Abstract
Embryonic stem cell (ES cell)‐based rat knockout technology, although successfully developed in 2010, has seen very limited usage to date due to low targeting efficiency and a lack of optimized procedures. In this study, we performed gene targeting in ES cells from the Sprague–Dawley (SD) and the Fischer 344 (F344) rat strains using an optimized procedure and the self‐excising neomycin (neo)‐positive selection cassette ACN to successfully generate Leptin and Trp53 knockout rats that did not carry the selection gene. These results demonstrate that our simplified targeting strategy using ACN provides an efficient approach to knock out many other rat genes.
Keywords: ES cells, gene targeting, homologous recombination, rat, selection‐gene‐free
Abbreviations
CRISPR, clustered regularly interspaced short palindromic repeats
DA, Dark Agouti
ES cell, embryonic stem cell
F344, Fischer 344
H&E, hematoxylin and eosin
neo, neomycin
SD, Sprague–Dawley
tk, thymidine kinase
WT, wild‐type
ZFN, zinc‐finger nucleases
Although rats have been widely used as research animal models, until recently, genetic tools in rats have lagged behind the mouse due to lack of authentic embryonic stem cells. Since 2008, successful isolation of rat embryonic stem cells (ES cells) and induced pluripotent stem cells (iPS cells) paved the way for rat genome engineering and rat model creation 1, 2, 3, 4. New tools represented by clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) have offered an alternative to conventional knockout technology (ES cells‐based gene targeting) to quickly generate gene‐modified rats 5, 6, 7, 8. Yet, it has become clear that conventional homologous recombination‐based gene targeting technology is still the best choice to generate precise gene‐modified models.
In conventional gene targeting or CRISPR‐assisted gene targeting in ES cells, to increase gene targeting efficiency, selection genes are used 9, 10. As shown in mice, the selection genes can result in unwanted consequences such as misregulation of adjacent genes or expression attenuation of the gene of interest 11, 12, 13, 14. Accordingly, several recombinase systems including Cre‐loxP, FLP/FRT, and PhiC31 have been developed to solve these issues 15, 16, 17, 18, 19, 20. Among these, researchers combined the Cre‐loxP system with testes‐specific promoter tACE and subsequently developed the ACN self‐excision cassette 21. The tACE promoter initiates transcription of Cre during spermatogenesis, followed by automatic excision of floxed selection gene as well as Cre. Since the first application of the ACN strategy in 1999, numerous research groups have used it to generate mouse mutant models. As more and more rat models are being generated, it would be advantageous to apply ACN self‐excision cassette as a rat gene targeting strategy.
In this study, we attempted to demonstrate that genetic reagents used in conventional mouse knockout technology can be used in rats. With an optimized gene targeting procedure, we generated knockout rats for Leptin and Trp53 (p53) genes with homologous recombination‐based vectors containing ACN cassette, and proved they were free of selection genes, indicating that the ACN self‐excision cassette works well in rat gene targeting.
Materials and methods
Animals
Male Dark Agouti (DA) rats were purchased from Shanghai Laboratory Animal Research Center (Shanghai, China). Sprague–Dawley (SD) rats were purchased from Charles River Laboratories (Beijing, China). All animal experiments were approved by Laboratory Animal Care and Use Committee of China Agricultural University. All rats used in this study were backcrossed to SD genetic background.
Vector construction
The Leptin‐ and p53‐targeting vectors were constructed according to previous protocol 22. The homologous arm were amplified from SD rat genomic DNA by PCR. We introduced Gluc as a reporter to indicate the expression level of Leptin, and ACN as a self‐excision cassette to generate selection‐gene‐free heterozygous mutant rats from chimeras 21.
ES cell lines
The SD and F344 ES cells were obtained from Rat Resource and Research Center (SD‐Tg (GFP) 2BalRrrc‐ES1/Rrrc, RRRC#: 561; F344‐Tg (UBC‐EGFP) F455Rrrc‐ES4011/Rrrc, RRRC#: 654) 23, 24. All ES cells were cultured in 3i/Lif medium, supplemented with 0.5 m A83‐01 (Tocris, San Diego, CA, USA), 3 mm CHIR99021 (Selleck Chemicals, Houston, TX, USA), 0.5 mm PD0325901 (Selleck Chemicals), and 1000 units·mL−1 rat Lif (ESGRO, Millipore, Bedford, MA, USA) in N2B27 (Invitrogen, Carlsbad, CA, USA) medium. β2 mouse fibroblasts with 40 Gy Co60 g‐ray treated were used as feeders.
