Abstract
BACKGROUND
Butyric acid is produced by degradation of dietary fibre by microbiota and is crucial for maintaining a healthy colon. The physicochemical properties are important for butyric acid formation, and this study aimed to evaluate the use of malting to tailor the functional characteristics of barley dietary fibre. The effect of different steeping conditions was evaluated in laboratory‐scale malting experiments with three different barley varieties.
RESULTS
Steeping at 35°C and with 0.4 % (v/v) lactic acid resulted in a higher content of β‐glucan and soluble fibre in malts than in those steeped at lower temperature and lower lactic acid concentration. Resistant starch increased, whereas the content of soluble arabinoxylan was lower. Dietary fibre components in Tipple were more affected by steeping conditions than the other varieties. The total contents of iron, phytate and amylose were little influenced by steeping conditions.
CONCLUSION
The selection of steeping conditions during malting influences composition and the characteristics of dietary fibre in barley. However, the choice of barley variety is also important for tailoring of functional ingredients beneficial for colonic health. © 2016 The Authors. Journal of the Science of Food and Agriculture published by JohnWiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: barley, malt, steeping, dietary fibres, temperature, lactic acid
INTRODUCTION
Colonic health is related to an increased consumption of fibre, which can be converted by the colonic microbiota into short‐chain fatty acids (SCFAs; mainly butyric, propionic and acetic acid). Butyric acid, in particular, provides energy to the colonocytes,1, 2 improving the nutritional status of the colonic mucosa and preventing diseases both locally and systemically. Notably, dietary fibre with different functional characteristics result in different amounts and profiles of SCFAs;3 β‐glucan and some types of resistant starch yield high amounts of butyric acid, whereas some authors argue that the arabinoxylan (AX), especially those of low molecular weight (MW) (66 vs. 354 kDa), are responsible for the increase of butyric acid formation.4, 5 Similarly, fructo‐oligosaccharides of low MW (degree of polymerization, DP 2–8) yield higher amounts of butyric acid than those of high MW (average DP 23).6 Further, dietary fibre polysaccharides could, due to their slower fermentation rate (wheat bran), shift the production of SCFA to the distal colon if they are mixed with a fibre that is more rapidly fermented (resistant starch).3 This is interesting because the majority of colonic disorders arise here.
Previous studies on barley and malt have reported a higher concentration of butyric acid in the colon of rats fed diets with malt than with untreated barley;7 however, the effects varied with different malt materials.8 The reason for this variation is unclear, but it could be due to several differences in fibre characteristics between the malt materials; the content of β‐glucan is lower in malt than in barley, but more soluble and possibly of lower MW. Further, the differences in barley composition depend on the variety and growing conditions.9
β‐Glucan and AX, major constituents of barley endosperm cell walls, are hydrolysed during malting, allowing release of the entrapped starch granules.10 As a consequence, soluble β‐glucan and AX increase viscosity in solutions, influenced by their physicochemical properties, such as MW and concentration,11 which may change the amount and pattern of SCFA.
Although the dietary fibre components of barley are usually hydrolysed during malting, previous studies have also reported that by modification of the steeping conditions malting can be a possible means to preserve β‐glucan content of barley malt.12
High‐amylose starch with low in vitro digestibility has been reported to increase hindgut fermentation and production of SCFA in pigs.13 Barley is also an important source of essential elements and contains between 25 and 63 µg iron, mostly located in the aleurone and scutellum,14 which may be important since patients with colonic diseases frequently have iron deficiency.
Protein from barley may also play a role in colonic health due to its high content of glutamine,15 which, followed by butyric acid, is one of the main energy sources for the colonocytes and a potential agent to accelerate mucosal healing and regeneration.16
Malts with high content of dietary fibre (β‐glucan, AX, resistant starch) of different MW, in addition to amylose and protein (glutamine), could potentially be a food ingredient to increase colonic health, whereas reducing phytate, a strong inhibitor of trace elements, could increase the bioavailability of metal ions.
The aim was to study the extent to which functional characteristics of dietary fibre could be modified by using different steeping conditions, for use in barley products promoting colonic health. For this purpose, total and soluble fibre and arabinoxylan, β‐glucan and β‐glucan MW, and resistant starch were investigated in laboratory‐scale malting experiments in barley varieties with different physicochemical properties.
To generate a more comprehensive characterization of the malt composition, other components of nutritional interest were also evaluated (amylose, protein, iron and phytate).
EXPERIMENTAL
Malting
Three barley varieties with different characteristics were provided by Lantmännen SW Seed AB, randomly selected from different field locations in the southern region of Sweden (50 kg, harvested in 2012): Tipple, widely used in beer production; Karmosé, with high content of amylose (30% amylose of total starch) and β‐glucan (∼5%); and Cinnamon, a waxy variety (6% amylose of total starch) with high β‐glucan content (∼5%).
