect of the study’s design and conduct; data collection, management, analysis, or interpretation of data; or the preparation, review, or approval of the manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Quality Enhancement Research Initiative’s Evidence Synthesis Program within the Office of Research and Development, Veterans Health Administration, Department of Veterans Affairs (QUERI: VA-ESP Project #09-009; 2013).
References
- Bass D. M., Clark P. A., Looman W. J., McCarthy C. A., Eckert S. (2003). The Cleveland Alzheimer’s managed care demonstration: Outcomes after 12 months of implementation. The Gerontologist, 43, 73-85. [DOI] [PubMed] [Google Scholar]
- Belle S. H., Burgio L., Burns R., Coon D., Czaja S. J., Gallagher-Thompson D., . . . Zhang S. (2006). Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: A randomized, controlled trial. Annals of Internal Medicine, 145, 727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois M. S., Schulz R., Burgio L. D., Beach S. (2002). Skills training for spouses of patients with Alzheimer’s disease: Outcomes of an intervention study. Journal of Clinical Geropsychology, 8, 53-73. [Google Scholar]
- Brodaty H., Arasaratnam C. (2012). Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. American Journal of Psychiatry, 169, 946-953. [DOI] [PubMed] [Google Scholar]
- Brodaty H., Mittelman M., Gibson L., Seeher K., Burns A. (2009). The effects of counseling spouse caregivers of people with Alzheimer disease taking donepezil and of country of residence on rates of admission to nursing homes and mortality. American Journal of Geriatric Psychiatry, 17, 734-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgener S. C., Bakas T., Murray C., Dunahee J., Tossey S. (1998). Effective caregiving approaches for patients with Alzheimer’s disease. Geriatric Nursing, 19, 121-126. [DOI] [PubMed] [Google Scholar]
- Burns R., Nichols L. O., Martindale-Adams J., Graney M. J., Lummus A. (2003). Primary care interventions for dementia caregivers: 2-year outcomes from the REACH study. The Gerontologist, 43, 547-555. [DOI] [PubMed] [Google Scholar]
- Camberg L., Woods P., Ooi W. L., Hurley A., Volicer L., Ashley J., . . . McIntyre K. (1999). Evaluation of simulated presence: A personalized approach to enhance well-being in persons with Alzheimer’s disease. Journal of the American Geriatrics Society, 47, 446-452. [DOI] [PubMed] [Google Scholar]
- Chang B. L. (1999). Cognitive-behavioral intervention for homebound caregivers of persons with dementia. Nursing Research, 48, 173-182. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Corbett A., Stevens J., Aarsland D., Day S., Moniz-Cook E., Woods R., . . . Ballard C. (2012). Systematic review of services providing information and/or advice to people with dementia and/or their caregivers. International Journal of Geriatric Psychiatry, 27, 628-636. [DOI] [PubMed] [Google Scholar]
- Elliott A. F., Burgio L. D., Decoster J. (2010). Enhancing caregiver health: Findings from the resources for enhancing Alzheimer’s caregiver health II intervention. Journal of the American Geriatrics Society, 58, 30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler J. E., Reese M., Mittelman M. S. (2013). Effects of the NYU caregiver intervention-adult child on residential care placement. The Gerontologist, 53, 985-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdner L. A., Buckwalter K. C., Reed D. (2002). Impact of a psychoeducational intervention on caregiver response to behavioral problems. Nursing Research, 51, 363-374. [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Corcoran M., Winter L., Boyce A., Hauck W. W. (2001). A randomized, controlled trial of a home environmental intervention: Effect on efficacy and upset in caregivers and on daily function of persons with dementia. The Gerontologist, 41, 4-14. [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Burke J., Chernett N., Dennis M. P., Hauck W. W. (2008). Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: A randomized pilot study. The American Journal of Geriatric Psychiatry, 16, 229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Corcoran M., Dennis M. P., Schinfeld S., Hauck W. W. (2003). Effects of the home environmental skill-building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH Initiative. The Gerontologist, 43, 532-546. [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Dennis M. P., Hodgson N., Hauck W. W. (2010a). A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: The COPE randomized trial. Journal of the American Medical Association, 304, 983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Dennis M. P., Hodgson N., Hauck W. W. (2010b). Targeting and managing behavioral symptoms in individuals with dementia: A randomized trial of a nonpharmacological intervention. Journal of the American Geriatrics Society, 58, 1465-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonyea J. G., O’Connor M. K., Boyle P. A. (2006). Project CARE: A randomized controlled trial of a behavioral intervention group for Alzheimer’s disease caregivers. The Gerontologist, 46, 827-832. [DOI] [PubMed] [Google Scholar]