Electroporation and screening
The targeting vectors containing the neo/tk double selection cassette were linearized with SwaI. Approximately, 6 μg linearized targeting vectors were electroporated to 2 × 106 ES cells using Lonza Amaxa Nucleofector‐2b program B‐016. Twenty‐four hours after nucleofection, transfected ES cells were selected for resistance to G418 (400 μg·mL−1; EMD Chemicals, Inc., San Diego, CA, USA) and FIAU (0.3 μm) for 5 days. About 10 days after electroporation, drug‐resistant colonies were picked and propagated for cryopreservation and screening. To screen successful homologous recombination clones, DNA was extracted from each isolated clone and amplified using TaKaRa LA Taq® DNA Polymerase under previously reported protocol 22. Primers WS1147 and WS1149 were used for PCR screening of Leptin gene‐targeted ES cells. Primers LH‐21 and LH‐20 were used for PCR screening of p53 gene‐targeted ES cells.
Targeted cell karyotyping
Targeted cell karyotypes were analyzed according to previous reports 23, 24. The chromosome number was counted by leica cytovision software (Leica Camera AG, Wetzlar, Germany). The karyotyping analysis was performed at passage of 6–10.
Generation of chimeras and breeding
Chimeras were generated by blastocyst injections as previously reported 23, 24. Heterozygous clones with correct chromosome number were injected into DA × SD hybrid blastocysts. Chimeric animals were identified from offspring by coat color chimerism. Male chimeric animals derived from successfully targeted cell lines were bred to SD mates to produce gene‐targeting animals.
Genotyping and Southern blot analysis
DNA was extracted from tail biopsies of chimeric males and their progenies. Fragments were amplified by using GoTaq® Flexi DNA Polymerase (Promega, Madison, AL, USA). Approximately, 200 ng genomic DNA was used for PCR amplification for 32 cycles in a 12.5‐μL reaction mixture. PCR products with different size were used to distinguish wild‐type, ACN removal, and ACN‐containing mutant rats. Genomic DNA (10 μg) was digested with appropriate enzymes overnight for Southern blot analysis. Probes were prepared by PCR amplification using PCR DIG Probe Synthesis Kit (Roche, Basel, Switzerland). Southern blotting was performed as described previously.
Western blot analysis
The total tissue proteins were extracted from white adipose tissue of Leptin mutant rats and their wild‐type littermates with RIPA lysis buffer (Beyotime, Shanghai, China; P0013B). One hundred micrograms of protein was denatured at 95–100 °C for 10 min in sample buffer, then separated by 10% SDS/PAGE and transferred onto polyvinylidene fluoride membrane (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The anti‐Leptin primary antibody (Abcam, Cambridge, MA, USA; ab3583) was used at 1 : 4000 dilution and anti‐tubulin primary antibody (Beyotime; AT819) was used at 1 : 10 000. The anti‐rabbit secondary antibody (Beyotime; A0208) was used at the dilution of 1 : 10 000. SuperSignal West‐Dura Extended Duration Substrate kit (Thermo Fisher Scientific, Waltham, MA, USA; #34075) was used to develop the signal.
Body weight and random blood glucose
Leptin mutant rats and their control littermates were weighed every month from 1 to 8 months old. Blood was collected by tail vein puncture and blood glucose was analyzed by Roche Accu‐CHEK Performa glucometer (Roche). Random blood glucose was measured at 9:00 a.m.
Histological analysis
Rats were euthanized, and the liver, pancreas, and abdominal adipose tissue were fixed in 4% formaldehyde and mounted in paraffin blocks. The sections were stained with hematoxylin and eosin (H&E).
Results
Generation of Leptin knockout rats using vectors containing ACN cassette
The introduction of the ACN self‐excision cassette into the gene targeting strategy significantly promotes the generation of selection‐gene‐free mouse models 21, 22, 25, 26. To test whether the same approach works in rats, we designed a Leptin targeting strategy by knocking IRES‐Gluc and ACN self‐excision cassette into the second exon (Fig. 1A). We next electrotransfected linearized targeting vectors into SD rat ES cells (male). Previously published reports have suggested that rat ES cells are sensitive to drug selection, and thymidine kinase (tk) could not be used as negative selection gene for rat gene targeting 27, 28. However, we performed both G418‐positive and 1‐(2‐deoxy‐2‐fluoro‐beta‐d‐arabinofuranosyl)‐5‐iodouracil (FIAU)‐negative drug selection against neomycin (neo) and tk at similar concentration as used for mouse ES cells for five successive days to enrich Leptin gene‐targeted rat ES cells. The colonies passed through the drug selection were picked and expanded for further analysis. To identify gene‐targeted clones, we used long‐range PCR to amplify regions flanking the Leptin‐modified region, and 3 of 18 (17%) colonies were confirmed to have undergone the desired gene targeting events (Fig. 1B).