The conditions selected for the steeping experiments were based on studies previously used to preserve β‐glucan content in barley.12, 17 The combination of high steeping temperature and addition of lactic acid to the steeping water was found to contribute to a low β‐glucanase activity and a preserved content of β‐glucan. The malting was performed based on a method previously described by us.18
The laboratory‐scale malting experiments were performed at Lantmännen SW Seed AB, Svalöv. For the steeping, perforated plastic bags were used containing 80 g of grains of each barley variety (Tipple, Karmosé or Cinnamon). The grains were soaked in containers with 800 mL water at different concentrations of lactic acid (0%, 0.2% or 0.4% v/v) and at 15 or 35°C, resulting in six different steeping conditions for each barley variety (Table 1). Each malting was carried out twice, resulting in a total of 36 malted samples. A portion of the untreated barley of each variety was also saved for later analyses. The steeping water was changed once when the grain moisture reached 30–35%, and the steeping continued under the same conditions until the moisture content of the grains reached approximately 42%. This resulted in a total steeping time of 32–37 h, depending on the variety. Laboratory‐scale malting equipment was used for the germination and kilning steps. The grains were randomly placed in their corresponding slots of the malting equipment and allowed to germinate at 15°C for 71 h. Afterwards, the germinated grains were kilned for a total of 26 h by gradually increasing the temperature from 55°C (10 h) to 70°C (8 h) and then 82°C (8 h).
Table 1.
Steeping conditions
| Steeping condition | Lactic acid | Temperature |
|---|---|---|
| (% v/v) | (°C) | |
| 1 | 0 | 15 |
| 2 | 0 | 35 |
| 3 | 0.2 | 15 |
| 4 | 0.2 | 35 |
| 5 | 0.4 | 15 |
| 6 | 0.4 | 35 |
Chemical characterization
Before analysis, samples were ground with an analytical mill (A11 basic, IKA) until a homogeneous flour was obtained. Dry matter content was determined using a moisture balance (Precisa HA 300, Dietikon, Switzerland). The characterization analyses were performed in both untreated barley and malted samples.
Dietary fibre components
Soluble and insoluble dietary fibres were quantified according to an enzymatic gravimetric method by precipitation of the soluble fibre with ethanol 80% (v/v) (Fibertec System E, Höganäs, Sweden), and the total fibre was calculated as their sum.19 The composition of the neutral sugar of the dietary fibre residues obtained from the gravimetric method was measured using a gas chromatographic method.20 In this method the dietary fibres are hydrolysed, filtered and derivatized into their alditol acetates. The AX content was estimated as the sum of arabinose and xylose. β‐Glucan content was assessed with the mixed‐linkage β‐glucan assay kit from Megazyme (K‐BGLU, Megazyme International, Ireland), measuring β‐glucan content including those of low MW. For determination of the average β‐glucan MW, the β‐glucan was extracted from samples (1 g) with 10 mL deionized water containing CaCl2 (0.3 mg mL−1) and 20 μL thermostable α‐amylase at 100°C for 90 min and analysed using high‐performance size exclusion chromatography with fluorescence detection (HPSEC‐FD) and Calcofluor post‐column complexation, with the low limit of detection around 10 kDa.21 Resistant starch was estimated by calculating the difference of absorbance between total (after KOH solubilization) and available starch (without KOH), which was obtained using an enzymatic assay with α‐amylase and amyloglucosidase.22, 23
Non‐dietary fibre components
The proportion of amylose (percentage of total starch) was measured spectrophotometrically with a commercial kit, using the Megazyme amylose/amylopectin assay according to the manufacturer's recommendation (K‐AMYL, Megazyme International). Total amylose (g kg−1) was obtained by multiplying the proportion of amylose by the total starch. The crude protein (total nitrogen) content was determined using an elemental analyser (Flash EA 1112, Thermo Fisher Scientific Inc., Waltham, MA, USA) with a nitrogen conversion factor of 5.83.24 Phytate (myo‐inositol hexaphosphate) content was measured by high‐performance ion chromatography with UV detection (HPIC‐UV) from samples extracted with HCl.25 Iron was quantified by HPLC after samples had been microwave‐digested with concentrated HNO3 and HCl.26
Calculations and statistical analyses
The analyses were made at least once in all replicas exposed to the different malting conditions, (i.e. in duplicate, since each malting was performed twice) and twice in the untreated barley. Protein, dietary fibre, β‐glucan MW, phytate and iron were assayed twice per sample. The data are shown as the mean and standard error of the mean (SEM). ANOVA and Tukey's post hoc tests were used to evaluate the difference between each steeping condition and untreated barley for each variety, as well as differences between the varieties of untreated barley (Tables 2 and 3). A general linear model was used to evaluate the significances and interaction effects of the steeping conditions (Table 4). A two‐tailed Pearson's test was used to evaluate correlations. Statistical significance was established when P < 0.05, and tendency as P < 0.1. Statistical analysis was performed using SPSS Statistics software. To study the variation in chemical composition and characteristics and to find correlations between different variables, a principal component analysis (PCA; SIMCA 13, Umetrics, Umeå, Sweden) was performed (Fig. 2).27
Table 2.