- Goy E., Kansagara D., Freeman M. (2010). A systematic evidence review of interventions for non-professional caregivers of individuals with dementia. Washington, DC: Department of Veterans Affairs; Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK49194/pdf/TOC.pdf [PubMed] [Google Scholar]
- Griffin J. M., Meis L., Greer N., Jensen A., MacDonald R., Rutks I., . . . Wilt T. J. (2013, April). Effectiveness of family and caregiver interventions on patient outcomes among adults with cancer or memory-related disorders: A systematic review. Washington, DC: Department of Veterans Affairs; Retrieved from http://www.hsrd.research.va.gov/publications/esp/caregiver-interventions.pdf [PubMed] [Google Scholar]
- Hebert L. E., Weuve J., Scherr P. A., Evans D. A. (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology, 80, 1778-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions (Version 5.1.0, updated March 2011). The Cochrane Collaboration; Retrieved from www.cochrane-handbook.org [Google Scholar]
- Hurd M. D., Martorell P., Delavande A., Mullen K. J., Langa K. M. (2013). Monetary costs of dementia in the United States. New England Journal of Medicine, 368, 1326-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirovec M. M., Templin T. (2001). Predicting success using individualized scheduled toileting for memory-impaired elders at home. Research in Nursing & Health, 24, 1-8. [DOI] [PubMed] [Google Scholar]
- Lee P. E., Gill S. S., Freedman M., Bronskill S. E., Hillmer M. P., Rochon P. A. (2004). A typical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: Systematic review. British Medical Journal, 329(7457), Article 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. S., O’Connor E., Rossom R. C., Perdue L. A., Eckstrom E. (2013). Screening for cognitive impairment in older adults: A systematic review for the U.S. preventive services task force. Annals of Internal Medicine, 159, 601-612. [DOI] [PubMed] [Google Scholar]
- Logsdon R. G., Gibbons L. E., McCurry S. M., Teri L. (2002). Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine, 64, 510-519. [DOI] [PubMed] [Google Scholar]
- Logsdon R. G., Pike K. C., McCurry S. M., Hunter P., Maher J., Snyder L., Teri L. (2010). Early-stage memory loss support groups: Outcomes from a randomized controlled clinical trial. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 65, 691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cook K., Davis B. A., Hynan L. S., Weiner M. F. (2005). A randomized, controlled study of an Alzheimer’s caregiver skills training program. American Journal of Alzheimer’s Disease & Other Dementias, 20, 204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach B. T., Coon D. W., Depp C., Rabinowitz Y. G., Wilson-Arias E., Kraemer H. C., . . . Gallagher-Thompson D. (2004). Ethnicity and time to institutionalization of dementia patients: A comparison of Latina and Caucasian female family caregivers. Journal of the American Geriatrics Society, 52, 1077-1084. [DOI] [PubMed] [Google Scholar]
- McCallion P., Toseland R. W., Freeman K. (1999). An evaluation of a family visit education program. Journal of the American Geriatrics Society, 47, 203-214. [DOI] [PubMed] [Google Scholar]
- McCurry S. M., Gibbons L. E., Logsdon R. G., Vitiello M. V., Teri L. (2005). Nighttime insomnia treatment and education for Alzheimer’s disease: A randomized, controlled trial. Journal of the American Geriatrics Society, 53, 793-802. [DOI] [PubMed] [Google Scholar]
- Mittelman M. S., Haley W. E., Clay O. J., Roth D. L. (2006). Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology, 67, 1592-1599. [DOI] [PubMed] [Google Scholar]
- Mittelman M. S., Roth D. L., Haley W. E., Zarit S. H. (2004). Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: Results of a randomized trial. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 59(1), P27-P34. [DOI] [PubMed] [Google Scholar]