Figure 1.
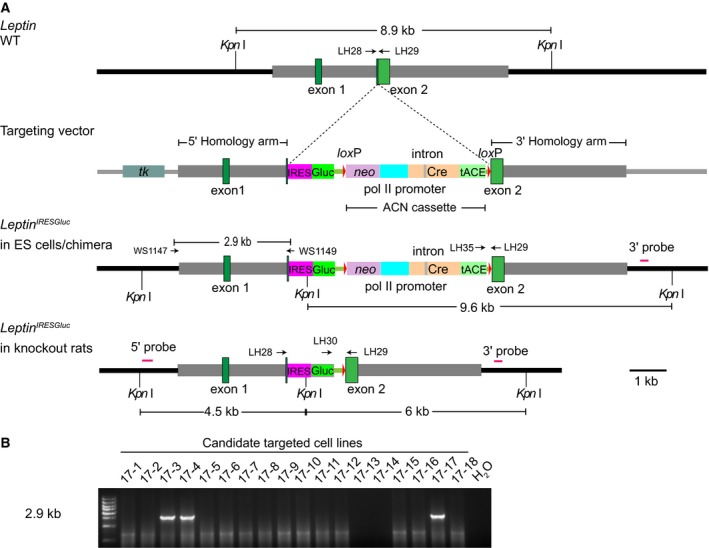
Gene targeting strategy for Leptin via homologous recombination. (A) Genomic structure of wild‐type Leptin allele (top), the Leptin targeting vector (the second line), targeted allele in rat ES cells (the third line), and targeted allele in mutant rats (bottom). Different colors represented different elements. The 5′ and 3′ probes used for Southern blot analysis were shown as purple lines. KpnI was used to digest genomic DNA from Leptin mutant rats and their littermates. The drug‐resistant genes neo and HSVtk were used for positive/negative selection in rat ES cells. The cassette containing tACE, Cre, polymerase II promoter, and neo flanked with loxP sites was named as ACN 21. Primers WS1147 and WS1149 are used to identify gene targeting event. Primers LH‐28, LH‐29, and LH‐30 are used to identify germline transmission. Primers LH‐29 and LH‐35 are used to test if ACN is removed. (B) PCR screening strategy of gene‐targeted ES cell clones. We amplified DNA of 12 isolated colonies and obtained three successfully targeted clones (No. 17‐3 17‐4 and 17‐17) with a product size of 2.9 kb. PCR with H2O template was used as a blank control. IRES, internal ribosome entry site; Gluc, Gaussia luciferase; tACE, the murine angiotensin‐converting enzyme; tk, herpes simplex virus thymidine kinase gene.
Next, Leptin gene‐targeted ES cells with correct karyotypes were injected into F1 blastocysts from Dark Agouti male crossed to SD female for generation of chimeras. Four males and three females were chimeric (identified by coat color) in a total of 22 pups obtained (Table 1). The male chimeras were mated to wild‐type SD female rats for offspring production, and 2 of 240 pups were genotyped as heterozygous, indicating the successful generation of Leptin knockout rats.
Table 1.
Generation of chimeras via blastocyst injection with Leptin and p53 homologous recombination cell lines and germline transmission
| Donor blastocyst | Recipient | Transferred embryos | Total pups | Coat color chimera | Offspring | No. of germline transmission | |
|---|---|---|---|---|---|---|---|
| Leptin | DA × SD | SD | 193 | 22 | 4M; 3F | 240 | 2 |
| p53 | DA × SD | SD | 169 | 42 | 6M; 5F | 210 | 2 |
M, male; F, female.