Dietary fibre content (total and soluble fibre, β‐glucan, total and soluble arabinoxylan and resistant starch), and β‐glucan molecular weight (MW) in untreated and malted barley after steeping at different temperatures and lactic acid concentrations (calculated on dry weight basis)
| Lactic acid (% v/v) | T (°C) | Total fibre | Soluble fibre | β‐Glucan | β‐Glucan MW | Total arabinoxylan | Soluble arabinoxylan | Resistant starch | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | SEM | g kg−1 | SEM | g kg−1 | SEM | × 106 g mol−1 | SEM | g kg−1 | SEM | g kg−1 | SEM | g kg−1 | SEM | |||
| Tipple | Untreated | x 208 b | 5 | x 37 c | 1 | x 39 d | 1 | x 1.56 a | 0.1 | x 73 ab | 2 | x 19 b | 0 | x 6 a | 1 | |
| 0 | 15 | 180 a | 0 | 17 a | 1 | 23 a | 0 | 1.54 a | 0.0 | 84 d | 1 | 16 b | 0 | 24 b | 2 | |
| 0 | 35 | 187 a | 3 | 21 ab | 0 | 23 a | 1 | 1.46 a | 0.1 | 83 bcd | 3 | 17 b | 2 | 34 bc | 1 | |
| 0.2 | 15 | 180 a | 0 | 23 ab | 2 | 24 ab | 1 | 1.48 a | 0.0 | 85 cd | 3 | 16 b | 3 | nd | ||
| 0.2 | 35 | 185 a | 6 | 22 ab | 0 | 30 c | 0 | 1.47 a | 0.0 | 85 cd | 2 | 16 b | 1 | nd | ||
| 0.4 | 15 | 182 a | 4 | 23 ab | 1 | 27 bc | 0 | 1.46 a | 0.0 | 82 abc | 1 | 14 b | 0 | 42 c | 4 | |
| 0.4 | 35 | 188 a | 1 | 26 b | 1 | 37 d | 0 | 1.43 a | 0.0 | 71 a | 0 | 3 a | 0 | 42 c | 4 | |
| Karmosé | Untreated | y 246 b | 0 | y 48 d | 1 | y 52 c | 3 | x 1.67 b | 0.0 | x 93 a | 3 | y 25 ab | 0 | y 39 a | 1 | |
| 0 | 15 | 241 ab | 7 | 28 a | 1 | 36 a | 0 | 1.54 ab | 0.1 | 114 a | 5 | 31 b | 1 | 27 a | 6 | |
| 0 | 35 | 240 ab | 1 | 35 abc | 1 | 39 ab | 1 | 1.43 ab | 0.0 | 105 a | 4 | 26 ab | 4 | 45 a | 3 | |
| 0.2 | 15 | 217 a | 4 | 34 ab | 2 | 38 a | 1 | 1.48 ab | 0.0 | 103 a | 2 | 25 ab | 1 | nd | ||
| 0.2 | 35 | 217 a | 3 | 40 bcd | 1 | 40 ab | 1 | 1.48 ab | 0.0 | 106 a | 3 | 24 ab | 0 | nd | ||
| 0.4 | 15 | 225 ab | 6 | 41 bcd | 1 | 40 ab | 1 | 1.47 ab | 0.0 | 104 a | 2 | 21 ab | 3 | 45 a | 1 | |
| 0.4 | 35 | 242 ab | 7 | 43 cd | 1 | 47 bc | 2 | 1.46 a | 0.1 | 97 a | 7 | 15 a | 2 | 35 a | 5 | |
| Cinnamon | Untreated | x 213 a | 5 | xy 42 b | 2 | xy 51 b | 2 | x 1.60 a | 0.0 | x 83 a | 1 | y 25 a | 0 | x 5 a | 0 | |
| 0 | 15 | 215 a | 2 | 33 a | 3 | 39 a | 1 | 1.59 a | 0.0 | 99 a | 3 | 19 a | 0 | 3 a | 0 | |
| 0 | 35 | 187 a | 5 | 37 ab | 0 | 37 a | 3 | 1.54 a | 0.0 | 95 a | 8 | 20 a | 1 | 16 a | 4 | |
| 0.2 | 15 | 198 a | 1 | 37 ab | 1 | 43 ab | 1 | 1.53 a | 0.0 | 94 a | 9 | 17 a | 5 | nd | ||
| 0.2 | 35 | 214 a | 3 | 40 ab | 0 | 46 ab | 3 | 1.55 a | 0.0 | 104 a | 5 | 21 a | 2 | nd | ||
| 0.4 | 15 | 210 a | 4 | 35 ab | 1 | 42 ab | 2 | 1.54 a | 0.0 | 93 a | 0 | 15 a | 3 | 13 a | 6 | |
| 0.4 | 35 | 217 a | 4 | 42 b | 2 | 47 ab | 0 | 1.53 a | 0.0 | 94 a | 8 | 13 a | 2 | 16 a | 3 | |
Values are expressed as the means ± SEM. Means in the same column and for the same variety and not sharing a letter (a–d) are significantly different (P < 0.05) and means for the different varieties not sharing a letter (x, y) are significantly different (P < 0.05). nd, not determined.
Table 3.