- Opie J., Rosewarne R., O’Connor D. W. (1999). The efficacy of psychosocial approaches to behaviour disorders in dementia: A systematic literature review. Australian & New Zealand Journal of Psychiatry, 33, 789-799. [DOI] [PubMed] [Google Scholar]
- Ostwald S. K., Hepburn K. W., Caron W., Burns T., Mantell R. (1999). Reducing caregiver burden: A randomized psychoeducational intervention for caregivers of persons with dementia. The Gerontologist, 39, 299-309. [DOI] [PubMed] [Google Scholar]
- Owens D. K., Lohr K. N., Atkins D., Treadwell J. R., Reston J. T., Bass E. B., . . . Helfand M. (2010). AHRQ series paper 5: Grading the strength of a body of evidence when comparing medical interventions—Agency for healthcare research and quality and the effective health-care program. Journal of Clinical Epidemiology, 63, 513-523. [DOI] [PubMed] [Google Scholar]
- Quayhagen M. P., Quayhagen M., Corbeil R. R., Hendrix R. C., Jackson J., Snyder L., Bower D. (2000). Coping with dementia: Evaluation of four non pharmacologic interventions. International Psychogeriatrics, 12, 249-265. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer Program]. (2014). (Version 5.3). Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration. [Google Scholar]
- Robison J., Curry L., Gruman C., Porter M., Henderson C. R., Jr., Pillemer K. (2007). Partners in caregiving in a special care environment: Cooperative communication between staff and families on dementia units. The Gerontologist, 47, 504-515. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M., Dyck D. G. (2014). Cognitive rehabilitation multi-family group intervention for individuals with mild cognitive impairment and their care-partners. Journal of the International Neuropsychological Society, 20, 897-908. [DOI] [PubMed] [Google Scholar]
- Sink K. M., Holden K. F., Yaffe K. (2005). Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. Journal of the American Medical Association, 293, 596-608. [DOI] [PubMed] [Google Scholar]
- Sorensen S., Pinquart M., Duberstein P. (2002). How effective are interventions with caregivers? An updated meta-analysis. The Gerontologist, 42, 356-372. [DOI] [PubMed] [Google Scholar]
- Teri L., Gibbons L. E., McCurry S. M., Logsdon R. G., Buchner D. M., Barlow W. E., . . . Larson E. B. (2003). Exercise plus behavioral management in patients with Alzheimer disease: A randomized controlled trial. Journal of the American Medical Association, 290, 2015-2022. [DOI] [PubMed] [Google Scholar]
- Teri L., Logsdon R. G., Uomoto J., McCurry S. M. (1997). Behavioral treatment of depression in dementia patients: A controlled clinical trial. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 52(4), P159-P166. [DOI] [PubMed] [Google Scholar]
- Teri L., McCurry S. M., Logsdon R., Gibbons L. E. (2005). Training community consultants to help family members improve dementia care: A randomized controlled trial. The Gerontologist, 45, 802-811. [DOI] [PubMed] [Google Scholar]
- Thompson C. A., Spilsbury K., Hall J., Birks Y., Barnes C., Adamson J. (2007). Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatrics, 7, Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti F. M., Jr., Gwyther L. P., Reed S. D., Friedman J. Y., Schulman K. A. (2004). A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer Disease & Associated Disorders, 18, 99-109. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. (2013). National plan to address Alzheimer’s disease: 2013 update. Retrieved from http://aspe.hhs.gov/daltcp/napa/NatlPlan2013.shtml
- Van’t Leven N., Prick A.-E. J. C., Groenewoud J. G., Roelofs P. D. D. M., de Lange J., Pot A. M. (2013). Dyadic interventions for community-dwelling people with dementia and their family caregivers: A systematic review. International Psychogeriatrics, 25, 1581-1603. [DOI] [PubMed] [Google Scholar]
- Visser-Meily A., van Heugten C., Post M., Schepers V., Lindeman E. (2005). Intervention studies for caregivers of stroke survivors: A critical review. Patient Education & Counseling, 56, 257-267. [DOI] [PubMed] [Google Scholar]
- Wray L. O., Shulan M. D., Toseland R. W., Freeman K. E., Vasquez B. E., Gao J. (2010). The effect of telephone support groups on costs of care for veterans with dementia. The Gerontologist, 50, 623-631. [DOI] [PubMed] [Google Scholar]
- Wright L. K., Litaker M., Laraia M. T., DeAndrade S. (2001). Continuum of care for Alzheimer’s disease: A nurse education and counseling program. Issues in Mental Health Nursing, 22, 231-252. [DOI] [PubMed] [Google Scholar]