Identification of self‐excision of ACN cassette in mutant offspring rats
To confirm the removal of the ACN cassette in mutant offspring rats, we designed a PCR strategy that produced a specific amplicon for wild‐type, gene‐targeted, or ACN‐removed alleles (Fig. 2A). The two Leptin heterozygous animals showed a 529 bp specific band indicating successful removal of ACN cassette (Fig. 2B). We further bred the heterozygous animals to produce homozygous Leptin knockout mutants. Southern blot analysis of genomic DNA extracted from offspring tails showed that all the mutants had the neo cassette removed (Fig. 2C).
Figure 2.
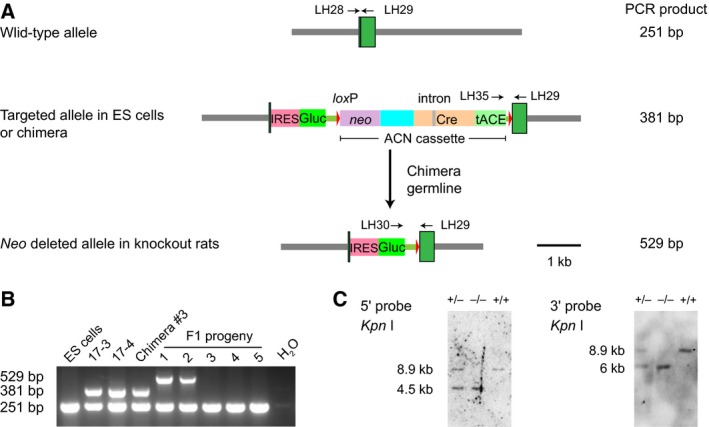
Removal of ACN cassette in Leptin knockout rats. (A) Structure of the wild‐type Leptin allele (top), the targeted allele in ES cells and chimera (middle), and the targeted allele in mutant rats (bottom). (B) PCR verification of ACN removal. DNA was extracted from wild‐type ES cells (ES), recombinant ES cell lines (17‐3 and 17‐4), tail biopsies of male chimera #3, and F1 progenies obtained from chimera #3 germline transmission. Product size: wild‐type, 251 bp; ACN‐removed allele: 529 bp; ACN‐containing allele: 381 bp. PCR with H2O template was used as a blank control. (C) Southern blot analysis of Leptin mutant rats using 5′ and 3′ probes. DNA extracted from a cross of heterozygous rats was digested with KpnI. The wild‐type band is 8.9 kb and Leptin mutant bands with 5′ and 3′ probes are 6 and 4.5 kb, respectively.
Taken together, the strategy of automatic removal of a selection gene using the ACN cassette worked efficiently in rats.
Characterization of Leptin mutant rats
Leptin is a hormone secreted by adipocyte that regulates energy metabolism. To examine Leptin function in rats, we analyzed the phenotype of our Leptin mutant rats, and found these Leptin −/− mutant rats were overtly obese (Fig. 3A). The western blot analysis of total protein also confirmed the loss of Leptin in white adipose tissue of Leptin mutant rats (Fig. 3B). Body weight of Leptin −/− rats and their littermates was measured beginning at 1 month of age. In comparison with control littermates, both male and female Leptin −/− rats showed significantly heavier body mass over time (Fig. 3C).
Figure 3.
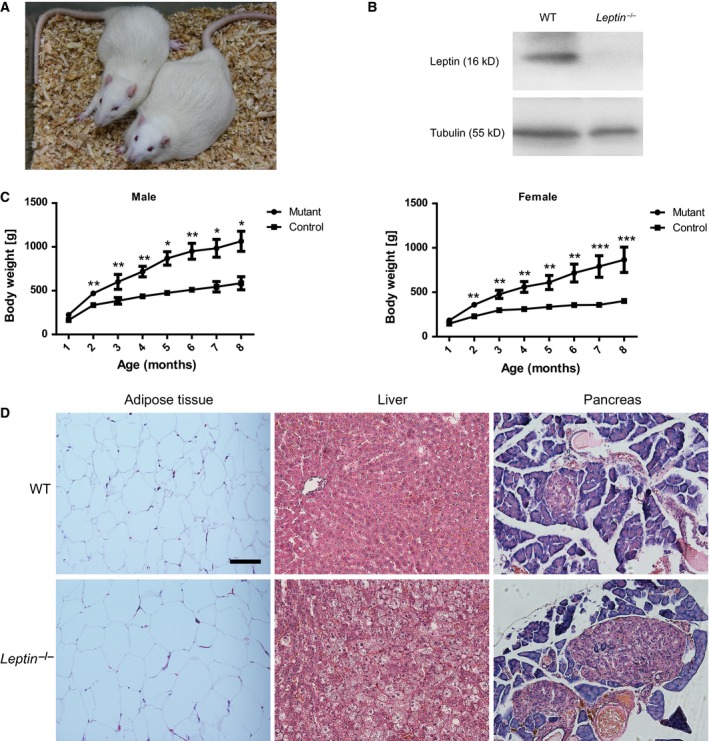
Leptin mutant rats are obese. (A) A representative Leptin mutant rat is overtly obese compared to its wild‐type (WT) littermate at 8 months old. (B) Western blot analysis for Leptin protein expression of white adipose tissues in WT and Leptin mutant rats. No Leptin protein was detected in mutant rats. Tubulin was used as a loading control. (C) Body weight was measured over 8 months for male control littermates (n = 3), male Leptin mutant rats (n = 2), female control littermate (n = 4), and female Leptin mutant rats (n = 4). Both male and female Leptin −/− rats demonstrated significantly heavier body mass than controls. (D) Hematoxylin and eosin (H&E) staining of the liver, pancreas, and adipose tissue revealed pathological changes in Leptin mutant rats. Scale bar = 200 μm. Data were represented as mean ± SEM, Student's t‐tests were performed using graphpad prism software (GraphPad Software, Inc. La Jolla, CA, USA). *P < 0.05, **P < 0.01, ***P < 0.001 versus control littermate rats.