Total content of non‐fibre components (amylose, protein, phytate and iron) in untreated and malted barley after steeping at different temperatures and lactic acid concentrations (calculated on dry weight basis)
| Lactic acid (% v/v) | T (°C) | Amylose | Protein | Phytate | Iron | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | SEM | g kg−1 | SEM | mmol kg−1 | SEM | mg kg−1 | SEM | |||
| Tipple | Untreated | y 120 ab | 2 | x 74 a | 1 | x 10.8 b | 0.2 | y 31.5 a | 0.3 | |
| 0 | 15 | 113 a | 2 | 79 b | 0 | 9.5 a | 0.2 | 33.2 a | 1.1 | |
| 0 | 35 | 133 b | 7 | 80 b | 0 | 9.5 a | 0.1 | 33.9 a | 0.2 | |
| 0.2 | 15 | nd | 83 b.c | 3 | 9.3 a | 0.0 | 33.7 a | 0.9 | ||
| 0.2 | 35 | nd | 86 c | 1 | 9.6 a | 0.1 | 31.3 a | 1.2 | ||
| 0.4 | 15 | 115 ab | 3 | 85 c | 0 | 9.8 a | 0.1 | 33.6 a | 1.2 | |
| 0.4 | 35 | 123 ab | 3 | 84 b.c | 1 | 9.9 a | 0.1 | 30.7 a | 1.7 | |
| Karmosé | Untreated | y 129 a | 4 | y 79 a | 0 | z 14.7 b | 0.2 | x 28.8 a | 0.3 | |
| 0 | 15 | 140 b | 2 | 80 ab | 2 | 12.6 a | 0.2 | 29.8 a | 0.0 | |
| 0 | 35 | 163 c | 0 | 81 ab | 1 | 12.7 a | 0.4 | 26.3 a | 1.5 | |
| 0.2 | 15 | nd | 85 b | 1 | 12.0 a | 0.1 | 30.0 a | 0.0 | ||
| 0.2 | 35 | nd | 84 ab | 2 | 12.2 a | 0.4 | 27.9 a | 0.9 | ||
| 0.4 | 15 | 156 c | 2 | 82 ab | 0 | 12.8 a | 0.1 | 30.1 a | 1.9 | |
| 0.4 | 35 | 158 c | 0 | 79 a | 1 | 12.9 a | 0.3 | 29.4 a | 0.4 | |
| Cinnamon | Untreated | x 13 a | 0 | y 81 a | 0 | y 13.2 c | 0.1 | z 34.4 a | 0.1 | |
| 0 | 15 | 23 b | 0 | 81 a | 1 | 10.7 a | 0.0 | 33.2 a | 0.1 | |
| 0 | 35 | 21 b | 0 | 87 b | 1 | 10.8 a | 0.0 | 35.2 a | 1.4 | |
| 0.2 | 15 | nd | 87 b | 1 | 10.7 a | 0.2 | 34.9 a | 1.3 | ||
| 0.2 | 35 | nd | 84 ab | 0 | 11.1ab | 0.3 | 33.9 a | 1.4 | ||
| 0.4 | 15 | 13 a | 1 | 81 a | 2 | 11.4 ab | 0.1 | 34.7 a | 0.3 | |
| 0.4 | 35 | 11 a | 2 | 82 a | 2 | 11.7 b | 0.0 | 38.1 a | 0.9 | |
Values are expressed as the means ± SEM. Means in the same column and for the same variety and not sharing a letter (a–c) are significantly different (P < 0.05) and means for the different varieties not sharing a letter (x–z) are significantly different (P < 0.05).
Table 4.
Significance of the effects of steeping conditions in the malted varieties (T, Tipple; K, Karmosé; C, Cinnamon) for each malt component (P < 0.05)
| Total fibre | Soluble fibre | β‐Glucan | β‐Glucan MW | AX | Soluble AX | Resistant starch | Amylose | Protein | Phytate | Iron | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | T* | T K* C | T K | ns | T | T K* | C* | T K | C | C* | C |
| Lactic acid | ns | T* K | T K C | ns | T | T K C* | T* | K C | T C* | T* C | ns |
| Interaction | ns | ns | T | ns | ns | T | ns | K | C | ns | ns |
Significant at 0.1 level. ns, not significant at 0.1 or 0.05 level.
Figure 2.
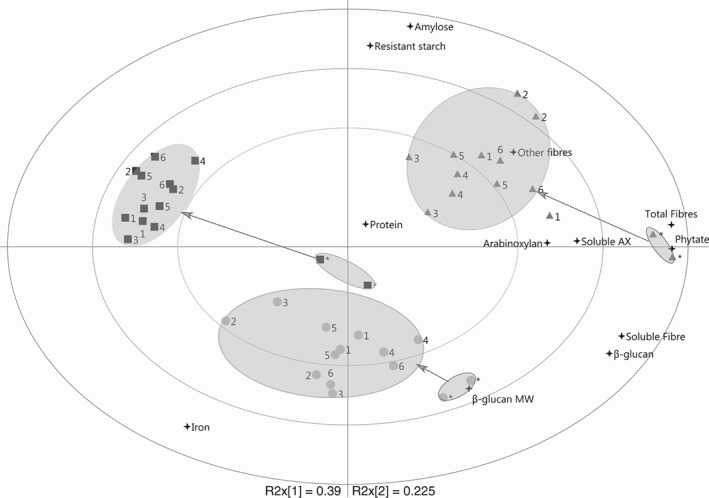
Bi‐plot of PCA based on the chemical characterization of untreated barley and barley malt. The positions for untreated (*) samples and each steeping condition (for details see Table 1) are shown and related to the chemical characteristics measured ( ).Data points belonging to the same group are within the marked areas. The data are grouped according to the barley variety (Tipple
).Data points belonging to the same group are within the marked areas. The data are grouped according to the barley variety (Tipple  , Karmosé
, Karmosé  , Cinnamon
, Cinnamon  ). The arrows show the change in chemical characteristics from untreated material to malt of each barley variety.
). The arrows show the change in chemical characteristics from untreated material to malt of each barley variety.
RESULTS
The composition of each barley variety before and after malting is shown in Table 2 (dietary fibre, β‐glucan, AX, β‐glucan MW, resistant starch) and Table 3 (amylose, protein, iron and phytate), whereas Fig. 1 illustrates the proportion of soluble fibre and soluble AX. The significance of the effects and interaction of steeping conditions for each component and barley variety are summarized in Table 4. The relationship between the components and the treatments is shown in Fig. 2. All values are presented on a dry weight basis.