We next investigated pathological changes of pancreas, liver, adipose tissue, and kidney. In comparison with their control littermates, the Leptin −/− rats showed obviously larger adipocytes and severe hepatic steatosis, and the pancreatic islets of the Leptin −/− rats displayed severe hypertrophy and vacuolation (Fig. 3D).
In conclusion, homozygous Leptin gene knockout rats exhibited a significant obesity phenotype, consistent with previous reports for mutant Leptin and Leptin receptor rats generated by zinc‐finger nucleases (ZFN) and CRISPR/Cas9 technologies 29, 30, 31.
Successful application of ACN self‐excision cassette in p53 gene‐targeting rats
To verify the ACN cassette also works in other gene loci, we designed the p53 gene‐targeting vector using a similar strategy to Leptin (Fig. 4A). The linearized p53 gene‐targeting vectors were introduced into F344 ES cells using nucleofection. After G418/FIAU drug selection, we identified 5 of 20 colonies with correct gene targeting events by long‐range PCR (Fig. 4B). Positive gene‐targeted ES cells with normal karyotypes were then used for chimeric animal generation. We obtained 42 pups in total and 11 (6 male and 5 female) were identified as chimeric animals by coat color (Table 1). All six male chimeras were bred with wild‐type SD females, and two were capable of germline transmission based on PCR analysis of their offspring.
Figure 4.
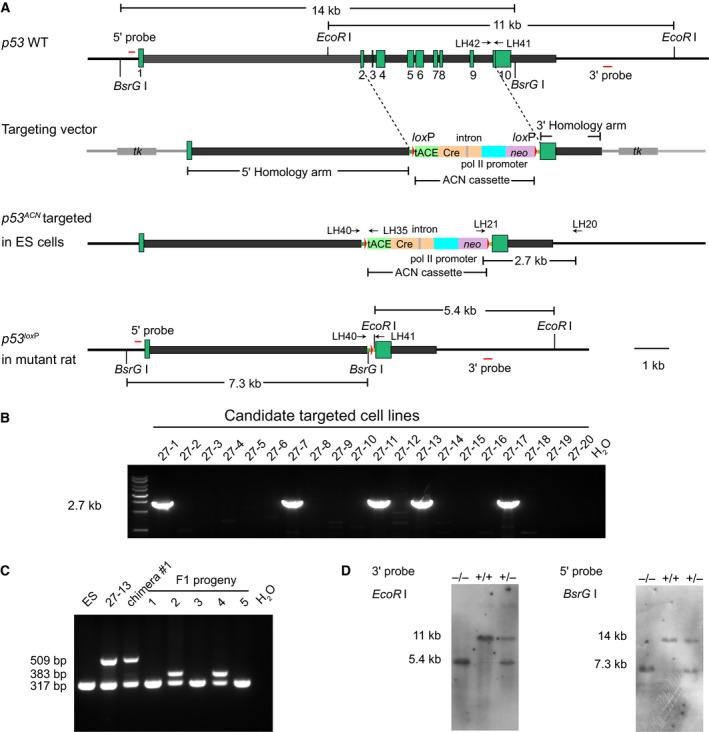
Gene targeting strategy for p53. (A) Genomic structure of the wild‐type p53 allele, p53 targeting vector, targeted allele in ES cells, and targeted allele in mutant rats. The 5′ and 3′ probes used for Southern blot analysis were shown as red lines, and the restriction enzyme BsrGI and EcoRI were used for digestion. Neo and HSVtk were used for positive/negative selection in rat ES cells. Primers LH‐20 and LH‐21 are used for screening positive targeted ES clones. Primers LH‐40, LH‐41, and LH‐42 are used to identify germline transmission. Primers LH‐40 and LH‐35 are used to test if ACN cassette is removed. (B) PCR analysis of the positive targeted ES cell clones. The positive targeted ES cell clones were 27‐1, 27‐7, 27‐11, 27‐13, and 27‐17 with the product size of 2.7 kb. PCR with H2O template was used as a blank control. (C) PCR analysis of ACN removal. DNA was extracted from wild‐type ES cells (ES), recombinant ES cell line (27‐13), tail biopsies of male chimera #1, and F1 progenies produced from chimera #1. Product size: wild‐type, 317 bp; ACN‐removed allele: 383 bp; ACN‐containing allele: 509 bp. (D) Southern blot analysis of p53 mutant rats using 5′ and 3′ probes. DNA extracted from offspring tails obtained by crossing heterozygous rats was digested with BsrGI and EcoRI. The wild‐type band is 14 kb and p53 mutant band is 7.3 kb with 5′ probe. The wild‐type band is 11 kb and p53 mutant band is 5.4 kb with 3′ probe.
To confirm the removal of selection gene in F1 heterozygous progeny, we designed a PCR strategy to distinguish wild‐type, gene‐targeted, or ACN‐removed alleles. The two p53 heterozygous animals showed a 383 bp band specific for the ACN‐removed allele demonstrating successful removal of ACN cassette (Fig. 4C). We also used Southern blot to analyze DNA extracted from offspring tails obtained by crossing heterozygous rats, and the result showed that the neo cassette was removed in all mutant alleles (Fig. 4D).
In summary, we generated p53 gene knockout rats without selection genes which suggested the ACN self‐excision cassette has broad application in rat gene targeting.
Discussion
Although germline competent rat ES cells were derived since 2008, few homologous recombination‐mediated gene knockout/knockin rats have been reported, and none of them mentioned the elimination of selection genes 1, 2, 27, 32, 33, 34. Rats are more superior animal models than mice in many areas of biomedical research, but applying genetic modification technologies in the rat is still in its infancy. Therefore, successfully extending mouse‐derived sophisticated gene‐targeting strategies to rats has great significance. Here, we have demonstrated the feasibility of ACN self‐excision in rat gene targeting strategy, and successfully generated Leptin and p53 gene knockout rats containing no selection genes. In addition, the Cre expression in the ACN in rat was very accurate and specific only during the spermatogenesis, and the efficiency of excision in vivo can reach 100%. Our study will greatly increase the possibility of successful application in other species.
In our study, the frequency of both Leptin and p53 gene targeting via homologous recombination was ~ 20% with SD and F344 ES cells, in comparison with previously reported typical efficiencies of 0.6–4.4% 27, 34. Although germline‐competent rat ES cells have been derived from various strains 1, 2, 23, 35, the knockout rats generated via homologous recombination have only been achieved in DA rats 27, 32, 34. It has also been reported that the gene targeting event resulted in the failure of germline transmission in SD rat ES cells 32. Previous reports attributed their low gene targeting frequency to ES cells sensitivity to drug screening 27. However, we were able to use the more strict double selection (neo and tk) typically used in mouse ES cells, thus excluding most nontargeted ES cells and increasing our overall targeting efficiency. Furthermore, for unknown reasons, cultured rat ES cells often have unstable karyotype and tedious subcloning was used to find clones with normal karyotypes. However, in our study, we were able to obtain gene‐targeted SD and F344 ES cells without tedious subcloning. In our procedure, the addition of A‐83‐01 (the inhibitor of TGF‐β type I receptor ALK5 kinase) in the culture medium could probably have helped maintain rat ES cells relatively free of karyotypes abnormality 3, 35.