Figure 1.
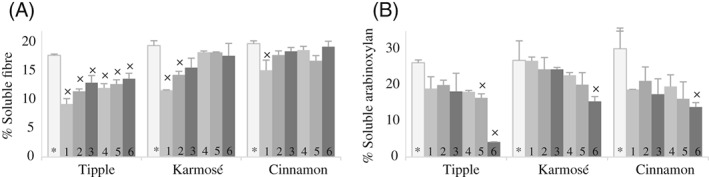
Proportions of (A) soluble fibre and (B) soluble arabinoxylan (mean and standard deviation) in untreated barley (*) and malts steeped under different conditions (for details see Table 1). Values belonging to the same variety and sharing the symbol × are significantly different relative to the values of untreated barley (*) (P < 0.05).
Untreated barley varieties
Karmosé contained the highest amount of total fibre (246 vs. 211 ± 2 g kg−1, P < 0.05), β‐glucan (52 vs. Tipple 39 g kg−1, P < 0.05), resistant starch (39 vs. 6 ± 2 g kg−1, P < 0.05) and phytate (14.7 vs. 12.0 ± 1.2 mmol kg−1, P < 0.05), while Cinnamon had the highest iron content (34.4 mg kg−1, P < 0.05) and lowest amylose content (13 g kg−1, P < 0.05) (Tables 2 and 3). Tipple had the lowest amount of soluble AX (19 g kg−1, P < 0.05), protein (74 g kg−1, P < 0.05) and phytate (10.8 mmol kg−1, P < 0.05).
Contents of β‐glucan MW (mean 1.61 ± 0.05 g mol−1), total AX (mean 83 ± 10 g kg−1), proportion of soluble fibre (mean 19 ± 1%), and proportion of AX (mean 28 ± 2%) were similar in all three untreated varieties (Fig. 1). Soluble fibre content was higher in Karmosé than in Tipple (48 vs. 37 g kg−1, P < 0.05).
Effects of malting on dietary fibre components
Total, insoluble fibre and soluble fibre
The steeping conditions did not affect the total fibre content in the malts to a great extent (Table 4). However, steeping with lactic acid at 0.2% reduced the total fibre content in Karmosé compared to untreated barley (from 246 to 217 g kg−1, P < 0.05) (Table 2). For Tipple, the total fibre content decreased after malting but independently of the different steeping conditions (from 208 to a mean of 184 ± 4 g kg−1, P < 0.05), whereas Cinnamon was unaffected by the malting process per se.
Insoluble fibre behaved similarly to the total fibre (data not shown), which was expected because total fibre content was correlated with the content of insoluble fibre (r = 0.91, P < 0.01) and comprises at least 80% of the total fibre.
Soluble fibre content decreased significantly after steeping at 15°C and without lactic acid (reduction of 20 g kg−1 for Tipple and Karmosé, and 9 g kg−1 for Cinnamon), which was also reflected in the decrease in the proportion of soluble fibre (Fig. 1). A high steeping temperature (35°C) combined with a high lactic acid concentration (0.4%) preserved the soluble fibre to a greater extent. Thus, the content of soluble fibre in malts steeped under this condition was the same as in untreated barley in Cinnamon (42 g kg−1) and only slightly lower for Karmosé (43 vs. 48 g kg−1 in untreated barley, P > 0.1), whereas it was still considerably lower for Tipple (26 vs. 37 g kg−1 in untreated barley, P < 0.05).
Arabinoxylan
The total AX content increased after malting more or less independent of steeping conditions for all varieties, but significance was achieved by Tipple (Table 2).
The content and the proportion of soluble AX were maintained or lower after malting (Table 2 and Fig. 1). In particular, Tipple malts steeped at 35°C and 0.4% lactic acid contained lower amounts and proportions than with the other malting conditions, for which an interaction effect of the steeping conditions was observed (Table 4).
β‐Glucan
Steeping at both high temperature (35°C) and lactic acid concentration (0.4%) preserved the β‐glucan, although differently depending on the variety. Tipple and Karmosé had the highest content of β‐glucan under these conditions (37 and 47 g kg−1, respectively), whereas Cinnamon also had a high β‐glucan content at 0.2% lactic acid (mean 43 g kg−1) (Table 2). The preservation of β‐glucan seems to be due to both an increase in the lactic acid concentration and the temperature for Karmosé, and with an interaction effect in Tipple, whereas the lactic acid concentration was most important for Cinnamon (Table 4).
After malting, the β‐glucan MW was slightly lower (Table 2), although without any significant effect of the steeping conditions (Table 4). An exception was Karmosé steeped at 35 °C and 0.4% lactic acid (1.46 × 106 vs. 1.67 × 106 g mol−1 for untreated barley, P < 0.05).
Resistant starch
After malting, there was a substantial increase in resistant starch (Table 2), especially in Tipple at 0.4% lactic acid concentration (40 g kg−1, P < 0.05).
Effects of malting on non‐dietary fibre components
Amylose
The amylose content was either maintained or increased after malting, but to a varying degree for each variety (Table 3). For Karmosé the increase in amylose was due to both lactic acid concentration and temperature, whereas for Tipple there was an effect only of temperature (Table 4). In Cinnamon, a significant increase in the amylose content was observed only without lactic acid.
Protein
During malting, proteins are mainly solubilized and hydrolysed into smaller peptides and amino acids through a range of proteolytic enzymes,28 but the absolute crude protein content was not expected to change significantly. There was a small relative increase in protein content for all varieties after malting (<10 g kg−1) (Table 3).