Compared to previously published rat knockout work in ES cells 27, 32, 34, our gene targeting procedure is optimized in multiple steps, including β2 mouse feeders, positive neomycin (neo) and negative FIAU (tk) selections, 3i culture medium, DA/SD hybrid recipient blastocysts, and so on. These resulted in higher targeting efficiency and germline transmission efficiency than other procedures. We believe that CRISPR and other gene editing tools can be perfectly combined with homologous recombination‐based gene targeting technology to further improve targeting efficiency.
In conclusion, ACN self‐excision cassette can be successfully used to obtain gene knockout rats and we speculate that the mature gene editing technology established in mouse may be applied to the rat as well as other species, including human.
Author contributions
SW conceived the project. HL, SL, ZG, HM, and YW performed the experiments and analyzed the data. HL, SL, and SW wrote the manuscript with help from all authors. NL, EB, MC, and SW supervised the project. HL and SL contributed equally.
Supporting information
Table S1. Primer list.
Acknowledgements
We thank Susan Tamowski, Sheila Barnett, Carol Lenz, Joan Shuhua for help in cell culture and embryo manipulation. We thank Tan Tan for providing the Leptin primary antibody. We are grateful for continuous support from the Wu lab members, especially Xiaolan Qi. This work was supported by the National Natural Science Foundation of China 31271598 and the Project for Extramural Scientists of State Key Laboratory of Agrobiotechnology 2015SKLAB6‐15. The Rat Resource and Research Center is supported by funding from the NIH (P40 OD011062).
Edited by Wilfried Ellmeier
References
- 1. Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL and Smith A (2008) Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298. [DOI] [PubMed] [Google Scholar]
- 2. Li P, Tong C, Mehrian‐Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, et al (2008) Germline competent embryonic stem cells derived from rat blastocysts. Cell 135, 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H and Ding S (2009) Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4, 16–19. [DOI] [PubMed] [Google Scholar]
- 4. Liao J, Cui C, Chen S, Ren J, Chen J, Gao Y, Li H, Jia N, Cheng L, Xiao H, et al (2009) Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell 4, 11–15. [DOI] [PubMed] [Google Scholar]
- 5. Ma Y, Ma J, Zhang X, Chen W, Yu L, Lu Y, Bai L, Shen B, Huang X and Zhang L (2014) Generation of eGFP and Cre knockin rats by CRISPR/Cas9. FEBS J 281, 3779–3790. [DOI] [PubMed] [Google Scholar]
- 6. Ma Y, Zhang X, Shen B, Lu Y, Chen W, Ma J, Bai L, Huang X and Zhang L (2014) Generating rats with conditional alleles using CRISPR/Cas9. Cell Res 24, 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan Y, Shao Y, Li D and Liu M (2014) Generation of site‐specific mutations in the rat genome via CRISPR/Cas9. Methods Enzymol 546, 297–317. [DOI] [PubMed] [Google Scholar]
- 8. Shao Y, Guan Y, Wang L, Qiu Z, Liu M, Chen Y, Wu L, Li Y, Ma X, Liu M, et al (2014) CRISPR/Cas‐mediated genome editing in the rat via direct injection of one‐cell embryos. Nat Protoc 9, 2493–2512. [DOI] [PubMed] [Google Scholar]
- 9. Thomas KR and Capecchi MR (1987) Site‐directed mutagenesis by gene targeting in mouse embryo‐derived stem cells. Cell 51, 503–512. [DOI] [PubMed] [Google Scholar]
- 10. Doetschman T, Gregg RG, Maeda N, Hooper ML, Melton DW, Thompson S and Smithies O (1987) Targeted correction of a mutant HPRT gene in mouse embryonic stem‐cells. Nature 330, 576–578. [DOI] [PubMed] [Google Scholar]
- 11. Pham CT, MacIvor DM, Hug BA, Heusel JW and Ley TJ (1996) Long‐range disruption of gene expression by a selectable marker cassette. Proc Natl Acad Sci USA 93, 13090–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olson EN, Arnold HH, Rigby PW and Wold BJ (1996) Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell 85, 1–4. [DOI] [PubMed] [Google Scholar]
- 13. Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang CW, Cheng SH, Macvinish LJ, Anderson JR, Cuthbert AW and Evans MJ (1995) Generation and characterization of a delta‐F508 cystic‐fibrosis mouse model. Nat Genet 10, 445–452. [DOI] [PubMed] [Google Scholar]
- 14. Meyers EN, Lewandoski M and Martin GR (1998) An Fgf8 mutant allelic series generated by Cre‐ and Flp‐mediated recombination. Nat Genet 18, 136–141. [DOI] [PubMed] [Google Scholar]
- 15. Dale EC and Ow DW (1991) Gene transfer with subsequent removal of the selection gene from the host genome. Proc Natl Acad Sci USA 88, 10558–10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broach JR and Hicks JB (1980) Replication and recombination functions associated with the yeast plasmid, 2‐mu circle. Cell 21, 501–508. [DOI] [PubMed] [Google Scholar]
- 17. Sternberg N and Hamilton D (1981) Bacteriophage P1 site‐specific recombination. I. Recombination between loxP sites. J Mol Biol 150, 467–486. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF and Dymecki SM (2000) High‐efficiency deleter mice show that FLPe is an alternative to Cre‐loxP. Nat Genet 25, 139–140. [DOI] [PubMed] [Google Scholar]
- 19. Thorpe HM and Smith MC (1998) In vitro site‐specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc Natl Acad Sci USA 95, 5505–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sangiorgi E, Shuhua Z and Capecchi MR (2008) In vivo evaluation of PhiC31 recombinase activity using a self‐excision cassette. Nucleic Acids Res 36, e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bunting M, Bernstein KE, Greer JM, Capecchi MR and Thomas KR (1999) Targeting genes for self‐excision in the germ line. Genes Dev 13, 1524–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu S, Ying G, Wu Q and Capecchi MR (2008) A protocol for constructing gene targeting vectors: generating knockout mice for the cadherin family and beyond. Nat Protoc 3, 1056–1076. [DOI] [PubMed] [Google Scholar]
- 23. Men H and Bryda EC (2013) Derivation of a germline competent transgenic Fischer 344 embryonic stem cell line. PLoS One 8, e56518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Men H, Bauer BA and Bryda EC (2012) Germline transmission of a novel rat embryonic stem cell line derived from transgenic rats. Stem Cells Dev 21, 2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen M, Wolfe A, Wang X, Chang C, Yeh S and Radovick S (2009) Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol Cell Biochem 321, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu Y, Wang G, Scott SA and Capecchi MR (2008) Hoxc10 and Hoxd10 regulate mouse columnar, divisional and motor pool identity of lumbar motoneurons. Development 135, 171–182. [DOI] [PubMed] [Google Scholar]
- 27. Tong C, Li P, Wu NL, Yan Y and Ying QL (2010) Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 467, 211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tong C, Huang G, Ashton C, Li P and Ying QL (2011) Generating gene knockout rats by homologous recombination in embryonic stem cells. Nat Protoc 6, 827–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bao D, Ma Y, Zhang X, Guan F, Chen W, Gao K, Qin C and Zhang L (2015) Preliminary characterization of a leptin receptor knockout rat created by CRISPR/Cas9 system. Sci Rep 5, 15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vaira S, Yang C, McCoy A, Keys K, Xue S, Weinstein EJ, Novack DV and Cui X (2012) Creation and preliminary characterization of a leptin knockout rat. Endocrinology 153, 5622–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. D'Souza AM, Asadi A, Johnson JD, Covey SD and Kieffer TJ (2014) Leptin deficiency in rats results in hyperinsulinemia and impaired glucose homeostasis. Endocrinology 155, 1268–1279. [DOI] [PubMed] [Google Scholar]
- 32. Meek S, Buehr M, Sutherland L, Thomson A, Mullins JJ, Smith AJ and Burdon T (2010) Efficient gene targeting by homologous recombination in rat embryonic stem cells. PLoS One 5, e14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobayashi T, Kato‐Itoh M, Yamaguchi T, Tamura C, Sanbo M, Hirabayashi M and Nakauchi H (2012) Identification of rat Rosa26 locus enables generation of knock‐in rat lines ubiquitously expressing tdTomato. Stem Cells Dev 21, 2981–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto S, Nakata M, Sasada R, Ooshima Y, Yano T, Shinozawa T, Tsukimi Y, Takeyama M, Matsumoto Y and Hashimoto T (2012) Derivation of rat embryonic stem cells and generation of protease‐activated receptor‐2 knockout rats. Transgenic Res 21, 743–755. [DOI] [PubMed] [Google Scholar]
- 35. Kawamata M and Ochiya T (2010) Generation of genetically modified rats from embryonic stem cells. Proc Natl Acad Sci USA 107, 14223–14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer list.