Phytate and iron
The phytate content decreased after malting (Table 3), but different steeping conditions had no significant effects, except in Cinnamon steeped with 0.4% lactic acid and 35°C (11.7 mmol kg−1, P < 0.05). Malting had no effect on iron content, but in Cinnamon the content tended to increase with temperature (Tables 3 and 4).
Multivariate data analyses
The PCA bi‐plot (Fig. 2) shows the relationship between each sample (before and after malting) and their chemical characteristics according to their proximity. The bi‐plot displays an arrangement of three groups based on the barley variety. The change in characteristics from barley to malt for each variety (depicted as arrows) shows that the malting process alters barley into a material with lower contents of total and soluble fibre, β‐glucan and phytate. The longer the arrows the greater the effects of malting; Cinnamon was less affected than Tipple and Karmosé by the malting process. Karmosé and Cinnamon malts were associated with higher contents of total and soluble fibre, β‐glucan, β‐glucan MW, total and soluble AX and phytate than Tipple. Further, Karmosé malts were different from Cinnamon malts due to their higher amylose and resistant starch content and were lower in iron.
Correlations between barley components
For all varieties, phytate, soluble fibre and β‐glucan are highly correlated (0.78 < r < 0.91, P < 0.01). This indicates that an increase in soluble fibre and β‐glucan contents would also imply an increase in phytate content.
High β‐glucan content was negatively associated with soluble AX content (−0.71 < r < −0.61, P < 0.05) for Tipple and Karmosé, whereas soluble fibre and β‐glucan contents were negatively associated with low total AX content (−0.83 < r < −0.55, P < 0.05). In Cinnamon, these correlations were not present.
Amylose and resistant starch contents were highly correlated in the malted products (r = 0.84, P < 0.01) but not in the untreated barley.
DISCUSSION
Barley malt has been shown to increase butyric acid production more than untreated barley, but not for all varieties.8, 29 This prompted us to investigate the possibility of modifying the malt composition into a product with functional characteristics that potentially favour the formation of butyric acid in the distal colon. This means a product with high content of soluble fibre, especially β‐glucan and AX. Furthermore, a reduction of phytate would be desirable for increased ion availability.
The positive correlation between the content of soluble fibre, β‐glucan and phytate was valid in all varieties, but the other components analysed seemed to be associated differently for each variety. The steeping conditions mostly affected the contents of soluble fibre, β‐glucan, soluble AX and amylose compared with the other measured components (Table 4), but to different extents depending on barley variety (Fig. 2). The different behaviour depending on the variety could be due to the intrinsic structure of the kernel, which influences the hydration and consequently the activation and production of enzymes for endosperm degradation.
The changes in fibre content after malting were mostly from soluble fibre. This was expected, since insoluble fibres found in the outer layer of the grain are probably less prone to degradation during malting.30, 31 On the other hand, there was an increase in the content of total AX, amylose, resistant starch, protein and iron after malting, most probably due to losses of dry matter. This was not measured in the present study, but leaching of soluble material into the steeping water or the formation of carbon dioxide during germination, leaving the grain with a higher proportion of those components, has been reported previously.32 It cannot be excluded that the increase in amylose content could also be due to the activity of amylase on amylopectin, forming dextrins with α‐1,4 linkages, which is the characteristic form of amylose.33 Further, other studies have found that iron and other minerals are not degraded but redistributed during malting within the kernel to rootlets and shoots. In this study, rootlets and shoots were not removed after malting, which may have contributed to a similar content to that before malting.34
A short time of germination can be used to decrease fibre degradation during malting; therefore a time of 3 days was used instead of the usual 3–7 days.10 This resulted in a limited degradation of total AX, β‐glucan MW and also phytate, since the activity of endo‐xylanase, 35 β‐glucan solubilase (increases β‐glucan availability for degradation)36 and phytase30 are higher after 3 days of germination. This can explain the small changes in the β‐glucan MW and MW distributions (data not shown), which was also reported in other studies.17, 18, 30 To properly understand the effect of the steeping conditions in the whole range of β‐glucan MW, a method with a lower MW limit of detection is required.
The changes in the contents and proportions of soluble fibre and soluble AX, and content of β‐glucan under the different steeping conditions, may be explained by endogenous enzymatic activities. The degradation products of these components might be either simple sugars or oligosaccharides. Since ethanol 80% (v/v) was used for quantification of dietary fibre, some oligosaccharides might fail to precipitate and are not taken into account in the analyses.
The soluble fibre and the β‐glucan content changed in an approximate ratio of 1:1with the different steeping conditions, indicating that the degradation of β‐glucan was largely responsible for the loss of soluble fibre, as reported elsewhere.30 For all varieties studied here, steeping at 35°C and 0.4% lactic acid resulted in a better preservation of the soluble fibre and β‐glucan content than under the other steeping conditions, most likely due to both the temperature and/or lactic acid concentrations; endo‐β‐glucanase activity is stable for long periods at 25°C (72–96 h) (www.brenda‐enzymes.info), but rapidly loses activity above 30°C.37, 38 This could explain a decrease in β‐glucanase activity at the temperature used in this study (35°C), and in agreement with previous studies in which steeping at 48°C combined with 0.8% lactic acid was found to reduce β‐glucan degradation, and suggested to be due to a decrease in β‐glucanase activity.12, 17 Moreover, β‐glucanase is sensitive to pH changes,38 and a small decrease in pH can occur in the first 2 days of germination,39 which could further explain the reduced activity for β‐glucanase in malts steeped with 0.4% lactic acid in steeping water. Similarly, the decrease in soluble AX in Tipple and Karmosé steeped at 35°C and 0.4% lactic acid might be caused by an optimal activity of xylanase at approximately 35°C,40 although the addition of lactic acid to the steeping water also influenced the soluble AX content (Table 4).
The phytate content was little influenced by the steeping temperature, as reported previously.12 In a previous study, steeping with 0.8% lactic acid was found to reduce phytate content, with the most extensive degradation after steeping at higher temperatures (48°C).17 Compared with our study, the steeping temperature (48°C) was closer to the optimal temperature for phytase activity41 than in the present study (15 and 35°C). In addition, since the germination time was longer and a higher lactic acid concentration was used, direct comparison between the studies is difficult. Furthermore, the combination of high lactic acid concentration and high temperature seemed to be more important than each factor alone.17
CONCLUSION
Steeping and germination conditions during malting can be modified to influence the composition and characteristics of barley malt to a certain extent. By adding lactic acid and/or increasing the temperature of the steeping water, malts with a higher content of soluble fibre and β‐glucan than conventionally steeped malts can be produced, increasing their potential to promote colonic health. However, for this purpose, the choice of barley variety is also crucial.
With the conditions chosen, the reduction of β‐glucan MW and phytate was not as extensive as expected, possibly due to too short a germination time. For further evaluation of the use of barley malt to improve colonic health, increased knowledge of AX MW and β‐glucan with MW below 10 kDa would also be valuable. In future experiments both the selection of raw materials and further modification of malting conditions should be considered to enhance the desired properties.
ACKNOWLEDGEMENTS
The authors thank Lantmännen SW Seed AB, Svalöv, for allowing the use of laboratory‐scale malting equipment. The study was financially supported by TvärLivs (Formas No. 222‐2011‐271 and Lantmännen Research Foundation).
REFERENCES
- 1. Roediger WEW, Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83:424–429 (1982). [PubMed] [Google Scholar]
- 2. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ and Brummer RJ, Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27:104–119 (2008). [DOI] [PubMed] [Google Scholar]
- 3. Jakobsdottir G, Nyman M and Fåk F, Designing future prebiotic fiber to target metabolic syndrome. Nutrition 30:497–502 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Knudsen KE, Jensen BB and Hansen I, Oat bran but not a beta‐glucan‐enriched oat fraction enhances butyrate production in the large intestine of pigs. J Nutr 123:1235–1247 (1993). [DOI] [PubMed] [Google Scholar]
- 5. Hughes SA, Shewry PR, Li L, Gibson GR, Sanz ML and Rastall RA, In vitro fermentation by human fecal microflora of wheat arabinoxylans. J Agric Food Chem 55:4589–4595 (2007). [DOI] [PubMed] [Google Scholar]
- 6. Nilsson U and Nyman M, Short‐chain fatty acid formation in the hindgut of rats fed oligosaccharides varying in monomeric composition, degree of polymerisation and solubility. Br J Nutr 94:705–713 (2005). [DOI] [PubMed] [Google Scholar]
- 7. Bränning CE and Nyman ME, Malt in combination with Lactobacillus rhamnosus increases concentrations of butyric acid in the distal colon and serum in rats compared with other barley products but decreases viable counts of cecal bifidobacteria. J Nutr 141:101–107 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhong Y, Teixeira C, Marungruang N, Sae‐Lim W, Tareke E, Andersson R et al, Barley malt increases hindgut and portal butyric acid, modulates gene expression of gut tight junction proteins and Toll‐like receptors in rats fed high‐fat diets, but high advanced glycation end‐products partially attenuate the effects. Food Funct 6:3165–3176 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Newman RK and Newman CW, Barley for Food and Health: Science, Technology, and Products. Wiley, Hoboken, NJ: (2008). [Google Scholar]
- 10. Briggs D, Malts and Malting. Blackie Academic, London: (1998). [Google Scholar]
- 11. Lazaridou A, Biliaderis CG, Micha‐Screttas M and Steele BR, A comparative study on structure–function relations of mixed‐linkage (1→3), (1→4) linear β‐d‐glucans. Food Hydrocolloids 18:837–855 (2004). [Google Scholar]
- 12. Rimsten L, Haraldsson AK, Andersson R, Alminger M, Sandberg AS and Aman P, Effects of malting on beta‐glucanase and phytase activity in barley grain. J Sci Food Agric 82:904–912 (2002). [Google Scholar]
- 13. Fouhse JM, Ganzle MG, Regmi PR, van Kempen TA and Zijlstra RT, High amylose starch with low in vitro digestibility stimulates hindgut fermentation and has a bifidogenic effect in weaned pigs. J Nutr 145:2464–2470 (2015). [DOI] [PubMed] [Google Scholar]
- 14. Shewry PR and Ullrich SE, Barley: Chemistry and Technology. AACC International, Eagan, MN: (2014). [Google Scholar]
- 15. Mohamed A, Hojilla‐Evangelista MP, Peterson SC and Biresaw G, Barley protein isolate: thermal, functional, rheological, and surface properties. J Am Oil Chem Soc 84:281–288 (2007). [Google Scholar]
- 16. Kaya E, Ceylan A, Kara N, Guven H and Yildiz L, The effect of l‐glutamine on mucosal healing in experimental colitis is superior to short‐chain fatty acids. Turkish J Gastroenterol 18:89–94 (2007). [PubMed] [Google Scholar]
- 17. Haraldsson AK, Rimsten L, Alminger ML, Andersson R, Andlid T, Aman P et al., Phytate content is reduced and beta‐glucanase activity suppressed in malted barley steeped with lactic acid at high temperature. J Sci Food Agric 84:653–662 (2004). [Google Scholar]
- 18. Rimsten L, Haraldsson A‐K, Andersson R, Alminger M, Sandberg A‐S and Åman P, Effects of malting on β‐glucanase and phytase activity in barley grain. J Sci Food Agric 82:904–912 (2002). [Google Scholar]
- 19. Asp NG, Johansson CG, Hallmer H and Siljestrom M, Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem 31:476–482 (1983). [DOI] [PubMed] [Google Scholar]
- 20. Theander O, Åman P, Westerlund E, Andersson R and Pettersson D, Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. J AOAC Int 78:1030–1044 (1995). [PubMed] [Google Scholar]
- 21. Rimsten L, Stenberg T, Andersson R, Andersson A and Åman P, Determination of β‐glucan molecular weight using SEC with calcofluor detection in cereal extracts. Cereal Chem J 80:485–490 (2003). [Google Scholar]
- 22. Bjorck IME and Siljestrom MA, In vivo and in vitro digestibility of starch in autoclaved pea and potato products. J Sci Food Agric 58:541–553 (1992). [Google Scholar]
- 23. Holm J, Bjorck I, Drews A and Asp NG, A rapid method for the analysis of starch. Starch–Starke 38:224–226 (1986). [Google Scholar]
- 24. FAO , Food energy: methods of analysis and conversion factors. FAO Food and Nutrition Paper; (2003). [Google Scholar]
- 25. Carlsson NG, Bergman EL, Skoglund E, Hasselblad K and Sandberg AS, Rapid analysis of inositol phosphates. J Agric Food Chem 49:1695–1701 (2001). [DOI] [PubMed] [Google Scholar]
- 26. Fredrikson M, Carlsson NG, Almgren A and Sandberg AS, Simultaneous and sensitive analysis of Cu, Ni, Zn, Co, Mn, and Fe in food and biological samples by ion chromatography. J Agric Food Chem 50:59–65 (2002). [DOI] [PubMed] [Google Scholar]
- 27. Eriksson L and Umetrics , Multi‐ and Megavariate Data Analysis: Basic Principles and Applications. MKS Umetrics, Malmö: (2013). [Google Scholar]
- 28. Baxter ED, Hordein in barley and malt: a review. J Inst Brewing 87:173–176 (1981). [Google Scholar]
- 29. Zhong Y, Nyman M and Fåk F, Modulation of gut microbiota in rats fed high‐fat diets by processing whole‐grain barley to barley malt. Mol Nutr Food Res 59:2066–2076 (2015). [DOI] [PubMed] [Google Scholar]
- 30. Hübner F, O'Neil T, Cashman KD and Arendt EK, The influence of germination conditions on beta‐glucan, dietary fibre and phytate during the germination of oats and barley. Eur Food Res Technol 231:27–35 (2010). [Google Scholar]
- 31. Nyman M, Siljestrom M, Pedersen B, Knudsen KEB, Asp N‐G, Johansson C‐G et al., Dietary fiber content and composition in six cereals at different extraction rates. Cereal Chem 61:14–19 (1984). [Google Scholar]
- 32. Briggs DE, Malts and Malting. Blackie Academic, London: (1998). [Google Scholar]
- 33. Greenwood CT and Thomson J, A comparison of the starches from barley and malted barley. J Inst Brewing 65:346–353 (1959). [Google Scholar]
- 34. Liu DJ, Pomeranz Y and Robbins GS, Mineral content of developing and malted barley. Cereal Chem 52:678–686 (1975). [Google Scholar]
- 35. Sungurtas J, Swanston JS, Davies HV and McDougall GJ, Xylan‐degrading enzymes and arabinoxylan solubilisation in barley cultivars of differing malting quality. J Cereal Sci 39:273–281 (2004). [Google Scholar]
- 36. Bamforth CW, Martin HL and Wainwright T, Role for carboxypeptidase in the solubilization of barley beta‐glucan. J Inst Brewing 85:334–338 (1979). [Google Scholar]
- 37. McCleary BV, Measurement of malt beta‐glucanase, in 19th Convention of the Institute of Brewing, Australia and New Zealand Section, pp. 181–187 (1986). [Google Scholar]
- 38. Woodward JR and Fincher GB, Substrate specificities and kinetic‐properties of 2 (1‐]3), (1‐]4)‐beta‐d‐glucan endo‐hydrolases from germinating barley (Hordeum vulgare). Carbohydr Res 106:111–122 (1982). [Google Scholar]
- 39. MacWilliam IC, pH in malting and brewing: a review. J Inst Brewing 81:65–70 (1975). [Google Scholar]
- 40. Benjavongkulchai E and Spencer MS, Purification and characterization of barley‐aleurone xylanase. Planta 169:415–419 (1986). [DOI] [PubMed] [Google Scholar]
- 41. Sung HG, Shin HT, Ha JK, Lai HL, Cheng KJ and Lee JH, Effect of germination temperature on characteristics of phytase production from barley. Bioresour Technol 96:1297–1303 (2005). [DOI] [PubMed] [Google Scholar]


